Analytical Workflow for Tracking Aquatic Biomass Responses to Sea Surface Temperature Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Input Data
2.2. Performing Principal Component Analysis on a Dataset
2.3. Analysis Workflow Structure
2.3.1. Step 1: Regression Model Definition
- R-squared (R2): Indicates the accuracy of the model, measuring the portion of the variance in observed data captured by the model. Its value ranges between 0 and 1. Values closer to 1 indicate a better performance of the data variance model, and vice versa, so values closer to 0 indicate a worse data variance model.
- Mean Absolute Error (MAE): Indicates the accuracy of the model measuring the average absolute difference between observed and forecast value. Low values indicate good model performance.
- Root Mean Squared Error (RMSE): Indicates the accuracy of the model by measuring the average of the squared difference between observed and forecast values. In this case, the accuracy is evaluated giving more importance to the larger errors. Low values indicate good model performance.
2.3.2. Step 2: SST, Chl-a and NPP Time Series Construction
2.3.3. Step 3: Temporal Projection of the Time Series
- Seasonal (S): this component refers to the repeating patterns (daily, monthly, yearly or other) in the time series.
- Autoregressive (AR): this component captures the relationship between the current data point and its previous values, accounting for the autocorrelation in the time series.
- Integrated (I): this element transforms a non-stationary time series into a stationary one by applying differencing to reduce trends or seasonality.
- Moving Average (MA): the MA component identifies short-term noise by analyzing the relationship between the current data point and past forecast errors.
3. Results
3.1. PCA
3.2. Regression Model Definition
3.3. Analytical Workflow
3.4. Projection of the Time Series into the Future
4. Discussion
- Long-term dynamics from 2003 to 2023, which may be driven by global warming, which poses a greater risk to the system, potentially leading to significant long-term disruptions if the trend continues.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chl-a | Chlorophyll-a |
| NPP | Net Primary Production |
| SST | Sea Surface Temperature |
| ENSO | El Niño–Southern Oscillation |
References
- Garrison, T.S. Oceanography: An Invitation to Marine Science; Thompson Brooks/Cole: Baltimore, MD, USA, 2005; Volume 4. [Google Scholar]
- Barbier, E.B. Marine ecosystem services. Curr. Biol. 2017, 27, R507–R510. [Google Scholar] [CrossRef] [PubMed]
- Costanza, R.; Fisher, B.; Mulder, K.; Liu, S.; Christopher, T. Biodiversity and ecosystem services: A multi-scale empirical study of the relationship between species richness and net primary production. Ecol. Econ. 2007, 61, 478–491. [Google Scholar] [CrossRef]
- Wirtz, K.; Smith, S.L.; Mathis, M.; Taucher, J. Vertically migrating phytoplankton fuel high oceanic primary production. Nat. Clim. Change 2022, 12, 750–756. [Google Scholar] [CrossRef]
- World Ocean Review. WOR 8 The Ocean—A Climate Champion? How to Boost Marine Carbon Dioxide Uptake. 2024. Available online: https://worldoceanreview.com/en/wor-8/the-role-of-the-ocean-in-the-global-carbon-cyclee/how-the-ocean-absorbs-carbon-dioxide/ (accessed on 10 January 2025).
- Canadell, J.G.; Monteiro, P.M.S.; Costa, M.H.; da Cunha, L.C.; Cox, P.M.; Eliseev, A.V.; Henson, S.; Ishii, M.; Jaccard, S.; Koven, C.; et al. Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 673–816. [Google Scholar] [CrossRef]
- Mapping Ocean Wealth. Esosystem Services. Available online: https://oceanwealth.org/ecosystem-services/ (accessed on 23 February 2025).
