Short-Term Effects of Minimum Tillage and Wood Distillate Addition on Plants and Springtails in an Olive Grove
Abstract
1. Introduction
2. Materials and Methods
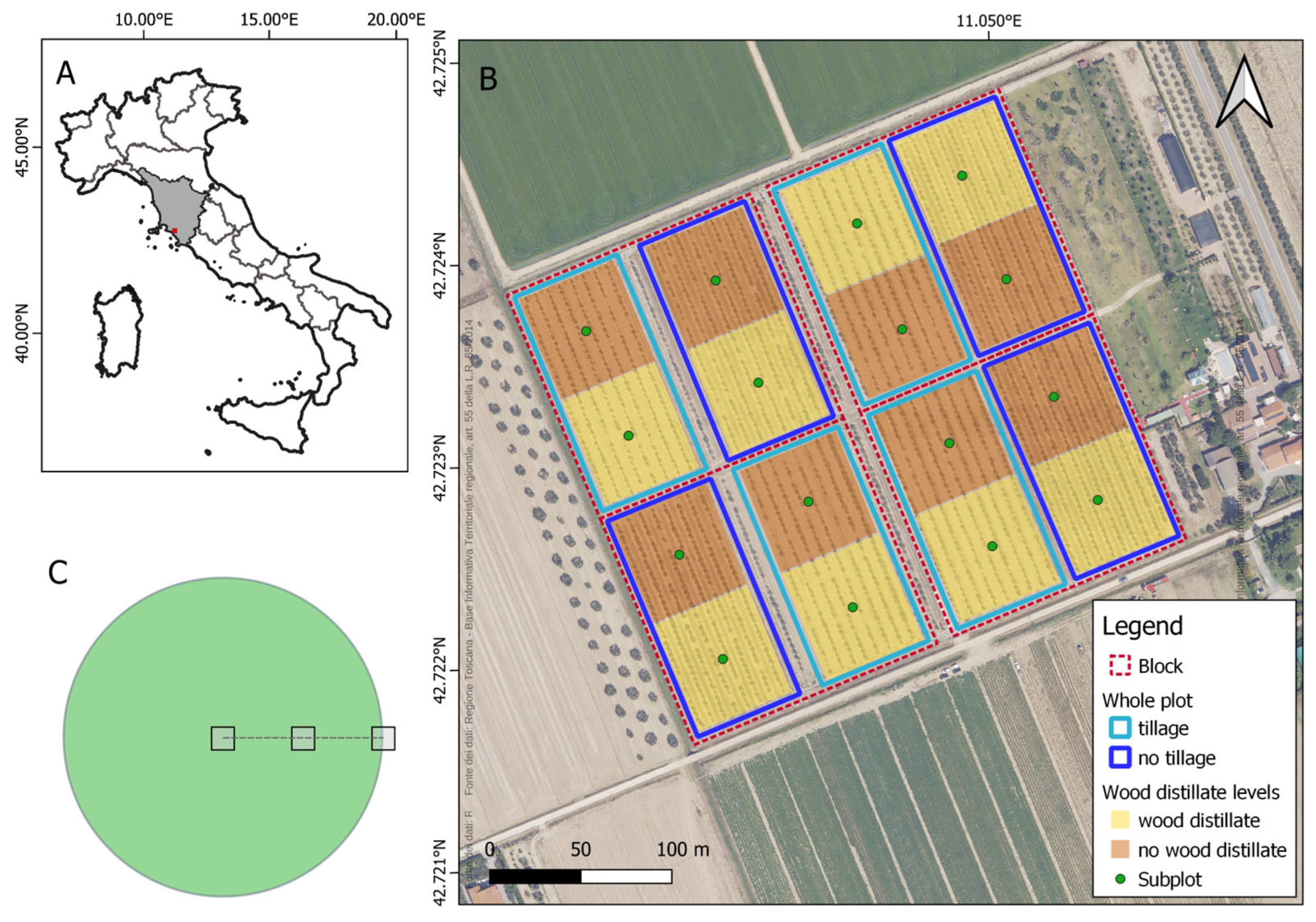
2.1. Study Area
2.2. Experimental Design
2.3. Biodiversity Sampling
2.4. Statistical Analyses
3. Results
3.1. Detected Biodiversity
3.2. Effects of Treatments on Biotic Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Zaks, D.P. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Murray, C.J. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Steffan-Dewenter, I. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Bàrberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Maccherini, S.; Angiolini, C.; De Simone, L.; Fiaschi, T.; Tassinari, A.; Bacaro, G. Drivers of diversity of arable plant communities in one of their European conservation hotspots. Biodivers. Conserv. 2023, 32, 2055–2075. [Google Scholar] [CrossRef]
- Richner, N.; Holderegger, R.; Linder, H.P.; Walter, T. Reviewing change in the arable flora of Europe: A meta-analysis. Weed Res. 2015, 55, 1–13. [Google Scholar] [CrossRef]
- Fried, G.; Dessaint, F.; Reboud, X. Local and Regional Changes in Taxonomic and FunctionalDiversity of Arable Weed Communities in Burgundy (France) between the 1970s and the 2000s. Bot. Lett. 2016, 163, 359–371. [Google Scholar] [CrossRef]
- Olivier, T.; Thébault, E.; Elias, M.; Fontaine, B.; Fontaine, C. Urbanization and agricultural intensification destabilize animal communities differently than diversity loss. Nat. Commun. 2020, 11, 2686. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Ulrich, W.; Batáry, P.; Baudry, J.; Beaumelle, L.; Bucher, R.; Birkhofer, K. From functional diversity to human well-being: A conceptual framework for agroecosystem sustainability. Agric. Syst. 2023, 208, 103659. [Google Scholar] [CrossRef]
- Besnard, G.; Terral, J.F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, N.G.; López, M.J.; Caudullo, G.; De Rigo, D. Olea europaea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; p. 01534b. [Google Scholar]
- Fanfarillo, E.; Scoppola, A.; Lososová, Z.; Abbate, G. Segetal plant communities of traditional agroecosystems: A phytosociological survey in central Italy. Phytocoenologia 2019, 49, 165–183. [Google Scholar] [CrossRef]
- Maccherini, S.; Santi, E.; Bonini, I.; Amici, V.; Pruscini, S.; Palazzo, D.; Selva, F.C. The impact of land abandonment on the plant diversity of olive groves. Biodivers. Conserv. 2013, 22, 3067–3083. [Google Scholar] [CrossRef]
- Calabrese, G.; Tartaglini, N.; Ladisa, G. Study on Biodiversity in Century-Old Olive Groves; CIHEAM-Mediterranean Agronomic Institute: Bari, Italy, 2012; pp. 1–108. [Google Scholar]
- Carpio, A.J.; Oteros, J.; Tortosa, F.S.; Guerrero-Casado, J. Land use and biodiversity patterns of the herpetofauna: The role of olive groves. Acta Oecologica 2016, 70, 103–111. [Google Scholar] [CrossRef]
- Gómez, J.A.; Campos, M.; Guzmán, G.; Castillo-Llanque, F.; Vanwalleghem, T.; Lora, Á.; Giráldez, J.V. Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ. Sci. Pollut. Res. 2018, 25, 977–989. [Google Scholar] [CrossRef]
