Abstract
Macrophytes are important components of aquatic ecosystems performing essential ecological functions. Their species composition and density reflect the ecological status of water bodies. The optimal ratio of morphological types of macrophytes is an important condition for preventing eutrophication. The aim of the study is to analyse the species composition, distribution, and density of macrophytes in the Vyzhyvka River (Ukraine) in a seasonal aspect (2023–2024) under constant physical and chemical characteristics of water. To assess the seasonal dynamics of water quality, changes in indicators in three representative areas were analysed. The MIR method of environmental indexation of watercourses was used to assess the ecological state of the river. The water quality in the Vyzhyvka River at all test sites corresponds to the second class of the “good” category with the trophic status of “mesotrophic”. This is confirmed by the identified species diversity, which includes 64 species of higher aquatic and riparian plants. Among the various morphological types of macrophytes, submerged rooted forms account for only 10.56% of the total species composition. To ensure a functional balance between submerged and other forms of macrophytes, a scientifically based approach is proposed, which involves the use of mineral raw materials of local origin, in particular, mining and quarrying wastes rich in silicon, calcium and other mineral components. The results obtained are of practical value for water management, environmental protection, and ecological reclamation and can be used to develop effective measures to restore river ecosystems.
1. Introduction
The increasing pressure on the planet’s freshwater resources due to intensified water use, rising pollution levels, and the effects of climate change has led to a widespread awareness of the key role of freshwater for sustainable development and the urgent need to improve water management mechanisms. At the same time, river ecosystems are among the most vulnerable to anthropogenic changes, as they accumulate significant amounts of pollutants of industrial, domestic, and diffuse origin [1]. In Ukraine, the state of freshwater ecosystems is further deteriorating under the influence of the effects of military operations. Studies have shown that hydromorphological disturbances in the Irpin River basin caused by military events could have led to an increase in the environmental burden on aquatic ecosystems, not only locally but also throughout the country [2].
Excessive intake of pollutants and nutrients into water bodies causes a decrease in the concentration of dissolved oxygen due to the intensification of the development of microbial communities and aquatic vegetation, which is a typical sign of eutrophication processes. This leads to massive growth of algae, plankton blooms, degradation of fish and other aquatic organisms, as well as accumulation of suspended particles and increased water turbidity. In addition to environmental impacts, such changes significantly reduce the recreational and aesthetic value of water bodies, worsening conditions for recreation and reducing the attractiveness of coastal areas and the economic efficiency of ecotourism development. The degradation of the aquatic environment also contributes to the development of pathogenic microorganisms, which increases the risk of spreading infectious diseases associated with water use [3].
Although traditional methods of contaminant removal, such as membrane bioreactors, activated sludge plants, rotating biological contact devices, and other biological treatment mechanisms are used in water treatment, most of them are costly and inefficient in terms of environmental sustainability [4]. In this regard, there is a growing interest in nature-based approaches to water environment purification. One of the most promising areas is the introduction of phytoremediation technologies based on the use of higher aquatic vegetation. This approach is considered to be an environmentally safe and cost-effective bioengineering tool for reducing the concentration of pollutants in the aquatic environment through biofiltration processes [5]. Due to their developed root system and large surface areas, macrophytes are particularly effective in aquatic phytoremediation.
Macrophytes are higher aquatic plants that grow in the water column or on the bottom of water bodies and are clearly visible to the naked eye. Based on morphological and functional characteristics, they are divided into four groups: emergent, floating-leaved, submerged, and free-floating forms [6]. Emergent macrophytes have roots anchored in bottom sediments and form above-water structures, which helps to stabilise the substrate [7]. Floating-leaved species absorb nutrients mainly through their root system, while photosynthesis is carried out by the leaves on the surface. Submerged macrophytes are completely submerged in water, sensitive to light and dissolved oxygen concentration, and absorb nutrients through both their roots and vegetative organs. Free-floating species are not anchored in the substrate and move freely across the surface of the water body [8].
Macrophytes are key components of freshwater ecosystems, actively participating in the processes of energy transformation, nutrient cycling, and sedimentation [9]. They provide a wide range of ecosystem services, including oxygen production, support of trophic networks, secretion of biologically active compounds, and creation of habitats for aquatic organisms. They also play an important role in regulating the penetration of light into the water column, which affects photosynthetic processes and phytoplankton productivity, as well as in stabilising the temperature regime and ensuring the transparency of the aquatic environment [10].
In addition to their ecological functions, macrophytes are of significant importance to society due to their recreational and aesthetic properties. Of particular value is their ability to naturally purify water—they participate in the filtration of pollutants, help reduce eutrophication, and maintain the ability of water bodies to clean themselves [11,12]. The absorption of a wide range of organic and inorganic pollutants from the aquatic environment by macrophytes has been confirmed by numerous studies [13,14,15]. Scientific data also indicate the prospects of using macrophytes in wastewater treatment systems to improve the overall treatment efficiency and reduce the risk of environmental contamination by antibiotic residues [16]. Other authors [17] have achieved over 97% removal of chemical oxygen demand (COD), ammonia, and phosphorus from wastewater.
Macrophytic vegetation helps to reduce water pollution through several physical, chemical, and biological processes. In particular, one of the key mechanisms is the absorption and accumulation of pollutants through the root system, followed by transport and retention in aboveground organs such as leaves and stems, which is in line with the principles of phytoremediation technologies [18]. Another way is rhizofiltration, when the roots of some species, such as Arundo donax, secrete compounds that can chemically fix heavy metals or stimulate the development of microflora involved in their immobilisation [19]. An important role is played by phytodegradation, in which microorganisms associated with the root zone (for example, in Scirpus grossus and Phragmites australis) break down organic matter through microbial transformation [20]. In addition, macrophytes contribute to the deposition of particles in water by reducing hydrodynamic activity, creating a natural barrier that facilitates the sedimentation of suspended particles that may contain pollutants [21]. The effectiveness of these processes is largely determined by such adaptive properties of macrophytes as intensive biomass formation [22], developed root morphology with a large absorption area [23], and a high level of resistance to toxicants [24].
In recent years, various regions of the world have been researching the biological monitoring of river ecosystems using macrophytes as bioindicators. Several water quality assessment systems based on macrophytes have been developed in Europe: the biological macrophyte index in France [25]; trophic and reference indices in Germany [26]; and the average trophic rank in Great Britain [27], which has been adapted in other countries. These methods are limited because they are based on the dependence of macrophytes on nutrients (phosphorus, nitrogen), while the influence of other factors (hydrology, substrate) is difficult to separate, which reduces the accuracy of assessments and requires improvement of methodologies.
Despite certain limitations of existing methods for assessing water quality using macrophytes, one of the most widely used and proven methods is the one developed in Poland in 2007. This approach, based on the calculation of the Macrophyte Index for Rivers (MIR), provides a comprehensive and reliable assessment of the ecological status of aquatic ecosystems based on the analysis of the quantitative and qualitative composition of freshwater plants. In the course of its application, the method has been improved based on the results of numerous studies and intercalibration within the framework of the EU Water Framework Directive, which has increased its accuracy and adaptability to various environmental conditions [28].
This method has also found practical application in Ukraine. In particular, the macrophyte approach was used to determine the species composition of aquatic and coastal plants and assess the ecological status of surface waters in the upper reaches of the Prypiat River (Turia and Tsyr Rivers) [29], the Luha River within the city of Volodymyr (Volyn Oblast) [30], and the Psyol River, a left tributary of the Dnipro River (Black Sea Basin) [31].
Most studies focus on individual populations of aquatic plants, while the role of macrophyte communities in improving water quality has not been sufficiently studied [32]. Monocultures increase the risk of secondary pollution due to the decomposition of biomass with the release of nutrients. Stable and diverse macrophyte communities ensure the continuous removal of pollutants and maintain ecological balance. For example, the author [27] showed that a combined system of Phragmites karka and Eichhornia crassipes reduced COD by 95%, while individual cultures reduced it by only 39% and 92%, respectively. The optimal ratio of macrophyte morphological types is key to minimising eutrophication.
The presence and distribution of macrophytes in aquatic ecosystems are determined by the interaction of physical, chemical, biological, and morphological factors. Physical factors, in particular water light penetration, affect photosynthesis and plant dominance [33]. Increased eutrophication, in particular excess phosphorus and nitrogen, reduces transparency and displaces submerged macrophytes, promoting the dominance of phytoplankton or free-floating forms [34]. Phytoplankton dominates in large water bodies, free-floating macrophytes in small ones, and submerged plants maintain ecological stability under low eutrophication. The chemical composition of water and bottom sediments regulates the availability of nutrients, biological factors influence species composition, and the morphology of the environment (substrate type, hydromorphology) determines the conditions for plant rooting [35].
Rooted macrophytes (emergent and submerged) have the highest productivity due to their ability to absorb resources from water and the atmosphere [36] and are key to maintaining water quality and the functioning of river ecosystems [37]. They take root in oxygen-deficient sediments and, thanks to their physiological characteristics, supply oxygen to microorganisms in the rhizoplane and rhizosphere, creating local aerobic zones [38]. Strong fibrous stems, such as those found in Zizania latifolia and Phragmites australis, transport oxygen and metabolites to the rhizosphere, supporting plants and forming oxygen micro-niches [39]. In these zones, close interactions between plants and microbiota occur, which increase the availability of nutrients and the resistance of plants to pathogens [40]. Maintaining the density of root macrophytes is critical for the stability of river ecosystems.
Optimal conditions for the development of root macrophytes are largely dependent on the pH of the water environment. Van Onsem and Triest [41] found that pH is one of the few abiotic factors that significantly affect the species composition and abundance of macrophytes (in contrast to total phosphorus, dissolved phosphorus, nitrogen concentration, and water depth, which had less of an impact).
The physical and chemical properties of water and bottom substrate significantly affect the viability of root macrophytes; therefore, various methods of improving the environment are used to restore river ecosystems [42]. In particular, the use of iron can stimulate the germination and growth of macrophytes (e.g., Vallisneria natans) and the activity of rhizosphere microbiota [43]. However, high iron concentrations (>5.0 mg/dm3) inhibit these processes, and the effect of a single application is unstable. Due to the costs and risks of toxicity, alternatives should be sought.
Another approach to maintaining the required level of basal macrophyte coverage in aquatic ecosystems is introduction, which is implemented by three main methods: sowing, direct transplantation, or sampling with subsequent cultivation and planting. However, sowing is often hampered by the limited availability of viable seeds [44], while direct transplantation can lead to significant environmental losses at collection sites due to the removal of large numbers of plants. Although there are successful examples of cultivation followed by introduction [45], it requires careful consideration of factors such as water depth and flow velocity to minimise biomass loss due to hydrodynamic drag. At the same time, the artificial cultivation of basal macrophytes or their introduction into new environments to ensure a high level of water coverage is accompanied by significant costs for materials, installation, and maintenance. Given this, the large-scale introduction of hydrophytes in river ecosystems is rare.