- Vuong, Q.-H.; Duong, M.-P.T.; Nguyen, Q.-Y.T.; La, V.-P.; Nguyen, P.-T.; Nguyen, M.-H. Ocean economic and cultural benefit perceptions as stakeholders’ constraints for supporting conservation policies: A multi-national investigation. Mar. Policy 2024, 163, 106134. [Google Scholar] [CrossRef]
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Peters, G.P.; Ciais, P.; Friedlingstein, P.; Jones, S.D.; Sitch, S.; Tans, P.; Arneth, A.; et al. Global carbon budget 2014. Earth Syst. Sci. Data 2015, 7, 47–85. [Google Scholar] [CrossRef]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Lungomela, C.; Nyamisi, P. Chapter 10: Phytoplankton and Ocean Primary Productivity. In State of the Coast, for mainland Tazania; Transitioning to Blue Economy: Contribution of Coastal and marine Environmental; Mangora, M.M., Msangameno, D.J., Woiso, J.F., Eds.; Western Indian Ocean Marine Science Association (WIOMSA): Zanzibar, Tanzania, 2024; ISBN 978-9912-9882-0-0. [Google Scholar]
- A Climate Change Dashboard. Available online: https://chpdb.it/_climate_dash/index.php?tag=temperatura (accessed on 15 July 2024).
- Woodhouse, A.; Swain, A.; Fagan, W.F.; Fraass, A.J.; Lowery, C.M. Late Cenozoic cooling restructured global marine plankton communities. Nature 2023, 614, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Racault, M.-F.; Sathyendranath, S.; Brewin, R.J.W.; Raitsos, D.E.; Jackson, T.; Platt, T. Impact of El Niño Variability on Oceanic Phytoplankton. Front. Mar. Sci. 2017, 4, 133. [Google Scholar] [CrossRef]
- Arteaga, L.A.; Rousseaux, C.S. Impact of Pacific Ocean heatwaves on phytoplankton community composition. Commun. Biol. 2023, 6, 263. [Google Scholar] [CrossRef]
- Sigman, D.M.; Hain, M.P. The Biological Productivity of the Ocean. Nat. Educ. Knowl. 2012, 3, 21. [Google Scholar]
- Cervantes-Duarte, R.; González-Rodríguez, E.; Funes-Rodríguez, R.; Ramos-Rodríguez, A.; Torres-Hernández, M.Y.; Aguirre-Bahena, F. Variability of Net Primary Productivity and Associated Biophysical Drivers in Bahía de La Paz (Mexico). Remote Sens. 2021, 13, 1644. [Google Scholar] [CrossRef]
- Semeraro, T.; Luvisi, A.; Lillo, A.; Aretano, R.; Buccolieri, R.; Marwan, N. Recurrence Analysis of Vegetation Indices for Highlighting the Ecosystem Response to Drought Events: An Application to the Amazon Forest. Remote Sens. 2020, 12, 907. [Google Scholar] [CrossRef]
- Semeraro, T.; Buccolieri, R.; Vergine, M.; De Bellis, L.; Luvisi, A.; Emmanuel, R.; Marwan, N. Analysis of Olive Grove Destruction by Xylella fastidiosa Bacterium on the Land Surface Temperature in Salento Detected Using Satellite Images. Forests 2021, 12, 1266. [Google Scholar] [CrossRef]
- Barbosa, C.C.A.; Atkinson, P.M.; Dearing, J.A. Remote sensing of ecosystem services: A systematic review. Ecol. Indic. 2015, 52, 430–443. [Google Scholar] [CrossRef]
- Amani, M.; Moghimi, A.; Mirmazloumi, S.M.; Ranjgar, B.; Ghorbanian, A.; Ojaghi, S.; Ebrahimy, H.; Naboureh, A.; Nazari, M.E.; Mahdavi, S.; et al. Ocean Remote Sensing Techniques and Applications: A Review (Part I). Water 2022, 14, 3400. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, R.; Duan, H.; Pahlevan, N.; Melack, J.; Shen, M.; Xue, K. A machine learning approach to estimate chlorophyll-a from Landsat-8 measurements in inland lakes. Remote Sens. Environ. 2020, 248, 111974. [Google Scholar] [CrossRef]
- Ocean Productivity. Available online: http://orca.science.oregonstate.edu/npp_products.php (accessed on 10 June 2024).