- Muñoz-Rojas, J.; García-Ruiz, R. Olive Groves (and Olive Oil) in the Mediterranean in 2023: A Heterogeneous and Dynamic Mosaic of Rural Landscapes and Ecosystems. In The Olive Landscapes of the Mediterranean; Muñoz-Rojas, J., García-Ruiz, R., Eds.; Landscape Series; Springer: Cham, Switzerland, 2024; Volume 36. [Google Scholar] [CrossRef]
- Harris, D.R. The Origins and Spread of Agriculture and Pastoralism in Eurasia; UCL Press: London, UK, 1996. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Giller, K.E.; Beare, M.H.; Lavelle, P.; Izac, A.M.; Swift, M.J. Agricultural intensification, soil biodiversity and agroecosystem function. Appl. Soil. Ecol. 1997, 6, 3–16. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B 2008, 363, 543–555. [Google Scholar] [CrossRef]
- Albrecht, H.; Cambecèdes, J.; Lang, M.; Wagner, M. Management options for the conservation of rare arable plants in Europe. Bot. Lett. 2016, 163, 389–415. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Kasperski, A.; Giuliani, A.; Abbate, G. Shifts of arable plant communities after agricultural intensification: A floristic and ecological diachronic analysis in maize fields of Latium (central Italy). Bot. Lett. 2019, 166, 356–365. [Google Scholar] [CrossRef]
- Colbach, N.; Cordeau, S. Are no-till herbicide-free systems possible? A simulation study. Front. Agron. 2022, 4, 823069. [Google Scholar] [CrossRef]
- DeClerck, F.A.; Koziell, I.; Benton, T.; Garibaldi, L.A.; Kremen, C.; Maron, M.; Winowiecki, L. A whole earth approach to nature-positive food: Biodiversity and agriculture. In Science and Innovations for Food Systems Transformation; Springer: Cham, Switzerland, 2023; pp. 469–496. [Google Scholar] [CrossRef]
- Storkey, J.; Meyer, S.; Still, K.S.; Leuschner, C. The impact of agricultural intensification and land-use change on the European arable flora. Proc. R. Soc. B Biol. Sci. 2012, 279, 1421–1429. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A hazard assessment. Front. Environ. Sci. 2021, 9, 643847. [Google Scholar] [CrossRef]
- Shi, T.S.; Collins, S.L.; Yu, K.; Peñuelas, J.; Sardans, J.; Li, H.; Ye, J.S. A global meta-analysis on the effects of organic and inorganic fertilization on grasslands and croplands. Nat. Commun. 2024, 15, 3411. [Google Scholar] [CrossRef] [PubMed]
- Fanfarillo, E.; Calabrese, D.; Angiolini, C.; Bacaro, G.; Biagiotti, S.; Castagnini, P.; Maccherini, S. Effects of conventional and organic management on plant and insect communities in a traditional elephant garlic crop. Community Ecol. 2022, 23, 417–427. [Google Scholar] [CrossRef]
- Di Giovanni, F.; Nardi, F.; Frati, F.; Migliorini, M. Below-ground arthropod diversity in conventional and organic vineyards: A review. Crop Prot. 2024, 180, 106666. [Google Scholar] [CrossRef]
- Italian Ministerial Decree 6793. Gazzetta Ufficiale della Repubblica Italiana. 5 September 2018. Available online: https://www.gazzettaufficiale.it/eli/id/2018/09/05/18A05693/sg (accessed on 15 October 2024).