Most problems can be solved using inexpensive and environmentally friendly local materials that increase the physical stability of the root system of macrophytes and the biogeochemical stability of ecosystems. For example, zeolite and expanded clay enrich water with silicon, a key element for the development of root macrophytes, which stimulates biomass accumulation and increases the ecological efficiency of plants [46]. Silicon improves nitrogen and phosphorus uptake, optimises photosynthesis, and replaces part of the carbon structures, reducing lignin content, which increases plant resistance to mechanical damage [47]. Silicon compounds are naturally present in water in concentrations of 1–30 mg/dm3, sometimes up to 50% of anions in groundwater. The main source of silicon is the chemical leaching of aluminosilicates under the influence of CO2, H+ or OH− ions, as well as the biochemical decomposition of flora and fauna residues.
One way to increase the density of root macrophytes in nutrient-poor rivers is to add limestone as a source of calcium. Calcium promotes the accumulation of nutrients in biomass and sediments, limiting the development of phytoplankton and free-floating macrophytes, providing better light conditions for the growth of submerged plants [48]. Limestone also regulates the pH of the water by neutralising hydrogen ions and increasing the buffer capacity of the system, which contributes to the stability of conditions for macrophytes [33]. The choice of substance to improve growth depends on the hydrochemical status of the water body and the objectives, so a comprehensive assessment of water quality, nutrients, and sediments should be carried out before application.
The purpose of the research was to assess the balance of the aquatic ecosystem of the Vyzhyvka River through the study of the species composition of macrophytes and the analysis of hydrochemical indicators of water quality in different seasons of the year. The research task was to develop recommendations on the possibility of regulating the chemical composition of water in individual sections of the river to support groups of rooted submerged macrophytes, which provide significant efficiency in the processes of self-purification of aquatic ecosystems. The emphasis of the research task was placed on the search for natural geomaterials that can optimise the balance of nutrients in the river water to maintain its good condition.
The scientific novelty of the work lies in identifying the patterns of distribution of morphoecological groups of macrophytes depending on the qualitative composition of the Vyzhyvka River water and substantiating the influence of chemical characteristics of the aquatic environment on the structural and functional organisation of aquatic vegetation. For the first time, the results of hydrochemical analysis and assessment of the species composition of macrophytes were integrated for a comprehensive determination of the state of the aquatic ecosystem, which allowed the development of environmentally and economically feasible recommendations for adjusting the chemical composition of water using geomaterials—waste from the mining industry. The use of such materials contributes to an increase in the content of silicon and calcium in water, creates favourable conditions for the development of submerged rooted macrophytes, reduces the risks of eutrophication, and ensures stabilization of the ecological balance of the river, opening up new opportunities for the restoration of aquatic ecosystems with minimal impact on the environment.
The practical value of the results lies in the development of a scientifically sound approach to optimising the structure of macrophyte vegetation by targeted introduction of rocks to achieve an ecological balance between surface and root forms. The results obtained can be used in water management activities, environmental protection practices, as well as to improve the recreational and aesthetic condition of water bodies and the development of ecological tourism.
2. Materials and Methods
2.1. Geographical Characteristics of the Vyzhyvka River Basin
The Vyzhyvka River was chosen for the study—a right tributary of the Pripyat River (Dnieper basin), flowing within the Starovyzhivskyi district of the Volyn region. The watercourse is a typical example of small plain rivers of the region and has representative hydrological characteristics. The length of the river is 21 km, and the area of the catchment basin is 155 km2 [49]. The slope of the riverbed is on average 1.3 m/km. The Vyzhyvka is fed by numerous nameless streams. The Vyzhyvka riverbed and its streams are characterised by moderate meandering and calm flow. The river basin also contains melioration channels with straightened sections of the riverbeds. The width of the valley varies from 50 to 300 m, and the floodplain part is approximately 100–200 m wide. During spring floods and heavy rainfall, the floodplain is usually flooded to a depth of 1.0–1.5 m. The river banks are mostly low and gentle, with areas of moderately steep slopes 1.0–1.5 m high. In many places, the banks are covered with shrub vegetation and have a peaty structure.
According to hydrological characteristics, the Vyzhyvka River belongs to watercourses with a mixed type of supply, where snow supply dominates. Snow and rainwater form up to 70% of the annual runoff, while the contribution of groundwater supply is about 30%. The hydrological regime of the Vyzhyvka is characterised by significant fluctuations in water levels and seasonal spills during spring floods and summer-autumn rains. The average annual amplitude of water level fluctuations is 0.5–1.5 m. During spring floods, the water level usually rises by 10–20 cm per day, and in some years, up to 30 cm. The duration of floods is 10–20 days. According to the size typology, the Vyzhyvka is classified as a small river with a catchment area of up to 500 km2. According to the geological typology, its basin is predominantly organic with significant areas of peatlands [49].
2.2. Geological and Structural Features of the Vyzhyvka River Basin
The Vyzhyvka River flows through the territory of the Volyn Oblast, which belongs to the Volyn–Podilsky Plate—the southwestern edge of the East European Platform. The geological structure of the region is associated with the buried crystalline basement of the Ukrainian Shield, which lies at different depths. Within the framework of tectonic zoning, the western slope of the shield, the Kovel protrusion and the Lviv Palaeozoic depression are distinguished here. On the Palaeozoic rocks, there are layers of the Cretaceous system (limestones, marls) and Paleogene formations (sands, clays, sandstones), which are covered by anthropogenic loess-like loams [50].
The geological composition of the Vyzhyvka basin is represented mainly by sedimentary rocks—clays, loams and sands, which form channel and floodplain areas. Peat deposits associated with periodic flooding are widespread in the floodplain. The presence of carbonate rocks, in particular chalk and limestone, contributes to the development of karst processes in some areas of the basin. There is no active economic activity directly within the Vyzhyvka basin. The river retains its natural hydrological regime and is not subject to significant anthropogenic load, which allows it to be considered as a representative example of relatively undisturbed aquatic ecosystems in the region, despite the widespread distribution of limestone, basalt, and granite deposits in Volyn, which determine active industrial mining in the surrounding areas.
2.3. Selection of Test Sites and Representative Observation Points
The water sampling points were determined taking into account the reference to the state monitoring observation points, which are characterised by different levels of anthropogenic load and meet the representativeness criteria for assessing the impact of point and diffuse sources of pollution. Three test sites were selected for the study: the village of Komariv (site No. 1), the village of Nova Vyzhva (site No. 2), and the village of Yakushiv (site No. 3), which are schematically presented in Figure 1. In all three test sites, the riverbed has undergone technical modification; in particular, in site No. 3, the banks are reinforced with concrete slabs.
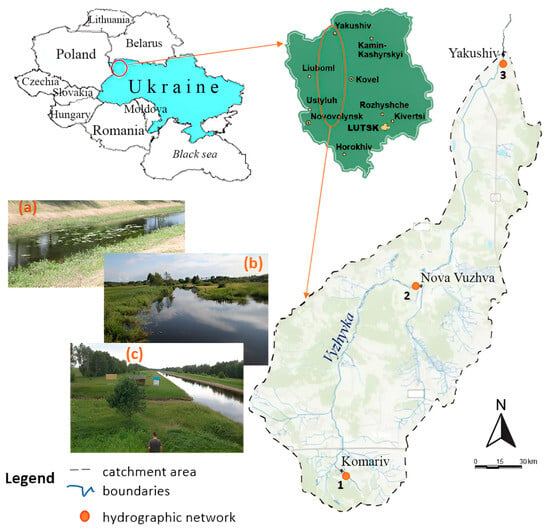
Figure 1.
Layout of test plots in the Vyzhyvka River basin and view of them: (a) village of Komariv, Site No 1; (b) village of Nova Vyzhva, Site No 2; (c) village of Yakushiv, Site No 3.
The numbering of test plots and corresponding representative sampling points is given in Table 1.

Table 1.
Characteristics of test plots of phytoindication studies and representative sections for sampling water of the Vyzhyvka River.
2.4. Study of the Seasonal Dynamics of Water Quality Changes in the Vyzhyvka River
To track the seasonal dynamics of surface water quality in the Vyzhyvka River, an analysis of changes in the absolute values of water quality indicators in representative sections was carried out in 2023–2024. The results were presented in the form of graphs showing the distribution of median values of physical and chemical indicators within the interquartile range (25–75%) and fluctuations between minimum and maximum values. Statistical significance was assessed by Student’s t-test (p ≤ 0.05) [51]. Water samples were collected serially in representative sections at quarterly intervals, which allowed us to obtain data on the chemical composition of water within each hydrological season. A total of 38 samples were collected in compliance with regulatory requirements [52]. All samples were obtained at a depth of 0.5 m below the water surface using clean polyethene bottles, pre-washed with hydrochloric acid and distilled water. Sampling was carried out until noon in the daylight hours, after which the samples were sealed and labelled and delivered to the sanitary and hygienic laboratory for further hydrochemical analysis on the same day [53]. The analysis was carried out by groups of substances, according to their functions in hydroecosystems [54]: water mineralisation (total mineralisation, chloride (Cl−) and sulphate (SO42−) concentrations); physicochemical parameters (suspended solids content, pH, dissolved oxygen (DO) concentration, permanganate (POX) and bichromate (BOX) oxidisability); indicators of organic pollution and nutrient content (BOD5, concentrations of ammonium (NH4+), nitrate (NO3−) and nitrite (NO2−) nitrogen, orthophosphate (PO43−), and total phosphorus (TP)).
2.5. Phytoindication of Water Quality in the Vyzhyvka River
To achieve the study objective, the Macrophyte River Assessment Methodology [28] was chosen, the main objective of which is to determine the ecological status of rivers based on the quantitative and qualitative characteristics of aquatic and coastal plants. The methodology takes into account such parameters as the number of indicator species, their projected coverage, as well as indices that characterise the trophic state of the ecosystem, in particular the trophic index L and the weighting factor W. The L-index varies from 1 (for waters with developed eutrophic processes) to 10 (for oligotrophic waters). The weighting factor W reflects the level of ecological tolerance of species: a value of 1 corresponds to eutrophic species, and a value of 3 to stenotopic species.
Based on these indicators, the Macrophyte Index of Rivers (MIR) is calculated, which is used to assess the ecological status of water bodies in accordance with the requirements of the EU Water Framework Directive [28]. The index is calculated using a list of 153 species of macrophyte bioindicators.
The methodology of macrophyte assessment of rivers allows determination of the watercourse degradation degree, in particular that caused by water pollution with nutrients. The choice of this approach for application in Ukraine is based on the high level of correspondence between the floristic lists of the studied river sections and the set of indicator macrophyte species used to calculate the MIR.
