Abstract
This review reports some aspects of soil contaminant chemistry and its fundamental role in shaping the soil–human health relationship. Exposure to soil contaminants can occur through direct pathways, such as ingestion, inhalation, and dermal contact, as well as indirect pathways, including food chain contamination via plant uptake or groundwater leaching. The mobility and persistence of organic and inorganic pollutants in soil are primarily controlled by sorption–desorption processes, which involve a complex interplay of physical and chemical mechanisms. Soil properties, such as pH, organic matter content, clay minerals, and oxide hydroxides, play a crucial role in regulating these processes and determining contaminant behavior. A high sorption capacity enhances the soil’s ability to mitigate pollutant mobility, thereby reducing their infiltration into groundwater and accumulation in the food chain. Soils rich in organic matter and fine-textured minerals, such as clay, can effectively immobilize contaminants, limiting their bioavailability and potential harm to human health. A deeper understanding of how soil characteristics influence contaminant mobility and bioavailability is critical to addressing the hazards of soil pollution for human health. Beyond merely assessing contaminant concentrations, it is essential to consider the dynamic processes governing pollutant fate in soil, as they ultimately shape exposure pathways and health risks. This knowledge is the key to developing more effective strategies for mitigating soil contamination and protecting public health.
1. Introduction
Soil, the outermost layer of the Earth’s crust, is a dynamic, multi-phase medium composed of minerals, organic materials, water, air, and living organisms. It provides numerous essential ecosystem services: supporting biomass production, enhancing biodiversity, acting as a major carbon reservoir, and being integral to ecological stability and human activities [1]. Despite being classified as a non-renewable resource, soil is increasingly threatened by human activities such as compaction, contamination, and urbanization, as well as related processes like erosion, flooding, and landslides. These impacts are further exacerbated by practices related to agriculture, industry, urban expansion, and land-use changes. As a result, soil degradation reduces fertility, carbon sequestration, water retention, and the efficiency of nutrient cycling, while diminishing the capacity to break down pollutants. Such degradation has far-reaching consequences, affecting water and air quality, amplifying climate change, and threatening food security and public health [2]. Soil has always been the basis of human existence, and it is critical for health, as it serves as the principal source for food production [3]. Ensuring the availability of food is an ongoing challenge, a longstanding issue. While multiple factors impact food supply, soil remains a vital resource in addressing this complex issue. Globally, rising food demand underscores the need for sustainable soil management and conservation. All countries are involved in mitigating soil degradation which can compromise long-term agricultural productivity both in developing and industrialized countries [4].
In Europe, the new “Soil Strategy for 2030” considers soil a key resource in European environmental policies, aiming to ensure healthy soils by 2050 [5]. This strategy is based on the principle that soils are the basis of human health. It is now established that there is a close relationship between soil quality and human health [6,7,8,9]. With 95% of food in the EU originating from soil, improving soil quality is a priority for safeguarding public health and the environment. Adopted in 2021, the strategy outlines measures for soil protection, including a dedicated legal framework to prevent soil degradation and contamination. Member states must take measures to avoid soil contamination and to remediate contaminated soils when concentrations of the contaminants pose a risk for human health or the environment.
Similar policies have been adopted in all industrialized countries since five million contaminated sites have been discovered all over the world with both metals and organic contaminants [10,11]. Most human activities are closely connected with the soil, therefore, there is continuous human exposure to contaminants present in the soil, both through direct contact and through the food chain. Soil chemistry plays an essential role in determining the distribution of contaminants across soil phases, their persistence, and their transfer along exposure pathways. As a result, soil chemistry is of fundamental importance to human health [12].
Understanding contaminant behavior in complex soil systems is essential for developing effective remediation strategies that minimize human exposure. Soil chemistry supports these efforts by updating remediation techniques that enhance efficiency and reduce risks while promoting sustainable soil restoration. However, the intricate nature of contaminant interactions within the soil environment—encompassing its various physical and chemical phases—pose challenges for defining comprehensive legislative frameworks.
Unlike air and water regulations, which assume that the total concentration of contaminants is fully bioavailable, soil-bound contaminants exhibit bioavailability that is highly dependent on soil properties. This variability complicates the modeling of exposure pathways and the establishment of health-based guidelines [13].
This article reviews key aspects of soil contaminant chemistry and its crucial role in the soil–human health relationship. A comprehensive understanding of this relationship requires going beyond the mere analytical assessment of contaminant concentrations. It is essential to investigate the dynamic processes occurring in soils, which are influenced by their specific properties and, in turn, affect the amount and persistence of contaminants along exposure pathways. Since soil is a living system, chemical factors are only one component of a complex interplay of biological, microbiological, and physical processes, all of which play a crucial role in determining the impact of contamination on human health.
We also briefly mention some emerging contaminants that will require further studies in the very near future, as well as the positive effects of certain elements that, despite being classified as potential pollutants, are essential for human health.
2. Exposure Pathways
Protecting human health is the primary objective in risk-based assessments of soil contamination. However, the extent of health damage caused by contaminated soil remains a topic of debate. To evaluate the risks associated with contaminated sites, several exposure models have been developed [14]. The most commonly used models follow a two-step approach: first, assessing contaminant distribution among soil phases, and second, analyzing the direct and indirect transfer of contaminants from soil to humans.
Soil chemical reactions regulate the distribution of contaminants across different phases (solid, liquid, and gaseous) determining their persistence in the environment and the exposure routes that pose health risks [15]. The solid phase of soil provides numerous surfaces that can retain contaminants, thereby influencing their release into the liquid phase, where they may become leachable to groundwater and bioavailable to plants and living organisms.
To assess the hazards posed by soil contamination, it is essential to understand the sources of pollutants and the pathways through which they reach human receptors. Soil contamination can originate from localized (point source) pollution or widespread (diffuse) pollution, with the primary distinction being how pollutants enter the soil. Point source pollution results from specific activities, such as industrial operations, landfills, and incinerators, where soil is directly exposed to contaminants. In contrast, diffuse pollution stems from broader processes, including natural events such as long-distance pollutant transport, atmospheric deposition, or sediment accumulation. Agricultural practices, waste recycling, and improper waste management also contribute significantly to diffuse contamination [16].
Human exposure to soil contaminants occurs through both direct pathways, including soil ingestion, inhalation, and dermal contact [15] and indirect pathways, such as food chain contamination (via plant uptake or groundwater leaching). It is estimated that over nine million human deaths are attributable to environmental pollution each year [17].
2.1. Direct Pathways
Ingesting contaminated soil can expose individuals to harmful substances, posing particular risks for children, who are more likely to ingest soil by placing soiled hands or objects in their mouths [18,19,20,21,22]. Among the toxic substances present in soil, heavy metals are of significant concern [23,24,25,26,27,28]. Lead exposure, in particular, poses severe neurotoxic risks to children, impairing the development of their nervous systems [29]. Ingestion is also a primary exposure route for agricultural workers, who are in frequent direct contact with soil [30,31].
Soil inhalation is another factor of concern in evaluating risks associated with contaminated environments [32,33,34]. Particles of soil dust inhaled by humans become trapped in the lungs, where they can contribute to the development of respiratory diseases. The severity of lung damage depends on the nature and amounts of the inhaled particles. Finest particles can penetrate deep in the pulmonary alveoli, triggering inflammation [35]. Further risks arise from inhaling particles from contaminated soil containing toxic substances, which can exacerbate health problems [36]. Also, agricultural soils are considered a source for toxics inhalation due to the frequent presence of heavy metals and persistent pesticides [37].
2.2. Indirect Pathways
The transfer of contaminants from soil to plants is the primary indirect exposure pathway for humans. This process is influenced by multiple factors, including the physical and chemical properties of contaminants, soil characteristics, and plant-specific traits. Contaminant absorption occurs via two main mechanisms: root absorption, followed by translocation to the aerial parts of the plant and leaf absorption, through direct contact with volatile compounds or contaminated dust [38].
Contaminant uptake by plants occurs through both active and passive mechanisms. Active uptake is specific to certain elements or compounds, while passive uptake depends on the movement of contaminants via the transpiration stream. For instance, heavy metals absorbed by plant roots can accumulate in edible plant tissues, ultimately entering the food chain and posing risks to human health. Root uptake is a crucial step in this process [39], with soil physicochemical properties—particularly pH—playing a fundamental role. Soil pH affects the solubility of inorganic elements, thereby determining their presence in the soil’s liquid phase, from which they can be absorbed by plants [40].
Concerning organic compounds, their uptake by plants can occur passively through transport across root membranes via the transpiration stream or may be actively absorbed by a transporter protein [41]. Once absorbed, plants may metabolize these compounds into secondary products that can accumulate in edible tissues, posing potential risks to the food chain [42].
Volatile compounds, characterized by Henry’s law constant greater than 10−4, tend to evaporate from soil surfaces. Instead of being absorbed through the roots, they are more likely to contaminate plants via leaf absorption. Understanding these processes provides a valuable framework for assessing contamination risks in polluted soils [43].
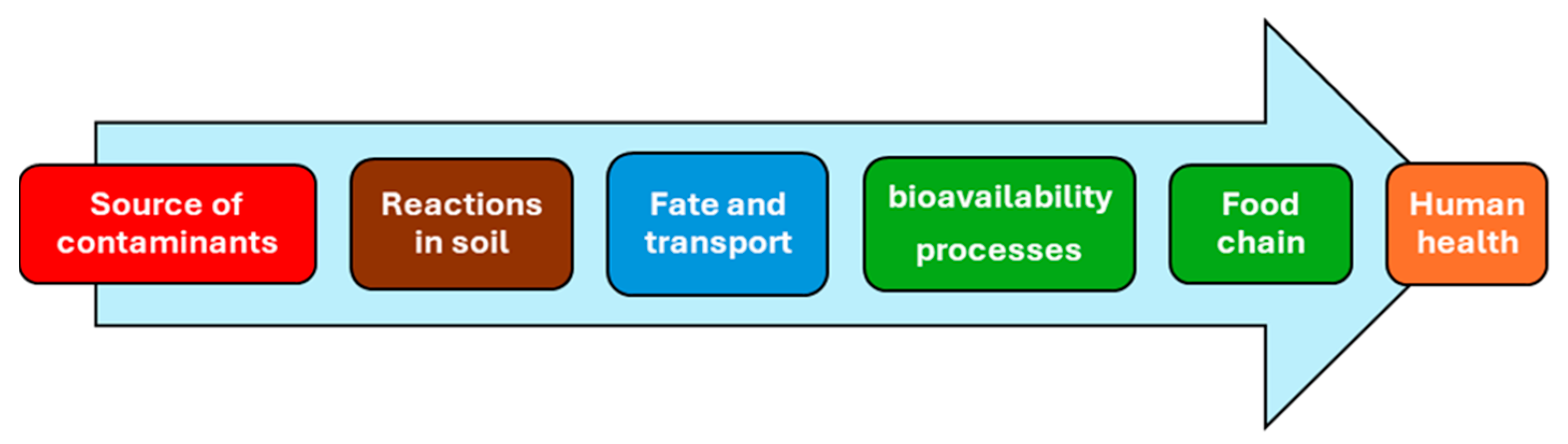
In Figure 1, the relation between soil contaminants, plant uptake, and human health is reported.