- Fedeli, R.; Vannini, A.; Djatouf, N.; Celletti, S.; Loppi, S. Can lettuce plants grow in saline soils supplemented with biochar? Heliyon 2024, 10, e26526. [Google Scholar] [CrossRef] [PubMed]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Zulkarami, B.; Ashrafuzzaman, M.; Husni, M.O.; Ismail, M.R. Effect of pyroligneous acid on growth, yield, and quality improvement of rockmelon in soilless culture. Aust. J. Crop Sci. 2011, 5, 1508–1514. [Google Scholar]
- Fedeli, R.; Dichiara, M.; Carullo, G.; Tudino, V.; Gemma, S.; Butini, S.; Loppi, S. Unlocking the potential of biostimulants in sustainable agriculture: Effect of wood distillate on the nutritional profiling of apples. Heliyon 2024, 10, e37599. [Google Scholar] [CrossRef]
- Fedeli, R.; Celletti, S.; Loppi, S. Wood Distillate Promotes the Tolerance of Lettuce in Extreme Salt Stress Conditions. Plants 2024, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Fedeli, R.; Mariotti, L.; Pisuttu, C.; Nali, C.; Pellegrini, E.; Loppi, S. Foliar application of wood distillate protects basil plants against ozone damage by preserving membrane integrity and triggering antioxidant mechanisms. Agronomy 2024, 14, 1233. [Google Scholar] [CrossRef]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-dependent and species-specific effects of wood distillate addition on the germination performance of threatened arable plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef]
- Barbato, D.; Perini, C.; Mocali, S.; Bacaro, G.; Tordoni, E.; Maccherini, S.; Salerni, E. Teamwork makes the dream work: Disentangling cross-taxon congruence across soil biota in black pine plantations. Sci. Total Environ. 2019, 656, 659–669. [Google Scholar] [CrossRef]
- Maccherini, S.; Salerni, E.; Mocali, S.; Bianchetto, E.; Landi, S.; De Meo, I.; Cantiani, P. Silvicultural management does not affect biotic communities in conifer plantations in the short-term: A multi-taxon assessment using a BACI approach. For. Ecol. Manag. 2021, 493, 119257. [Google Scholar] [CrossRef]
- Bazzato, E.; Lallai, E.; Caria, M.; Schifani, E.; Cillo, D.; Ancona, C.; Marignani, M. Focusing on the role of abiotic and biotic drivers on cross-taxon congruence. Ecol. Indic. 2023, 151, 110323. [Google Scholar] [CrossRef]
- Hawes, C.; Squire, G.R.; Hallett, P.D.; Watson, C.A.; Young, M. Arable plant communities as indicators of farming practice. Agric. Ecosyst. Environ. 2010, 138, 17–26. [Google Scholar] [CrossRef]
- Cassani, M.T.; Sabatté, M.L.; González Arzac, A.; Massobrio, M.J. Mesofauna as an indicator of agroecosystem stability: Degree of artificialization effect on land uses in Azul district, Argentina. SN Appl. Sci. 2020, 2, 324. [Google Scholar] [CrossRef]
- Hagner, M.; Kuoppala, E.; Fagernäs, L.; Tiilikkala, K.; Setälä, H. Using the copse snail Arianta arbustorum (Linnaeus) to detect repellent compounds and the quality of wood vinegar. Int. J. Environ. Res. 2015, 9, 53–60. [Google Scholar] [CrossRef]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- LaMMA Consortium. Clima di Grosseto. 2024. Available online: https://www.lamma.toscana.it/clima-e-energia/climatologia/clima-grosseto (accessed on 15 October 2024).
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.M.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Kasperski, A. An index of ecological value for European arable plant communities. Biodivers. Conserv. 2021, 30, 2145–2164. [Google Scholar] [CrossRef]
- Fedeli, R.; Loppi, S.; Cruz, C.; Munzi, S. Evaluating seawater and wood distillate for sustainable hydroponic cultivation: Implications for crop growth and nutritional quality. Sustainability 2024, 16, 7186. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Twardowski, J.P.; Hurej, M.; Gruss, I. Diversity and abundance of springtails (Hexapoda: Collembola) in soil under 90-year potato monoculture in relation to crop rotation. Arch. Agron. Soil. Sci. 2016, 62, 1158–1168. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017–2019; Volume 1–4. [Google Scholar]
- Portal to the Flora of Italy v. 2024.2. Floritaly. Available online: http://dryades.units.it/floritaly (accessed on 15 October 2024).
