Inactivation of Continuously Released Airborne Virus by Upper-Room UVC LED Irradiation Under Realistic Testing Conditions
Abstract
1. Introduction
2. Materials and Methods
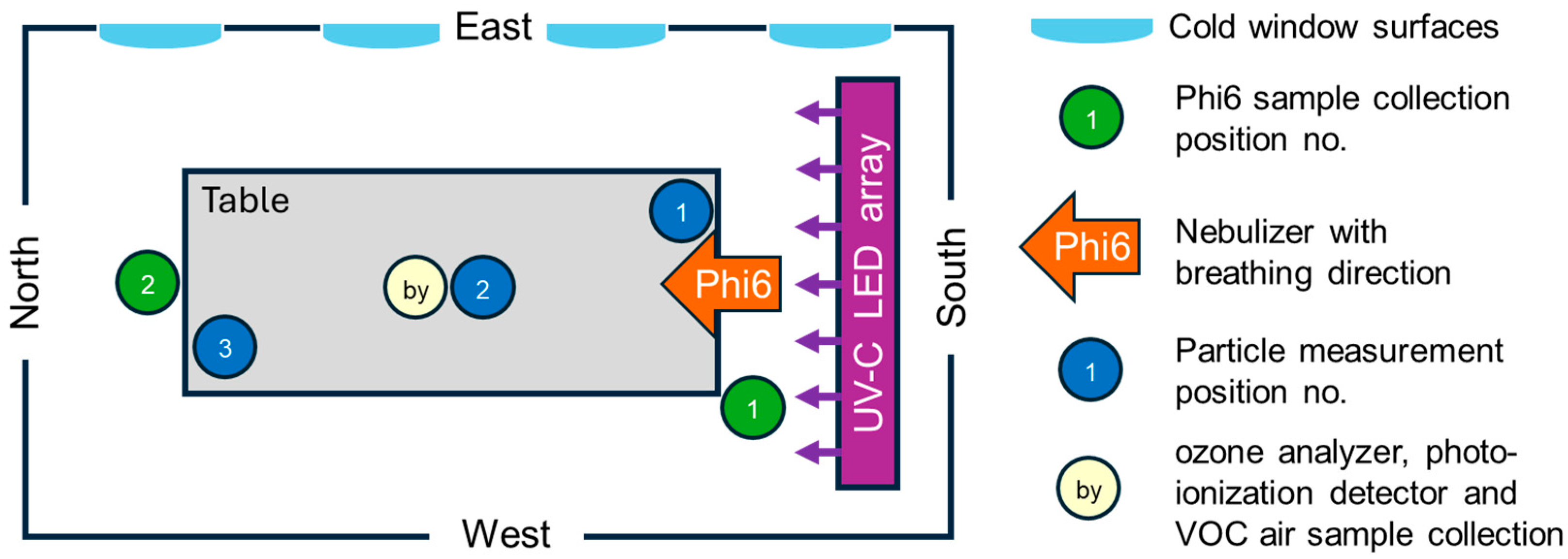
2.1. Test Room and Setup
- Indoor air temperature: 22.5 °C (±0.5 K)
- Relative humidity: 39% (±5%) for the dynamic equilibrium measurement and 32% (±5%) for the exponential decay measurement
- Air pressure 947 mbar
- Air exchange rate 0 h−1 (static, no air exchange applied in the test facility)
2.2. Materials, Equipment, and Methods
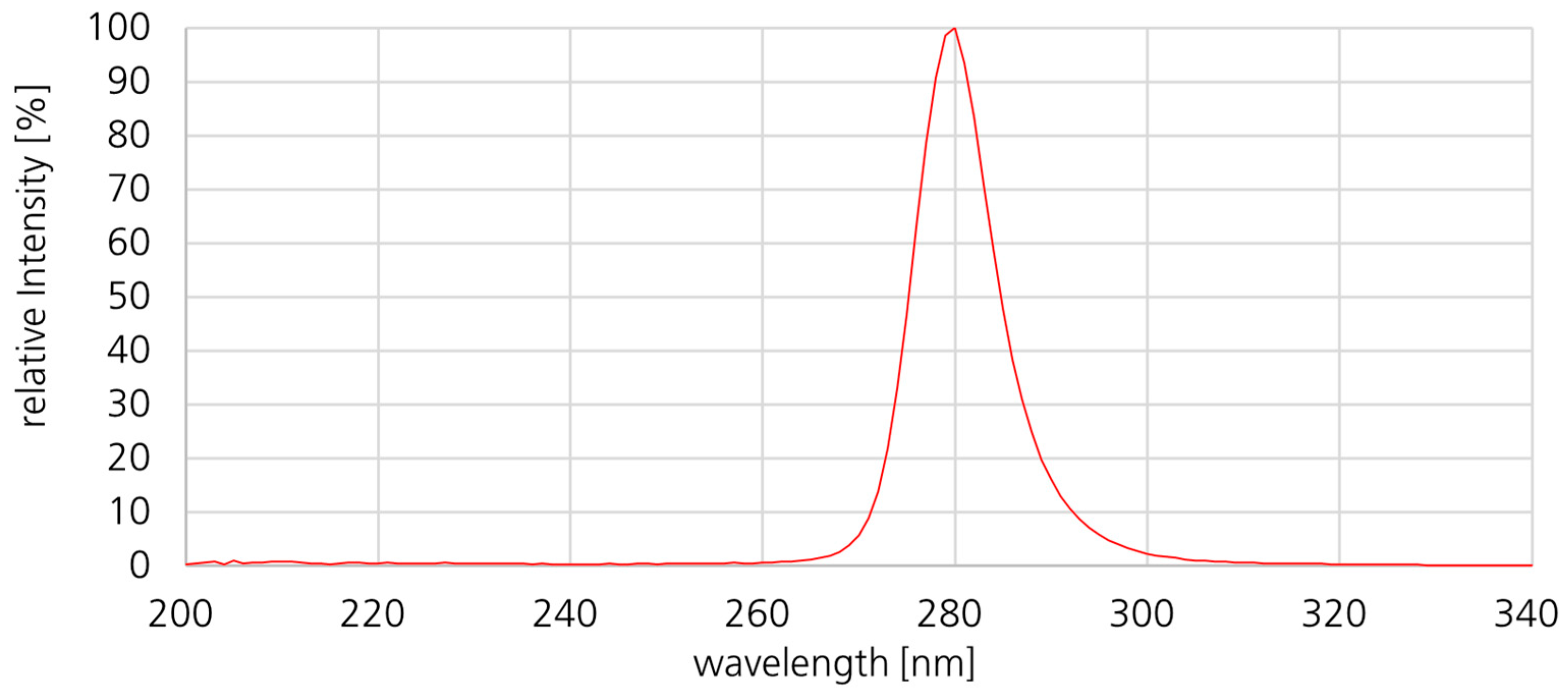
2.3. Description of the Investigated Upper-Room UVC Irradiation System
2.4. Implementation of the Investigations (Time Schedule, Ventilation)
2.5. General Considerations on Modeling the Concentration Profiles for Continuous Phi6 Release
- The process is dominated by the mixing. This condition applies to the aerosol loss coefficient. (k ≤ 0.2 h−1 --> → e−0.2×0.2 = e−0.04 = 96%)
- The process is dominated by the loss term. This condition applies to the inactivation Phi6 loss coefficient. (k ≥ 20 h−1 → e−0.2×20 = e−4 = 2%)
- The process is influenced by both loss term and mixing. This condition applies to the natural Phi6 loss coefficient. (k ≅ 3.5 h−1 → e−0.2×3.5 = e−0.7 = 50%)
2.6. Modeling for Continuous Phi6 Release in Combination with a Concentration Gradient
2.6.1. Calculation of Concentration Profile with Gradient Model
2.6.2. Determination of kAC Using the Gradient Model
2.6.3. Calculation of the Uncertainties for the Gradient Model
2.7. Modeling for Continuous Phi6 Release in Combination with Ideal Well-Mixed Conditions
2.7.1. Calculation of Concentration Profile with Well-Mixed Model
2.7.2. Determination of kAC for Well-Mixed Conditions
2.7.3. Calculation of the Uncertainties for the Well-Mixed Model
2.8. Testing Without Continuous Phi6 Release (Exponential Decay)
2.9. Modeling the Particle Concentration Profiles
3. Results
3.1. Loss Coefficient kAC of the UR-UVGI System Determined with Continuous Phi6 Release
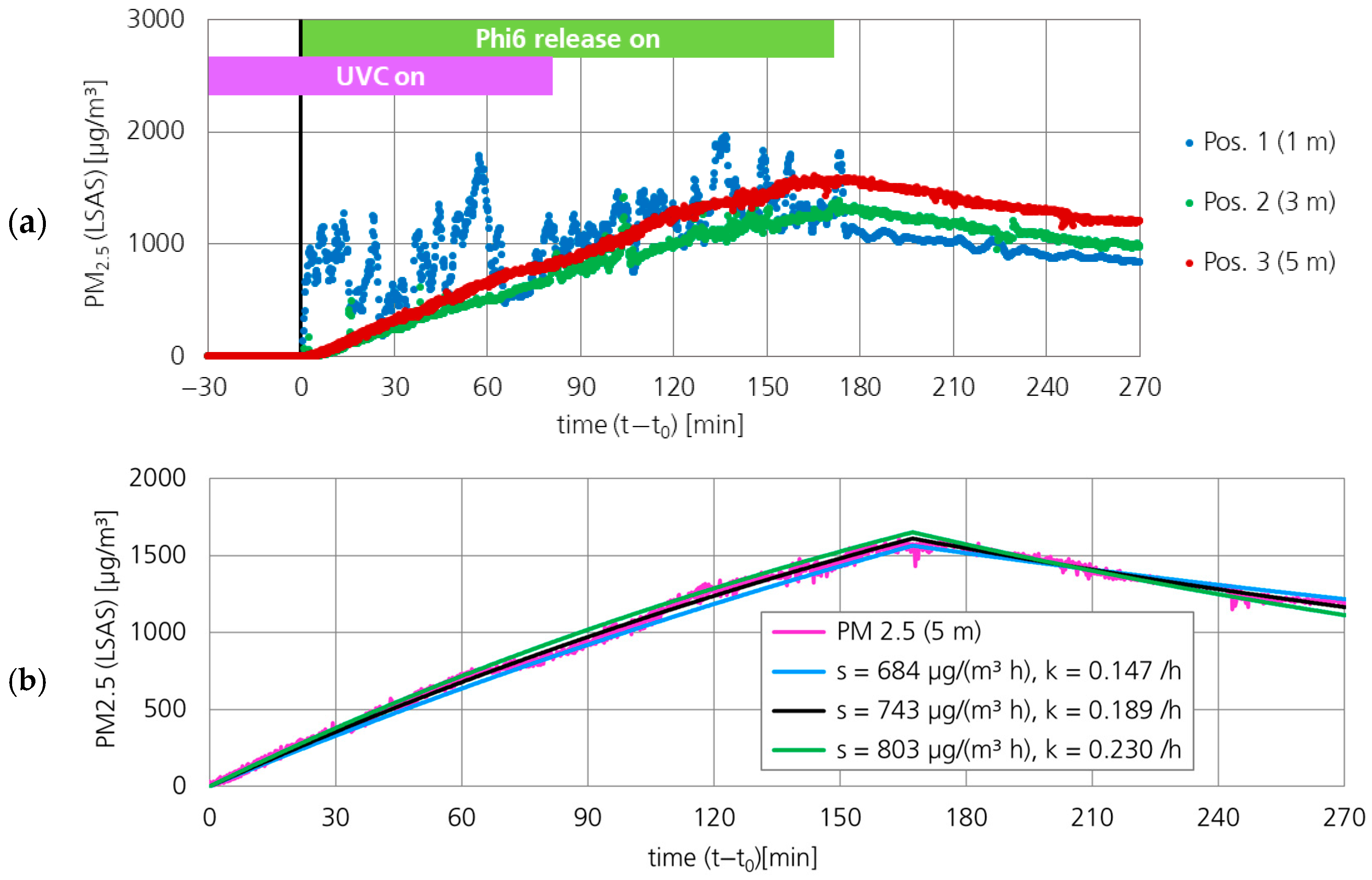
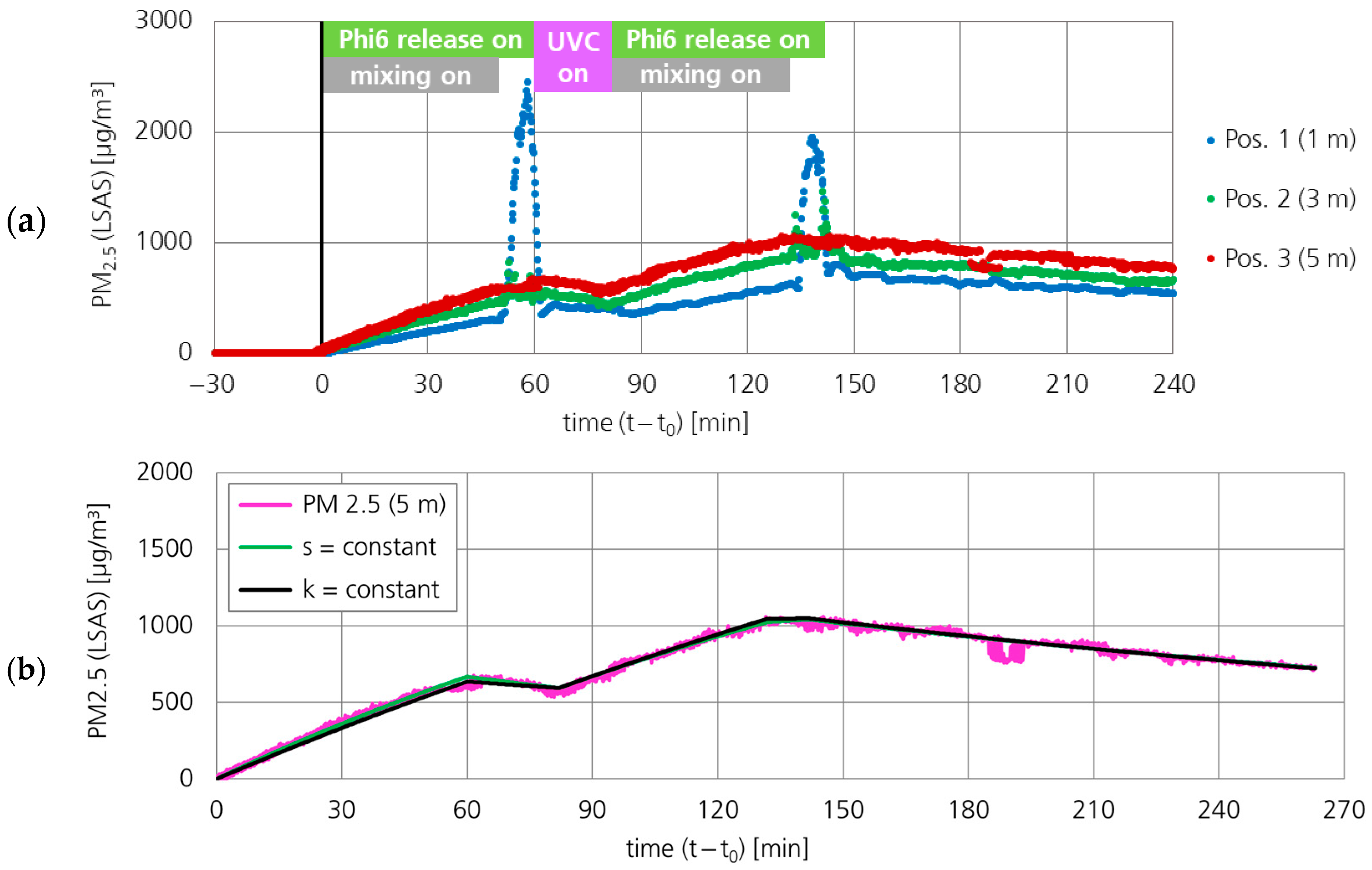
3.1.1. Particle Concentration (PM2.5)
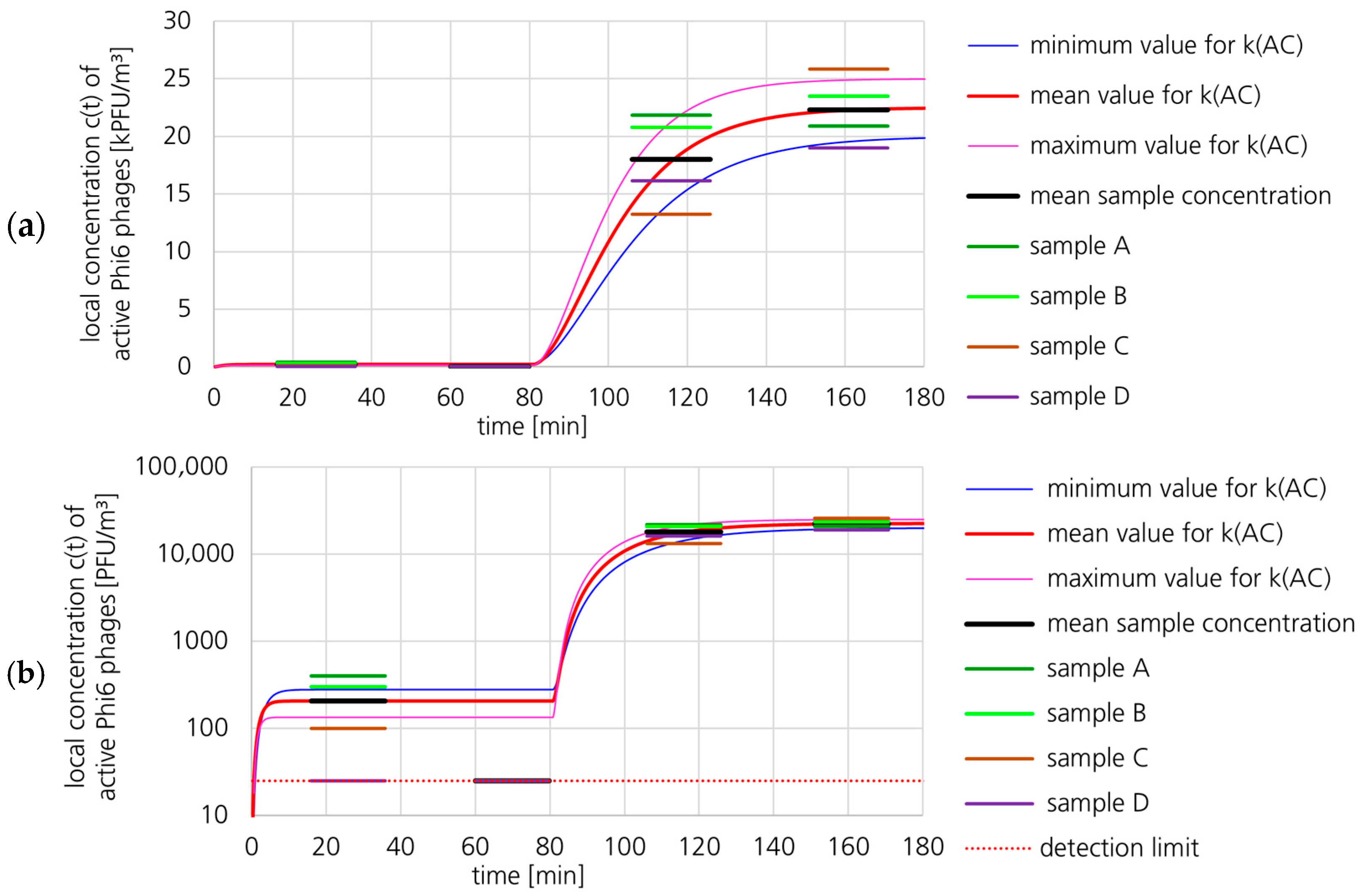
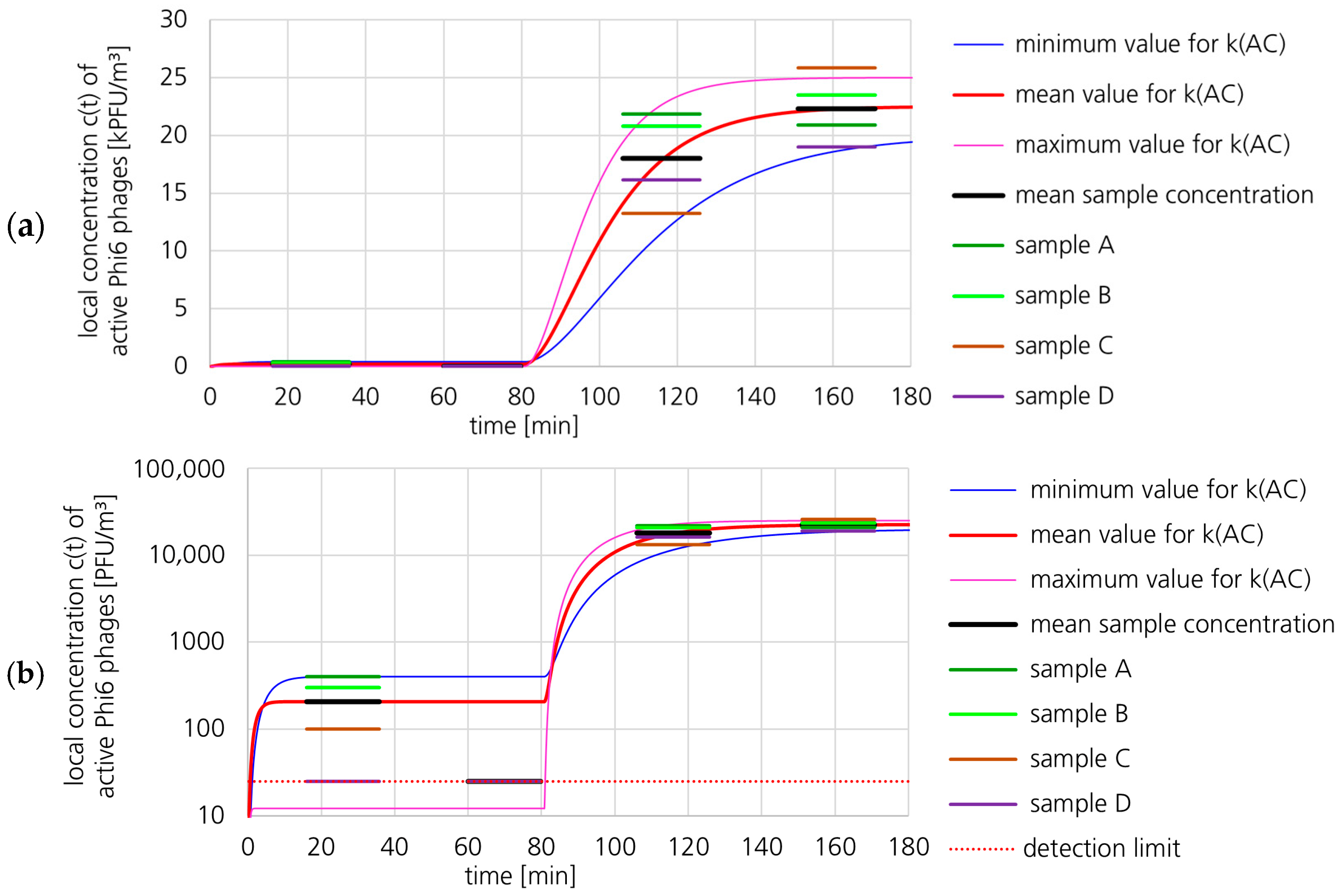
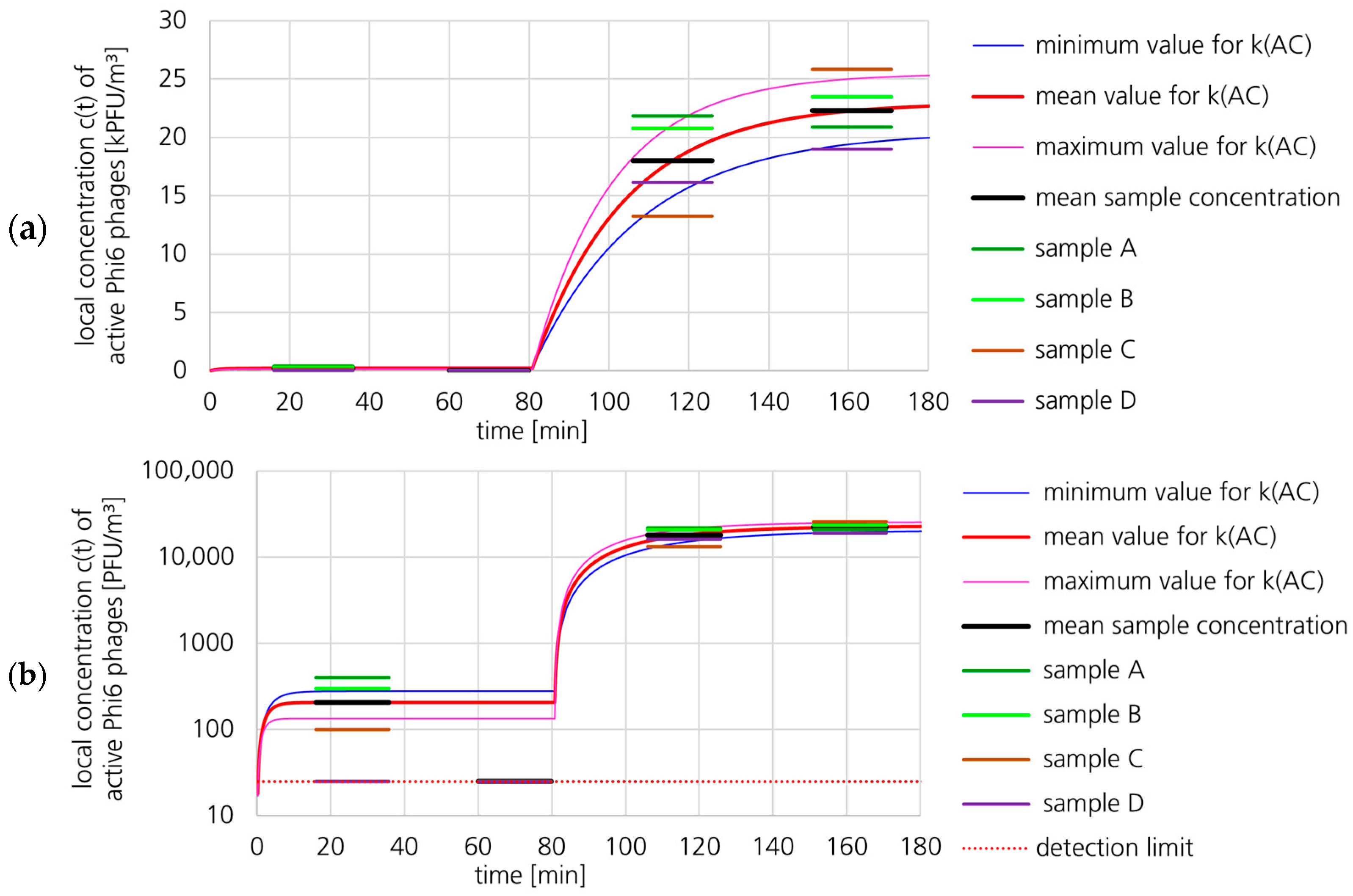
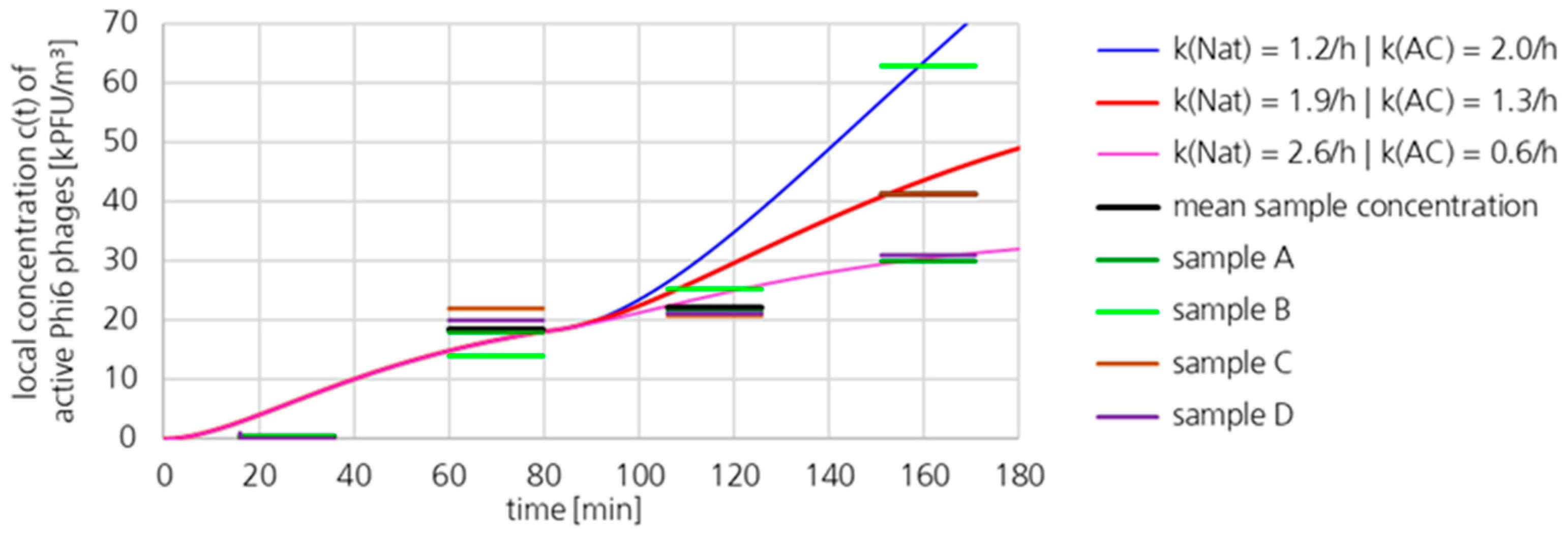
3.1.2. Active Phi6 Concentration at Pos. 2 with Gradient Model and Moderate Uncertainty
3.1.3. Active Phi6 Concentration at Pos. 2 with Gradient Model and High Uncertainty
3.1.4. Active Phi6 Concentration at Pos. 2 with Well-Mixed Model and Moderate Uncertainty
3.1.5. Active Phi6 Concentration at Pos. 1
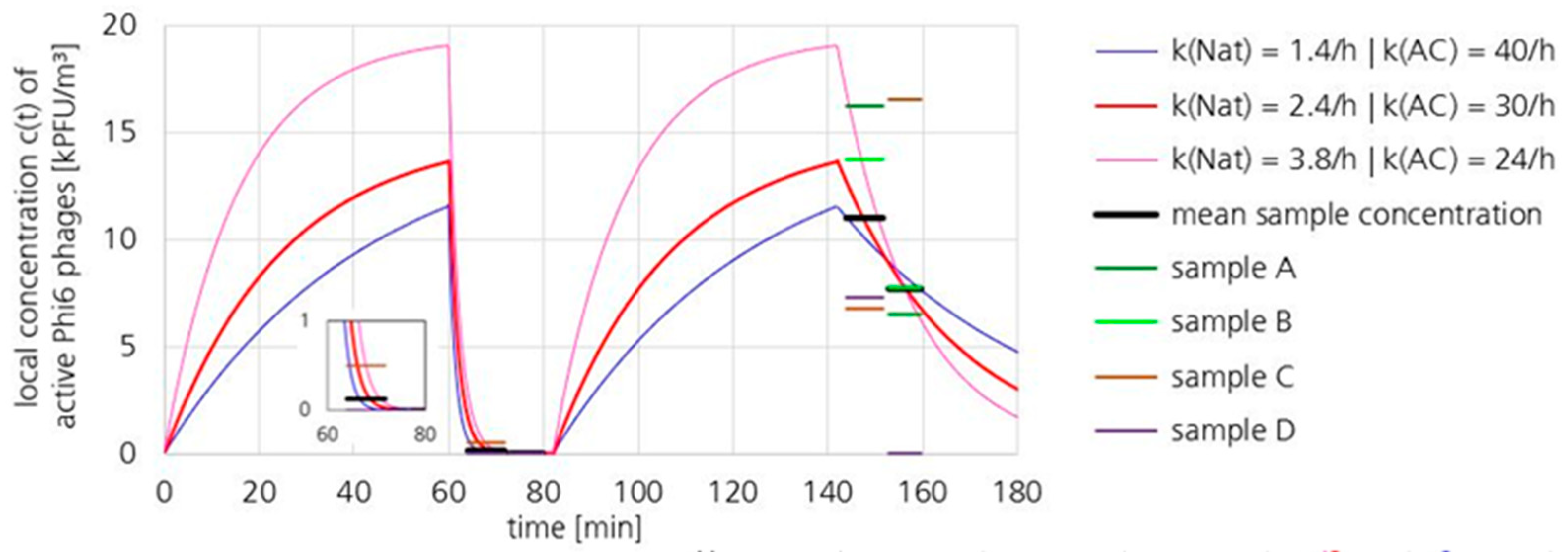
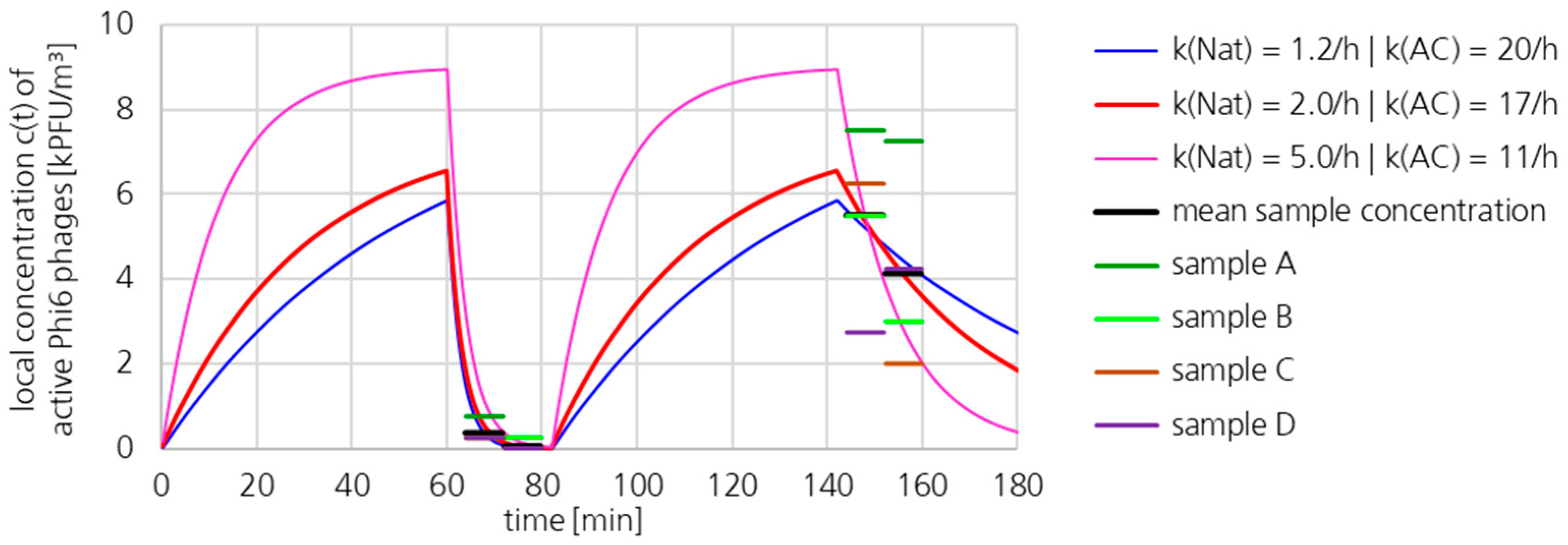
3.2. Loss Coefficient kAC of the UR-UVGI System Determined by Exponential Decay
3.2.1. Particle Concentration (PM2.5)
3.2.2. Active Phi6 Concentration at Pos. 2
3.2.3. Active Phi6 Concentration at Pos. 1
3.3. Summary of Determined Loss Coefficients
4. Discussion
4.1. Fundamental Considerations
4.2. Interpretation of the Test Results
4.3. Results Regarded as By-Products
4.4. UVC LED Technoloqy and Energy Concerns
4.5. UVC LED Technoloqy and Safety Concerns
4.6. Transfer of the Test Results to Other Interiors and Environmental Conditions
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, M.; Bartolomeu, M.; Vieira, C.; Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Photoinactivation of Phage Phi6 as a SARS-CoV-2 Model in Wastewater: Evidence of Efficacy and Safety. Microorganisms 2022, 10, 659. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Braz, M.; Costa, P.; Duarte, J.; Pereira, C.; Almeida, A. Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6. Microorganisms 2022, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 Survival on Surfaces and the Effect of UV-C Light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef]