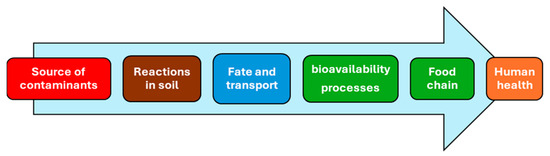
Figure 1.
The continuum from soil contaminants–plants–human health.
As illustrated in Figure 1, soil chemistry plays a key role in defining site-specific reactions based on the unique properties of each site. This understanding leads to the development of “fate and transport” models, which rely on site-specific experimental evidence.
Soil chemistry contributes to determining bioavailability processes. Over the years, various methodologies have been developed to assess bioavailability, including chemical extractions and other analytical approaches. However, the evaluation of bioavailability must also consider the biological characteristics of the target organism. For instance, plants utilize their physiological mechanisms to absorb a certain amount of contaminants present in the soil in bioavailable forms [13]. This aspect is crucial, as food quality can directly affect human health through the consumption of plant-based products or indirectly through the consumption of animal-derived products, such as meat, where bioaccumulation processes may occur.
2.3. Bioaccessibility and Bioavailability
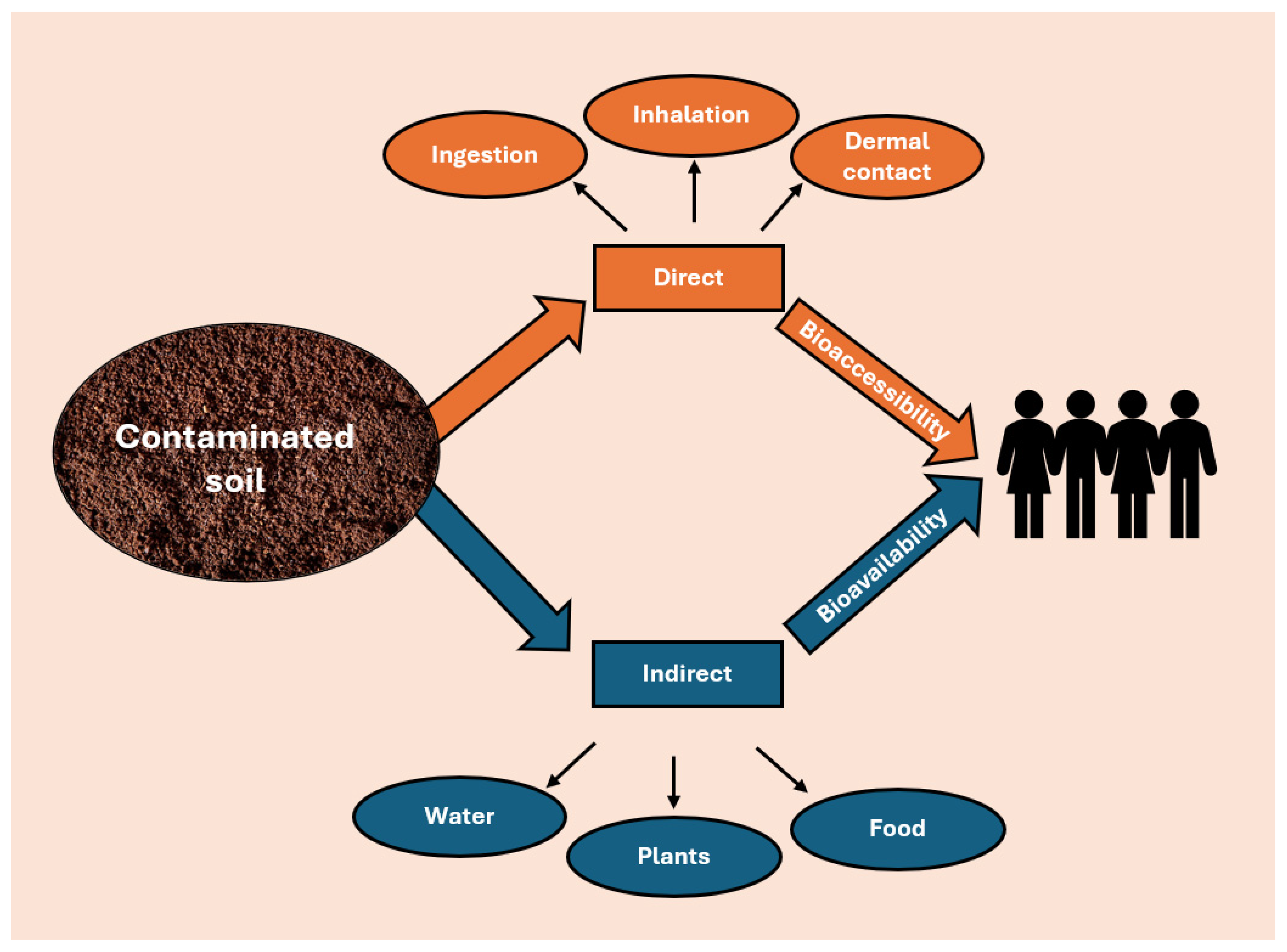
From a chemical perspective, direct and indirect pathways can be described in different ways. While direct ingestion and inhalation of soil are directly related to the total concentration of a contaminant in the soil, the transfer of contaminants from soil to food depends more on the bioavailable concentration rather than the total contaminant content (Figure 2). This distinction underscores the need for a deeper understanding of contaminant behavior in soil. Consequently, a comprehensive health risk assessment cannot rely solely on total concentration measurements but must also consider bioavailability pathways. Therefore, the health risk pathways can be described by two different quantities: bioaccessibility and bioavailability [44]. Bioaccessibility refers to the fraction of contaminants that dissolve in the gastrointestinal tract and become available for absorption by the human body. This parameter is essential for assessing exposure through soil ingestion, a particularly critical pathway for children due to hand-to-mouth behaviors [45]. Bioavailability, also defined as environmental availability, is determined by the concentration of contaminants in the soil solution, and it is essential for identifying the amount of contaminants that can be absorbed by plants, ultimately determining potential human exposure through food consumption [46].
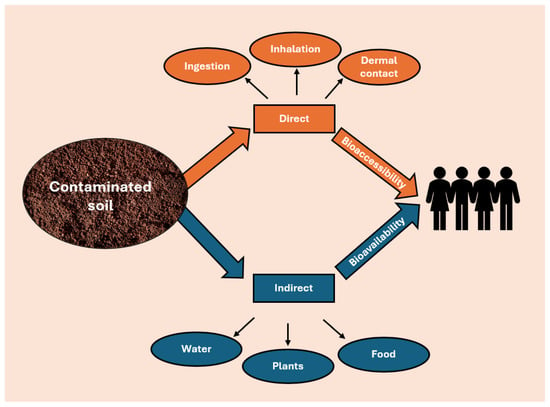
Figure 2.
Transfer contaminants from soil to humans.
From an analytical perspective, bioaccessibility is generally estimated in the <250 μm soil fraction which is considered the most representative of the hands-to-mouth transfer. There are various in vitro methods such as ISO 17924:2018 method which have also been validated in vivo studies [47]. One of the key advantages of in vitro testing is the elimination of animal use, along with the reduced time and cost associated with analysis.
The determination of the bioavailable fraction of soil contaminants has been extensively studied, with various procedures proposed and applied depending on the nature of the contaminants [13]. From a chemical standpoint, methodologies primarily differ based on pH conditions. In bioaccessibility tests, the highly acidic pH facilitates the extraction of contaminants largely correlated with total concentration. In contrast, bioavailability tests employ milder extractants with pH values closer to neutrality, leading to an extraction process that is more influenced by the soil’s chemical and physical properties. The different types of extraction therefore lead to different results between the two parameters, bioavailability and bioaccessibility, as observed in various soil types [44,48,49].
3. Overview of Relevant Contaminants and Their Behavior in Soil
3.1. Organic Contaminants
Many organic compounds widely used in industrial processes and agriculture degrade very slowly, persisting in the soil for extended periods. Among them, persistent organic pollutants (POPs) are of particular concern, as they can bioaccumulate under certain environmental conditions, leading to concentrations that pose serious risks to ecosystems and human health [50].
POPs of various origins have been associated with serious health problems, including cardiovascular, neurological and immunologic illnesses [17,50,51], reproductive disorders, and various forms of cancer [52]. Currently, the list of POPs consists of 22 compounds of substances (Table 1) characterized by the presence of very strong carbon chlorine and carbon fluorine bonds, which make them highly resistant to degradation. Due to their lipophilic nature, and chemical stability, these compounds persist in the environment and accumulate in living organisms.

Table 1.
POPs main sources of soil contamination and related risks. The numbers in brackets indicate the references describing the effects of each compound on human health.
The regulation of POPs is primarily governed by the Stockholm Convention, which establishes control measures for these substances. As new hazardous compounds are identified, the convention is updated to include additional pollutants. Member states are encouraged to develop and implement safer alternatives to mitigate the environmental and health risks associated with POPs.
The movement and persistence of organic contaminants, including POPs, in the soil, are primarily governed by sorption–desorption processes, which involve multiple physical and chemical mechanisms. The term sorption refers to the accumulation of contaminants in the solid phase of soil, regardless of the specific mechanisms involved [68]. These processes significantly influence contaminant persistence, degradation, and bioavailability, in the soil, affecting their potential to enter the food chain.
Sorption plays a crucial role in slowing down the transport of organic contaminants in both the liquid and gaseous phases. This delays contaminant leaching, preventing their migration to groundwater, and reduces mass loss through volatilization. In addition, sorption can also influence the rate of biodegradation and the bioavailability of organic contaminants.
Thus, these processes play an important role in mitigating environmental pollution because they slow down the leaching of harmful substances into water sources, which are often used for drinking and irrigation. Additionally, sorption can limit the uptake of toxic compounds by crops, reducing the risk of human exposure through food consumption.
The sorption processes depend both on contaminant characteristics and soil properties. The key properties of organic contaminants that influence their interaction with soil surfaces include their hydrophobic nature, water solubility, the presence of specific functional groups, and molecular size. A widely used indicator [69] for assessing hydrophobicity is the n-octanol-water partition coefficient (Kow). Kow > 4.0 indicates highly hydrophobic compounds (strong soil sorption) and Kow < 4.0 more water-soluble compounds (weaker sorption). Even within the same class of organic contaminants, Kow values can vary significantly, influencing environmental behavior and health risks.