- PANGAEA. Data Publisher for Earth & Environmental Science. Available online: https://www.pangaea.de/ (accessed on 10 January 2025).
- Redalje, D.G.; Laws, E.A. A new method for estimating phytoplankton growth rates and carbon biomass. Mar. Biol. 1981, 62, 73–79. [Google Scholar] [CrossRef]
- Hein, M.; Riemann, B. Nutrient limitation of phytoplankton biomass or growth rate: An experimental approach using marine enclosures. J. Exp. Mar. Biol. Ecol. 1995, 188, 67–180. [Google Scholar] [CrossRef]
- Steeman Nielsen, E.; Jensen, E.A. The autotrophic production of organic matter in the oceans. Calathea Rep. 1957, 1, 49–124. [Google Scholar]
- Marra, J. Net and gross productivity: Weighting in with 14C. Aquat. Microb. Ecol. 2009, 56, 123–131. [Google Scholar] [CrossRef]
- Siswanto, E.; Ye, H.; Yamazaki, D.; Tang, D. Detailed spatiotemporal impacts of El Niño on phytoplankton biomass in the South China Sea. J. Geophys. Res. Ocean. 2017, 12, 2709–2723. [Google Scholar] [CrossRef]
- World Health Organization. El Niño Southern Oscillation (ENSO). 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/el-nino-southern-oscillation-(enso) (accessed on 8 September 2024).
- Christopher, J. Internal wave detection using the Moderate Resolution Imaging Spectroradiometer (MODIS). J. Geophys. Res. 2007, 112, C11012. [Google Scholar] [CrossRef]
- EARTHDATA. Ocean Color. Available online: https://oceancolor.gsfc.nasa.gov/ (accessed on 1 December 2024).
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 2003, 84, 2347–2363. [Google Scholar] [CrossRef]
- Elhaik, E. Principal Component Analyses (PCA)-based findings in population genetic studies are highly biased and must be reevaluated. Sci. Rep. 2022, 12, 14683. [Google Scholar] [CrossRef]
- Ilin, A.; Raiko, T. Practical approaches to Principal Component Analysis in the presence of missing values. J. Mach. Learn. Res. 2010, 11, 1957–2000. [Google Scholar]
- LifeWatchItaly. DataLabs: LifeWatch’s Collaborative Coding Platform for Biodiversity and Ecosystem Research. Available online: https://datalabs.lifewatchitaly.eu/ (accessed on 15 July 2024).
- Rodriguez-Galiano, V.; Sanchez-Castillo, M.; Chica-Olmo, M.; Chica-Rivas, M. Machine learning predictive models for mineral prospectivity: An evaluation of neural networks, random forest, regression trees and support vector machines. Ore Geol. Rev. 2015, 71, 804–818. [Google Scholar] [CrossRef]
- Ramezan, C.A.; Warner, T.A.; Maxwell, A.E. Evaluation of Sampling and Cross-Validation Tuning Strategies for Regional-Scale Machine Learning Classification. Remote Sens. 2019, 11, 185. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Zhao, W.; Shi, T.; Hu, Z.; Li, Q.; Wu, G. Comparison of Machine Learning Techniques in Inferring Phytoplankton Size Classes. Remote Sens. 2018, 10, 191. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Marwan, N.; Carmen Romano, M.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, K.; Yan, W.; Cheng, C. Forecasting the incidence of tuberculosis in China using the seasonal auto-regressive integrated moving average (SARIMA) model. J. Infect. Public Health 2018, 11, 707–712. [Google Scholar] [CrossRef]
- Adams, S.O.; Mustapha, B.; Alumbugu, A.I. Seasonal Autoregressive Integrated Moving Average (SARIMA) Model for the Analysis of Frequency of Monthly Rainfall in Osun State, Nigeria. Phys. Sci. Int. J. 2019, 22, 1–9. Available online: https://ssrn.com/abstract=4338971 (accessed on 10 August 2019). [CrossRef]
- Zhang, X.; Pang, Y.; Cui, M.; Stallones, L.; Xiang, H. Forecasting mortality of road traffic injuries in China using seasonal autoregressive integrated moving average model. Ann. Epidemiol. 2015, 25, 101–106. [Google Scholar] [CrossRef] [PubMed]
- The NOAA Physical Sciences Laboratory. Available online: https://psl.noaa.gov/about/ (accessed on 25 January 2025).