- Belland, K.; Garcia, D.; DeJohn, C.; Allen, G.R.; Mills, W.D.; Glaudel, S.P. Safety and Effectiveness Assessment of Ultraviolet-C Disinfection in Aircraft Cabins. Aerosp. Med. Hum. Perform. 2024, 95, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.R.; Benner, K.J.; Bahnfleth, W.P. Inactivation of Pathogens in Air Using Ultraviolet Direct Irradiation Below Exposure Limits. J. Res. Natl. Inst. Stand. Technol. 2021, 126, 126052. [Google Scholar] [CrossRef]
- Sliney, D.H.; Stuck, B.E. A Need to Revise Human Exposure Limits for Ultraviolet UV-C Radiation†. Photochem. Photobiol. 2021, 97, 485–492. [Google Scholar] [CrossRef]
- Sliney, D. Balancing the risk of eye irritation from UV-C with infection from bioaerosols. Photochem. Photobiol. 2013, 89, 770–776. [Google Scholar] [CrossRef]
- Bang, J.-I.; Kim, J.-H.; Choi, A.; Sung, M. The Wavelength-Based Inactivation Effects of a Light-Emitting Diode Module on Indoor Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 9659. [Google Scholar] [CrossRef]
- Zhu, S.; Srebric, J.; Rudnick, S.N.; Vincent, R.L.; Nardell, E.A. Numerical investigation of upper-room UVGI disinfection efficacy in an environmental chamber with a ceiling fan. Photochem. Photobiol. 2013, 89, 782–791. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, T.; Wang, L.; Nardell, E.A.; Vincent, R.L.; Srebric, J. Ceiling impact on air disinfection performance of Upper-Room Germicidal Ultraviolet (UR-GUV). Build. Environ. 2022, 224, 109530. [Google Scholar] [CrossRef]
- Yıldırım, G.; Kılıç, H.; Karakaş, H.M. The antimicrobial efficacy of shielded ultraviolet germicidal irradiation in CT rooms with intense human circulation. Diagn. Interv. Radiol. 2021, 27, 293–301. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Z.; Liu, H.; Jiang, C.; Li, Y.; Liu, J.; He, J. Air disinfection performance of upper-room ultraviolet germicidal irradiation (UR-UVGI) system in a multi-compartment dental clinic. J. Hazard. Mater. 2024, 477, 135383. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, A.C.; Wu, C. Study on the disinfection efficiency of multiple upper-room ultraviolet germicidal fixtures system on airborne microorganisms. Build. Environ. 2016, 103, 99–110. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhang, H.H.; Chan, C.K.; Lee, P.; Lai, A. Experimental study of the disinfection performance of a 222-nm Far-UVC upper-room system on airborne microorganisms in a full-scale chamber. Build. Environ. 2023, 236, 110260. [Google Scholar] [CrossRef]
- Shen, J.; Kong, M.; Dong, B.; Birnkrant, M.J.; Zhang, J. Airborne transmission of SARS-CoV-2 in indoor environments: A comprehensive review. Sci. Technol. Built Environ. 2021, 27, 1331–1367. [Google Scholar] [CrossRef]
- Riley, R.L.; Permutt, S.; Kaufman, J.E. Room air disinfection by ultraviolet irradiation of upper air. Further analysis of convective air exchange. Arch. Environ. Health 1971, 23, 35–39. [Google Scholar] [CrossRef]
- Riley, R.L.; Permutt, S. Room air disinfection by ultraviolet irradiation of upper air. Air mixing and germicidal effectiveness. Arch. Environ. Health 1971, 22, 208–219. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Park, S.; Mistrick, R.; Rim, D. Performance of upper-room ultraviolet germicidal irradiation (UVGI) system in learning environments: Effects of ventilation rate, UV fluence rate, and UV radiating volume. Sustain. Cities Soc. 2022, 85, 104048. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Zhang, H.; Lai, A.C.K. Comparison of disinfection performance of UVC-LED and conventional upper-room UVGI systems. Indoor Air 2020, 30, 180–191. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Zhang, H.H.; Lai, A.C.K. A novel upper-room UVC-LED irradiation system for disinfection of indoor bioaerosols under different operating and airflow conditions. J. Hazard. Mater. 2020, 396, 122715. [Google Scholar] [CrossRef]