- Migliorini, M.; Fanciulli, P.P.; Bernini, F. Comparative analysis of two edaphic zoocoenoses (Acari Oribatida; Hexapoda Collembola) in the area of Orio al Serio Airport (Bergamo, northern Italy). Pedobiologia 2003, 47, 9–18. [Google Scholar] [CrossRef]
- Rusek, J. Eine Präparationstechnik für Sprungschwänze und ähnliche Gliederfüsser. Mikrokosmos 1975, 12, 376–381. [Google Scholar]
- Gisin, H. Collembolenfauna Europas, 1st ed.; Museum d’Histoire Naturelle: Geneva, Switzerland, 1960. [Google Scholar]
- Jordana, R.; Arbea, J.; Simón, C.; Luciañez, M.J. Collembola, Poduromorpha. In Fauna Ibérica; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1997; Volume 8, Available online: https://www.fauna-iberica.mncn.csic.es/publicaciones/fi8.php (accessed on 26 March 2025).
- Pomorski, R.J. Onychiurinae of Poland (Collembola: Onychiuridae); Genus-Supplement; Polish Taxonomical Society: Warsaw, Poland, 1998; Volume 9, 201p. [Google Scholar]
- Bretfeld, G. Symphypleona, Synopses on Palaearctic Collembola; Senckenberg Museum of Natural History: Görlitz, Germany, 1999. [Google Scholar]
- Potapov, M. Isotomidae, Synopses on Palaearctic Collembola; Senckenberg Museum of Natural History: Görlitz, Germany, 2001. [Google Scholar]
- Thibaud, J.M.; Schulz, H.J.; Gama Assalino, M.M. Hypogastruridae, Synopses on Palaearctic Collembola; Senckenberg Museum of Natural History: Görlitz, Germany, 2004. [Google Scholar]
- Dunger, W.; Schlitt, B. Synopses on Palaearctic Collembola, Volume 6/1. Tullbergiidae. Soil Org. 2011, 83, 1–168. [Google Scholar]
- Jordana, R. Capbrynae & Entomobryni, Synopses on Palaearctic Collembola; Senckenberg Museum of Natural History: Görlitz, Germany, 2012. [Google Scholar]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Pape, T.; Hobern, D.; et al. Catalogue of Life, Version 2024-11-18; Catalogue of Life: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Anderson, M. Permutational test for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R. PRIMER v6: Users Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.; Ter Braak, C. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Santi, E.; Maccherini, S.; Rocchini, D.; Bonini, I.; Brunialti, G.; Favilli, L.; Chiarucci, A. Simple to sample: Vascular plants as surrogate group in a nature reserve. J. Nat. Conserv. 2010, 18, 2–11. [Google Scholar] [CrossRef]
- Cici, S.Z.H.; Van Acker, R.C. A review of the recruitment biology of winter annual weeds in Canada. Can. J. Plant Sci. 2009, 89, 575–589. [Google Scholar] [CrossRef]
- Block, W.; Zettel, J. Activity and dormancy in relation to body water and cold tolerance in a winter-active springtail (Collembola). Eur. J. Entomol. 2003, 100, 305–312. [Google Scholar] [CrossRef]
- Boscutti, F.; Sigura, M.; Gambon, N.; Lagazio, C.; Krüsi, B.O.; Bonfanti, P. Conservation tillage affects species composition but not species diversity: A comparative study in northern Italy. Environ. Manage. 2015, 55, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Plaza, E.; Kozak, M.; Navarrete, L.; González-Andújar, J.L. Tillage system did not affect weed diversity in a 23-year experiment in Mediterranean dryland. Agric. Ecosyst. Environ. 2011, 140, 102–105. [Google Scholar] [CrossRef]