- Hassani, H.; Yeganegi, M.R. Selecting optimal lag order in Ljung–Box test. Phys. A Stat. Mech. Its Appl. 2020, 541, 123700. [Google Scholar] [CrossRef]
- Smith, P.F.; Ganesh, S.; Liu, P. A comparison of random forest regression and multiple linear regression for prediction in neuroscience. J. Neurosci. Methods 2013, 220, 85–91. [Google Scholar] [CrossRef]
- Xie, X.; Wu, T.; Zhu, M.; Jiang, G.; Xu, Y.; Wang, X.; Pu, L. Comparison of random forest and multiple linear regression models for estimation of soil extracellular enzyme activities in agricultural reclaimed coastal saline land. Ecol. Indic. 2021, 120, 106925. [Google Scholar] [CrossRef]
- Wang, C.; Fiedler, P.C. ENSO variability and the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 239–266. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, W.; Lin, X.; Li, Z.; Zhang, Y. Nonlinear El Niño impacts on the global economy under climate change. Nat. Commun. 2022, 14, 5887. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Curci, L.M.; Leggieri, A.; Lenucci, M.; Basset, A.; Santino, A.; Piro, G.; De Caroli, M. Shading effects in agrivoltaic systems can make the difference in boosting food security in climate change. Appl. Energy 2024, 358, 122565. [Google Scholar] [CrossRef]