The properties of the soil that significantly contribute to quantitatively determining the behavior and transformations of organic contaminants in the soil environment are the organic matter, and the mineral components with various particles sizes.
In addition, physicochemical parameters of the liquid soil phase such as pH, temperature, and the ionic strength can play key roles in the sorption of organic contaminants reactions [70,71].
The characteristics of organic matter (OM) largely determine the sorption behavior of organic compounds. OM is a heterogeneous sorbent, and its reactivity depends on its composition. Soil OM, a complex mixture of organic compounds with various functional groups and properties, is considered a hydrophobic polymeric phase where hydrophobic organic contaminants, such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs), undergo reactions through both internal surfaces (partitioning) and external surfaces (adsorption) [72,73,74,75].
The diverse molecular composition of humic substances enables the coexistence of both hydrophilic and hydrophobic regions, which in turn imparts notable sorptive capa-bilities toward organic pollutants spanning a broad range of polarities. In partitioning, hydrophobic organic compounds become homogeneously distributed within the nonpolar regions of OM, such as hydrophobic domains in humic substances. This process is driven by the tendency of hydrophobic molecules to minimize their interaction with water while maximizing contact with other nonpolar molecules [71].
The nonpolar regions of OM, as well as certain functional groups like aromatic rings and carbon–carbon double bonds, can provide sites for the sorption of hydrophobic compounds. Humic substances exhibit a high sorption capacity due to the macromolecular aliphatic chains and aromatic components which constitute their hydrophobic domains, and a significant porosity that promotes the sorption and retention of organic contaminants. Weak van der Waals forces, including London dispersion forces, can occur between the nonpolar regions of OM and hydrophobic compounds. Additionally, aromatic structures enhance sorption affinity through specific linkages involving the aromatic system [76,77].
The sorption of organic contaminants in soil is directly correlated with OM content and is often described by the partition coefficient (Kow) [76]. For example, the sorption of perfluorinated alkyl substances (PFAS) and non-ionic herbicides is also largely influenced by soil OM, generally increasing with higher OM content [74,78,79,80,81]. However, the chemical characteristics of humic substances vary between soil types, leading to differences in sorption capacity even among soils with similar OM content [82,83]. These variations arise from differences in OM structural conformation and the degree of humification [84,85,86].
OM also contains polar functional groups capable of hydrogen bonding, which can reduce the sorption of hydrophobic organic contaminants due to increased electrostatic repulsion [87]. Conversely, the polarity of OM functional groups enhances the sorption of polar organic contaminants, such as atrazine-like herbicides [88].
The interaction of organic contaminants with mineral soil components, such as clay minerals and oxy-hydroxides, plays a crucial role in sorption processes. The sorption of organic compounds depends on the specific properties of the involved clays and is particularly significant when the clay-to-OM ratio is sufficiently high (i.e., >10–30), especially for polar organic compounds [89]. Organochlorine pesticides have been found to sorb and be retained by montmorillonite and kaolinite clays through both hydrophobic interactions and charge–dipole interactions [90]. Studies on pesticide sorption indicate that these organic compounds are primarily associated with smaller size soil particles, where high surface areas facilitate strong binding, reducing leachability [91].
In non-swelling clays, sorption primarily occurs on the outer mineral surfaces. In contrast, swelling clays allow additional sorption within their interlayer spaces, increasing their overall capacity to retain organic contaminants [92,93]. The molecular structure of organic contaminants significantly influences their interactions with clay minerals. While smaller molecules can penetrate the interlayer regions of swelling clays, larger molecules are restricted to sorption on external surfaces [94].
Mineral surface charge also plays a key role in organic contaminant sorption. Polar organic compounds, such as cationic herbicides and organochlorine pesticides, exhibit strong interactions with minerals that possess permanent negative charges, often through cation exchange mechanisms [95,96,97]. Additionally, polar organic contaminants can interact with oxygen atoms in mineral structures via hydrogen bonding [88,98,99].
Hydrophobic organic contaminants, such as PAH, tend to exhibit high sorption due to their link with the neutral surfaces of clay minerals [94,100]. The sorption of nonpolar organic contaminants is largely governed by hydrophobic associations, van der Waals forces, and electron donor-acceptor mechanisms. The strength of these interactions depends on the molecular size and shape of the organic contaminants as well as the available sorption sites on mineral surface area [101,102].
Additionally, aluminum and iron oxy-hydroxides significantly contribute to organic compounds sorption in soils through hydrogen bonds and ligand exchange [98]. Also, inner-sphere complexes can be formed between hydrophobic organics and oxide surfaces [99]. It must be considered that OM can form stable complexes on the surface of minerals [100,101,102] which influence the sorption processes of organic compounds by changing the availability of the sorption sites [98,103,104].
Organic pollutants in soil pose significant risks to human health, with consequences depending on the type of contaminant, level of exposure, and individual susceptibility [51]. Certain POPs, such as dioxins and PCBs, are classified as carcinogens and exhibit endocrine-disrupting and immunotoxic properties. Long-term exposure has been linked to an increased risk of various cancers, including liver cancer, breast cancer, and non-Hodgkin lymphoma. Due to their stable chemical structure, these compounds accumulate in fatty tissues, leading to chronic health effects [105].
Organochlorine pesticides (e.g., DDT, chlordane, and dieldrin) are well known for their endocrine-disrupting effects, which can result in reproductive issues and developmental disorders in newborns and children. Some have also been associated with an increased risk of tumors [106]. These pesticides persist in the environment and bioaccumulate through the food chain, leading to human exposure via direct contact with contaminated soil or water, and indirect exposure through the consumption of contaminated food. Other organic pesticides and derivatives, such as hexachlorobenzene (HCB), have been associated with neurotoxic effects and may contribute to immune and endocrine dysfunctions. Their high persistence and bioaccumulation potential result in chronic exposure, even at low concentrations, leading to long-term health effects [107].
It is important to note that the toxicity of these compounds depends on concentration, duration of exposure, and the specific route of entry (inhalation, ingestion, or skin contact). Additionally, the presence of multiple organic contaminants can lead to synergistic or additive effects, further complicating their toxicological profiles and associated health risks [108].
3.2. Inorganic Pollutants
Among inorganic contaminants, heavy metals are the most critical elements in contaminated soil. Soil contamination by heavy metals has escalated significantly since the Industrial Revolution. Due to their non-biodegradable nature, these elements persist in soils, accumulating over time. Heavy metals constitute a major class of soil pollutants due to their potential to enter the food chain, thereby reducing food quality and posing risks to human health. Their ability to bioaccumulate exacerbates these risks, as they concentrate on living organisms over time, often exceeding safe levels [109]. This occurs because the absorption rates of heavy metals frequently surpass their excretion rates.
Heavy metals cannot be degraded or eliminated and may enter the soil through natural processes, such as soil formation, as well as human activities, including the application of fertilizers [110], biosolids [111], and mining operations [112]. Additionally, various sources continuously release airborne heavy metals into the environment [113]. Some trace elements, such as copper (Cu), zinc (Zn), cobalt (Co), nickel (Ni), and chromium (Cr), serve as essential nutrients when present in appropriate concentrations and chemical forms. However, others, including mercury (Hg), lead (Pb), and cadmium (Cd), are considered highly toxic and pose severe environmental and human health risks [114,115]. The United States Agency for Toxic Substances and Disease Registry (ATSDR) has classified arsenic (an anionic contaminant), lead, and mercury as the three most hazardous pollutants in its 2022 substances priority list [116].
Arsenic is a well-known, highly toxic carcinogen [117,118,119,120]. Moreover, it is a dangerous endocrine-disrupting chemical with a broad range of health risks [121]. Ingestion through contaminated water and food has been linked to cancers of the skin [122], bladder [123], lungs [124], and liver [86]. Furthermore, arsenic exposure has been associated with circulatory and neurological complications [125] as well as chronic liver and kidney diseases [126].
Lead (Pb) is known to cause numerous adverse health effects, including reduced cognitive function, infertility, and behavioral deficiencies in children [127]. Additionally, lead exposure has been linked to anemia, hypertension, renal dysfunction [128], and cardiovascular disease. It also poses significant risks to fetal development, as it can cross the placenta, potentially leading to premature birth [129]. At high exposure levels, lead is classified as a carcinogen [130] and has been associated with cardiovascular diseases [131].
Mercury (Hg) has been a focal point of research due to its toxicity and high mobility in the soil and the environment [132]. As a highly hazardous element, no safe level of mercury exposure has been established [133]. Even minimal exposure can have detrimental effects, particularly by disrupting the central nervous system [134]. Mercury toxicity extends to various systems, affecting the cardiovascular, pulmonary, renal, and reproductive functions [135,136].
Cadmium (Cd) exposure can disrupt calcium metabolism, leading to kidney dysfunction, osteoporosis, bone disease, and lung cancer [137]. It has also been implicated in neurodegenerative disorders [138], cardiovascular disease [139], osteoarthritis [140], diabetes, and breast and prostate cancers. Furthermore, cadmium negatively impacts fertility and neonatal health [141,142].
The potential health risks posed by heavy metals are closely linked to their bioavailability. For these contaminants to exert toxic effects, they must first be absorbed, a process influenced by exposure pathways and the organism’s unique physiological traits.
As with organic pollutants, the behavior of heavy metals and metalloids in soil strongly depends on their interactions with organic and inorganic soil constituents. These interactions, primarily governed by sorption processes, play a crucial role in distributing inorganic elements among soil phases, thus regulating their mobility and bioavailability. Since bioavailability determines the transfer of elements from the soil’s liquid phase to plants, it is a key factor in assessing human health risks associated with food chain contamination.
The distribution of an element among soil phases depends on several factors, including the following:
- (i)
- The intrinsic properties of the metal source, which may originate from primary minerals in soil parent material or anthropogenic contributions such as industrial activities, sewage sludge, mine tailings, and atmospheric deposition;
- (ii)
- The specific affinity of individual metal ions for soil sorption surfaces and soluble ligands in the soil solution;
- (iii)
- The characteristics of the soil itself, including pH, organic matter content, clay minerals, metal oxides and hydroxides, redox potential, moisture content, temperature, and biological activity [143].
Inorganic contaminants in mobile and bioavailable forms exist in soil solutions or are reversibly sorbed onto solid phases, from which they can be released in response to shifts in soil solution equilibrium. In the liquid phase of soil, metals occur in various chemical forms, including soluble complexes with inorganic and organic ligands and dissolved organic matter. Additionally, all forms present in the soil solution are subject to leaching through drainage water. In contrast, metals and metalloids irreversibly retained by the soil solid phase are not involved in bioavailability processes [40].