- Shemdoe, R.S.; Mbago, F.M.; Kikula, I.S.; Van Damme, P.L. Weed species diversity on arable land of the dryland areas of central Tanzania: Impacts of continuous application of traditional tillage practices. GeoJournal 2008, 71, 107–115. [Google Scholar] [CrossRef]
- Armengot, L.; Blanco-Moreno, J.; Bàrberi, P.; Bocci, G.; Carlesi, S.; Aendekerk, R.; Berner, A.; Celette, F.; Grosse, M.; Huiting, H.; et al. Tillage as a driver of change in weed communities: A functional perspective. Agric. Ecosyst. Environ. 2016, 222, 276–285. [Google Scholar] [CrossRef]
- Travlos, I.S.; Cheimona, N.; Roussis, I.; Bilalis, D.J. Weed-species abundance and diversity indices in relation to tillage systems and fertilization. Front. Environ. Sci. 2018, 6, 11. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Lang, B.; Russell, D.J. Reducing tillage intensity benefits the soil micro- and mesofauna in a global meta-analysis. Eur. J. Soil. Sci. 2022, 73, e13321. [Google Scholar] [CrossRef]
- Van de Bund, C.F. Influence of crop and tillage on mites and springtails in arable soil. Neth. J. Agric. Sci. 1970, 18, 308–314. [Google Scholar] [CrossRef]
- Fiera, C.; Ulrich, W.; Popescu, D.; Buchholz, J.; Querner, P.; Bunea, C.I.; Zaller, J.G. Tillage intensity and herbicide application influence surface-active springtail (Collembola) communities in Romanian vineyards. Agric. Ecosyst. Environ. 2020, 300, 107006. [Google Scholar] [CrossRef]
- Bokova, A.I.; Panina, K.S.; Dridiger, V.K.; Gadzhiumarov, R.G.; Kuznetsova, N.A.; Potapov, M.B. Soil-dwelling springtails as indicators of the efficiency of No-till technologies with different amounts of mineral fertilizers in the crop rotation on chernozem soils. Soil. Tillage Res. 2023, 232, 105760. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; Wu, D.; Sun, X. Effect of tillage and farming management on Collembola in marsh soils. Appl. Soil. Ecol. 2013, 64, 112–117. [Google Scholar] [CrossRef]
- Lang, M.; Kollmann, J.; Prestele, J.; Wiesinger, K.; Albrecht, H. Reintroduction of rare arable plants in extensively managed fields: Effects of crop type, sowing density and soil tillage. Agric. Ecosyst. Environ. 2021, 306, 107187. [Google Scholar] [CrossRef]
- Pereira, A.J.; Porto, M.; Correia, O.; Beja, P. Traditional ploughing is critical to the conservation of threatened plants in Mediterranean olive groves. Agric. Ecosyst. Environ. 2024, 359, 108775. [Google Scholar] [CrossRef]
- Graco-Roza, C.; Wong, M.; Barber, N.; Brodersen, J.; Callisto, M.; Castro, D.; Dröse, W.; Etard, A.; Gonçalves-Souza, T.; Graham, C.; et al. Human pressure homogenises species and traits globally. Preprint 2024. [Google Scholar] [CrossRef]
- Ozpinar, S. Effects of Tillage Systems on WeedPopulation and Economics for Winter Wheat Productionunder the Mediterranean Dryland Conditions. Soil Tillage Res. 2006, 87, 1–8. [Google Scholar] [CrossRef]
- Alarcón, R.; Hernández-Plaza, E.; Navarrete, L.; Sánchez, M.J.; Escudero, A.; Hernanz, J.L.; Sánchez, A.M. Effects of no-tillage and non-inversion tillage on weed community diversity and crop yield over nine years in a Mediterranean cereal-legume cropland. Soil. Tillage Res. 2018, 179, 54–62. [Google Scholar] [CrossRef]