- Nunayon, S.S.; Wang, M.; Zhang, H.H.; Lai, A.C.K. Evaluating the efficacy of a rotating upper-room UVC-LED irradiation device in inactivating aerosolized Escherichia coli under different disinfection ranges, air mixing, and irradiation conditions. J. Hazard. Mater. 2022, 440, 129791. [Google Scholar] [CrossRef]
- Noakes, C.J.; Beggs, C.B.; Sleigh, P.A. Modelling the Performance of Upper Room Ultraviolet Germicidal Irradiation Devices in Ventilated Rooms: Comparison of Analytical and CFD Methods. Indoor Built Environ. 2004, 13, 477–488. [Google Scholar] [CrossRef]
- Nardell, E.; Vincent, R.; Sliney, D.H. Upper-room ultraviolet germicidal irradiation (UVGI) for air disinfection: A symposium in print. Photochem. Photobiol. 2013, 89, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Nardell, E.A. Indoor environmental control of tuberculosis and other airborne infections. Indoor Air 2016, 26, 79–87. [Google Scholar] [CrossRef]
- Miller, S.L. Upper Room Germicidal Ultraviolet Systems for Air Disinfection Are Ready for Wide Implementation. Am. J. Respir. Crit. Care Med. 2015, 192, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Am. J. Infect. Control. 2010, 38, S13–S24. [Google Scholar] [CrossRef]
- McPhaul, K.M. Indoor Air Disinfection: Answering Questions About Germicidal Ultraviolet Lights. Workplace Health Saf. 2022, 70, 431. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Milton, D.K.; Rudnick, S.N.; First, M.W. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLoS ONE 2008, 3, e3186. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Tseng, C.-H.; Wang, H.-C.; Dai, C.-F.; Shih, Y.-H. The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol. Int. J. Environ. Res. Public Health 2019, 16, 2557. [Google Scholar] [CrossRef]
- Linnes, J.C.; Rudnick, S.N.; Hunt, G.M.; McDevitt, J.J.; Nardell, E.A. Eggcrate UV: A whole ceiling upper-room ultraviolet germicidal irradiation system for air disinfection in occupied rooms. Indoor Air 2014, 24, 116–124. [Google Scholar] [CrossRef]
- Lee, L.D.; Lie, L.; Bauer, M.; Bolanos, B.; Olmsted, R.N.; Varma, J.K.; Parada, J.P. Reduction of airborne and surface-borne bacteria in a medical center burn intensive care unit using active, upper-room, germicidal ultraviolet (GUV) disinfection. Infect. Control Hosp. Epidemiol. 2024, 45, 367–373. [Google Scholar] [CrossRef]
- Landry, S.A.; Jamriska, M.; Menon, V.J.; Lee, L.Y.Y.; Magnin-Bougma, I.; Subedi, D.; Barr, J.J.; Monty, J.; Kevin, K.; Gunatilaka, A.; et al. Ultraviolet radiation vs air filtration to mitigate virus laden aerosol in an occupied clinical room. J. Hazard. Mater. 2025, 487, 137211. [Google Scholar] [CrossRef]
- Kujundzic, E.; Matalkah, F.; Howard, C.J.; Hernandez, M.; Miller, S.L. UV air cleaners and upper-room air ultraviolet germicidal irradiation for controlling airborne bacteria and fungal spores. J. Occup. Environ. Hyg. 2006, 3, 536–546. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S.; OAR. What Is Upper-Room Ultraviolet Germicidal Irradiation (UVGI)? What Is HVAC UVGI? Can Either Be Used to Disinfect the Air and Help Protect Myself from COVID? | US EPA. Available online: https://www.epa.gov/indoor-air-quality-iaq/what-upper-room-ultraviolet-germicidal-irradiation-uvgi-what-hvac-uvgi-can (accessed on 1 February 2025).
- Davidson, B.L. Bare-bulb Upper-Room Germicidal Ultraviolet-C (GUV) Indoor Air Disinfection for COVID-19†. Photochem. Photobiol. 2021, 97, 524–526. [Google Scholar] [CrossRef]
- Challener, D.W.; Tande, A.J.; Koutras, C.; Wade, R.L.; McIntee, M.A.; Strauss, D.M.; Yao, X.; Chang, Y.-H.; Berbari, E. Evaluation of germicidal ultraviolet-C disinfection in a real-world outpatient health care environment. Am. J. Infect. Control 2024, 52, 1030–1034. [Google Scholar] [CrossRef]
- Bergman, R.S. Germicidal UV Sources and Systems†. Photochem. Photobiol. 2021, 97, 466–470. [Google Scholar] [CrossRef]
- Bergman, R.; Brenner, D.; Buonanno, M.; Eadie, E.; Forbes, P.D.; Jensen, P.; Nardell, E.A.; Sliney, D.; Vincent, R.; Welch, D.; et al. Air Disinfection with Germicidal Ultraviolet: For this Pandemic and the Next. Photochem. Photobiol. 2021, 97, 464–465. [Google Scholar] [CrossRef]