Among soil properties, pH plays a particularly critical role in determining metal concentrations in soil solution, influencing precipitation and dissolution dynamics [144]. Generally, heavy metal solubility decreases as pH increases, as alkaline conditions promote the formation of solid phases that reduce metal ion concentrations in the soil solution. Furthermore, higher pH levels promote metal hydrolysis and the formation of metal-hydroxo complexes, which significantly reduce metal ion solubility. Conversely, under acidic conditions, sorption declines due to acid-catalyzed oxide dissolution, and the complexation capacity of organic matter decreases.
Clay minerals also play a crucial role in metal ion retention through ion exchange and specific sorption mechanisms [145]. Metals may bind to hydroxyl ions adsorbed on clay surfaces or directly to sites formed by proton removal. Retention capacity varies among clay minerals; expandable clays, such as smectite and vermiculite, exhibit superior metal retention in their interlayer spaces compared to non-expandable clays like kaolinite. Thus, soil texture and mineral composition influence metal bioavailability, with sandy soils generally promoting greater metal solubility and availability to plants compared to clay-rich soils [146].
Despite being less abundant, organic matter greatly impacts metal mobility and availability. Metals bind to humic substances in both the solid and liquid phases, forming complexes that either increase or decrease their mobility. Soluble complexes with fulvic acids enhance mobility, whereas high-molecular-weight humic acids form very strong linkages with metals that reduce their bioavailability. Metal retention by organic matter occurs through complexation and sorption, driven by ion exchange and inner-sphere reactions, with functional groups such as carboxyl, phenol, and amino playing essential roles. During soil humification, an increase in these functional groups enhances the stability of metal-organic complexes, particularly under alkaline conditions [147,148].
Soil cation exchange capacity (CEC), determined by the density of negative charges on colloidal surfaces, is another key factor influencing heavy metal retention. Soils with high clays and high organic matter content exhibit greater CEC values due to their substantial net negative charges. Heavy metals can displace cations from these surfaces through exchange reactions, facilitated by weak electrostatic bonds [149,150].
Redox potential further modulates the solubility of heavy metals. Aerated, dry soils typically have high redox potential values, while waterlogged or organic-rich soils exhibit low values. Under low redox conditions, metal solubility often increases because Fe-Mn oxyhydroxides dissolve, releasing previously adsorbed metals. Conversely, in anaerobic environments, metal sulfides may form from sulfates, reducing solubility. Variations in soil characteristics lead to differing effects of redox potential on metal mobility [151,152].
Oxides and hydroxides of iron and manganese significantly contribute to heavy metal retention in soils through precipitation and specific sorption processes. All oxi-hydroxides are effective at immobilizing heavy metals in contaminated soils, with a sorption preference that generally follows the order: Pb > Cu >> Zn > Cd [146].
Beyond these primary factors, temperature and ionic strength also influence metal solubility. Higher temperatures may accelerate organic matter decomposition, altering metal mobility and plant uptake [153]. Increased ionic strength in soil solutions reduces heavy metal sorption due to competition from alkaline ions, increasing their mobility [154]. Biological activity also exerts a significant influence, as plant roots and microorganisms can alter metal solubility through the release of exudates that acidify the soil or act as complexing agents. Microbial processes may precipitate metals as sulfides or promote their retention through sorption onto newly formed organic surfaces. Understanding all the interconnection among metals and soil properties is essential for developing strategies to mitigate the environmental and health risks associated with heavy metal contamination in soils.
The behavior of contaminants such as arsenic and chromium, which exist as anions in soil, differs significantly from that of heavy metals due to their negative charge. Also, the mobility, bioavailability, and toxicity of anionic contaminants in soil are influenced by factors such as pH, organic matter, and mineral components, all of which affect their sorption onto the solid phase of the soil.
Arsenic (As) can be taken as an example of the behavior of anionic contaminants in soil. Arsenic pollution is widely recognized as one of the most critical global environmental threats also for the chemical similarity to Phosphor (P) which facilitates the replacement of P with As in plants and has been extensively studied over the past 30 years [155,156,157].
In the case of arsenic (As), soil pH is a key factor in determining its mobility and availability. Arsenic exists primarily as arsenate (AsV) in oxidizing conditions and arsenite (As III) in reducing conditions, with its sorption behavior being highly pH dependent. At neutral to alkaline pH, arsenate sorption onto soil minerals, particularly iron and aluminum oxides, decreases, leading to higher As mobility. Conversely, at lower pH levels, arsenate forms stronger bonds with these minerals, reducing its potential for leaching. Arsenite, being less negatively charged, generally exhibits weaker sorption and higher solubility across a broader pH range [146].
Redox conditions play a major role in determining arsenic behavior in soils. Under oxidizing conditions, arsenic is primarily present as arsenate, which has a higher affinity for sorption onto metal oxides, thereby reducing its mobility. In contrast, under reducing conditions, arsenate is converted to arsenite, which is more soluble and weakly adsorbed, leading to an increased risk of leaching and groundwater contamination. Additionally, the dissolution of Fe-Mn oxides under low redox conditions released previously adsorbed arsenic into the soil solution, further elevating its bioavailability [157].
Iron, manganese, and aluminum oxides are among the most critical soil components controlling arsenic retention. These oxides act as strong sorbents, particularly in oxidizing environments where arsenate forms inner-sphere complexes with their surfaces. However, under reducing conditions, the dissolution of Fe-Mn oxides releases arsenic into solution, significantly increasing its mobility. The presence of competing anions, such as phosphate, can also displace arsenic from oxide surfaces, further influencing its environmental fate [146].
Soil organic matter influences arsenic dynamics through complexation, redox interactions, and competitive sorption. Humic and fulvic acids can bind arsenic species, forming soluble organic-metal complexes that enhance arsenic mobility in soil solutions. In addition, microbial degradation of organic matter can alter redox conditions, affecting arsenic speciation. High molecular weight organic compounds may enhance arsenic retention by stabilizing metal oxides essential for arsenic sorption. Under reducing conditions, microbial activity may promote the conversion of arsenate to the more mobile arsenite form [158].
Clay minerals play a crucial role in controlling the retention of anionic contaminants through electrostatic interactions and ligand exchange processes. Unlike cations, anions are typically repelled by the negatively charged surfaces of clay minerals unless specific sorption sites are available. Iron- and aluminum-rich clays, such as allophane and kaolinite, can facilitate arsenic retention by forming inner-sphere complexes. However, competition with other anions like phosphate can significantly reduce arsenic sorption capacity, leading to increased mobility in soil solutions [146].
Unlike cationic contaminants, anions are not directly influenced by cation exchange capacity (CEC) but can still be affected by the soil’s charge properties. In highly weathered soils with variable-charge minerals, arsenic sorption is pH-dependent and often occurs through ligand exchange rather than electrostatic attraction. The presence of positively charged sorption sites, particularly in acidic soils rich in iron and aluminum oxides, enhances arsenic retention. However, in soils with high CEC dominated by negatively charged surfaces, anionic contaminants are more likely to remain in solution and be transported through the soil profile [157].
The effects of soil properties on the sorption and bioavailability of heavy metal and anionic contaminants are summarized in Table 2 using Cd and As as examples.

Table 2.
Different effects of some soil properties on Cd and As sorption and bioavailability.
In conclusion, the greater the soil’s sorption capacity, the more effective it is in protecting human health. Soils with high organic matter content and fine-textured minerals, such as clay, can significantly reduce the mobility of both organic and inorganic pollutants preventing their infiltration into groundwater and accumulation in the food chain. Understanding the sorption mechanisms are fundamental in environmental protection strategies. Strengthening soil’s natural filtration properties is therefore a crucial strategy for environmental protection and human health preservation. By enhancing the sorption potential of soils through sustainable land management practices, remediation techniques, and the use of adsorbent materials like biochar and activated clay can help to reduce human exposure to toxic substances.
3.3. The Long-Term Impact of Soil Quality on Human Health—Some Case Studies
A deeper understanding of how soil characteristics influence contaminant behavior is also essential for addressing many of the challenges targeted by the One Health and Planetary Health framework [159]. One of the most important tools to reach this target is cohort studies. Cohort studies represent a fundamental methodological approach for assessing the long-term health effects associated with environmental exposures, including those arising from contaminated soils. By monitoring defined populations over extended periods, these studies enable the identification of potential causal relationships between chronic exposure to soil pollutants and the incidence of various diseases, including cancers, respiratory illnesses, and metabolic or endocrine disorders.
Nevertheless, the study of soil-related health risks entails substantial methodological and conceptual complexity, primarily due to the inherently multidisciplinary nature of the topic. A comprehensive understanding requires the integration of soil chemistry—to characterize contaminant types, concentrations, bioavailability, and mobility—with epidemiological methods that quantify exposure levels, latency periods, and disease risk across diverse population groups. In addition, insights from toxicology, exposure science, spatial analysis, and public health are often essential to contextualize and interpret findings effectively.
These challenges are further confounded by heterogeneous exposure pathways, the variability in human behavior and vulnerability, and the long latency of many chronic diseases. Despite these obstacles, cohort studies remain an essential tool in environmental health research, providing robust evidence to inform risk assessments, regulatory actions, and public health interventions aimed at mitigating the impacts of soil contamination. A few case studies related to soil contamination by specific contaminants and human health outcomes are briefly reported below.
Concerning dioxins, risk of non-Hodgkin lymphoma derived by soil contamination was investigated [160] in three electoral districts (population: 170,000) near the municipal solid waste incinerator of Besançon in Eastern France, which has been operating since 1971. The facility failed to comply with emission regulations, notably in 1997, when exhaust gases were not maintained at sufficiently high temperatures, leading to dioxin emissions. Once released, dioxins and related organochlorines quickly settle into surface soils, where they persist for extended periods. The main human exposure route was through consumption of contaminated animal-derived foods (meat, dairy, and eggs) from the impacted area. The study analyzed blood samples from individuals diagnosed with non-Hodgkin lymphoma and healthy controls, revealing a correlation between serum organochlorine levels and lymphoma incidence.
The health effects of cadmium and lead contamination of soil was explored [161] near the Mbeubeuss landfill, located about 30 km from Dakar. Operating since 1970, the site receives over 395,000 tons of household waste annually, resulting in heavily polluted soils. The study measured cadmium and lead levels in blood and urine of residents living near the site for over five years, compared to those from less-exposed areas. Results showed significantly elevated metal concentrations in the exposed group. The observed toxicity was likely linked to oxidative stress triggered by increased reactive oxygen species (ROS), which can impair antioxidant defenses and cause lipid peroxidation. Biomarkers’ analysis also indicated early signs of kidney damage among the exposed population. These findings suggest that even low to moderate exposure to Cd and Pb can cause genotoxic effects through ROS pathways.