By the chosen methodology, at the preparatory stage, cartographic materials of the Volyn region were processed at various scales with a high level of accuracy and test sites for phytoindicative research were selected. At the second stage, field surveys of the test sites were conducted to determine the width, depth, and speed of the river flow. The surveys were carried out in areas that corresponded to representative water sampling sites (Table 1). The third stage involved a geobotanical description of macrophytes. Macrophytes were surveyed on river sections over 100 m long, taking into account underwater, free-floating and emergent taxa. Additionally, four abiotic parameters were recorded: current velocity, shade level, substrate type, and average depth.
Macrophytes were identified at the species level. The degree of coverage of each taxon was estimated as a percentage using a nine-point scale according to Holmes et al.’s methodology [55], where 1 corresponded to 0.1%; 2 to 0.1–1%; 3 to 1–2.5%; 4 to 2.5–5%; 5 to 5–10%; 6 to 10–25%; 7 to 25–50%; 8 to 50–75%; and 9 to more than 75%. The assessment also covered macrophytic species growing or rooting in riparian areas of the channel that are under water for more than 85% of the annual cycle. Sampling was carried out at each test site from early June to late August in 2023–2024, taking into account the specifics of the vegetation phases of different taxa. Some of the plants were identified directly in the field, while others were identified in the laboratory. The modern literature sources were used to establish species affiliation, and the scientific names of macrophytes were given by The Plant List and Euro+Med PlantBase databases [56].
The MIR was calculated based on the results of field studies using the following formula:
where is the value of the macrophyte index of the river at the sampling site; is the number of species at the sampling site; is the value of the index for the i-th taxon; is the weighting factor for the i-th taxon; and is the coverage factor for the i-th taxon, determined on a 9-point scale [28].
The calculated values of the MIR were used to assess the ecological status of the rivers in the study; the index values range from 10, which corresponds to a high level of eutrophication and degradation of the watercourse, to 100, which is typical for water bodies with the best ecological status. For plain rivers, the maximum MIR values usually do not exceed 60 [57]. Specific groups of indicator species correspond to each type of river. The determined values of the MIR index were classified according to five water quality classes according to the criteria of the EU Water Framework Directive.
3. Results and Discussion
3.1. Analysis of the Seasonal Dynamics of Water Quality in the Study River
The Vyzhyvka River flows mainly within natural landscapes and has no industrial facilities or significant sources of pollution in the immediate vicinity. Its catchment area covers a sparsely populated area, and the impact of anthropogenic load is minimal, which creates conditions for maintaining a relatively stable hydrochemical regime. To study the seasonal dynamics of water quality in the Vyzhyvka River, samples were taken at three texture sites, the results of which were averaged due to the slight variation between them. The visualisation is presented in the form of boxplot graphs showing the distribution of values by season: winter (Win), spring (Spr), summer (Sum), and autumn (Aut) (Figure 2 and Figure 3). Several physicochemical parameters were analysed to assess the water quality of the Vyzhyvka River.
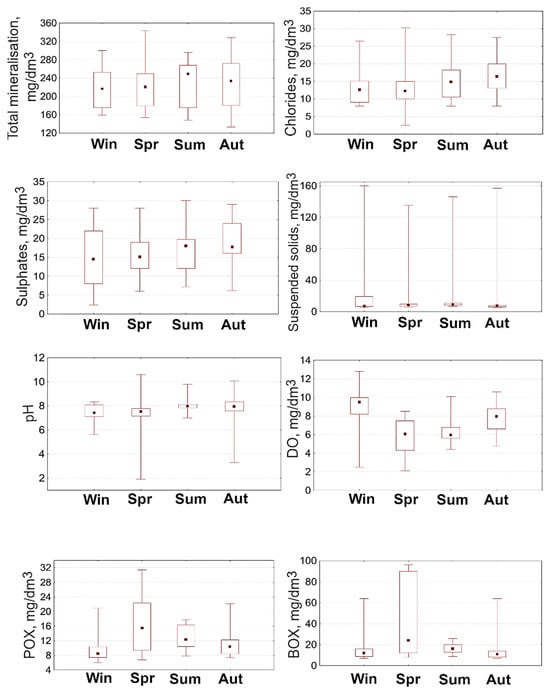
Figure 2.
Seasonal dynamics of physical and chemical parameters of water in representative sections of the Vyzhyvka River (2023–2024).
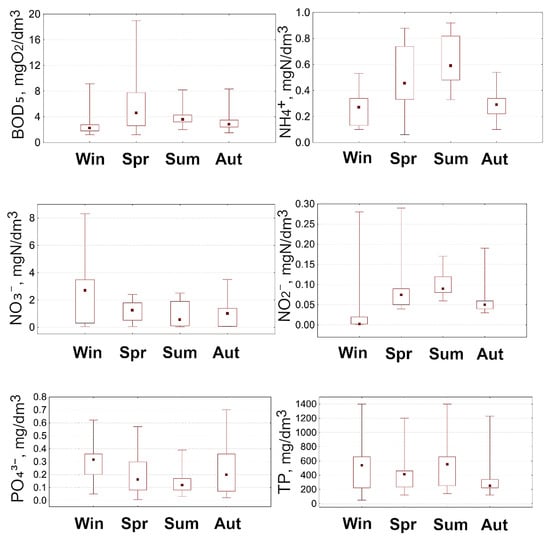
Figure 3.
Indicators of organic pollution and nutrient content in the water of the Vyzhyvka River (2023–2024).
According to the results of hydrochemical analysis, the highest values of total water salinity in the Vyzhyvka River were recorded in summer (230–240 mg/dm3), which is likely due to more intensive evaporation and, accordingly, an increased concentration of dissolved salts. The lowest values were observed in winter and autumn, about 200 mg/dm3. Despite seasonal fluctuations, the overall variation remained relatively stable throughout the year.
Chloride values remained stable during all seasons and amounted to 10–12 mg/dm3, indicating the absence of local anthropogenic load or industrial discharges. A similar situation is observed for SO42−: in winter, spring, and summer, the median values range from 17–20 mg/dm3, while in autumn, there is a slight increase to 22–24 mg/dm3. The overall range of fluctuations throughout the year is 10–30 mg/dm3. Thus, the seasonal variation in SO42− content is insignificant, and all the values obtained remain within the natural background.
The highest concentrations of suspended solids were observed in spring, which is likely due to active snowmelt and intensified erosion processes that cause intense surface runoff. In winter and autumn, the concentrations remained very low (median value of 2 mg/dm3), and in summer, there was a slight increase to 5 mg/dm3. Thus, the seasonal dynamics of suspended solids clearly reflect natural hydrological processes, and the total content remained within the range expected for small rivers in a temperate climate zone.
The reaction of the water environment of the Vyzhyvka River remained stable throughout the entire observation period, being within the neutral-low-alkaline range (pH 7.0–8.5), which is considered optimal for most aquatic organisms [34] and is a characteristic feature of a relatively clean water environment. In the spring, a slight increase in pH values to 8.5 was observed, which is probably due to the intensification of biological processes, in particular photosynthesis. The lowest average values were recorded in autumn (7.0), while the overall pH variation ranged from 7.0 in winter to 8.5 in summer.
An increase in the reaction of the aquatic environment towards alkalinity (pH > 7) can significantly affect the structure of aquatic ecosystems, in particular, macrophyte species that are sensitive to changes in chemical parameters. As noted by Lacoul and Freedman [58], pH is one of the leading abiotic factors that determine the composition and functioning of aquatic vegetation. Similar observations are presented in the work of Ljevanaić-Mašić [59], which emphasises the crucial importance of acid–base balance for the formation of macrophyte biocenoses. Svitok et al. [60] found that in watercourses with a pH above 8.4, the level of species richness of the macrophyte layer with free-floating leaves was significantly reduced compared to areas with less alkaline conditions. The authors attribute the reason for this phenomenon to the peculiarities of the metabolism of aquatic plants: some species can assimilate carbon only in the form of dissolved CO2. When the pH rises above 8.3, the availability of carbon dioxide decreases due to a shift in chemical equilibrium towards the hydrocarbonate form, which is less efficiently used in photosynthesis by certain taxa [60].
The lowest content of DO in the water of the Vyzhyvka River was recorded in spring (4–6 mg/dm3), which is associated with an increase in water temperature, intensification of biochemical processes of oxygen consumption and a decrease in turbulence. The highest DO concentrations were observed in winter (9–10 mg/dm3) and autumn (6–7 mg/dm3). According to the average seasonal values of this indicator, the water of the Vyzhyvka River belongs to the first quality class (very good category), which indicates its ecological condition. Low levels of DO are an important indicator of water body pollution and can negatively affect the state of aquatic ecosystems [61]. DO is a critical indicator of the ecological state of water, as it is essential for the existence of aquatic organisms. Many species of macrophytes are sensitive to a decrease in oxygen concentration in hydrosystems. Oxygen enters the water through diffusion from the atmosphere, as well as through the photosynthetic activity of aquatic plants that produce oxygen as a by-product [62].
BOD5 is an indicator of the amount of oxygen consumed by microorganisms during the oxidation of organic compounds in water [63]. Other studies [61] show that aquatic plants contribute to the reduction in BOD5 and chemical oxygen demand (COD), which improves water quality. In the Vyzhyvka River, the lowest BOD5 values were observed in summer, autumn and winter (2–3 mg/dm3), while the highest values were recorded in spring (4.5–5 mg/dm3). The increase in BOD5 levels in spring indicates an increased biological load, in particular the decomposition of organic materials.
The content of nitrogen compounds (NH4+, NO3− and NO2−) is an important indicator of the trophic state of a water body and an indicator of anthropogenic load [64]. In the Vyzhyvka River, the NH4+ concentration shows a clear seasonal trend: the minimum values were recorded in winter (0.1 mg N/dm3), while in summer, the median concentrations increased to 0.25–0.28 mg N/dm3. The peak in spring reached 0.35 mg N/dm3. This increase may be due to the active ammonification of organic matter at high temperatures and enhanced biodegradation. In autumn, the concentration decreases to 0.15 mg N/dm3.
The concentration of NO3− in the water of the Vyzhyvka River remains relatively constant throughout the year, which indicates a stable trophic state of the water body. In winter, the median value is about 0.55 mg N/dm3 with maximum peaks of up to 0.75 mg N/dm3. In spring and summer, the concentrations are kept within the range of 0.4–0.5 mg N/dm3, and in autumn, the indicators remain at around 0.4 mg N/dm3. This stability of the nitrate background indicates that there is no significant anthropogenic impact, including inputs from agricultural landscapes or untreated domestic wastewater, which is typical for non-urbanised or poorly transformed basins. According to the ecological classification of nitrate nitrogen, the water in the Vyzhyvka River belongs to the first quality class (very good category). It has been established [65] that macrophytic vegetation can contribute to the reduction in nitrate concentrations in the aquatic environment, as it can produce organic matter that is used by denitrifying bacteria as a source of carbon for the reduction in nitrate to molecular nitrogen.