- Beggs, C.B.; Avital, E.J. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: A feasibility study. PeerJ 2020, 8, e10196. [Google Scholar] [CrossRef]
- Bang, J.-I.; Park, J.; Choi, A.; Jeong, J.-W.; Kim, J.Y.; Sung, M. Evaluation of UR-UVGI System for Sterilization Effect on Microorganism Contamination in Negative Pressure Isolation Ward. Sustainability 2018, 10, 3192. [Google Scholar] [CrossRef]
- Nardell, E.A. Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential†. Photochem. Photobiol. 2021, 97, 493–497. [Google Scholar] [CrossRef]
- Nguyen, T.T.; He, C.; Carter, R.; Ballard, E.L.; Smith, K.; Groth, R.; Jaatinen, E.; Kidd, T.J.; Nguyen, T.-K.; Stockwell, R.E.; et al. The Effectiveness of Ultraviolet-C (UV-C) Irradiation on the Viability of Airborne Pseudomonas aeruginosa. Int. J. Environ. Res. Public Health 2022, 19, 13706. [Google Scholar] [CrossRef]
- Riley, R.L.; Permutt, S.; Kaufman, J.E. Convection, air mixing, and ultraviolet air disinfection in rooms. Arch. Environ. Health 1971, 22, 200–207. [Google Scholar] [CrossRef]
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Protocol for Determining Ultraviolet Light Emitting Diode (UV-LED) Fluence for Microbial Inactivation Studies. Environ. Sci. Technol. 2018, 52, 7390–7398. [Google Scholar] [CrossRef]
- Parry-Nweye, E.; Liu, Z.; Dhaouadi, Y.; Guo, X.; Huang, W.; Zhang, J.; Ren, D. Persistence of Phi6, a SARS-CoV-2 surrogate, in simulated indoor environments: Effects of humidity and material properties. PLoS ONE 2025, 20, e0313604. [Google Scholar] [CrossRef]
- French, A.J.; Longest, A.K.; Pan, J.; Vikesland, P.J.; Duggal, N.K.; Marr, L.C.; Lakdawala, S.S. Environmental Stability of Enveloped Viruses Is Impacted by Initial Volume and Evaporation Kinetics of Droplets. mBio 2023, 14, e03452-22. [Google Scholar] [CrossRef]
- Niazi, S.; Short, K.R.; Groth, R.; Cravigan, L.; Spann, K.; Ristovski, Z.; Johnson, G.R. Humidity-Dependent Survival of an Airborne Influenza A Virus: Practical Implications for Controlling Airborne Viruses. Environ. Sci. Technol. Lett. 2021, 8, 412–418. [Google Scholar] [CrossRef]
- Prussin, A.J.; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity, and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef]
- Plano LMde Franco, D.; Rizzo, M.G.; Zammuto, V.; Gugliandolo, C.; Silipigni, L.; Torrisi, L.; Guglielmino, S.P.P. Role of Phage Capsid in the Resistance to UV-C Radiations. Int. J. Mol. Sci. 2021, 22, 3408. [Google Scholar] [CrossRef]
- Masjoudi, M.; Mohseni, M.; Bolton, J.R. Sensitivity of Bacteria, Protozoa, Viruses, and Other Microorganisms to Ultraviolet Radiation. J. Res. Natl. Inst. Stand. Technol. 2021, 126, 126021. [Google Scholar] [CrossRef]
- Oh, C.; Sun, P.P.; Araud, E.; Nguyen, T.H. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222 nm. Water Res. 2020, 186, 116386. [Google Scholar] [CrossRef]
- Claus, H. Ozone Generation by Ultraviolet Lamps†. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef]
- Sørensen, S.B.; Dalby, F.R.; Olsen, S.K.; Kristensen, K. Influence of Germicidal UV (222 nm) Lamps on Ozone, Ultrafine Particles, and Volatile Organic Compounds in Indoor Office Spaces. Environ. Sci. Technol. 2024, 58, 20073–20080. [Google Scholar] [CrossRef]
- Ishida, K.; Onoda, Y.; Kadomura-Ishikawa, Y.; Nagahashi, M.; Yamashita, M.; Fukushima, S.; Aizawa, T.; Yamauchi, S.; Fujikawa, Y.; Tanaka, T.; et al. Development of a standard evaluation method for microbial UV sensitivity using light-emitting diodes. Heliyon 2024, 10, e27456. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Kaneko, C.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid Inactivation of SARS-CoV-2 Variants by Continuous and Intermittent Irradiation with a Deep-Ultraviolet Light-Emitting Diode (DUV-LED) Device. Pathogens 2021, 10, 754. [Google Scholar] [CrossRef]