- Gonçalves, M.F.; Pereira, J.A. Abundance and diversity of soil arthropods in the olive grove ecosystem. J. Insect Sci. 2012, 12, 20. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Wu, D.; Ai, Z.; Xu, Q.; Sun, X.; Chang, L. No tillage increases soil microarthropod (Acari and Collembola) abundance at the global scale. Soil. Ecol. Lett. 2024, 6, 230208. [Google Scholar] [CrossRef]
- Pfingstmann, A.; Paredes, D.; Buchholz, J.; Querner, P.; Bauer, T.; Strauss, P.; Zaller, J. Contrasting effects of tillage and landscape structure on spiders and springtails in vineyards. Sustainability 2019, 11, 2095. [Google Scholar] [CrossRef]
- Arbea, J.I.; Jordana, R. Ecología de las poblaciones de colémbolos edáficos en un prado y un pinar de la región submediterránea de Navarra. Mediterránea Ser. Biol. 1990, 12, 139–148. [Google Scholar] [CrossRef]
- Vanhée, B.; Devigne, C. Differences in collembola species assemblages (Arthropoda) between spoil tips and surrounding environments are dependent on vegetation development. Sci. Rep. 2018, 8, 18067. [Google Scholar] [CrossRef]
- Vinod, P.; Sanal Kumar, M.G.; Bini, B. Sex ratio of Cryptopygus thermophilus in rubber plantations of Chengannur Thaluk of Alappuzha District, Kerala, India. Int. J. Sci. Res. 2017, 6, 1360–1362. [Google Scholar]
- Petersen, H. Sex ratios and the extent of parthenogenetic reproduction in some collembolan populations. In First International Seminary on Apterygota; Dallai, R., Ed.; Accademia delle Scienze di Siena detta de’ Fisiocritici: Siena, Italy, 1978; pp. 19–35. [Google Scholar]
- Luciàñez, M.J.J.; Perez, M.; Simon, C. Contribution to the Ecological Study of Collembola Fauna from an Oak Forest in the Comunidad de Madrid (in Spanish); Alemanny, A., Ed.; Historia Natural 91; 1992; pp. 203–210. [Google Scholar]
- Valle, B.; Porco, D.; Skarżyński, D.; Frati, F.; Caccianiga, M.; Rodriguez-Prieto, A.; Zeni, M.; Gobbi, M. Alpine blooming of “snow fleas”: The importance of snow for Alpine springtails (Hexapoda: Collembola) ecology and biodiversity. Rend. Fis. Acc. Lincei 2024, 35, 163–180. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Fedeli, R.; Fiaschi, T.; de Simone, L.; Vannini, A.; Angiolini, C.; Maccherini, S. Effects of wood distillate on seedling emergence and first-stage growth in five threatened arable plants. Diversity 2022, 14, 669. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Bagavathiannan, M.; Yu, J. The use of wood vinegar as a non-synthetic herbicide for control of broadleaf weeds. Ind. Crops Prod. 2021, 173, 114105. [Google Scholar] [CrossRef]
- Hyvönen, T.T.; Hagner, M.M.; Hurme, T.K.; Lindqvist, B.E.; Ojanen, H.J. Control of Heracleum mantegazzianum with pyrolysis liquid products. Weed Res. 2023, 63, 311–316. [Google Scholar] [CrossRef]
- Bianchi, E.; Benesperi, R.; Giordani, P.; Martire, L.; Favero-Longo, S.E.; Loppi, S. Wood distillate as an alternative bio-based product against lichens on sandstone. Int. Biodeterior. Biodegrad. 2022, 170, 105386. [Google Scholar] [CrossRef]

| November | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Richness | Composition | ||||||||
| Source of Variation | df | SS | MS | Pseudo-F | SS | MS | Pseudo-F | ||
| Block | 3 | 23.25 | 7.75 | 0.70 | 3303.60 | 1101.20 | 0.97 | ||
| Tillage | 1 | 8 | 8 | 0.72 | 994.89 | 994.89 | 0.89 | ||