NO2−, an intermediate form of nitrogen in the nitrification and denitrification processes, also has seasonal fluctuations. In winter, low values were observed (0.05–0.08 mg N/dm3), and in spring, the median increased to 0.15 mg N/dm3 with peaks of up to 0.2 mg N/dm3, which is likely due to the activation of microbiological processes. In summer and autumn, the values stabilise within the range of 0.07–0.1 mg N/dm3. Elevated ammonium concentrations can have a toxic effect on aquatic vegetation, in particular on macrophytes. This is confirmed by the literature [66], which indicates the sensitivity of many species to ammonium nitrogen. The study [67] recorded a decrease in the biomass of Myriophyllum spicatum L. at a NH4+ concentration of 0.847 mg/dm3, which indicates the negative impact of excessive ammonium loading.
Phosphorus compounds are key biogenic elements involved in the processes of eutrophication of aquatic ecosystems. The results of the study of the PO43− content in the water of the Vyzhyvka River revealed seasonal dynamics, which indicate their predominantly natural origin. In winter, the concentration was 0.1–0.12 mgP/dm3, which corresponds to the II class of water quality, a good category. In the spring, a sharp increase to a median value of 0.3 mgP/dm3 was recorded with individual peaks of up to 0.6 mgP/dm3, which is likely to be due to surface runoff during snowmelt. In summer, the PO43− level decreased to 0.1 mgP/dm3, and in autumn, it remained stable with a median of ≈0.15 mgP/dm3.
TP concentrations showed similar dynamics: winter values were at 0.1–0.12 mgP/dm3; in spring, the median reached 0.4 mgP/dm3 with a variation of up to 0.6 mgP/dm3; and in summer and autumn, the indicator stabilised at 0.15–0.2 mgP/dm3. According to the water quality classification, such concentrations of TP also correspond to class II, good quality. The high spring values are of natural origin. Scientific research [68] shows that an increase in phosphate content promotes the growth of aquatic vegetation, in particular macrophyte species such as Lemna minor and Ceratophyllum demersum, which confirms the potential impact of phosphorus compounds on the trophic state of the reservoir.
The generalised results of the study indicate that the ecological status of the Vyzhyvka River is classified as “good”. This is confirmed by the moderate content of nutrients, stable physical and chemical characteristics, and low levels of organic pollution. The data obtained indicate the natural seasonal dynamics of water quality in the absence of significant anthropogenic impact.
3.2. Taxonomic Diversity, Species Composition and Cover Density of Macrophytes
In June-August 2023 and 2024, geobotanical surveys were conducted in the Vyzhyvka River basin, and the ecological status of the river was assessed using the Modul MacroPhyte MMP method. The classification table for four types of rivers used in the Polish methodology [28] was used for the calculation.
Based on surveys of the Vyzhyvka River, 64 macrophyte species belonging to 23 families were identified. Near the village of Komariv, 23 species were recorded, 17 of which are indicators of ecological status. These included floating-leaf plants such as Nuphar lutea (yellow water lily), emergent species like Sagittaria sagittifolia (common arrowhead), submerged plants including Elodea canadensis (Canadian elodea), and free-floating species such as Glyceria maxima (large glyceria) (Figure 4).

Figure 4.
Macrophytes of the Vyzhyvka River at site 1 near Komariv village: (a) Elodea canadensis Michx.); (b) Nuphar lutea (L.) Smith.; and Sagittaria sagittifolia L.
The macrophyte index for the entire observation period (2023–2024) ranged from 34.5 to 42.6, which corresponds to the “good” category according to the MMP methodology (Table 2).

Table 2.
MOR calculation for the Vyzhyvka River, near Komariv village, site 1.
In plot 2, 22 macrophyte species were identified, 21 of which are indicators of ecological status. The species composition was dominated by floating-leaf species such as Nuphar lutea (yellow water lily), emergent forms like Stratiotes aloides, and coastal plants including Glyceria maxima (Figure 5).

Figure 5.
Macrophytes of the Vyzhyvka River at site 2: (a) water cuttlefish (Stratiotes aloides L.); (b) Glyceria maxima (Hartm.).
During 2023–2024, the macrophyte index ranged from 36.2 to 42.4, which corresponds to the “good” condition category (Table 3).

Table 3.
Calculation of MIR for the Vyzhyvka River, near the village of Nova Vyzhva, site 2.
The species composition of higher aquatic plants at site 3 of the Vyzhyvka River, near the village of Yakushiv, was dominated by species with floating leaves, particularly Nuphar lutea (yellow water lily) and semi-submerged plants, among which Sagittaria sagittifolia (common arrowhead) prevailed. A significant part of the vegetation cover also consisted of free-floating species such as Lemna minor (small duckweed), Spirodela polyrhiza, and Carex acuta (slender sedge), which were frequently observed during the surveyed periods with high cover density (Figure 6).

Figure 6.
Macrophytes at site 3 of the Vyzhyvka River, near the village of Yakushiv: (a) Nuphar lutea (L.) Smith, Carex acuta L.; (b) Lemna minor L.
The macrophyte index ranged from 37.3 to 38.2, which indicates a consistently “good” state of the ecosystem of this site (Table 4).

Table 4.
Calculation of MOR for the Vyzhyvka River, near the village of Yakushiv, site 3.
Cicuta virosa L. was occasionally observed in all sections of the Vyzhyvka River. Species such as Myosotis scorpioides L., Ranunculus aquatilis L., Vallisneria spiralis L., and Eleocharis palustris (L.) were also rarely observed, mainly in the upper part of the riverbed. In all sections, abundant species with high cover densities included Lemna minor L., Sagittaria sagittifolia L., and Spirodela polyrhiza (L.) Schleid.
Water quality at all test sites was classified as good, with a mesotrophic trophic status, indicating favourable environmental conditions for the development of higher aquatic plants along the entire river course. This is confirmed by the diverse flora comprising 64 species of higher aquatic and coastal plants, 29 of which serve as indicators of ecological status. These species belong to 31 genera, 20 families, 14 orders, 3 classes (Equisetopsida, Liliopsida, and Magnoliopsida), and 2 divisions (Equisetophyta and Magnoliophyta) (Table 5).

Table 5.
Taxonomic composition of macrophytes of the Vyzhyvka River.
The flora of the Vyzhyvka River includes only one species of Equisetopsida—Equisetum palustre, which accounts for 2.78% of the total species number. The division Magnoliophyta comprises 35 species (97.22%). Among the class Magnoliopsida, there are 11 families (55% of the total families), including 15 species (41.66% of macrophytes). The biodiversity within Magnoliopsida is limited to 1–2 species per family (Table 5). The class Liliopsida consists of 8 families represented by 20 species (55.56%). Thus, while most families belong to Magnoliopsida, Liliopsida prevails in species number.
Thus, the overwhelming majority of the flora of the Vyzhyvka River is made up of floating plants represented by species of the Liliopsida class, including the Hydrocharitaceae family (four species, 11.11%). A small part of the flora is formed by semi-submerged plants of the Magnoliopsida class, which includes such families as Cyperaceae (13.89% of the total number of species) and Alismataceae (three species each, 8.33%). Other families include one to two species, including representatives of floating leaves and submerged species. Of all the macrophyte taxa listed, the genus Nuphar had the highest species diversity. Several species were recorded along the Vyzhyvka River, with yellow pitcherwort (Nuphar lutea) dominating. This species does not survive in waters with low nutrient levels and high flow velocities [69]. In addition, free-floating plants, such as large glyceria (Glyceria maxima), are also present in the river, indicating that there are ecologically favourable conditions for their growth.
According to the analysis of life forms [56], the macrophyte flora of the Vyzhyvka River consists mainly of herbaceous polycarpics, among which hemicryptophytes and cryptophytes predominate, accounting for 52.78% and 47.22%, respectively (Table 6).

Table 6.
Biomorphological structure of macrophytes in the Vyzhyvka River.
The biomorphological structure of the macrophytes of the Vyzhyvka River includes four main life forms: aquatic macrophytes (including free-floating, submerged and floating-leaved species), helophytes or air–water plants, moisture-loving riparian forms (hygrohelophytes), and bank hygrophytes.
The aquatic macrophytes (hydrophytes) of the Vyzhyvka River include 11 species (30.56% of the total number of flora species), which are classified into four groups according to their life forms. Free-floating species make up the largest percentage (11.66%) and are represented by representatives of the families Araceae (Spirodela polyrrhiza) and Hydrocharitaceae (Hydrocharis morsus-ranae). Hydrocharis morsus-ranae is highly adaptable to various environmental conditions, which allows it to successfully inhabit a wide range of aquatic environments. This species can form dense masses in which leaves and roots are intertwined, potentially leading to reduced light penetration to lower water levels. This situation can hurt other species of root macrophytes, limiting their growth and biodiversity. In addition, these mats can reduce water flow, limit the availability of nutrients and dissolved gases to other plants, and reduce DO concentrations, which can have an impact on aquatic life, including fish, and overall ecosystem function [70].
The group of submerged rooted macrophytes includes four species (10.56%), of which three belong to the Hydrocharitaceae family (including Elodea canadensis) and one to the Haloragaceae family (Myriophyllum spicatum). An increase in the number of Elodea canadensis can significantly change the ecological balance of aquatic environments. The intensive spread of this species can cause eutrophication processes, and at the end of the growing season, its decomposition leads to secondary eutrophication [71]. This activates bacterial metabolism, which results in anaerobic conditions that contribute to a decrease in the level of dissolved oxygen in water. Under such conditions, mineralisation processes slow down and fermentation end products accumulate, which can be toxic to other aquatic plants. The widespread distribution of Elodea canadensis has a negative impact on local aquatic flora, significantly reducing biodiversity in ecosystems where this species has become dominant.
Species with floating leaves are represented by Nuphar lutea and Persicaria amphibia (5.56%). The macrophyte Nuphar lutea (L.) Smith, known as the yellow pitcher, is a typical representative of aquatic plants with distinct leaf duality. The floating leaves of this plant create a dense cover on the water surface, which significantly reduces the penetration of light into the underwater environment, limiting the photosynthetic activity of underwater macrophytes. However, the submerged leaves of Nuphar lutea continue to play an important role, unaffected by seasonal changes. Although its photosynthetic activity is not as pronounced as that of surface leaves, submerged leaves remain an important component of the plant’s biomass during the growing season. Studies [72] have shown that the submerged leaves of N. lutea have significant engineering potential in the context of plant interaction with water flows. It contributes to a local reduction in flow velocity, which in turn stimulates the processes of sedimentation and deposition of organic and inorganic particles, including organic matter that enriches bottom sediments.