- Takamure, K.; Sakamoto, Y.; Iwatani, Y.; Amano, H.; Yagi, T.; Uchiyama, T. Characteristics of collection and inactivation of virus in air flowing inside a winding conduit equipped with 280 nm deep UV-LEDs. Environ. Int. 2022, 170, 107580. [Google Scholar] [CrossRef]
- Takamure, K.; Iwatani, Y.; Amano, H.; Yagi, T.; Uchiyama, T. Inactivation characteristics of a 280 nm Deep-UV irradiation dose on aerosolized SARS-CoV-2. Environ. Int. 2023, 177, 108022. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Bhopal, R.; Nguyen Tien, H. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-COV-2: Comparison with other respiratory viruses. Epidemiol. Infect. 2021, 149, e96. [Google Scholar] [CrossRef]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef]
- Sankaran, A.; Hain, R.; Matheis, C.; Fuchs, T.; Norrefeldt, V.; Grün, G.; Kähler, C.J. Investigation on the thermal budget and flow field of a manikin and comparison with human subject in different scenarios. Build. Environ. 2024, 252, 111290. [Google Scholar] [CrossRef]
- VDI-EE 4300-Blatt 14; Messen Von Innenraumluftverunreinigungen—Anforderungen an Mobile luftreiniger Zur Reduktion der Aerosolgebundenen Übertragung Von Infektionskrankheiten. Measurement of Indoor Pollution—Requirements for Mobile Air Purifiers to Reduce Aerosol-Borne Transmission of Infectious Diseases. VDI: Düsseldorf, Germany, 2021.
- ANSI/AHAM AC-1-2020; Method for Measuring Performance of Portable Household Electric Room Air Cleaners. American National Standards Institute and White Goods Manufacturers Association: Washington, DC, USA, 2020.
- de Aquino Carvalho, N.; Stachler, E.N.; Cimabue, N.; Bibby, K. Evaluation of Phi6 Persistence and Suitability as an Enveloped Virus Surrogate. Environ. Sci. Technol. 2017, 51, 8692–8700. [Google Scholar] [CrossRef]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rose, L. Persistence of Bacteriophage Phi 6 on Porous and Nonporous Surfaces and the Potential for Its Use as an Ebola Virus or Coronavirus Surrogate. Appl. Environ. Microbiol. 2020, 86, e01482-20. [Google Scholar] [CrossRef]
- Weyersberg, L.; Klemens, E.; Buehler, J.; Vatter, P.; Hessling, M. UVC, UVB and UVA susceptibility of Phi6 and its suitability as a SARS-CoV-2 surrogate. AIMS Microbiol. 2022, 8, 278–291. [Google Scholar] [CrossRef]
- Greenwald, R.; Hayat, M.J.; Dons, E.; Giles, L.; Villar, R.; Jakovljevic, D.G.; Good, N. Estimating minute ventilation and air pollution inhaled dose using heart rate, breath frequency, age, sex and forced vital capacity: A pooled-data analysis. PLoS ONE 2019, 14, e0218673. [Google Scholar] [CrossRef]
- AHAM AC-5-2022; Method for Assessing the Reduction Rate of Key Bioaero-Sols by Portable Air Cleaners Using an Aerobiology Test Chamber. American National Standards Institute and White Goods Manufacturers Association: Washington, DC, USA, 2022.
- DIN ISO 16000-16:2009-12; Innenraumluftverunreinigungen-Teil_16: Nachweis Und Zählung Von Schimmelpilzen—Probenahme Durch Filtration (ISO_16000-16:2008). DIN Media GmbH: Berlin, Germany, 2009.
- DIN ISO 16000-17:2010-06; Innenraumluftverunreinigungen-Teil_17: Nachweis Und Zählung Von Schimmelpilzen—Kultivierungsverfahren (ISO_16000-17:2008). DIN Media GmbH: Berlin, Germany, 2010.
- Baer, A.; Kehn-Hall, K. Viral concentration determination through plaque assays: Using traditional and novel overlay systems. J. Vis. Exp. JoVE 2014, 93, e52065. [Google Scholar] [CrossRef]
- DIN EN 13610:2003-06; Chemische Desinfektionsmittel—Quantitativer Suspensionsversuch zur Bestimmung der Viruziden Wirkung gegenüber Bakteriophagen von Chemischen Desinfektionsmitteln in den Bereichen Lebensmittel und Industrie - Prüfverfahren und Anforderungen (Phase 2, Stufe 1). DIN Media GmbH: Berlin, Germany, 2003. [CrossRef]
- DIN ISO 16000-6:2012-11; Innenraumluftverunreinigungen—Teil 6: Bestimmung von VOC in der Innenraumluft und in Prüfkammern, Probenahme auf Tenax TA®, thermische Desorption und Gaschromatographie mit MS oder MS-FID (ISO_16000-6:2011). Beuth Verlag GmbH: Berlin, Germany, 2012. [CrossRef]