| Whole-plot error | 3 | 33.25 | 11.08 | 3398.90 | 1133.00 | ||||
| Whole-plot total | 7 | 64.5 | 7697.50 | ||||||
| Plants | WD | 1 | 2.25 | 2.25 | 0.23 | 560.89 | 560.89 | 0.52 | |
| Tillage x WD | 1 | 20.25 | 20.25 | 2.04 | 1959.80 | 1959.80 | 1.84 | ||
| Sub-plot error | 6 | 59.5 | 9.92 | 6404.10 | 1067.40 | ||||
| Total | 15 | 211 | 24,320.00 | ||||||
| Source of Variation | df | SS | MS | Pseudo-F | SS | MS | Pseudo-F | ||
| Block | 3 | 3.34 | 1.11 | 0.37 | 1308.60 | 436.21 | 0.92 | ||
| Tillage | 1 | 0.03 | 0.03 | 0.01 | 627.27 | 627.27 | 1.33 | ||
| Whole-plot error | 3 | 9.09 | 3.03 | 1417.10 | 472.35 | ||||
| Whole-plot total | 7 | 12.47 | 3353 | ||||||
| Springtails | WD | 1 | 0.06 | 0.06 | 0.01 | 360.18 | 360.18 | 0.28 | |
| Tillage x WD | 1 | 0.06 | 0.06 | 0.01 | 544.19 | 544.19 | 0.43 | ||
| Sub-plot error | 6 | 28.37 | 4.73 | 7575.70 | 1262.60 | ||||
| Total | 15 | 53.44 | 15,186 | ||||||
| April | |||||||||
| Richness | Composition | ||||||||
| Source of Variation | df | SS | MS | Pseudo-F | SS | MS | Pseudo-F | ||
| Block | 3 | 20.34 | 6.78 | 0.54 | 2087.90 | 695.98 | 0.89 | ||
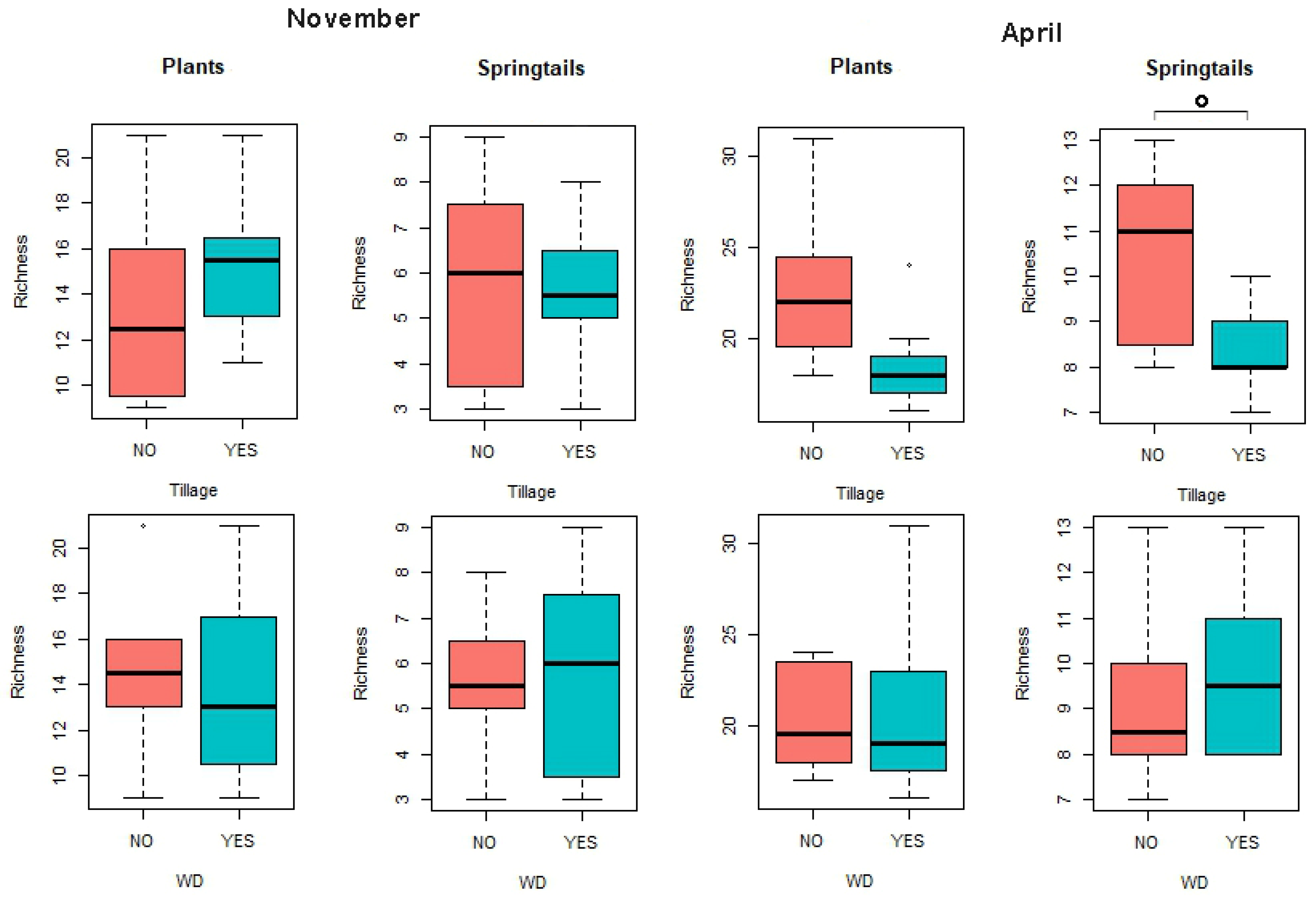
| Tillage | 1 | 34.03 | 34.03 | 2.70 | 3695.70 | 3695.70 | 4.75 * | ||
| Whole-plot error | 3 | 37.84 | 12.62 | 2331.40 | 777.14 | ||||
| Whole-plot total | 7 | 92.22 | 8115.10 | ||||||
| Plants | WD | 1 | 0.56 | 0.56 | 0.09 | 1295.10 | 1295.10 | 1.33 | |
| Tillage x WD | 1 | 14.06 | 14.06 | 2.17 | 1021.70 | 1021.70 | 1.05 | ||
| Sub-plot error | 6 | 38.88 | 6.48 | 5860.90 | 976.82 | ||||
| Total | 15 | 237.94 | 24,408 | ||||||
| Source of Variation | df | SS | MS | Pseudo-F | SS | MS | Pseudo-F | ||
| Block | 3 | 7.09 | 2.36 | 2.29 | 2210.80 | 736.95 | 3.21 | ||
| Tillage | 1 | 9.03 | 9.03 | 8.75 ° | 1578.20 | 1578.20 | 6.88 * | ||
| Whole-plot error | 3 | 3.09 | 1.03 | 688.13 | 229.38 | ||||
| Springtails | Whole-plot total | 7 | 19.21 | 4477.20 | |||||
| WD | 1 | 1.56 | 1.56 | 0.79 | 155.11 | 155.11 | 0.29 | ||
| Tillage x WD | 1 | 0.06 | 0.06 | 0.03 | 578.65 | 578.65 | 1.09 | ||
| Sub-plot error | 6 | 11.87 | 1.98 | 3185.90 | 530.99 | ||||
| Total | 15 | 51.94 | 12,874 |
| November | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | df | SS | MS | Pseudo-F | ||
| Block | 3 | 5847.6 | 1949.2 | 0.97 | ||
| Tillage | 1 | 488.28 | 488.28 | 0.24 | ||
| Whole-plot error | 3 | 6048.3 | 2016.1 | |||
| Number of springtail individuals | Whole-plot total | 7 | 12,384 | |||
| WD | 1 | 1242.6 | 1242.6 | 0.52 | ||
| Tillage x WD | 1 | 2730.1 | 2730.1 | 1.15 | ||
| Sub-plot error | 6 | 14,228 | 2371.3 | |||
| Total | 15 | 42,969 | ||||
| April | ||||||
| Source of Variation | df | SS | MS | Pseudo-F | ||
| Block | 3 | 106,130 | 35,378 | 2.57 | ||
| Tillage | 1 | 64,890 | 64,890 | 4.71 | ||
| Whole-plot error | 3 | 41,332 | 13,777 | |||
| Number of springtail individuals | Whole-plot total | 7 | 212,360 | |||
| WD | 1 | 2626.6 | 2626.6 | 0.44 | ||
| Tillage x WD | 1 | 23,180 | 23,180 | 3.9 | ||
| Sub-plot error | 6 | 35,663 | 5943.8 | |||
| Total | 15 | 486,180 | ||||
| Plants (Average Dissimilarity = 77.36) | ||||||
|---|---|---|---|---|---|---|
| No Tillage | Tillage | Av. Diss | ||||
| Av. Abund | Av. Abund | Diss/SD | Contrib% | Cum% | ||