While most research on polycyclic aromatic hydrocarbons (PAHs) has focused on occupational exposure, these compounds also pose risks to the general population from soil contamination, primarily through diet. A study [162] aimed to assess the relationship between dietary PAH intake and mortality in middle-aged women within the French E3N prospective cohort. A total of 72,513 participants completed a detailed food frequency questionnaire in 1993, covering 208 food items. Dietary PAH exposure was estimated using contamination data from the French Agency for Food, Environmental and Occupational Health & Safety (Anses) and focused on four PAHs (PAH4): benzo[a]pyrene (BaP), chrysene (CHR), benzo[a]anthracene (BaA), and benzo[b]fluoranthene (BbF). Regression models were used to calculate hazard ratios (HRs) for all-cause, cancer-related, and cardiovascular mortality, including specific causes such as breast, lung/tracheal, and colorectal cancer, as well as stroke and coronary heart disease. Between 1993 and 2011, 4620 deaths were recorded, including 2726 from cancer and 584 from cardiovascular disease. The median PAH4 intake was 66.1 ng/day. No significant associations were found between dietary PAHs and overall mortality or most specific causes. However, a statistically significant link was identified between higher PAH4 intake and increased mortality from lung and tracheal cancers—particularly among current smokers. These findings suggest that while dietary PAH exposure may not influence most mortality risks, it could elevate the risk of lung/tracheal cancer, especially in active smokers.
A further study of relevant importance related to soil quality and human health was carried out in UK [163]. Due to the logistical and financial constraints of conducting large-scale longitudinal cohort studies, this research proposed an alternative approach by integrating routinely collected medical outcome data with comprehensive soil quality surveys. This study utilized data from the Geochemical Baseline Survey of the Environment (G-BASE), which provides detailed information on inorganic soil chemistry across England and Wales using X-ray fluorescence analysis. Concentrations of 15 soil elements were estimated for each postcode area. These geochemical data were then linked to residential postcodes recorded in The Health Improvement Network (THIN), a large UK database of primary care medical records, enabling individual-level exposure assessment. To evaluate representativeness, observed exposure distributions within the THIN cohort were compared to UK national estimates. The linked dataset offers an unprecedented opportunity to explore the health impacts of soil-based elemental exposure at a population level, with strong statistical power. With appropriate methodological adjustments, the findings derived from this resource were expected to be both generalizable and representative of the broader population in England and Wales.
4. Contaminants of Emerging Concern (ECs)
The relationship between soil pollution and human health is considered an important problem of contemporary society. In the near future, evaluating the potential evolution of this problem and developing strategies to address the potential growth of these problems linked to the discovery of new emerging contaminants (ECs) are crucial steps in safeguarding public health.
In recent years, the number of substances identified as hazardous to human health in soil has increased. Recognizing the sources and distribution of these new contaminants is critical for identifying areas where their presence poses risks.
ECs refer to substances that are either newly identified or raising novel concerns due to their potential health risks. These materials originate from both human activities, where they are intentionally engineered, and natural phenomena such as volcanic eruptions, forest fires, mineral weathering, and atmospheric dust transport.
EC production and application remain inadequately regulated, their environmental interactions are largely unexplored, and comprehensive assessments of their toxicological and health impacts are lacking. Determining their sources and environmental distribution is essential for evaluating associated risks. The behavior of ECs in soil, including their transport and transformation, is influenced by numerous biotic and abiotic factors.
Industrial expansion in pharmaceuticals, household products, and plastics has led to the increasing presence of ECs in consumer goods such as cosmetics, skincare products, and medical treatments [164]. Improper disposal of these products has contributed to the widespread contamination of natural environments including soils [165].
The main ECs of concern include the following:
- Pharmaceuticals and Personal Care Products represent one of the largest groups of ECs, encompassing a wide range of compounds with diverse chemical and physical properties [166];
- Nanoparticles: Engineered nanomaterials used in various industrial products [167,168,169,170] are present in large range of commercial goods;
- Microplastics: Small plastic particles from breakdown or production processes. Increase in plastic production and consumption has contributed to the proliferation of micro/nano-plastics in various ecosystems [171,172];
- Per- and Polyfluoroalkyl Substances (PFAS) and similar compounds are persistent chemicals with widespread environmental presence [173,174];
- Antibiotics and Antimicrobial Compounds: Often originating from agricultural runoff and wastewater [175,176,177];
- Critical minerals such as rare earth elements, tungsten, graphite, and antimony [144,178,179].
The interactions between organic and inorganic contaminants and the properties of the soil, previously briefly described, also concern the behavior of ECs in soil. Thus, ECs significantly interact with organic matter, mineral constituents, dissolved ions, pH, redox conditions, and microbial activity.
ECs from industrial activities, agricultural practices, and improper waste management can interact with other pollutants in soil, potentially amplifying their toxicity, creating complex exposure pathways for humans [180]. Also, these contaminants can influence human health through multiple routes, including the consumption of polluted food and water, inhalation, and skin contact with contaminated soils [181,182,183]. ECs persistence in the environment, coupled bioaccumulation potential, amplifies the risk of human exposure and associated health impacts [182,183].
Understanding the health risks from ECs, however, remains a significant challenge due to their various characteristics, and complex chemical interactions, which complicate the traditional monitoring and risk assessment approaches [182]. To better understand the impact of ECs on the living organisms in the soil environment, it is necessary to evaluate their reactions in soil related to the characteristics of both, the soils and the substances. Current research predominantly relies on high-dosage exposure experiments, while in soil concentrations tend to be substantially lower, necessitating refined methodologies for accurate risk assessments. Significant advancements have been made in recent years regarding the environmental health research of ECs. Innovative pretreatment and detection methods, especially for inorganic ECs, have facilitated their identification and quantification in various ecosystems. Additionally, controlled laboratory studies have helped elucidate the mechanisms governing ECs transformation and mobility across different environmental media. These findings provide valuable theoretical frameworks for predicting ECs behavior and long-term environmental fate [167,172,184,185]. These recent evidence suggest links between ECs exposure and a range of health conditions, including development of antibiotic resistance and increased susceptibility to infectious diseases, hormonal imbalances and reproductive health disorders, cardiovascular and respiratory diseases, neurological damage and impacts on the immune system, including allergic responses [186].
Addressing the public health risks posed by ECs requires urgent and coordinated action. Addressing ECs-related health risks requires urgent, coordinated efforts. Research should focus on improving surveillance systems for contamination and health outcomes and developing innovative analytical and epidemiological methods to assess exposure and toxicological effects. Additionally, implementing effective regulatory frameworks, raising public awareness, and fostering interdisciplinary collaboration are essential to mitigate the health impacts of ECs and ensure public safety.
5. Microelements as Source for Humans
While some soils have accumulated toxic elements, many other soils around the world suffer from deficiencies of mineral elements essential for the health of animals and humans. Microelements perform several functions in both plants [187] and humans [188,189,190], playing a crucial role despite their low requirements. The presence of mineral elements in an available form in soil, which is the main source, is essential for plant growth [191]. Micronutrients, such as Zn, Fe, Mn, and Cu, are important for plant nutrition because they affect the growth, development, and quality of crops [192]. Microelements deficiencies in plants not only limit agricultural production [193] but also affect human nutrition as plant food is the main source of dietary intake [194,195]. Over two billion people are affected by one or more micronutrient deficiency diseases which can significantly affect human health, particularly among children, pregnant women, and the elderly, leading to hidden hunger [15]. Increasing the concentration of essential microelements, especially zinc, iron, selenium, and iodine, in food crops during crop cultivation is known as biofortification [196,197,198]. The ability of some crops to accumulate micronutrients is crucial for human nutrition and health. Plants are the first link in the food chain which ultimately ends with humans. Increasing the microelement content in plant products, including leafy and fruity vegetables, fruit crops, and cereals, without exceeding the toxic threshold, is an effective way to enhance the intake of elements for both animals and humans, potentially benefiting them long-term. Biofortification positively affects the accumulation of micronutrients in the edible plant parts and provides a rapid approach to achieving this without compromising crop yield [192,199,200].
There are several methods for enriching plants including the addition of microelements to soil, soaking seeds in a microelement-enriched solution before sowing, foliar or fruit spraying, and hydroponic cultivation with a nutrient solution containing minerals [201,202,203,204,205].
The addition of fertilizers to the soil is an effective way to biofortify large quantities of food products and increase the micronutrient content in soils that are deficient in these elements. However, in the case of selenium (Se), substantial amounts need to be applied to the soil to achieve plant Se concentrations comparable to other fertilization methods. The soil content can be increased by fertilizing with salt or by incorporating Se-hyperaccumulator plants into the soil [206]. Some Se-hyperaccumulator, such as Stanleya pinnata, can also be used for the phytoremediation of soil with high Se concentrations, and the Se-enriched plant material can be incorporated into the soil, thus combining phytoremediation and biofortification [207]. Fertilization with nickel (Ni) can positively affect plant development due to the role of this micronutrient in nitrogen (N) metabolism.
Foliar application of microelements has proven effective for the biofortification of horticultural crops, cereals, and legumes. The concentration of Se and other microelements in sprayed plants has been found to be higher compared to plants grown in enriched soil or nutrient solution [208]. Additionally, as for iron in alkaline soils [209], foliar spraying of selenium is preferable to soil application due to the lower amounts of elements generally used, and the absence of residual effects.
Micronutrients, such as zinc and copper, are vital for both plants and animals. While some plants can survive with minimal amounts, deficiencies in humans can lead to serious public health problems, including cognitive impairment, birth defects, and malnutrition, particularly in underserved communities; that could be mitigated through soil restoration and agricultural improvements [210].
6. Conclusions
Soil plays an essential role in sustaining life on Earth, acting as a critical source of nutrients and the foundation for agriculture. However, it also serves as a reservoir for pollutants, which can enter the human food chain through agricultural products and dietary intake. Despite its importance, the link between soil quality and human health remains understudied, likely due to the interdisciplinary expertise required to address this complex issue.
A fertile and balanced soil is essential for ensuring uncontaminated water, and nutrient-rich food. As a fundamental component of terrestrial ecosystems, soil plays a crucial role in food security and, consequently, human health. To develop effective strategies for its restoration and preservation, it is essential to deeply understand the behavior of contaminants in soil. Investing in sustainable soil management practices is a key step in promoting long-term soil ecosystems services and protecting human health.
The complexity and heterogeneity of soils make it challenging to predict contaminant concentrations and their reactions over time. Various chemical, physical, and biological factors influence the fate of contaminants, complicating the identification of primary risk drivers. Addressing soil-related health risks requires a holistic approach that considers a continuum the entire pathway from soil contamination to human exposure through the food chain. Research on contaminated sites has traditionally focused on simplistic correlations between pollutant concentrations in soil and human absorption. However, these correlations are influenced by complex pathways involving soil’s chemical and physical properties, its interactions with contaminants, and its dynamic nature as a thermodynamically open system.