Submerged rootless macrophytes are represented by only one species, Ceratophyllum demersum (2.78%). This plant has no root system and absorbs nutrients mainly through finely divided leaves. C. demersum is characterised by rapid growth in aquatic ecosystems with low light levels. Due to its ability to actively vegetatively reproduce, Ceratophyllum demersum can quickly form significant biomass covers, which allows it to adapt to different environmental conditions and actively colonise new territories [73].
Helophytes, or emergent (air–water) plants, account for 22.22% of the total number of species (eight species). Among them, five species (13.89%) belong to low-growing helophytes, represented by the families Alismataceae, Cyperaceae, and Ranunculaceae. Tall helophytes include three species (8.33%) and belong to the families Poaceae and Typhaceae. Moisture-loving coastal macrophytes (hygrophilous riparian species) constitute the most numerous group among aquatic vegetation, comprising 30.55% of the species composition (11 species). This group includes representatives of the families Apiaceae, Acoraceae, Brassicaceae, Cyperaceae, Plantaginaceae, Polygonaceae, and Ranunculaceae. The group of hygrophytes, i.e., semi-aquatic species capable of partially entering the aquatic environment, includes six species (16.67%) and is represented by the families Equisetaceae, Boraginaceae, Lamiaceae, Primulaceae, and Juncaceae.
3.3. Use of Geogenic Minerals to Stimulate the Development of Submerged Macrophytes in the Vyzhyvka River
The water body under study is currently not used for recreational purposes and does not serve an aesthetic function, but it meets sanitary and epidemiological requirements. At the same time, an analysis of macrophyte diversity in the Vyzhyvka River revealed an imbalance in the distribution of morphoecological groups, in particular, a low proportion of submerged root forms compared to other types of aquatic vegetation. Despite the classification of the water as “good”, such structural asymmetry may indicate potential threats to the stability of the ecosystem in the medium and long term.
Given the growing need to develop recreational areas near populated areas, especially in western Ukraine, which is receiving a significant number of internally displaced persons from regions where fighting continues, it is important to find solutions that combine environmental efficiency and social expediency. In this context, it is advisable to apply bottom-up regulatory mechanisms aimed at improving the physical and chemical conditions of the environment to stimulate the development of submerged aquatic vegetation and enhance the ecological and recreational value of the water body.
One promising and economically sound solution is the use of natural minerals, in particular mining and quarry waste containing silicon, calcium and other biophilic components. These substances are non-toxic, do not deteriorate water quality, do not inhibit the functioning of the biocenosis, and at the same time can stimulate the development of root macrophytes, stabilise bottom sediments, and reduce the bioavailability of nutrients. In addition to improving the quality of the aquatic environment, this strategy addresses the problem of industrial waste disposal, which is relevant for regions with a long history of mineral extraction. In particular, residues generated during the cutting and processing of geomaterials can be converted into by-products with high environmental potential that comply with the principles of clean production and a closed-loop economy. Unlike expensive technologies, such as the use of lanthanum-modified bentonite, which requires significant capital investment and energy consumption, the use of geogenic minerals is considered a financially accessible alternative [74].
The environmental effectiveness of this approach has already been demonstrated in studies conducted in the Ustyia River (Rivne, Ukraine) [75] and has the potential for application in the Vyzhyvka River basin. The authors proposed a combination of chemical and physical methods for restoring water bodies using regionally abundant natural minerals—carbonates and quartz-glauconite sand, which improve water quality by stabilising pH, and filtration properties and reducing pollutant concentrations. Experimental data confirmed that the use of these materials contributes to the improvement of the ecological state of river ecosystems and creates the conditions for their aesthetic and recreational restoration, with the possibility of adapting coastal areas for public spaces.
Near the Vyzhyvka River basin, there are enterprises specialising in the extraction and processing of mineral raw materials suitable for ecological use in aquatic ecosystems. In particular, PJSC “Ternopil Quarry” is one of the leading producers of limestone products in Ukraine. The enterprise develops limestone deposits with their subsequent processing into construction and technical materials, and the annual production capacity reaches 2 million tons. The Ivano–Dolinsky quarry provides the extraction of aluminosilicates, in particular bentonite, kaolin and crushed basalt, which can be used in environmental remediation measures, in particular to stabilise bottom sediments and stimulate the development of submerged aquatic vegetation. An important source of silicon-containing raw materials is also a granite quarry located in the Rokytniv district of the Rivne region. A general view of the extraction sites of natural materials, as well as their geographical location, is shown in Figure 7.
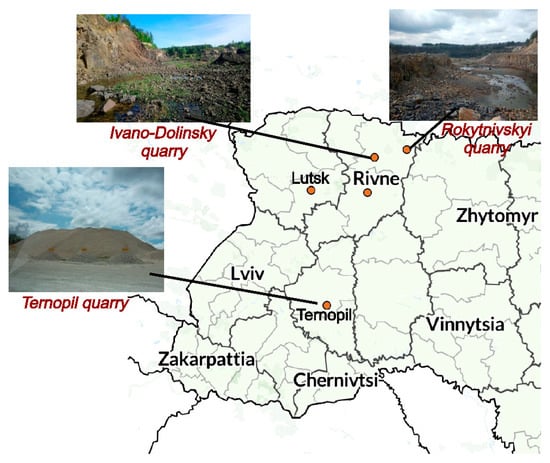
Figure 7.
General view of natural material extraction sites and their geographical location.
Given the availability of mineral resources within the Vyzhyvka River basin, it is advisable to consider the environmentally safe use of quarry waste as a potentially effective and economically viable means of maintaining the favourable ecological status of the reservoir. Limestone, granite, and basalt waste contains silicon, calcium, and other elements that participate in the biogeochemical processes of water bodies. Their introduction can contribute to the stabilisation of bottom sediments, reduce the bioavailability of phosphates, and improve conditions for the development of submerged macrophytes—an important element of ecological balance.
Studies [76] have shown that the addition of silicon improves the morphological and physiological properties of macrophytes, in particular Schoenoplectus spp., increasing tissue strength and reducing herbivory. Another study [77] showed that the addition of calcium hydroxide contributes to a reduction in phosphate concentration and phytoplankton production, while the presence of macrophytes has a positive effect on the structure of zooplankton, enhancing biocontrol.
Aluminosilicates are natural minerals that effectively absorb excess nutrients, reducing eutrophication in water bodies. They improve conditions for the growth of submerged macrophytes and stabilise bottom sediments by binding fine particles, reducing their mobility and the risk of resuspension [78]. This helps to maintain water transparency and the necessary level of light for photosynthesis. Aluminosilicates also act as a buffer, stabilising pH and creating favourable conditions for plants that are sensitive to fluctuations in acid–base balance [79]. Their use should be combined with biomanipulation, which regulates trophic links to limit phytoplankton [80], as well as with the management of bottom sediments by introducing stabilising materials (clay, lime, crushed basalt) and controlling their properties.
Bentonite is a sedimentary rock whose main component is montmorillonite (over 80% by weight; 60–80% bentonite clay) [81]. Its typical chemical composition includes 64.63% SiO2, 13.70% Al2O3, 2.72% Fe2O3, 3.94% CaO, 2.26% MgO, 0.16% K2O, and 2.32% Na2O [82]. Bentonite clay has high sorption capacity and water retention properties due to hydroxyl groups and negative charge, which ensures effective adsorption of cations, in particular heavy metals and organic compounds. Its use in water bodies helps to reduce turbidity, stabilises bottom sediments, and improves conditions for the growth of aquatic vegetation. In a study [83], bentonite had a positive effect on the development of Vallisneria spiralis: at bentonite/sediment ratios of 1:1 and 1:5, biomass growth was 18.78% and 11.79%, respectively. The activation of rhizosphere microbiota and changes in the physical and chemical characteristics of the sediment were also noted. One of the factors stimulating the growth of macrophytes was the release of nutrients from bentonite.
Crushed basalt, as a primary magmatic rock, is considered a promising mineral resource for improving the chemical composition of the aquatic environment and stimulating the growth of submerged plants. Studies [84] have shown that among geogenic substrates, basalt provides the most intense release of biophilic elements (Ca, Mg, Mn, Si), contributing to the formation of enriched biomass. Its effectiveness as a mineral fertiliser has also been confirmed in terrestrial ecosystems, in particular on Urochloa brizantha [85], where the application of basalt powder improved the agrochemical properties of the soil (increase in pH, macroelement content, reduction in phytotoxic Al), and increased plant productivity. Thus, crushed basalt shows potential as an environmentally safe mineral source of nutrients, capable of increasing soil fertility and stimulating plant growth in various types of ecosystems.
Limestone is a sedimentary rock, the basis of which is the minerals calcite and aragonite, various crystalline forms of calcium carbonate. It is advisable to use it to increase the buffer capacity and stabilise the pH of the aquatic environment. In addition, limestone particles can serve as a substrate for the rooting of submerged macrophytes, promoting their growth. According to the study [86], the limestone barrier created along the coastline contributed to the increase in the species diversity of coastal vegetation and improved conditions for the development of macrophytes, in particular Schoenoplectus lacustris and Glyceria maxima, which showed a high ability to absorb phosphorus a year after planting. At the same time, a stable decrease in the concentration of phosphorus in the water was recorded, which confirms the effectiveness of limestone as a natural filter in surface runoff treatment systems and maintaining the water’s ecological balance.
The use of limestone for surface water treatment is an effective measure aimed at regulating the acid–base balance, in particular, increasing the pH of the environment and reducing the concentrations of phosphates and heavy metals [87]. Due to its slow dissolution in water, limestone provides a gradual change in chemical properties without the risk of toxic effects on aquatic biota [88]. Its dissolution products, in particular carbonate ions, can serve as a source of inorganic carbon for photosynthetic processes, and also contribute to the formation of poorly soluble forms of heavy metals, reducing their environmental hazard [89].
The pH level of the aquatic environment is one of the key factors affecting the structure of aquatic vegetation. Submerged basal macrophytes can use bicarbonates as a carbon source; therefore, their productivity increases in conditions of increased alkalinity. Dissolution of limestone contributes to an increase in the concentration of these ions, which creates favourable conditions for the development of submerged species [90]. Increasing pH also limits the competitive capabilities of free-floating macrophytes, as their metabolic activity decreases at pH >9.5, and nutrient uptake, particularly phosphate and nitrate, becomes less efficient [91]. In addition, in alkaline environments, the bioavailability of trace elements such as iron, manganese, and phosphorus decreases due to their precipitation as poorly soluble compounds [92].