| Poa annua | 10.63 | 1.88 | 13.88 | 0.93 | 17.95 | 17.95 |
| Medicago arabica | 3.89 | 0.28 | 7.46 | 0.78 | 9.64 | 27.59 |
| Crepis sancta | 5.75 | 0.64 | 6.47 | 0.81 | 8.36 | 35.95 |
| Trifolium campestre | 5.38 | 0.29 | 6.1 | 0.66 | 7.88 | 43.83 |
| Cynodon dactylon | 3.13 | 0 | 5.89 | 0.37 | 7.61 | 51.44 |
| Cerastium glomeratum | 3.51 | 0.66 | 4.33 | 1.18 | 5.59 | 57.03 |
| Helminthotheca echioides | 3.89 | 1.03 | 4.25 | 1.06 | 5.5 | 62.53 |
| Geranium dissectum | 2.01 | 0.15 | 3 | 0.57 | 3.87 | 66.4 |
| Convolvulus arvensis | 1.88 | 0.09 | 2.98 | 1.19 | 3.85 | 70.25 |
| Hordeum murinum subsp. leporinum | 2.26 | 0.63 | 2.89 | 0.83 | 3.73 | 73.98 |
| Symphyotrichum squamatum | 1.5 | 0.04 | 2.18 | 2.2 | 2.81 | 76.8 |
| Beta vulgaris | 1.76 | 0.88 | 1.9 | 0.92 | 2.46 | 79.26 |
| Erigeron sumatrensis | 1.13 | 0.13 | 1.62 | 1.09 | 2.09 | 81.35 |
| Veronica arvensis | 1.15 | 0.04 | 1.46 | 0.8 | 1.89 | 83.24 |
| Lolium multiflorum | 1.88 | 1.25 | 1.35 | 0.85 | 1.74 | 84.98 |
| Senecio vulgaris | 0.78 | 0.18 | 1.32 | 0.8 | 1.71 | 86.69 |
| Centaurium tenuiflorum | 0.54 | 0 | 1.08 | 0.77 | 1.39 | 88.08 |
| Veronica persica | 1.13 | 0.51 | 1 | 0.97 | 1.29 | 89.37 |
| Allium vineale | 0.39 | 0.01 | 0.63 | 0.4 | 0.82 | 90.18 |
| Springtails (Average Dissimilarity = 65.22) | ||||||
| No Tillage | Tillage | |||||
| Av. Abund | Av. Abund | Av. Diss | Diss/SD | Contrib% | Cum.% | |
| Hypogastrura assimilis | 145.36 | 9 | 25.75 | 1.91 | 39.49 | 39.49 |
| Hemisotoma thermophila | 60.94 | 95.63 | 17.91 | 1.08 | 27.46 | 66.95 |
| Pseudanurophorus isotoma | 51.04 | 10.38 | 8.55 | 1.24 | 13.12 | 80.07 |
| Isotomurus maculatus | 31.88 | 13 | 5.13 | 1.03 | 7.87 | 87.94 |
| Ceratophysella engadinensis | 5.38 | 4.5 | 1.58 | 0.64 | 2.43 | 90.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanfarillo, E.; Angiolini, C.; Capitani, C.; De Pasquale Picciarelli, M.; Fedeli, R.; Fiaschi, T.; Jepkogei, P.; Pafumi, E.; Valle, B.; Maccherini, S. Short-Term Effects of Minimum Tillage and Wood Distillate Addition on Plants and Springtails in an Olive Grove. Environments 2025, 12, 204. https://doi.org/10.3390/environments12060204
Fanfarillo E, Angiolini C, Capitani C, De Pasquale Picciarelli M, Fedeli R, Fiaschi T, Jepkogei P, Pafumi E, Valle B, Maccherini S. Short-Term Effects of Minimum Tillage and Wood Distillate Addition on Plants and Springtails in an Olive Grove. Environments. 2025; 12(6):204. https://doi.org/10.3390/environments12060204
Chicago/Turabian StyleFanfarillo, Emanuele, Claudia Angiolini, Claudio Capitani, Margherita De Pasquale Picciarelli, Riccardo Fedeli, Tiberio Fiaschi, Prudence Jepkogei, Emilia Pafumi, Barbara Valle, and Simona Maccherini. 2025. "Short-Term Effects of Minimum Tillage and Wood Distillate Addition on Plants and Springtails in an Olive Grove" Environments 12, no. 6: 204. https://doi.org/10.3390/environments12060204
APA StyleFanfarillo, E., Angiolini, C., Capitani, C., De Pasquale Picciarelli, M., Fedeli, R., Fiaschi, T., Jepkogei, P., Pafumi, E., Valle, B., & Maccherini, S. (2025). Short-Term Effects of Minimum Tillage and Wood Distillate Addition on Plants and Springtails in an Olive Grove. Environments, 12(6), 204. https://doi.org/10.3390/environments12060204