- DIN ISO 16000-3:2013-01; Innenraumluftverunreinigungen—Teil 3: Messen von Formaldehyd und anderen Carbonylverbindungen in der Innenraumluft und in Prüfkammern—Probenahme mit einer Pumpe (ISO 16000-3:2011). Beuth Verlag GmbH: Berlin, Germany, 2013. [CrossRef]
- Prohaska, R.; Wieser, A.; Muschaweck, J. Lamp and System with Wall-Type Radiation Fields for Preventing or Minimising the Spread of Pathogens in Indoor Air. WO/2021/249668, 16.12.2021. US20230218791A1, 13 July 2023. [Google Scholar]
- Schmohl, A.; Buschhaus, M.; Norrefeldt, V.; Johann, S.; Burdack-Freitag, A.; Scherer, C.R.; Vega Garcia, P.A.; Schwitalla, C. Incremental Evaluation Model for the Analysis of Indoor Air Measurements. Atmosphere 2022, 13, 1655. [Google Scholar] [CrossRef]
- Schumacher, S.; Asbach, C.; Schmid, H.-J. Effektivität von Luftreinigern zur Reduzierung des COVID-19-Infektionsrisikos/Efficacy of air purifiers in reducing the risk of COVID-19 infections. Gefahrstoffe 2021, 81, 16–28. [Google Scholar] [CrossRef]
- Eadie, E.; Hiwar, W.; Fletcher, L.; Tidswell, E.; O’Mahoney, P.; Buonanno, M.; Welch, D.; Adamson, C.S.; Brenner, D.J.; Noakes, C.; et al. Far-UVC (222 nm) efficiently inactivates an airborne pathogen in a room-sized chamber. Sci. Rep. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Burdack-Freitag, A.; Buschhaus, M.; Grün, G.; Hofbauer, W.K.; Johann, S.; Nagele-Renzl, A.M.; Schmohl, A.; Scherer, C.R. Particulate Matter versus Airborne Viruses—Distinctive Differences between Filtering and Inactivating Air Cleaning Technologies. Atmosphere 2022, 13, 1575. [Google Scholar] [CrossRef]
- Chiappa, F.; Frascella, B.; Vigezzi, G.P.; Moro, M.; Diamanti, L.; Gentile, L.; Lago, P.; Clementi, N.; Signorelli, C.; Mancini, N.; et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: A systematic review. J. Hosp. Infect. 2021, 114, 63–78. [Google Scholar] [CrossRef]
- Dziubenko, O.; Arhun, S.; Hnatov, A.; Bogdan, D.; Patlins, A. Device for Inactivation of SARS-CoV-2 Using UVC LEDs. Elektron. Elektrotechnika 2022, 28, 55–61. [Google Scholar] [CrossRef]
- Gerchman, Y.; Mamane, H.; Friedman, N.; Mandelboim, M. UV-LED disinfection of Coronavirus: Wavelength effect. J. Photochem. Photobiol. B Biol. 2020, 212, 112044. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Teng, Y.-T.; Yeh, Y.-W.; Fan, X.; Chu, K.-H.; Lin, S.-H.; Yeh, K.-K.; Lee, P.-T.; Lin, Y.; Chen, Z.; et al. Perspectives on UVC LED: Its Progress and Application. Photonics 2021, 8, 196. [Google Scholar] [CrossRef]
- Kapse, S.; Rahman, D.; Avital, E.J.; Venkatesan, N.; Smith, T.; Cantero-Garcia, L.; Motallebi, F.; Samad, A.; Beggs, C.B. Conceptual Design of a UVC-LED Air Purifier to Reduce Airborne Pathogen Transmission—A Feasibility Study. Fluids 2023, 8, 111. [Google Scholar] [CrossRef]
- Ploydaeng, M.; Rajatanavin, N.; Rattanakaemakorn, P. UV-C light: A powerful technique for inactivating microorganisms and the related side effects to the skin. Photodermatol. Photoimmunol. Photomed. 2021, 37, 12–19. [Google Scholar] [CrossRef]
- Görlitz, M.; Justen, L.; Rochette, P.J.; Buonanno, M.; Welch, D.; Kleiman, N.J.; Eadie, E.; Kaidzu, S.; Bradshaw, W.J.; Javorsky, E.; et al. Assessing the safety of new germicidal far-UVC technologies. Photochem. Photobiol. 2024, 100, 501–520. [Google Scholar] [CrossRef]
- Brickner, P.W.; Vincent, R.L. Ultraviolet germicidal irradiation safety concerns: A lesson from the Tuberculosis Ultraviolet Shelter Study: Murphy’s Law affirmed. Photochem. Photobiol. 2013, 89, 819–821. [Google Scholar] [CrossRef]
- European Council. DIRECTIVE 2006/42/EC on Machinery Lays Down Health and Safety Requirements for the Design and Construction of Machinery, Placed on the European Market. Available online: https://eur-lex.europa.eu/eli/dir/2006/42/oj/eng (accessed on 3 July 2025).