Soil chemistry plays a crucial role in linking retrospective epidemiological studies, which assess the health effects of soil contamination, with prospective risk assessments that estimate the hazards posed by contaminants in polluted sites. To fully understand the environmental-health relationship, it is essential to move beyond viewing chemistry as merely an analytical tool. Only by addressing the complexity of interactions between contaminants and specific soil properties can we accurately assess contamination levels and exposure pathways. This approach enhances our ability to evaluate potential health risks, providing a stronger scientific foundation for environmental pollution prevention and mitigation strategies.
A fruitful interdisciplinary collaboration between soil chemistry and health sciences is essential to promote a sustainable development that is closely linked to human health.
Author Contributions
Conceptualization, G.P., B.P. and F.P.; methodology, G.P., B.P. and F.P.; validation, G.P., B.P. and F.P.; investigation, G.P., B.P. and F.P.; writing—original draft preparation, G.P., B.P. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Poesen, J.; Lugato, E.; Scarpa, L.; Montanarella, L.; Borrelli, P. A soil erosion indicator for supporting agricultural, environmental and climate policies in the European Union. Remote Sens. 2020, 12, 1365. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point. Main Report. Rome. 2022. Available online: https://openknowledge.fao.org/handle/20.500.14283/cb9910en (accessed on 1 March 2025).
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions EU Soil Strategy for 2030 Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate (COM/2021/699 Final). Available online: https://op.europa.eu/en/publication-detail/-/publication/c44ca1fb-4876-11ec-91ac-01aa75ed71a1/language-en (accessed on 1 March 2025).
- Brevik, E.C.; Pereg, L.; Steffan, J.J.; Burgess, L.C. Soil ecosystem services and human health. Curr. Opin. Environ. Sci. Health 2018, 5, 87–92. [Google Scholar] [CrossRef]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Abrahams, P.W. Soil, geography and human disease: A critical review of the importance of medical cartography. Prog. Phys. Geogr. 2006, 30, 490–512. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Elsakhawy, T.; Omara, A.E.D.; Amer, M.; Mohamed Abowaly, M.; El-Henawy, A.; Prokisch, J. Soil and Humans: A Comparative and A Pictorial Mini-Review. Egypt. J. Soil Sci. 2022, 62, 101–122. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef]
- FAO; UNEP. Global Assessment of Soil Pollution: Report; FAO: Rome, Italy; UNEP: Rome, Italy, 2021. [Google Scholar]
- Duckworth, O.W.; Polizzotto, M.L.; Thompson, A. Bringing soil chemistry to environmental health science to tackle soil contaminants. Front. Environ. Sci. 2022, 10, 981607. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tian, Y.X.; Cai, Y.X.; Liu, Q.Y.; Ma, J.; Wei, Y.; Yang, A.F. A 50-year systemic review of bioavailability application in Soil environmental criteria and risk assessment. Environ. Pollut. 2023, 335, 122272. [Google Scholar] [CrossRef]
- Mahammedi, C.; Mahdjoubi, L.; Booth, C.A.; Akram, H.; Butt, T.E. A systematic review of risk assessment tools for contaminated sites—Current perspectives and future prospects. Environ. Res. 2020, 191, 110180. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- Payá Pérez, A.; Rodríguez Eugenio, N. Status of Local Soil Contamination in Europe: Revision of the Indicator “Progress in the Management Contaminated Sites in Europe”; EUR 29124 EN; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress 50 update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Doabi, S.A.; Karami, M.; Afyuni, M.; Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf. 2018, 163, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Tripti; Maiti, S.K. Health Risk Assessment of Children Exposed to the Soil Containing Potentially Toxic Elements: A Case Study from Coal Mining Areas. Metals 2022, 12, 1795. [Google Scholar] [CrossRef]
- Chen, K.; Huang, L.; Yan, B.Z.; Li, H.B.; Sun, H.; Bi, J. Effect of Lead pollution control on environmental and childhood blood Lead level in Nantong, China: An interventional study. Environ. Sci. Technol. 2014, 48, 12930–12936. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F.; Rosellini, I. Bioavailability and bioaccessibility in soil: A short review and a case study. AIMS Environ. Sci. 2020, 7, 208–225. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Hu, G.C.; Chen, L.G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef]
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- Huang, J.; Guo, S.; Zeng, G.M.; Li, F.; Gu, Y.; Shi, Y.; Shi, L.; Liu, W.; Peng, S. A new exploration of health risk assessment quantification from sources of soil heavy metals under different land use. Environ. Pollut. 2018, 243 Pt A, 49–58. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Sun, J.; Li, X.; Geng, X.; Zhao, M.; Sun, T.; Fanet, Z. Health risk assessment of heavy metal(loid)s in park soils of the largest megacity in China by using Monte Carlo simulation coupled with positive matrix factorization model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Guo, D.; Ali, A.; Mi, S.; Liu, T.; Ren, C.; Li, R.; Zhang, Z. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jin, Y.; Zeng, G. Introduction of heavy metals contamination in the water and soil: A review on source, toxicity and remediation methods. Green Chem. Lett. Rev. 2024, 17, 2404235. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, J.; Wang, H.; Han, X.; Ma, J.; Ma, Y.; Luan, H. Distribution and health risk assessment of potentially toxic elements in soils around coal industrial areas: A global meta-analysis. Sci. Total Environ. 2020, 713, 135292. [Google Scholar] [CrossRef] [PubMed]
- Lupolt, S.N.; Agnew, J.; Burke, T.A.; Kennedy, R.D.; Nachman, K.E. Key considerations for assessing soil ingestion exposures among agricultural workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 481–492. [Google Scholar] [CrossRef]
- Lupolt, S.N.; Kim, B.F.; Agnew, J.; Ramachandran, G.; Burke, T.A.; Kennedy, R.D.; Nachman, K.E. Application and demonstration of meso-activity exposure factors to advance estimates of incidental soil ingestion among agricultural workers. J. Expo. Sci. Environ. Epidemiol. 2024, 35, 303–314. [Google Scholar] [CrossRef]
- Li, Y.; Ajmone-Marsan, F.; Padoan, E. Health risk assessment via ingestion and inhalation of soil PTE of an urban area. Chemosphere 2021, 281, 130964. [Google Scholar] [CrossRef]
- Weeks, J.J.; Hettiarachchi, G.M.; Santos, E.; Tatarko, J. Potential human inhalation exposure to soil contaminants in urban gardens on brownfields sites: A breath of fresh air? J. Environ. Qual. 2021, 50, 782–790. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Parikh, S.J. Chemical composition of soil-associated ash from the southern California Thomas Fire and its potential inhalation risks to farmworkers. J. Environ. Manag. 2021, 278 Pt 2, 111570. [Google Scholar] [CrossRef]
- Sing, D.; Sing, C.F. Impact of direct soil exposures from airborne dust and geophagy on human health. Int. J. Environ. Res. Public Health 2010, 7, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Barajas, B.; Kleinman, M.; Wang, X.; Bennett, B.J.; Gong, K.W.; Navab, M.; Harkema, J.; Sioutas, C.; Lusis, A.J.; et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008, 102, 589–596. [Google Scholar] [CrossRef]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F.; Rosellini, I.; Barbafieri, M. The Bioavailability Processes as a Key to Evaluate Phytoremediation Efficiency. In Phytoremediation; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer: Cham, Switzerland, 2015; pp. 31–43. [Google Scholar] [CrossRef]
- Muerdter, C.P.; Powers, M.M.; Webb, D.T.; Chowdhury, S.; Roach, K.E.; LeFevre, G.H. Functional Group Properties and Position Drive Differences in Xenobiotic Plant Uptake Rates, but Metabolism Shares a Similar Pathway. Environ. Sci. Technol. Lett. 2023, 10, 596–603. [Google Scholar] [CrossRef]
- Wan, W.; Huang, H.; Lv, J.; Han, R.; Zhang, S. Uptake, translocation, and biotransformation of organophosphorus esters in wheat (Triticum aestivum L.). Environ. Sci. Technol. 2017; 51, 13649–13658. [Google Scholar]
- Trapp, S.; Legind, C.N. Uptake of Organic Contaminants from Soil into Vegetables and Fruits. In Dealing with Contaminated Sites; Swartjes, F., Ed.; Springer: Dordrecht, The Netherland, 2011; pp. 369–408. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils. Environments 2024, 11, 26. [Google Scholar] [CrossRef]
- Billmann, M.; Hulot, C.; Pauget, B.; Badreddine, R.; Papin, A.; Pelfrêne, A. Oral bioaccessibility of PTEs in soils: A review of data, influencing factors and application in human health risk assessment. Sci. Total Environ. 2023, 896, 165263. [Google Scholar] [CrossRef]
- National Research Council (NRC). Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- ISO 17924; Soil Quality. Assessment of Human Exposure from Ingestion of Soil and Soil Material—Procedure for the Estimation of the Human Bioaccessibility/Bioavailability of Metals in Soil. ISO: Geneva, Switzerland, 2018.