Mining and quarry wastes have significant potential to improve the conditions for the development of basal macrophytes in aquatic ecosystems. Due to their ability to absorb pollutants, buffer pH and regulate the content of nutrients in the aquatic environment, mineral materials can create a stable and favourable environment for macrophytes. The use of these materials in ecological restoration processes provides new opportunities for maintaining aquatic biodiversity and improving the condition of degraded river ecosystems.
Regulation of the optimal ratio of macrophytes in river ecosystems can significantly improve water quality, reducing eutrophication, suppressing the development of undesirable aquatic vegetation and contributing to the restoration of natural balance. At the same time, this has not only ecological, but also important recreational and aesthetic significance. It is precisely water spaces with well-balanced aquatic vegetation that have an attractive appearance, contribute to the formation of a favourable microclimate and become centres for recreation, walks and sports. Modern projects for the revitalisation of urban rivers involve not only improving water quality but also creating multifunctional public spaces, where ecosystem balance is combined with comfort and accessibility for residents [75]. Thus, the ecological restoration of rivers by regulating the structure of aquatic vegetation opens up new opportunities for the development of ecotourism and strengthening the connection between man and the natural environment.
The research results are applicable not only for the improvement of natural rivers. They can also be used for green structures, integrating living plants and building structures. This is promising technology for the improvement [92,93] of the outdoor [94,95] and indoor environment, rainwater management [96,97,98], recovery of historical heritage [99,100], food production [101], etc. Usually, we use green roofs, green walls, vertical greening, etc. But there are some green roofs combined with artificial water features. An example is the Park Baku Residence in Baku, Azerbaijan (Figure 8), with artificial rivers.

Figure 8.
A natural river in the Park Baku Residence, Baku, Azerbaijan (author’s pictures).
The rivers have controlled depth and speed. The main purposes of them are the aesthetic improvement and ecoregulation—stress mitigation by interaction with nature. Such objects can increase the estimation by the green certification standards, including the Ukrainian SOU OEM 08.002.41.032 [102]. As is known, water can absorb air pollutants. Thus, we can improve the artificial rivers’ functions for cleaning the air environment by introducing the appropriate macrophytes. Their cleaning function is also important for the longer usage of the circulating water. There are some challenges, for example, the need for enough nutrients without breaking pumps, low depth, etc. It is possible to use most of the minerals in Section 3.3 for the bottom of the river, as they release the nutrients very slowly at the microelement level. Therefore, this direction will be the topic for future research.
4. Conclusions
The results of a comprehensive hydrobiological and hydrochemical study of the Vyzhyvka River in Ukraine confirmed its good ecological status. Throughout the year, the main physical and chemical indicators remained within natural background levels, indicating an absence of significant anthropogenic impact. According to the MIR index, all surveyed sections of the river are classified as Class II quality with a mesotrophic trophic status. The phytoindication analysis identified 64 species of macrophytes, with coastal life forms being the most dominant: hygro-helophytes (30.6%), helophytes (22.2%), and hygrophytes (16.7%). In contrast, the share of hydrophytes stands at only 30.6%, including four free-floating species, four submerged rooted species, two that have floating leaves, and one submerged unrooted species. This distribution pattern suggests a limited presence of submerged rooted forms, which are essential for regulating water transparency, binding sediments, and facilitating nutrient cycling. The low abundance of root-bound submerged macrophytes may be linked to a deficiency in key biogenic elements, particularly silicon and calcium. Although the overall ecological status of the river is assessed as good, the imbalance in life forms indicates potential risks to the ecosystem’s stability in the medium and long term. Therefore, a practical recommendation is proposed: to use locally sourced mineral raw materials (such as aluminosilicates, limestone, and geomaterials obtained from mining waste) as natural amendments to improve conditions for the growth of root forms. Further research should focus on experimentally testing the impact of various geomaterials on the development of macrophytes, especially root-bound submerged species, while considering their ecological sensitivity. Additionally, it is important to study the interactions between silicon and other nutrients to optimise the approach to the eco-technical improvement of water bodies. The results obtained from this research can serve as a foundation for developing an effective environmental strategy for managing aquatic ecosystems.
Author Contributions
Conceptualization, Y.T. and M.K.; methodology, O.B.; software, R.T. and V.M.; validation, T.T., Y.T. and M.K.; formal analysis, O.T.; investigation, M.B.; resources, I.S.; data curation, O.B.; writing—original draft preparation, M.K.; writing—review and editing, T.T.; visualization, V.M.; supervision, Y.T.; project administration, V.M.; funding acquisition, O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Nezbrytska, I.; Bilous, O.; Sereda, T.; Ivanova, N.; Pohorielova, M.; Shevchenko, T.; Dubniak, S.; Lietytska, O.; Zhezherya, V.; Polishchuk, O.; et al. Effects of War-Related Human Activities on Microalgae and Macrophytes in Freshwater Ecosystems: A Case Study of the Irpin River Basin, Ukraine. Water 2024, 16, 3604. [Google Scholar] [CrossRef]
- Sayanthan, S.; Hasan, H.A.; Abdullah, S.R.S. Floating Aquatic Macrophytes in Wastewater Treatment: Toward a Circular Economy. Water 2024, 16, 870. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, S.; Han, B.; Hua, Z.; Fu, D.; Li, P. Impacts of Water Flow on Epiphytic Microbes and Nutrients Removal in Constructed Wetlands Dominated by Vallisneria Natans with Decreasing Temperature. Bioresour. Technol. 2020, 318, 124058. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, L. Treatment Technology of Microbial Landscape Aquatic Plants for Water Pollution. Adv. Mater. Sci. Eng. 2021, 2021, 4409913. [Google Scholar] [CrossRef]
- Chaurasia, S. Role of Macrophytes: A Review. Adv. Zool. Bot. 2022, 10, 75–81. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Taib, S.M.; Dahalan, F.A.; Songip, A.R.; Singh, L.; Kamyab, H. The Efficient Role of Aquatic Plant (Water Hyacinth) in Treating Domestic Wastewater in Continuous System. Int. J. Phytoremediation 2016, 18, 679–685. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A Review on the Sustainability of Constructed Wetlands for Wastewater Treatment: Design and Operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Kuhar, U.; Germ, M.; Gaberščik, A.; Urbanič, G. Development of a River Macrophyte Index (RMI) for Assessing River Ecological Status. Limnologica 2011, 41, 235–243. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Cunha, E.R.D. The Role of Macrophytes in Habitat Structuring in Aquatic Ecosystems: Methods of Measurement, Causes and Consequences on Animal Assemblages’ Composition and Biodiversity. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Zapata, A.G.F.; Rodrigues, D.F.; Rodriguez, E.S.; Anjos, J.F.P.D.; Olímpio, R.G.; Lima, T.F.A.D.; Hayden, V.S.C.B.; Ferreira, Z.A.R.; Santos, M.D.; Santos, G.F.D.D.; et al. The use of floating macrophytes as a biological alternative for water quality improvement in eutrophic environments. Rev. Acadêmica Online 2025, 11, e1418. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Yang, J.; Zhou, S.; Du, J.; Zhang, M.; An, S. Mutual Promotion of Submerged Macrophytes and Biofilms on Artificial Macrophytes for Nitrogen and COD Removal Improvement in Eutrophic Water. Environ. Pollut. 2021, 277, 116718. [Google Scholar] [CrossRef] [PubMed]
- Adelodun, A.A.; Hassan, U.O.; Nwachuckwu, V.O. Environmental, Mechanical, and Biochemical Benefits of Water Hyacinth (Eichhornia Crassipes). Environ. Sci. Pollut. Res. 2020, 27, 30210–30221. [Google Scholar] [CrossRef] [PubMed]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Water Hyacinth (Eichhornia Crassipes) for Organic Contaminants Removal in Water—A Review. J. Hazard. Mater. Adv. 2022, 7, 100092. [Google Scholar] [CrossRef]
- Madikizela, L.M. Removal of Organic Pollutants in Water Using Water Hyacinth (Eichhornia Crassipes). J. Environ. Manag. 2021, 295, 113153. [Google Scholar] [CrossRef]
- Marques, R.Z.; Oliveira, P.G.D.; Barbato, M.L.; Kitamura, R.S.A.; Maranho, L.T.; Brito, J.C.M.; Nogueira, K.D.S.; Juneau, P.; Gomes, M.P. Green Solutions for Antibiotic Pollution: Assessing the Phytoremediation Potential of Aquatic Macrophytes in Wastewater Treatment Plants. Environ. Pollut. 2024, 357, 124376. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Said, N.S.M.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Macrophytes as Wastewater Treatment Agents: Nutrient Uptake and Potential of Produced Biomass Utilization toward Circular Economy Initiatives. Sci. Total Environ. 2021, 790, 148219. [Google Scholar] [CrossRef]