- European Council. DIRECTIVE 2014/35/EU on the Harmonisation of the Laws of the Member States Relating to the Making Available on the Market of Electrical Equipment Designed for Use Within Certain Voltage Limits. 2014. Available online: https://eur-lex.europa.eu/eli/dir/2014/35/oj/eng (accessed on 3 July 2025).
- DIN EN ISO 12100:2011-03; Sicherheit Von Maschinen—Allgemeine Gestaltungsleitsätze—Risikobeurteilung und Risikominderung (ISO 12100:2010); Deutsche Fassung EN ISO 12100:2010. DIN Media GmbH: Berlin, Germany, 2011.
- DIN EN 60335-2-65:2013-02; Household and Similar Electrical Appliances—Safety—Part 2–65: Particular Requirements for Air-Cleaning Appliances. DIN Media GmbH: Berlin, Germany, 2013.
- European Council. DIRECTIVE 2006/25/EC on the Minimum Health and Safety Requirements Regarding the Exposure of Workers to Risks Arising from Physical Agents (Artificial Optical Radiation). 2024. Available online: https://eur-lex.europa.eu/eli/dir/2006/25/oj/eng (accessed on 3 July 2025).
- DIN EN 62471:2009-03; Photobiological Safety of Lamps and Lamp Systems. DIN Media GmbH: Berlin, Germany, 2009.
- DIN EN IEC 62471-6:2024-05; Photobiological Safety of Lamps and Lamp Systems—Part 6: Ultraviolet Lamp Products. DIN Media GmbH: Berlin, Germany, 2024.
- Harmon, M.; Lau, J. The Facility Infection Risk Estimator™: A web application tool for comparing indoor risk mitigation strategies by estimating airborne transmission risk. Indoor Built Environ. 2022, 31, 1339–1362. [Google Scholar] [CrossRef]
- Matheis, C.; Norrefeldt, V.; Will, H.; Herrmann, T.; Noethlichs, B.; Eckhardt, M.; Stiebritz, A.; Jansson, M.; Schön, M. Modeling the Airborne Transmission of SARS-CoV-2 in Public Transport. Atmosphere 2022, 13, 389. [Google Scholar] [CrossRef]
- Leithäuser, C.; Norrefeldt, V.; Thiel, E.; Buschhaus, M.; Kuhnert, J.; Suchde, P. Predicting aerosol transmission in airplanes: Benefits of a joint approach using experiments and simulation. Numer. Methods Fluids 2024, 96, 991–1010. [Google Scholar] [CrossRef]
- Nicas, M.; Miller, S.L. A multi-zone model evaluation of the efficacy of upper-room air ultraviolet germicidal irradiation. Appl. Occup. Environ. Hyg. 1999, 14, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.L.; Nardell, E.A. Clearing the air. The theory and application of ultraviolet air disinfection. Am. Rev. Respir. Dis. 1989, 139, 1286–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Vallance, R.; Hii, K.-F.; Gordon, P.; Zong, Y.; Miller, C. Stamped metallic optical reflectors for ultraviolet light emitting diodes. In Light-Emitting Devices, Materials, and Applications XXVI; Proceedings SPIE 12022; SPIE: San Francisco, CA, USA, 2022; 120220N (3 March 2022); pp. 1–12. [Google Scholar] [CrossRef]
- European Council. DIRECTIVE 2011/65/EU on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. 2011. Available online: https://eur-lex.europa.eu/eli/dir/2011/65/oj/eng (accessed on 3 July 2025).
- European Union. REGULATION (EU) 2017/852 on Mercury, and Repealing Regulation (EC) No 1102/2008. 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/852/oj/eng (accessed on 3 July 2025).
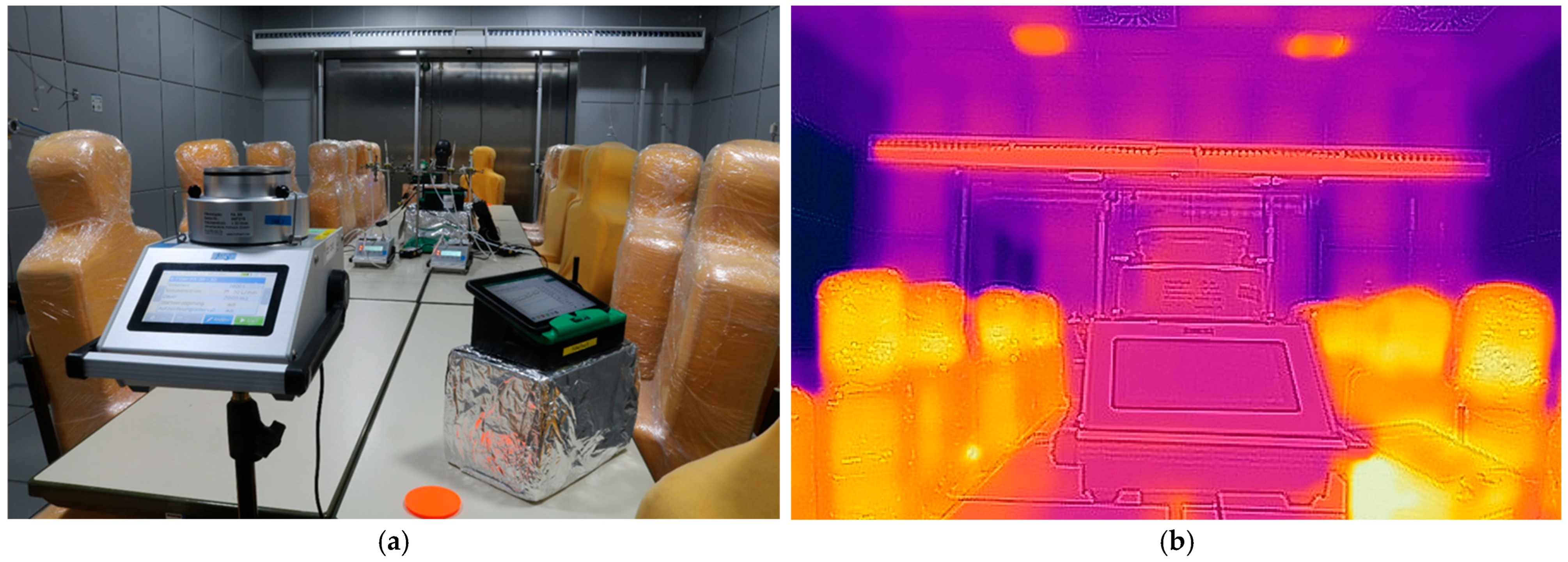
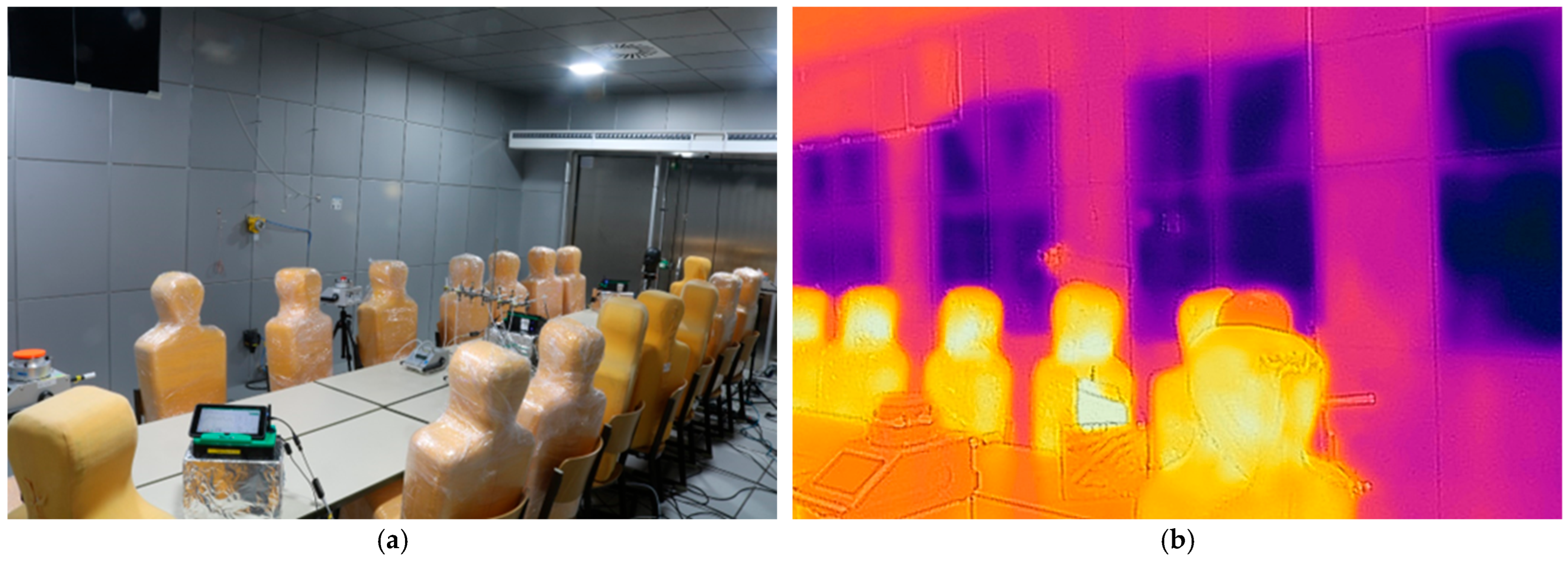
| Location in the Test room | Surface Temperature [°C] | Flow Velocity [m/s] |
|---|---|---|
| east wall | 21 | 0.06–0.13 |
| “windows” (cooled segments on east wall) | 17.5 | 0.15–0.30 |
| west wall | 21 | 0.06–0.12 |
| north wall | 23 | 0.50–0.60 |
| south wall | 23 | 0.05–0.07 |
| ceiling | 23 | - |
| floor | 23 | - |
| Thermal manikins on the north side | ≈30 | 0.14–0.19 |
| Thermal manikins on the east side | ≈30 | 0.10–0.18 |
| Thermal manikins on the south side | ≈30 | 0.10–0.18 |
| “breathing” Sheffield-Head (south side) | ≈23 | 0.48–1.00 |
| UVC LED radiation module (south side) | - | 0.18–0.20 |
| Time of Day | tevent [h] | Event |
|---|---|---|
| 08:30 | −0.50 | Start of particle measurement |
| 08:30 | −0.50 | UR-UVGI system was switched on. |
| 09:00 | 0.00 | Aerosol release was switched on. (0 min) |
| 09:16 | 0.27 | Collection of first air sample until 09:36. |
| 10:00 | 1.00 | Collection of second air sample until 10:20. |
| 10:21 | 1.35 | UR-UVGI system was switched off. |
| 10:46 | 1.77 | Collection of third air sample until 11:05. |
| 11:31 | 2.52 | Collection of fourth air sample until 11:51. |
| 11:53 | 1.88 | Aerosol release was switched off. (173 min) |
| 13:50 | 4.83 | End of particle measurement |
| Time of Day | tevent [h] | Event |
|---|---|---|
| 08:26 | −0.57 | Start of particle measurement |
| 08:29 | −0.52 | Start of air mixing (two fans oriented against each other) |
| 09:00 | 0.00 | Aerosol release was switched on (0 min) |
| 09:50 | 0.83 | End of air mixing |