- Barber, R.T.; Chavez, F.P. Biological Consequences of El Niño. Science 1983, 222, 1203–1210. [Google Scholar] [CrossRef]
- Shokri, M.; Cozzoli, F.; Vignes, F.; Bertoli, M.; Pizzul, E.; Basset, A. Metabolic rate and climate change across latitudes: Evidence of mass-dependent responses in aquatic amphipods. J. Exp. Biol. 2022, 225, jeb244842. [Google Scholar] [CrossRef]
- Shokri, M.; Lezzi, L.; Basset, A. The seasonal response of metabolic rate to projected climate change scenarios in aquatic amphipods. J. Therm. Biol. 2024, 124, 103941. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.; Cozzoli, F.; Basset, A. Metabolic rate and foraging behaviour: A mechanistic link across body size and temperature gradients. Oikos 2025, 2025, e10817. [Google Scholar] [CrossRef]
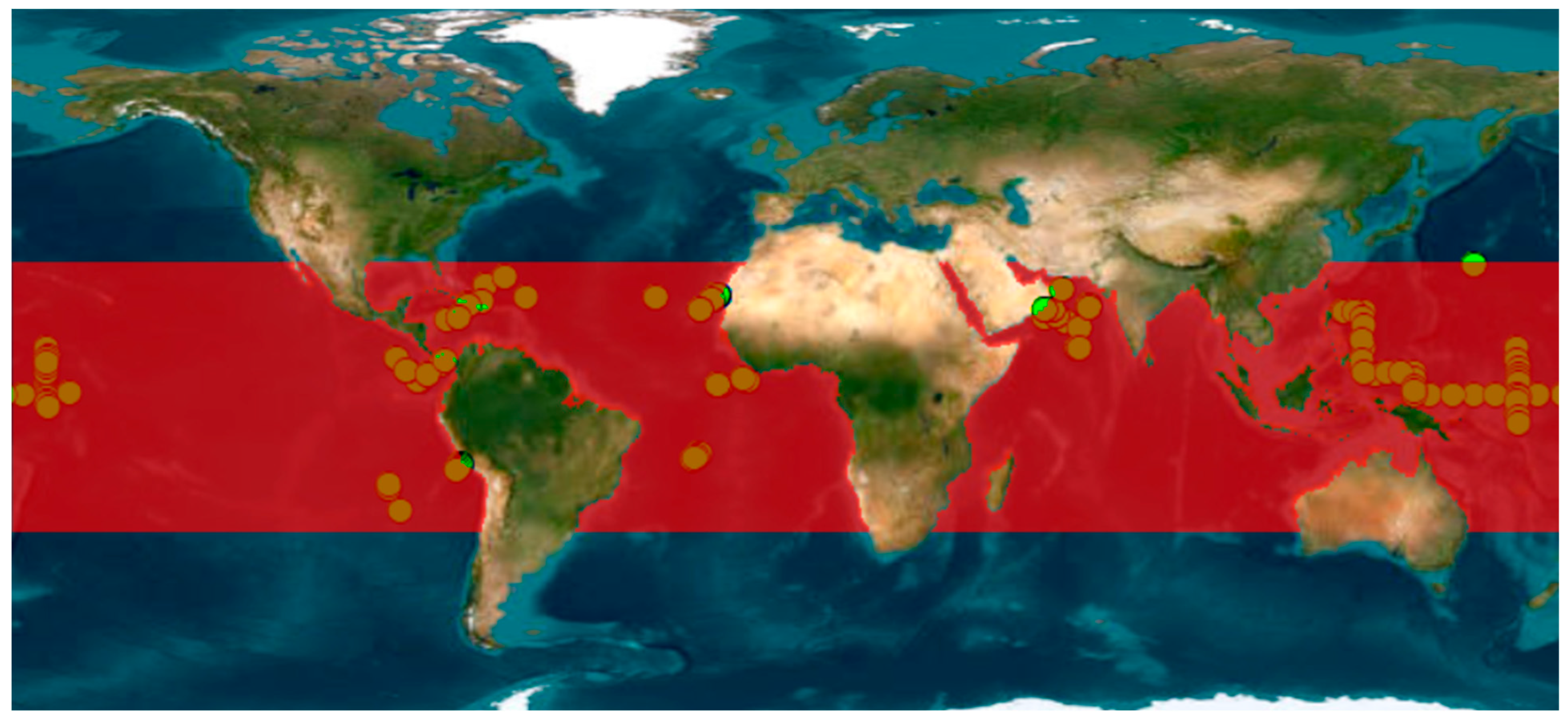




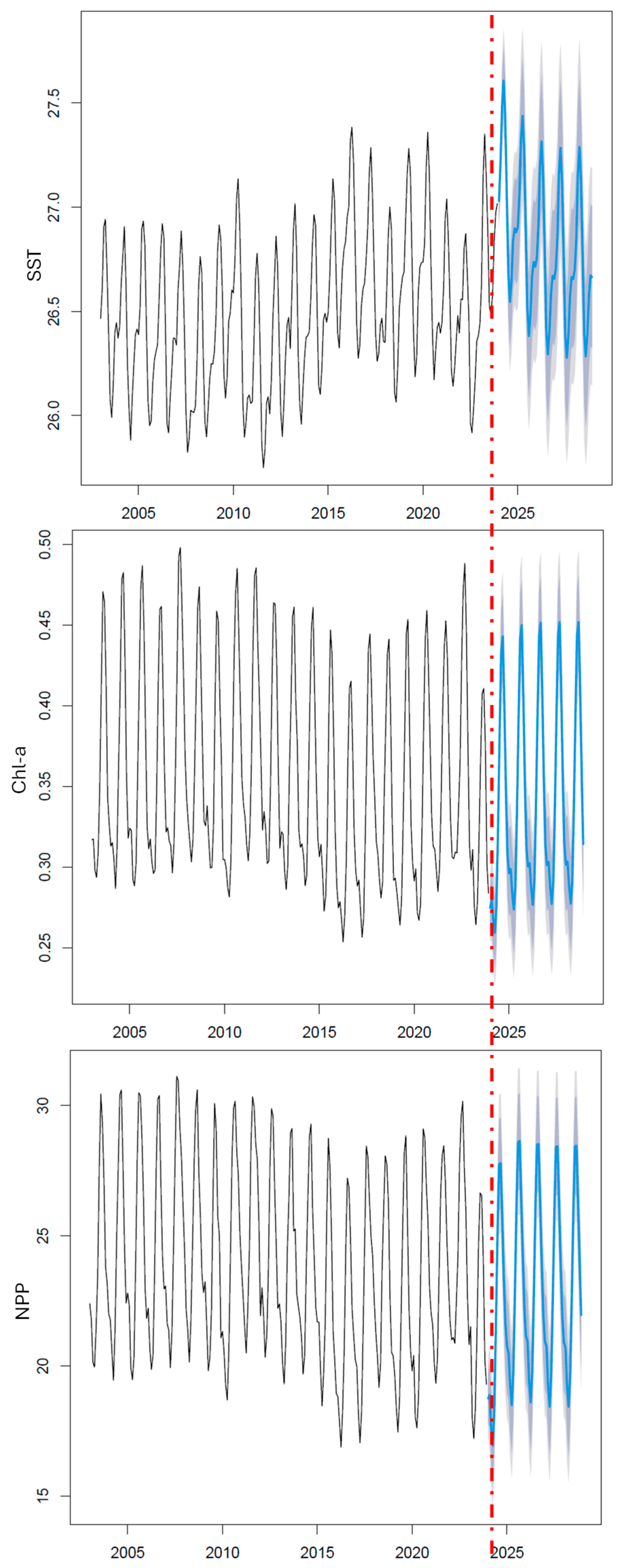
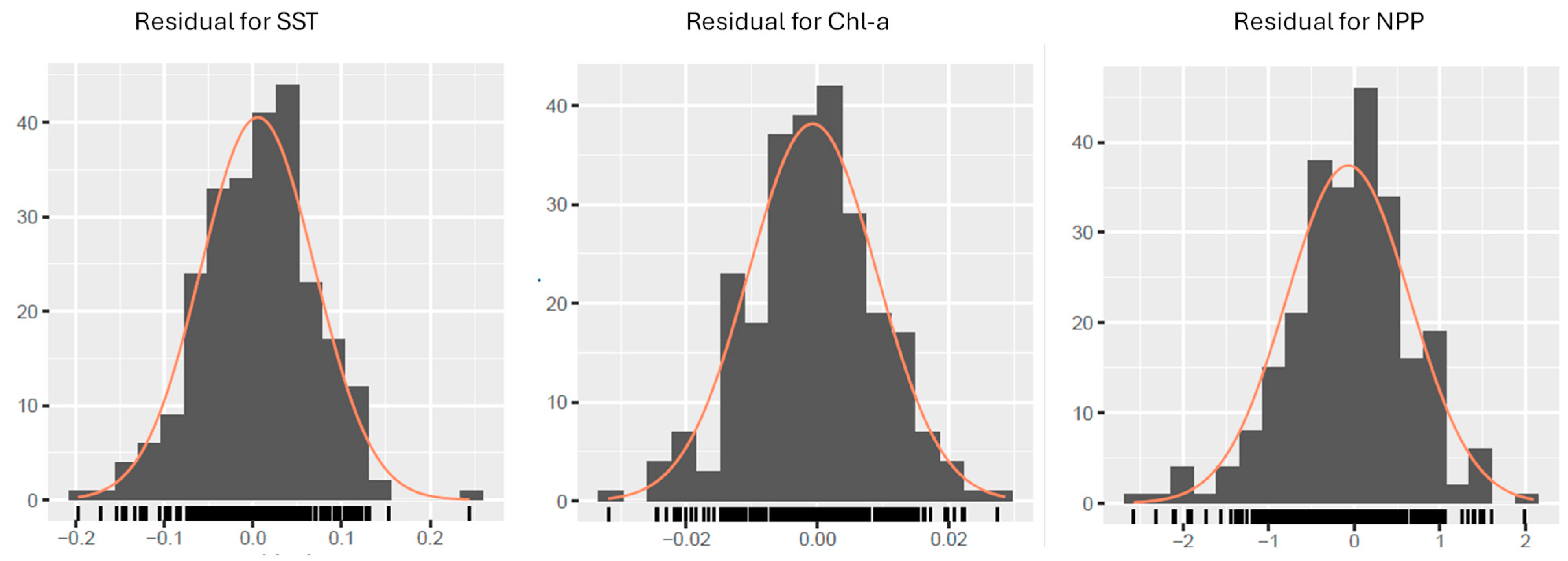
| Model | Biotic Parameters | R2 | MAE | RMSE |
|---|---|---|---|---|
| Linear regression | Chl-a | 0.6019373 | 0.7751063 | 0.9543401 |