- Akowanou, A.V.O.; Deguenon, H.E.J.; Balogoun, K.C.; Daouda, M.M.A.; Aina, M.P. The Combined Effect of Three Floating Macrophytes in Domestic Wastewater Treatment. Sci. Afr. 2023, 20, e01630. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Roulet, E.; Mench, M. Rhizofiltration of a Bordeaux Mixture Effluent in Pilot-Scale Constructed Wetland Using Arundo Donax L. Coupled with Potential Cu-Ecocatalyst Production. Ecol. Eng. 2017, 105, 296–305. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Anuar, N.; Suja, F.; Mushrifah, I. Phytodegradation of Total Petroleum Hydrocarbon (TPH) in Diesel-Contaminated Water Using Scirpus Grossus. Ecol. Eng. 2015, 74, 463–473. [Google Scholar] [CrossRef]
- Schulz, M.; Kozerski, H.-P.; Pluntke, T.; Rinke, K. The Influence of Macrophytes on Sedimentation and Nutrient Retention in the Lower River Spree (Germany). Water Res. 2003, 37, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Saralegui, A.B.; Willson, V.; Caracciolo, N.; Piol, M.N.; Boeykens, S.P. Macrophyte Biomass Productivity for Heavy Metal Adsorption. J. Environ. Manag. 2021, 289, 112398. [Google Scholar] [CrossRef]
- Ali, F.; Jilani, G.; Fahim, R.; Bai, L.; Wang, C.; Tian, L.; Jiang, H. Functional and Structural Roles of Wiry and Sturdy Rooted Emerged Macrophytes Root Functional Traits in the Abatement of Nutrients and Metals. J. Environ. Manag. 2019, 249, 109330. [Google Scholar] [CrossRef]
- Mesquita, Y.W.; Mengatto, M.F.; Nagai, R.H. Where and How? A Systematic Review of Microplastic Pollution on Beaches in Latin America and the Caribbean (LAC). Environ. Pollut. 2022, 314, 120231. [Google Scholar] [CrossRef] [PubMed]
- Haury, J.; Peltre, M.-C.; Trémolières, M.; Barbe, J.; Thiébaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A New Method to Assess Water Trophy and Organic Pollution—The Macrophyte Biological Index for Rivers (IBMR): Its Application to Different Types of River and Pollution. In Macrophytes in Aquatic Ecosystems: From Biology to Management; Caffrey, J.M., Dutartre, A., Haury, J., Murphy, K.J., Wade, P.M., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 2006; Volume 190, pp. 153–158. ISBN 978-1-4020-5389-4. [Google Scholar]
- Schneider, S.; Melzer, A. The Trophic Index of Macrophytes (TIM)—A New Tool for Indicating the Trophic State of Running Waters. Int. Rev. Hydrobiol. 2003, 88, 49–67. [Google Scholar] [CrossRef]
- Różańska-Boczula, M.; Sender, J. Exploring the Relationships between Macrophyte Groups and Environmental Conditions in Lake Ecosystems. Sci. Rep. 2025, 15, 11162. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2019, 12, 108. [Google Scholar] [CrossRef]
- Malovanyy, M.S.; Boiaryn, M.; Muzychenko, O.; Tsos, O. Assessment of the environmental state of surface waters of right-bank tributaries of the upper reaches of the Pripet River by macrophyte index MIR. J. Water Land Dev. 2022, No. 55 (X–XII), 97–103. [Google Scholar] [CrossRef]
- Tsos, O.; Radzii, V.; Kocun, L.; Sukhomlin, K.; Melnyk, O. Assessment of the Ecological State of Surface Waters of the Luga River, in the Town of Volodymyr, by Macrophytes. Agron. Sci. 2024, 79, 125–135. [Google Scholar] [CrossRef]
- Babko, R.; Diachenko, T.; Zaburko, J.; Danko, Y.; Kuzmina, T.; Szulżyk-Cieplak, J.; Czarnota, J.; Łagód, G. Macrophyte Communities as Bioindicator of Stormwater Pollution in Rivers: A Quantitative Analysis. PeerJ 2023, 11, e15248. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Xiong, J.; Li, L.; Zhao, B.; Sohail, I.; He, Z. A Constructed Wetland System with Aquatic Macrophytes for Cleaning Contaminated Runoff/Storm Water from Urban Area in Florida. J. Environ. Manag. 2021, 280, 111794. [Google Scholar] [CrossRef] [PubMed]
- Szabó, S.; Koleszár, G.; Zavanyi, G.; Nagy, P.T.; Braun, M.; Hilt, S. Disentangling the Mechanisms Sustaining a Stable State of Submerged Macrophyte Dominance against Free-Floating Competitors. Front. Plant Sci. 2022, 13, 963579. [Google Scholar] [CrossRef] [PubMed]
- Szabó, S.; Csizmár, A.; Koleszár, G.; Oláh, V.; Birk, S.; Peeters, E.T.H.M. Density-Dependent Facilitation and Inhibition between Submerged and Free-Floating Plants. Hydrobiologia 2024, 851, 2749–2760. [Google Scholar] [CrossRef]
- Ciecierska, H.; Kolada, A. ESMI: A Macrophyte Index for Assessing the Ecological Status of Lakes. Environ. Monit. Assess. 2014, 186, 5501–5517. [Google Scholar] [CrossRef]
- Ijoma, G.N.; Lopes, T.; Mannie, T.; Mhlongo, T.N. Exploring Macrophytes’ Microbial Populations Dynamics to Enhance Bioremediation in Constructed Wetlands for Industrial Pollutants Removal in Sustainable Wastewater Treatment. Symbiosis 2024, 92, 323–354. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Li, Z.; Wang, G.; Liu, Y.; Wang, H.; Xie, J. Optimal Submerged Macrophyte Coverage for Improving Water Quality in a Temperate Lake in China. Ecol. Eng. 2021, 162, 106177. [Google Scholar] [CrossRef]
- Clairmont, L.K.; Stevens, K.J.; Slawson, R.M. Site-Specific Differences in Microbial Community Structure and Function within the Rhizosphere and Rhizoplane of Wetland Plants Is Plant Species Dependent. Rhizosphere 2019, 9, 56–68. [Google Scholar] [CrossRef]
- Toyama, T.; Furukawa, T.; Maeda, N.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Accelerated Biodegradation of Pyrene and Benzo[a]Pyrene in the Phragmites Australis Rhizosphere by Bacteria–Root Exudate Interactions. Water Res. 2011, 45, 1629–1638. [Google Scholar] [CrossRef]
- Ofek-Lalzar, M.; Sela, N.; Goldman-Voronov, M.; Green, S.J.; Hadar, Y.; Minz, D. Niche and Host-Associated Functional Signatures of the Root Surface Microbiome. Nat. Commun. 2014, 5, 4950. [Google Scholar] [CrossRef]
- Van Onsem, S.; Triest, L. Turbidity, Waterfowl Herbivory, and Propagule Banks Shape Submerged Aquatic Vegetation in Ponds. Front. Plant Sci. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Nabi, F.; Peng, Y.; Kama, R.; Sajid, S.; Memon, F.U.; Ma, C.; Li, H. Enhanced Nutrient Removal from Aquaculture Wastewater Using Optimized Constructed Wetlands: A Comprehensive Screening of Microbial Complexes, Substrates, and Macrophytes. J. Water Process Eng. 2025, 69, 106634. [Google Scholar] [CrossRef]
- Yan, P.; Peng, Y.; Fan, Y.; Zhang, M.; Chen, J.; Gu, X.; Sun, S.; He, S. Effects of Ferrous Addition to Vallisneria Natans: An Attempt to Apply Ferrous to Submerged Macrophyte Restoration. Environ. Res. 2023, 237, 117022. [Google Scholar] [CrossRef]
- Rodrigo, M.A. Wetland Restoration with Hydrophytes: A Review. Plants 2021, 10, 1035. [Google Scholar] [CrossRef]
- Van Der Cruysse, L.; De Cock, A.; Lock, K.; Boets, P.; Goethals, P.L.M. Introduction of Native Submerged Macrophytes to Restore Biodiversity in Streams. Plants 2024, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic Plasticity for Plant Development, Function and Life History. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Lin, J.; Wang, X.; Sun, S.; Hao, Q.; Wu, L.; Zhou, J.; Xia, S.; Ran, X.; et al. Silicon Promotes Biomass Accumulation in Phragmites Australis under Waterlogged Conditions in Coastal Wetland. Plant Soil 2024, 503, 503–516. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Martinsen, K.T.; Jakobsen, A.L.; Sø, J.S.; Madsen-Østerbye, M.; Kjær, J.E.; Kristensen, E.; Kragh, T. Large Pools and Fluxes of Carbon, Calcium and Phosphorus in Dense Charophyte Stands in Ponds. Sci. Total Environ. 2021, 765, 142792. [Google Scholar] [CrossRef]
- Nekos, A.; Boiaryn, M.; Karpyuk, Z.; Kotsun, L.; Andreyeva, V.; Lugowska, M. Evaluation of the Efficiency of Functioning of the Nature Reserve Fund in the Pripet River Basin in the Volyn Region. Visnyk VN Karazin Kharkiv Natl. Univ. Ser. Geol. Geogr. Ecol. 2024, 60, 389–398. [Google Scholar] [CrossRef]
- Fesyuk, V.O.; Moroz, I.A.; Kirchuk, R.V.; Polianskyi, S.V.; Fedoniuk, M.A. Soil Degradation in Volyn Region: Current State, Dynamics, Ways of Reduction. J. Geol. Geogr. Geoecology 2021, 30, 239–249. [Google Scholar] [CrossRef]
- Saalidong, B.M.; Aram, S.A.; Otu, S.; Lartey, P.O. Examining the Dynamics of the Relationship between Water pH and Other Water Quality Parameters in Ground and Surface Water Systems. PLoS ONE 2022, 17, e0262117. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.; Mishra, P.; Pandey, G. Application of Student’s t-Test, Analysis of Variance, and Covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Chidiac, S.; El Najjar, P.; Ouaini, N.; El Rayess, Y.; El Azzi, D. A Comprehensive Review of Water Quality Indices (WQIs): History, Models, Attempts and Perspectives. Rev. Environ. Sci. Biotechnol. 2023, 22, 349–395. [Google Scholar] [CrossRef]
- Carter, J.B.; Huffaker, R.; Singh, A.; Bean, E. HUM: A Review of Hydrochemical Analysis Using Ultraviolet-Visible Absorption Spectroscopy and Machine Learning. Sci. Total Environ. 2023, 901, 165826. [Google Scholar] [CrossRef]
- Holmes, N.T.H.; Newman, J.R.; Chadd, S. Mean Trophic Rank: A User’s Manual; Environment Agency: Copenhagen, Denmark, 1999; ISBN 978-1-85705-092-9. [Google Scholar]
- Von Raab-Straube, E.; Raus, T. Euro+Med-Checklist Notulae, 15. Willdenowia 2022, 52, 273–299. [Google Scholar] [CrossRef]
- Ciecierska, H.; Dynowska, M. (Eds.) Biologiczne Metody Oceny Stanu Środowiska: Podręcznik Metodyczny. T. 2: Ekosystemy Wodne; Wydawnictwo Mantis: Olsztyn, Poland, 2013; ISBN 978-83-62860-19-7. [Google Scholar]
- Lacoul, P.; Freedman, B. Environmental Influences on Aquatic Plants in Freshwater Ecosystems. Environ. Rev. 2006, 14, 89–136. [Google Scholar] [CrossRef]
- Ljevnaić-Mašić, B.; Džigurski, D.; Nikolić, L.; Brdar-Jokanović, M.; Čabilovski, R.; Ćirić, V.; Petrović, A. Assessment of the Habitat Conditions of a Rare and Endangered Inland Saline Wetland Community with Bolboschoenus Maritimus (L.) Palla Dominance in Southeastern Europe: The Effects of Physical–Chemical Water and Soil Properties. Wetl. Ecol. Manag. 2020, 28, 421–438. [Google Scholar] [CrossRef]