- Luo, X.S.; Jing, D.; Bo, X.; Wang, Y.J.; Li, H.B.; Shen, Y. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef]
- Naidu, R.; Juhasz, A.; Mallavarapu, M.; Smith, E.; Lombi, E.; Bolan, N.S.; Wong, M.; Harmsen, J. Chemical bioavailability in the terrestrial environment—Recent advances. J. Hazard. Mater. 2013, 261, 685–686. [Google Scholar] [CrossRef]
- Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants 1289 Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. [Google Scholar] [CrossRef] [PubMed]
- Rokni, L.; Rezaei, M.; Rafieizonooz, M.; Khankhajeh, E.; Mohammadi, A.A.; Rezania, S. Effect of Persistent Organic Pollutants on Human Health in South Korea: A 73 Review of the Reported Diseases. Sustainability 2023, 15, 10851. [Google Scholar] [CrossRef]
- Negrete, D.B.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent organic pollutants: The trade-off between potential risks and sustainable remediation methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef]
- Jorgenson, J.L. Aldrin and dieldrin: A review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ. Health Perspect. 2001, 109, 113–139. [Google Scholar] [CrossRef]
- Ware, G.W. Chlordane. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1988; Volume 104. [Google Scholar] [CrossRef]
- Beard, J. Australian Rural Health Research Collaboration. DDT and human health. Sci. Total Environ. 2006, 355, 78–89. [Google Scholar] [CrossRef]
- Kolankaya, D. Organochlorine pesticide residues and their toxic effects on the environment and organisms in Turkey. Int. J. Environ. Anal. Chem. 2006, 86, 147–160. [Google Scholar] [CrossRef]
- Reed, L.; Buchner, V.; Tchounwou, P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health 2007, 22, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Kueberuwa, S.; Smith, L.; DeRosa, C. ATSDR evaluation of health effects of chemicals. II. Mirex and chlordecone: Health effects, toxicokinetics, human exposure, and environmental fate. Toxicol. Ind. Health. [CrossRef]
- de Geus, H.J.; Besselink, H.; Brouwer, A.; Klungsoyr, J.; McHugh, B.; Nixon, E.; Rimkus, G.G.; Wester, P.G.; de Boer, J. Environmental Occurrence, Analysis, and Toxicology of Toxaphene Compounds. Environ. Health Perspect. 1999, 107 (Suppl. S1), 115–144. [Google Scholar]
- Multigner, L.; Kadhel, P.; Rouget, F.; Blanchet, P.; Cordier, S. Chlordecone exposure and adverse effects in French West Indies populations. Environ. Sci. Pollut. Res. Int. 2016, 23, 3–8. [Google Scholar] [CrossRef]
- Ajaz, A.; Masood, A. Deciphering the toxic effects of organochlorine pesticide, dicofol on human RBCs and lymphocytes. Pestic. Biochem. Physiol. 2017, 143, 127–134. [Google Scholar] [CrossRef]
- Carpenter, D.O. Polychlorinated biphenyls (PCBs): Routes of exposure and effects on human health. Rev. Environ. Health 2006, 21, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ. Int. 2019, 126, 598–610. [Google Scholar] [CrossRef]
- Poston, R.G.; Saha, R.N. Epigenetic Effects of Polybrominated Diphenyl Ethers on Human Health. Int. J. Environ. Res. Public Health 2019, 16, 2703. [Google Scholar] [CrossRef]
- White, S.S.; Birnbaum, L.S. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Bakhiya, N.; Appel, K.E. Toxicity and carcinogenicity of furan in human diet. Arch. Toxicol. 2010, 84, 563–578. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.C.; Hursthouse, A.; van Hullebusch, E.D. Sorption vs adsorption: The words they are a-changin’, not the phenomena. Sci. Total Environ. 2022, 838, 156545. [Google Scholar] [CrossRef] [PubMed]
- Nendza, M.; Kosfeld, V.; Schlechtriem, C. Consolidated octanol/water partition coefficients: Combining multiple estimates from different methods to reduce uncertainties in log KOW. Environ. Sci. Eur. 2025, 37, 44. [Google Scholar] [CrossRef]
- Belfort, G.; Altshuler, G.L.; Thallam, K.K.; Feerick, C.P., Jr.; Woodfield, K.L. Selective adsorption of organic homologues onto activated carbon from dilute aqueous solutions: Solvophobic interaction approach IV. Effect of simple structural modifications with aliphatics. AIChE J. 1984, 30, 197–207. [Google Scholar] [CrossRef]
- Rao, P.S.C.; Bellin, C.A.; Lee, L.S. Sorption and biodegradation of organic contaminants in soils: Conceptual representation of process coupling. In Environmental Impact of Soil Component Interactions; Huang, P.M., Berthelin, J., Bollag, J.M., McGill, W.B., Page, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 259–270. [Google Scholar]
- Cui, Z.; Song, S.; Liu, J. PCBs adsorption to soils: Isotherm modeling and influencing factors. Ecol. Environ. Sci. 2010, 19, 325–329. [Google Scholar]
- Frankki, S.; Persson, Y.; Tysklind, M.; Skyllberg, U. Partitioning of CPs, PCDEs, and PCDD/Fs between particulate and experimentally enhanced dissolved natural organic matter in a contaminated soil. Environ. Sci. Technol. 2006, 40, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Persson, Y.; Hemstrom, K.; Oberg, L.; Tysklind, M.; Enell, A. Use of a column leaching test to study the mobility of chlorinated HOCs from a contaminated soil and the distribution of compounds between soluble and colloid phase. Chemosphere 2008, 71, 35–42. [Google Scholar] [CrossRef]
- Badea, S.L.; Mustafa, M.; Lundstedt, S.; Tysklind, M. Leachability and desorption of PCBs from soil and their dependency on pH and dissolved organic matter. Sci. Total Environ. 2014, 499, 220–227. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, L.; Wang, X.L.; Xing, B.S.; Tao, S. Effects of composition and domain arrangement of biopolymer components of soil organic matter on the bioavailability of phenanthrene. Environ. Sci. Technol. 2010, 44, 3339–3344. [Google Scholar] [CrossRef]
- Milinovic, J.; Lacorte, S.; Vidal, M.; Rigol, A. Sorption behaviour of perfluoroalkyl substances in soils. Sci. Total Environ. 2015, 511, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Benoit, P.; Madrigal, I.; Preston, C.M.; Chenu, C.; Barriuso, E. Sorption and desorption of non-ionic herbicides onto particulate organic matter from surface soils under different land uses. Eur. J. Soil Sci. 2008, 59, 178–189. [Google Scholar] [CrossRef]
- Wei, C.; Song, X.; Wang, Q.; Hu, Z. Sorption kinetics, isotherms and mechanisms of PFOS on soils with different physicochemical properties. Ecotoxicol. Environ. Saf. 2017, 142, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mingli, W. Evaluation on adsorption capacity of low organic matter soil for hydrophobic organic pollutant. J. Environ. Chem. Eng. 2022, 10, 107561. [Google Scholar] [CrossRef]
- Adeyinka, G.C.; Moodley, B. Kinetic and thermodynamic studies on partitioning of polychlorinated biphenyls (PCBs) between aqueous solution and modeled individual soil particle grain sizes. J. Environ. Sci. 2019, 76, 100–110. [Google Scholar] [CrossRef]
- Mechlińska, A.; Gdaniec-Pietryka, M.; Wolska, L.; Namieśnik, J. Evolution of models for sorption of PAHs and PCBs on geosorbents. TrAC Trends Anal. Chem. 2009, 28, 466–482. [Google Scholar] [CrossRef]
- Han, L.F.; Sun, K.; Jin, J.; Wei, X.; Xia, X.H.; Wu, F.C.; Gao, B.; Xing, B.S. Role of structure and microporosity in phenanthrene sorption by natural and engineered organic matter. Environ. Sci. Technol. 2014, 48, 11227–11234. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sun, K.; Wang, Z.Y.; Han, L.F.; Pan, Z.Z.; Wu, F.C.; Liu, X.T.; Zhao, Y.; Xing, B.S. Characterization and phthalate esters sorption of organic matter fractions isolated from soils and sediments. Environ. Pollut. 2015, 206, 24–31. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, S.; Zhang, D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014, 135, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.M.; Yan, W.; Jing, C.Y. Experimental and molecular dynamic simulation study of perfluorooctane sulfonate adsorption on soil and sediment components. J. Environ. Sci. (China) 2015, 29, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Yang, Q.; Zhou, W.J.; Zhu, L.Z. Sorption characteristics and contribution of organic matter fractions for atrazine in soil. J. Soils Sediments 2015, 15, 2210–2219. [Google Scholar] [CrossRef]
- Boyd, S.A.; Sheng, G.; Teppen, B.J.; Johnston, C.T. Mechanisms for the adsorption of substituted nitrobenzenes by smectite clays. Environ. Sci. Technol. 2001, 35, 4227–4234. [Google Scholar] [CrossRef]
- Peng, X.; Wang, J.; Fan, B.; Luan, Z. Sorption of endrin to montmorillonite and kaolinite clays. J. Hazard. Mater. 2009, 168, 210–214. [Google Scholar] [CrossRef]
- Vilanova, R.M.; Fernandez, P.; Grimalt, J.O. Polychlorinated biphenyl partitioning in the waters of a remote mountain lake. Sci. Total Environ. 2001, 279, 51–62. [Google Scholar] [CrossRef]
- Polati, S.; Angioi, S.; Gianotti, V.; Gosetti, F.; Gennaro, M.C. Sorption of pesticides on kaolinite and montmorillonite as a function of hydrophilicity. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2006, 41, 333–344. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Awad, A.M.; Shifa, M.R.S.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Li, S.; Han, R.; Wang, G. Insights into tetracycline adsorption onto kaolinite and montmorillonite: Experiments and modelling. Environ. Sci. Pol. 2015, 22, 17031–17040. [Google Scholar] [CrossRef] [PubMed]
- Masini, J.C.; Abate, G. Guidelines to Study the Adsorption of Pesticides onto Clay Minerals Aiming at a Straightforward Evaluation of Their Removal Performance. Minerals 2021, 11, 1282. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018, 610–611, 1154–1163. [Google Scholar] [CrossRef]
- Xu, J.; Gu, X.; Guo, Y.; Tong, F.; Chen, L. Adsorption behavior and mechanism of glufosinate onto goethite. Sci. Total Environ. 2016, 560, 123–130. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Hu, E.; Zhao, X.; Pan, S.; Ye, Z.; He, F. Sorption of non-ionic aromatic organics to mineral micropores: Interactive effect of cation hydration and mineral charge density. Environ. Sci. Technol. 2019, 53, 3067–3077. [Google Scholar] [CrossRef]
- Zacháry, D.; Filep, T.; Jakab, G.; Ringer, M.; Balázs, R.; Németh, T.; Szalai, Z. The effect of mineral composition on soil organic matter turnover in temperate forest soils. J. Soils Sediments 2023, 23, 1389–1402. [Google Scholar] [CrossRef]
- Arroyave, J.M.; Waiman, C.C.; Zanini, G.P.; Avena, M.J. Effect of humic acid on the adsorption/ desorption behavior of glyphosate on goethite. Isotherms and kinetics. Chemosphere 2016, 145, 34–41. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, P.; Bao, X.; Xiong, M.; Liu, F. The influence of humic substances on the sorption of three organic contaminants with different structure and polarity to clay minerals. Water Air Soil Pollut. 2017, 228, 199. [Google Scholar] [CrossRef]
- Cachada, A.; Pato, P.; Rocha-Santos, T.; Ferreira da Silva, E.; Duarte, A.C. Levels, sources and potential human health risks of organic pollutants in urban soils. Sci. Total Environ. 2012, 430, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Arora, Y.; Kumar, M.; Kumar, D. The role of Pesticides in Cancer initiation and progression: A comprehensive review. Chem. Biol. Lett. 2024, 11, 669. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Hansen, K.E.A.; Johanson, S.M.; Steppeler, C.; Sødring, M.; Østby, G.C.; Berntsen, H.F.; Zimmer, K.E.; Aleksandersen, M.; Paulsen, J.E.; Ropstad, E. A mixture of Persistent Organic Pollutants (POPs) and Azoxymethane (AOM) show potential synergistic effects on intestinal tumorigenesis in the A/J Min/+ mouse model. Chemosphere 2019, 214, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, Q.; Liu, C.; Sun, Q.; Zeng, X. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics, 2023; 11, 322. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of Macronutrients in Plant Growth and Acclimation: Recent Advances and Future Prospective. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M., Azooz, M., Phan Tran, L.S., Eds.; Springer: New York, NY, USA, 2014; pp. 197–216. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids Management and Utilizations: A Review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring Techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The Fate of Heavy Metals During Combustion and Gasification of Contaminated Biomass—A Brief Review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR) National Priorities List (NPL) Sites 2022. Available online: https://www.epa.gov/superfund/agency-toxic-substances-and-disease-registry-atsdr-national-priorities-list-npl-sites (accessed on 15 February 2025).