| NPP | 0.4081017 | 1.047433 | 1.240625 | |
| Random Forest | Chl-a | 0.752684 | 0.5669773 | 0.7623114 |
| NPP | 0.6112026 | 0.754811 | 0.9819766 |
| Variable | MAE | RMSE |
|---|---|---|
| SST | 0.05089551 | 0.0644293 |
| Chl-a | 0.007572948 | 0.009766999 |
| NPP | 0.7226367 | 0.5497908 |
| Lag | SST p-Value | Chl-a p-Value | NPP p-Value |
|---|---|---|---|
| 6 | 0.04985 | 0.2026 | 0.071 |
| 12 | 0.1604 | 0.1501 | 0.07359 |
| 24 | 0.5431 | 0.2027 | 0.01291 |
| 36 | 0.6684 | 0.2958 | 0.006218 |
| 48 | 0.8242 | 0.4298 | 0.01163 |
| 60 | 0.9421 | 0.3563 | 0.005601 |
| 72 | 0.8531 | 0.213 | 0.006164 |
| 84 | 0.7614 | 0.1574 | 0.004539 |
| 96 | 0.8554 | 0.1895 | 0.003743 |
| 108 | 0.825 | 0.1151 | 0.001579 |
| 120 | 0.8683 | 0.2214 | 0.003643 |
| 132 | 0.8352 | 0.04086 | 0.0001627 |
| 148 | 0.8627 | 0.05703 | 0.0001433 |
| 150 | 0.8863 | 0.06034 | 0.0001357 |
| 162 | 0.8791 | 0.05593 | 0.0001735 |
| 174 | 0.93 | 0.07611 | 0.0004293 |
| 186 | 0.9317 | 0.0702 | 0.000794 |
| 198 | 0.9675 | 0.0746 | 0.001189 |
| 210 | 0.8796 | 0.1172 | 0.003056 |
| 222 | 0.8191 | 0.2312 | 0.005376 |