- Svitok, M.; Hrivnák, R.; Kochjarová, J.; Oťaheľová, H.; Paľove-Balang, P. Environmental Thresholds and Predictors of Macrophyte Species Richness in Aquatic Habitats in Central Europe. Folia Geobot. 2016, 51, 227–238. [Google Scholar] [CrossRef]
- Qureshimatva, U.M.; Maurya, R.R.; Gamit, S.B.; Patel, R.D.; Solanki, H.A. Determination of Physico-Chemical Parameters and Water Quality Index (Wqi) of Chandlodia Lake, Ahmedabad, Gujarat, India. J. Environ. Anal. Toxicol. 2015, 5, 288. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Radhakrishnan, K.; Aanand, S.; Rajaram, R. Influence of Physicochemical Water Quality on Aquatic Macrophyte Diversity in Seasonal Wetlands. Appl. Water Sci. 2019, 9, 12. [Google Scholar] [CrossRef]
- Shah, M.; Hashmi, H.N.; Ali, A.; Ghumman, A.R. Performance Assessment of Aquatic Macrophytes for Treatment of Municipal Wastewater. J. Environ. Health Sci. Eng. 2014, 12, 106. [Google Scholar] [CrossRef]
- Veraart, A.J.; De Bruijne, W.J.J.; De Klein, J.J.M.; Peeters, E.T.H.M.; Scheffer, M. Effects of Aquatic Vegetation Type on Denitrification. Biogeochemistry 2011, 104, 267–274. [Google Scholar] [CrossRef]
- Clarke, E.; Baldwin, A.H. Responses of Wetland Plants to Ammonia and Water Level. Ecol. Eng. 2002, 18, 257–264. [Google Scholar] [CrossRef]
- Bytyçi, P.; Shala-Abazi, A.; Zhushi-Etemi, F.; Bonifazi, G.; Hyseni-Spahiu, M.; Fetoshi, O.; Çadraku, H.; Feka, F.; Millaku, F. The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo. Plants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Daldorph, P.W.G.; Thomas, J.D. The Effect of Nutrient Enrichment on a Freshwater Community Dominated by Macrophytes and Molluscs and Its Relevance to Snail Control. J. Appl. Ecol. 1991, 28, 685. [Google Scholar] [CrossRef]
- Germ, M.; Janež, V.; Gaberščik, A.; Zelnik, I. Diversity of Macrophytes and Environmental Assessment of the Ljubljanica River (Slovenia). Diversity 2021, 13, 278. [Google Scholar] [CrossRef]
- Zhu, B.; Ottaviani, C.C.; Naddafi, R.; Dai, Z.; Du, D. Invasive European Frogbit (Hydrocharis Morsus-Ranae L.) in North America: An Updated Review 2003–16. J. Plant Ecol. 2018, 11, 17–25. [Google Scholar] [CrossRef]
- Zehnsdorf, A.; Hussner, A.; Eismann, F.; Rönicke, H.; Melzer, A. Management Options of Invasive Elodea Nuttallii and Elodea Canadensis. Limnologica 2015, 51, 110–117. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A.; Golob, A.; Germ, M. Evaluation of Nuphar Lutea as Bioindicator of Metal Pollution in Freshwater Ecosystems. Ecol. Indic. 2022, 136, 108633. [Google Scholar] [CrossRef]
- Qadri, H.; Uqab, B.; Javeed, O.; Dar, G.H.; Bhat, R.A. Ceratophyllum Demersum-An Accretion Biotool for Heavy Metal Remediation. Sci. Total Environ. 2022, 806, 150548. [Google Scholar] [CrossRef]
- Han, Y.; Jeppesen, E.; Lürling, M.; Zhang, Y.; Ma, T.; Li, W.; Chen, K.; Li, K. Combining Lanthanum-Modified Bentonite (LMB) and Submerged Macrophytes Alleviates Water Quality Deterioration in the Presence of Omni-Benthivorous Fish. J. Environ. Manage. 2022, 314, 115036. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Melnychuk, G.; Mazur, Ł.; Podlasek, A.; Vaverková, M.D.; Koda, E. Using Local Mineral Materials for the Rehabilitation of the Ustya River—A Case Study. Desalination Water Treat. 2021, 232, 346–356. [Google Scholar] [CrossRef]
- Sloey, T.M.; Hester, M.W. Impact of Nitrogen and Importance of Silicon on Mechanical Stem Strength in Schoenoplectus Acutus and Schoenoplectus Californicus: Applications for Restoration. Wetl. Ecol. Manag. 2018, 26, 459–474. [Google Scholar] [CrossRef]
- Frau, D.; Spies, M.E.; Battauz, Y.; Medrano, J.; Sinistro, R. Approaches for Phosphorus Removal with Calcium Hydroxide and Floating Macrophytes in a Mesocosm Experiment: Impacts on Plankton Structure. Hydrobiologia 2019, 828, 287–299. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water. Air. Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P.; Reeves, P. Restoring Shallow Lakes Impaired by Eutrophication: Approaches, Outcomes, and Challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1199–1246. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Gajewska, M. Phosphorus Removal by Application of Natural and Semi-Natural Materials for Possible Recovery According to Assumptions of Circular Economy and Closed Circuit of P. Sci. Total Environ. 2019, 650, 249–256. [Google Scholar] [CrossRef]
- Biliani, I.; Tsavatopoulou, V.; Zacharias, I. Comparative Study of Ammonium and Orthophosphate Removal Efficiency with Natural and Modified Clay-Based Materials, for Sustainable Management of Eutrophic Water Bodies. Sustainability 2024, 16, 10214. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Kong, L.; Bai, G.; Luo, F.; Liu, Z.; Wang, C.; Ding, Z.; He, F.; Wu, Z.; et al. Effects of Bentonite on the Growth Process of Submerged Macrophytes and Sediment Microenvironment. J. Environ. Manag. 2021, 287, 112308. [Google Scholar] [CrossRef]
- Burghelea, C.; Zaharescu, D.G.; Dontsova, K.; Maier, R.; Huxman, T.; Chorover, J. Mineral Nutrient Mobilization by Plants from Rock: Influence of Rock Type and Arbuscular Mycorrhiza. Biogeochemistry 2015, 124, 187–203. [Google Scholar] [CrossRef]
- Rodrigues, M.; Bortolini, P.C.; Neto, C.K.; De Andrade, E.A.; Dos Passos, A.I.; Pacheco, F.P.; Nanni, M.R.; De Melo Teixeira, L. Unlocking Higher Yields in Urochloa Brizantha: The Role of Basalt Powder in Enhancing Soil Nutrient Availability. Discov. Soil 2024, 1, 4. [Google Scholar] [CrossRef]
- Frątczak, W.; Michalska-Hejduk, D.; Zalewski, M.; Izydorczyk, K. Effective Phosphorous Reduction by a Riparian Plant Buffer Zone Enhanced with a Limestone-Based Barrier. Ecol. Eng. 2019, 130, 94–100. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Trach, R. Removal of Cationic and Anionic Pollutants from Water Solutions Using Ukrainian Limestones: A Comparative Analysis. Desalination Water Treat. 2022, 275, 24–34. [Google Scholar] [CrossRef]
- Angeler, D.G.; Drakare, S.; Johnson, R.K.; Köhler, S.; Vrede, T. Managing Ecosystems without Prior Knowledge: Pathological Outcomes of Lake Liming. Ecol. Soc. 2017, 22, art44. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Cametti, G.; Vanhecke, D.; Churakov, S.V. The Role of Interfaces in Controlling Pb2+ Removal by Calcium Carbonate Minerals. Cryst. Growth Des. 2020, 20, 6157–6169. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Stadnyk, O.; Trach, R.; Bujakowski, F.; Kiersnowska, A.; Rutkowska, G.; Skakun, L.; Szer, J.; Koda, E. The Possibility of Implementation of West Ukrainian Paleogene Glauconite–Quartz Sands in the Building Industry: A Case Study. Sustainability 2023, 15, 1489. [Google Scholar] [CrossRef]
- Szabó, S.; Fedor, N.; Koleszár, G.; Braun, M.; Korponai, J.; Kočić, A.; Hilt, S.; Oláh, V. Submerged Macrophytes Can Maintain Stable Dominance Over Free-Floating Competitors Through High pH. Freshw. Biol. 2025, 70, e14363. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater Photosynthesis of Submerged Plants—Recent Advances and Methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef]
- Vranayova, Z.; Tkachenko, T.; Lis, A.; Savchenko, O.; Vranay, F. Green Buildings in Pursuit of Healthy and Safe Human Living Environment. Syst. Saf. Hum.—Tech. Facil.—Environ. 2023, 5, 204–211. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V.; Konovaliuk, V.; Kravchenko, M.; Satin, I. Biotechnical Approach for a Continuous Simultaneous Increase of Indoor and Outdoor Air Quality. IOP Conf. Ser. Earth Environ. Sci. 2023, 1254, 012074. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V. Capturing Carbon Dioxide from Human-Driven Vehicles by Green Structures for Carbon Neutrality. IOP Conf. Ser. Earth Environ. Sci. 2022, 1111, 012056. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V.; Ujma, A. Field Study of Air Quality Improvement by a “Green Roof” in Kyiv. Syst. Saf. Hum.—Tech. Facil.—Environ. 2019, 1, 419–424. [Google Scholar] [CrossRef]
- Kravchenko, M.; Wrzesiński, G.; Pawluk, K.; Lendo-Siwicka, M.; Markiewicz, A.; Tkachenko, T.; Mileikovskyi, V.; Zhovkva, O.; Szymanek, S.; Piechowicz, K. Improving Urban Stormwater Management Using the Hydrological Model of Water Infiltration by Rain Gardens Considering the Water Column. Water 2024, 16, 2339. [Google Scholar] [CrossRef]
- Kravchenko, M.; Trach, Y.; Trach, R.; Tkachenko, T.; Mileikovskyi, V. Behaviour and Peculiarities of Oil Hydrocarbon Removal from Rain Garden Structures. Water 2024, 16, 1802. [Google Scholar] [CrossRef]
- Kravchenko, M.; Trach, Y.; Trach, R.; Tkachenko, T.; Mileikovskyi, V. Improving the Efficiency and Environmental Friendliness of Urban Stormwater Management by Enhancing the Water Filtration Model in Rain Gardens. Water 2024, 16, 1316. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V.; Ujma, A.; Hajiyev, M. Treatment of Out-of-Context Buildings During the Restoration of Historical Territories Using Green Structures. Int. J. Conserv. Sci. 2024, 15, 129–140. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V.; Dziubenko, V.; Tkachenko, O. Improvement of the Safety of Multi-Floor Housing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 907, 012064. [Google Scholar] [CrossRef]
- Tkachenko, T.; Mileikovskyi, V.; Kravchenko, M.; Konovaliuk, V. Simulation of Illumination and Wind Conditions for Green and Fed Cities Using CFD Software. IOP Conf. Ser. Earth Environ. Sci. 2023, 1275, 012014. [Google Scholar] [CrossRef]
- All-Ukraine NGO “Living Planet”. System for Environmental Certification and Ecolabelling According to DSTU ISO 14024:2018 (ISO 14024:2018, IDT). Public Buildings. Environmental Criteria and Method for Life Cycle Assessment; SOU OEM 08.002.41.032:20XX; Kyiv, 2025. Electronic Resource. Available online: https://livingplanet.org.ua/images/2025/28-02-2025-druga-redakciya.pdf (accessed on 7 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).