| 10:00 | 1.00 | Aerosol release was switched off (60 min) |
| 10:00 | 1.00 | UR-UVGI system was switched on. |
| 10:04 | 1.07 | Collection of first air sample until 10:12. |
| 10:12 | 1.20 | Collection of second air sample until 10:20. |
| 10:22 | 1.37 | UR-UVGI system was switched off. |
| 10:22 | 1.37 | Start of air mixing (two fans oriented against each other) |
| 10:22 | 1.37 | Aerosol release was switched on (82 min) |
| 11:12 | 2.20 | End of air mixing (132 min) |
| 11:22 | 2.37 | Aerosol release was switched off (142 min) |
| 11:26 | 2.43 | Collection of third air sample until 11:34. |
| 11:34 | 2.57 | Collection of fourth air sample until 11:52. |
| 13:24 | 4.40 | End of particle measurement |
| Mean Value | Deviation Absolute | Deviation Relative | Partial or Total Uncertainty for kAC [h−1] | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|
| kNat [h−1] | 5.0 | 0.75 | 15% | 7.1 (1) | 4.25 | 5.75 |
| c1eq [PFU/m3] | 206 | 72 | 35% | 9.1 (1) | 134 | 278 |
| c2eq [PFU/m3] | 22,500 | 2500 | 11% | 2.9 (1) | 20,000 | 25,000 |
| kAC [h−1] | 47.3 | n.a. (2) | 25% | 11.9 (3) | 32 (4) | 73 (5) |
| Mean Value | Deviation Absolute | Deviation Relative | Partial or Total Uncertainty for kAC [h−1] | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|
| kNat [h−1] | 5.0 | 1.75 | 35% | 16.5 (1) | 3.25 | 6.75 |
| c1eq [PFU/m3] | 206 | 194 | 94% | 24.6 (1) | 12 | 400 |
| c2eq [PFU/m3] | 22,500 | 2500 | 11% | 2.9 (1) | 20,000 | 25,000 |
| kAC [h−1] | 47.3 | n.a. (2) | 63% | 29.8 (3) | 19.7 (4) | 301 (5) |
| Mean Value | Deviation Absolute | Deviation Relative | Partial or Total Uncertainty for kAC [h−1] | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|
| kNat [h−1] | 2.60 | 0.39 | 15% | 43 (1) | 2.21 | 2.99 |
| c1eq [PFU/m3] | 206 | 72 | 35% | 102 (1) | 134 | 278 |
| c2eq [PFU/m3] | 23,000 | 2500 | 11% | 32 (1) | 20,500 | 25,500 |
| kAC [h−1] | 288 | n.a. (2) | 40% | 115 (3) | 161 (4) | 566 (5) |
| Mean Value | Deviation Absolute | Deviation Relative | Partial or Total Uncertainty for kAC [h−1] | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|
| kNat [h−1] | 2.60 | 0.39 | 15% | 3.7 (1) | 2.21 | 2.99 |
| c1eq [PFU/m3] | 206 | 72 | 35% | 4.8 (1) | 134 | 278 |
| c2eq [PFU/m3] | 23,000 | 2500 | 11% | 1.5 (1) | 20,500 | 25,500 |
| kAC [h−1] | 24.9 | n.a. (2) | 25% | 6.3 (3) | 17 (4) | 38 (5) |
| Line in Figure 9 | Fit Parameter | 0–60 min | 60–82 min | 82–132 min | 132–142 min | 142–260 min |
|---|---|---|---|---|---|---|
| s = constant | k [h−1] | 0.322 | 0.322 | 0.322 | 0.664 | 0.178 |
| (green line) | s [µg/(m3 h)] | 781 | 0 | 781 | 781 | 0 |
| k = constant | k [h−1] | 0.185 | 0.185 | 0.185 | 0.185 | 0.185 |
| (black line) | s [µg/(m3 h)] | 696 | 0 | 696 | 201 | 0 |
| Position in the Test Room | Dynamic Equilibrium | Exponential Decay | ||||
|---|---|---|---|---|---|---|
| (Compare Figure 3) | kAC [h−1] | kNat [h−1] | FR (1) | kAC [h−1] | kNat [h−1] | FR (2) |
| Pos. 2 | 47 (32–73) (3) 288 (161–566) (4) 25 (17–38) (5) | 5.0 (4.25–5.75) (3) 2.6 (2.21–2.99) (4) 2.6 (2.21–2.99) (5) | 109 (3) 111 (4) 111 (5) | 30 (24–40) | 2.4 (1.4–3.8) | 182 |
| Pos. 1 | 1.3 (0.6–2.0) (6) | 1.9 (1.2–2.6) (6) | <2.8 (6) | 17 (11–20) | 2.0 (1.2–5.0) | 90 |
| Parameter | Natural Loss (Without UVC) | UVC Loss Pos. 2 Exponential Decline | UVC loss Pos. 2 (1) Dynamic Equilibrium | UVC Loss Pos. 2 (2) Dynamic Equilibrium |
|---|---|---|---|---|
| Loss coefficient k [h−1] | 2.8 (1.2–5.75) | 30 (24–40) | 47 (32–73) | 25 (17–38) |
| Log reduction in 10 min [-] | 0.2 (0.1–0.4) | 2.2 (1.7–2.9) | 3.4 (2.3–5.3) | 1.8 (1.2–2.8) |
| Log reduction in 30 min [-] | 0.6 (0.3–1.2) | 6.5 (5.2–8.7) | 10.2 (6.9–15.9) | 5.4 (3.7–8.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmohl, A.; Nagele-Renzl, A.; Buschhaus, M. Inactivation of Continuously Released Airborne Virus by Upper-Room UVC LED Irradiation Under Realistic Testing Conditions. Environments 2025, 12, 233. https://doi.org/10.3390/environments12070233
Schmohl A, Nagele-Renzl A, Buschhaus M. Inactivation of Continuously Released Airborne Virus by Upper-Room UVC LED Irradiation Under Realistic Testing Conditions. Environments. 2025; 12(7):233. https://doi.org/10.3390/environments12070233
Chicago/Turabian StyleSchmohl, Andreas, Anna Nagele-Renzl, and Michael Buschhaus. 2025. "Inactivation of Continuously Released Airborne Virus by Upper-Room UVC LED Irradiation Under Realistic Testing Conditions" Environments 12, no. 7: 233. https://doi.org/10.3390/environments12070233
APA StyleSchmohl, A., Nagele-Renzl, A., & Buschhaus, M. (2025). Inactivation of Continuously Released Airborne Virus by Upper-Room UVC LED Irradiation Under Realistic Testing Conditions. Environments, 12(7), 233. https://doi.org/10.3390/environments12070233