| 234 | 0.797 | 0.242 | 0.005095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeraro, T.; Titocci, J.; Liberatore, L.; Monti, F.; De Leo, F.; Ingrosso, G.; Shokri, M.; Basset, A. Analytical Workflow for Tracking Aquatic Biomass Responses to Sea Surface Temperature Changes. Environments 2025, 12, 210. https://doi.org/10.3390/environments12070210
Semeraro T, Titocci J, Liberatore L, Monti F, De Leo F, Ingrosso G, Shokri M, Basset A. Analytical Workflow for Tracking Aquatic Biomass Responses to Sea Surface Temperature Changes. Environments. 2025; 12(7):210. https://doi.org/10.3390/environments12070210
Chicago/Turabian StyleSemeraro, Teodoro, Jessica Titocci, Lorenzo Liberatore, Flavio Monti, Francesco De Leo, Gianmarco Ingrosso, Milad Shokri, and Alberto Basset. 2025. "Analytical Workflow for Tracking Aquatic Biomass Responses to Sea Surface Temperature Changes" Environments 12, no. 7: 210. https://doi.org/10.3390/environments12070210
APA StyleSemeraro, T., Titocci, J., Liberatore, L., Monti, F., De Leo, F., Ingrosso, G., Shokri, M., & Basset, A. (2025). Analytical Workflow for Tracking Aquatic Biomass Responses to Sea Surface Temperature Changes. Environments, 12(7), 210. https://doi.org/10.3390/environments12070210