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Shankar, S.; Shanker, U.; Shikha. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Fatoki, J.O.; Jelili, A.B. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Demissie, S.; Mekonen, S.; Awoke, T.; Mengistie, B. Assessing Acute and Chronic Risks of Human Exposure to Arsenic: A Cross-Sectional Study in Ethiopia Employing Body Biomarkers. Environ. Health Insights 2024, 18, 11786302241257365. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef]
- Surdu, S. Non-melanoma skin cancer: Occupational risk from UV light and arsenic exposure. Rev. Environ. Health 2014, 29, 255–265. [Google Scholar] [CrossRef]
- Radosavljevic, V.; Jakovljevic, B. Arsenic and bladder cancer: Observations and suggestions. J. Environ. Health 2008, 71, 40–42. [Google Scholar]
- Celik, I.; Gallicchio, L.; Boyd, K.; Lam, T.K.; Matanoski, G.; Tao, X.; Shiels, M.; Hammond, E.; Chen, L.; Robinson, K.A. Arsenic in drinking water and lung cancer: A systematic review. Environ. Res. 2008, 108, 48–55. [Google Scholar] [CrossRef]
- Mandal, B.K.; Ogra, Y.; Suzuki, K.T. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem. Res. Toxicol. 2001, 14, 371–378. [Google Scholar] [CrossRef]
- Chen, Y.; Parvez, F.; Gamble, M.; Islam, T.; Ahmed, A.; Argos, M.; Graziano, J.H.; Ahsan, H. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: Review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol. Appl. Pharmacol. 2009, 239, 184–192. [Google Scholar]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Jin, J.; Li, W.; Liang, J.; Zhou, S.; Weng, Z.; Zhou, Y.; Liao, X.; Gu, A. Early-life exposure to lead changes cardiac development and compromises long-term cardiac function. Sci. Total Environ. 2023, 904, 166667. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, P.; Adams, F. Toxicity, Sources and Biogeochemical Cycle of Mercury. J. Phys. IV 2004, 121, 185–193. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Azevedo, B.F.; Furieri, L.B.; Peçanha, F.M.; Wiggers, G.A.; Vassallo, P.F.; Simões, M.R.; Fiorim, J.; Rossi de Batista, P.; Fioresi, M.; Rossoni, L.; et al. Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems. J. Biomed. Biotechnol. 2012, 2012, 949048. [Google Scholar]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Johnson-Arbor, K.; Tefera, E.; Farrell, J. Characteristics and Treatment of Elemental Mercury Intoxication: A Case Series. Health Sci. Rep. 2021, 4, e293. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Yao, T.M.; Zhu, Z.L.; Wang, C.; Ji, L.N. Impacts of Cd (II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta Proteins Proteom. 2007, 1774, 1414–1421. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium exposure and incident cardiovascular disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Cuevas, Y.Z.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, C.; Gutiérrez-Ruiz, M.C.; Del Castillo, E.R. Toxicity of cadmium in musculoskeletal diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release Processes in Soil—The Basis of Phytoremediation Efficiency. In Phytoremediation; Ansari, A., Gill, S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 91–112. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. The Dynamics of Tungsten in Soil: An Overview. Environments 2021, 8, 66. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Hu, J.; Zheng, Q.; Zhao, Y.; Lv, G.; Liao, L. Clay minerals and clay-based materials for heavy metals pollution control. Sci. Total Environ. 2024, 954, 176193. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Al-Hamandi, H.M. Organic Matter and Heavy Metals Sorption. Tikrit J. Agric. Sci. 2022, 22, 158–165. [Google Scholar] [CrossRef]
- Li, X.; Schindler, M.; Zhou, J.; Samaradiwakara, S.; Wu, L. Interaction between metal(loid)s and soil mineral-organic matter associations. Crit. Rev. Environ. Sci. Technol. 2024, 55, 624–647. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, E.F.; Andrade, M.L.; Vega, F.A. Retention and Mobility of Copper and Lead in Soils as Influenced by Soil Horizon Properties. Pedosphere 2011, 21, 603–614. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Rahman, S.U.; Abbas, A.; Lia, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-manganese (oxyhydro) oxides, rather than oxidation of sulfides, determine mobilization of cd during soil drainage in paddy soil systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Stroud, J.L.; Ma, J.F.; McGrath, S.P.; Zhao, F.J. Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ. Sci. Technol. 2009, 43, 3778–3783. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Tian, C.; Wang, R.; Dai, J. Effect of pH, Temperature, and the Role of Ionic Strength on the Adsorption of Mercury(II) by Typical Chinese Soils. Commun. Soil Sci. Plant Anal. 2012, 43, 1599–1613. [Google Scholar] [CrossRef]
- El-Bayaa, A.A.; Badawy, N.A.; AlKhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, J.J.; Niu, L.L.; Chen, Q.; Zhou, Q.; Xiao, N.; Man, J.; Ma, J.Q.; Wei, C.L.; Zhang, S.H.; et al. Escalating arsenic contamination throughout Chinese soils. Nat. Sustain. 2024, 7, 766–775. [Google Scholar] [CrossRef]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.L.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef]
- Singh, S.B.; Srivastava, P.K. Bioavailability of arsenic in agricultural soils under the influence of different soil properties. SN Appl. Sci. 2020, 2, 153. [Google Scholar] [CrossRef]
- Sevak, P.; Pushkar, B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis. J. Environ. Manag. 2024, 349, 119504. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Floret, N.; Deconinck, E.; Focant, J.F.; De Pauw, E.; Cahn, J.Y. Increased risk of non-Hodgkin lymphoma and serum organochlorine concentrations among neighbors of a municipal solid waste incinerator. Environ. Int. 2011, 37, 449–453. [Google Scholar] [CrossRef]
- Cabral, M.; Toure, A.; Garçon, G.; Diop, C.; Bouhsina, S.; Dewaele, D.; Cazier, F.; Courcot, D.; Tall-Dia, A.; Shirali, P.; et al. Effects of environmental cadmium and lead exposure on adults neighboring a discharge: Evidences of adverse health effects. Environ. Pollut. 2015, 206, 247–255. [Google Scholar] [CrossRef]
- Marques, C.; Fiolet, T.; Frenoy, P.; Severi, G.; Mancini, F.R. Association between polycyclic aromatic hydrocarbons (PAH) dietary exposure and mortality risk in the E3N cohort. Sci. Total Environ. 2022, 840, 156626. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.E.; Ander, E.L.; Cave, M.; Bath-Hextall, F.; Musah, A.; Leonardi-Bee, J. Linkage of national soil quality measurements to primary care medical records in England and Wales: A new resource for investigating environmental impacts on human health. Popul. Health Metr. 2018, 16, 12. [Google Scholar] [CrossRef]
- Montgomery, D.R.; Rabinowitz, P.; Sipos, Y.; Wheat, E.E. Soil health: A common focus for one health and planetary health interventions. One Health 2024, 18, 100673. [Google Scholar] [CrossRef]
- Johnson, C.; Bell, S.J. Linking emerging contaminants to production and consumption practices. WIREs Water 2022, 10, e1615. [Google Scholar] [CrossRef]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.H. Source, fate, transport and modelling of selected emerging contaminants in the aquatic environment: Current status and future perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Wang, Z.; Xing, B. Roadmap of environmental health research on emerging contaminants: Inspiration from the studies on engineered nanomaterials. Eco-Environ. Health 2022, 1, 181–197. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Bernd, N. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook. 2022. Available online: https://www.oecd.org/content/dam/oecd/en/publications/reports/2022/02/global-plastics-outlook_a653d1c9/de747aef-en.pdf (accessed on 15 February 2025).
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, S.Y.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Shi, Q.; Gan, J. Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2745–2776. [Google Scholar] [CrossRef]
- Lesmeister, L.; Lange, F.T.; Breuer, J.; Biegel-Engler, A.; Giese, E.; Scheurer, M. Extending the knowledge about PFAS bioaccumulation factors for agricultural plants—A review. Sci. Total Environ. 2021, 766, 142640. [Google Scholar] [CrossRef]
- Anwar, M.; Iqbal, Q.; Saleem, F. Improper disposal of unused antibiotics: An often overlooked driver of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 2020, 18, 697–699. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Caioni, G.; Benedetti, E.; Perugini, M.; Amorena, M.; Merola, C. Personal care products as a contributing factor to antimicrobial resistance: Current state and novel approach to investigation. Antibiotic 2023, 12, 724. [Google Scholar] [CrossRef]
- Berthet, E.; Lavalley, J.; Anquetil-Deck, C.; Ballesteros, F.; Stadler, K.; Soyta, U.; Hauschild, M.; Laurent, A. Assessing the social and environmental impacts of critical mineral supply chains for the energy transition in Europe. Glob. Environ. Change 2024, 86, 102841. [Google Scholar] [CrossRef]
- European Commission: Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Fang, M.; Hu, L.; Chen, D.; Guo, Y.; Liu, J.; Lan, C.; Jicheng Gong, J.; Wang, B. Exposome in human health: Utopia or wonderland? Innovation 2021, 2, 100172. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Barceló, D. Microplastics and other emerging contaminants in the environment after COVID-19 pandemic: The need of global reconnaissance studies. Curr. Opin. Environ. Sci. Health 2023, 33, 100468. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of Emerging Contaminants and Associated Human Health Effects. BioMed Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Brooks, B.W. Pharmaceuticals and personal care products in the environment: What progress has been made in addressing the big research questions? Environ. Toxicol. Chem. 2024, 43, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lin, C.; Nguyen, H.L.; Nguyen, T.Q.H.; La, D.D.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions. Sci. Total Environ. 2023, 899, 165323. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiang, L.; Leung, K.S.Y.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging contaminants: A One Health perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, N.; Siddique, K.H.M.; Nayyar, H. Beneficial Elements for Agricultural Crops and Their Functional Relevance in Defense against Stresses. Arch. Agron. Soil Sci. 2016, 62, 905–920. [Google Scholar] [CrossRef]
- Rotter, I.; Kosik-Bogacka, D.I.; Dołęgowska, B.; Safranow, K.; Kuczyńska, M.; Laszczyńska, M. Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ. Geochem. Health 2016, 38, 749–761. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef]
- Nedić, O. Iodine: Physiological importance and food sources. eFood 2023, 4, e63. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.; Bozdar, B.; et al. Micronutrients and their effects on Horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Tiwari, S.; Patel, A.; Pandey, N.; Raju, A.; Singh, M.; Prasad, S.M. Deficiency of Essential Elements in Crop Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Mishra, K., Tandon, P.K., Srivastava, S., Eds.; Springer: Singapore, 2020; pp. 19–52. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. (Eds.) Soil Components and Human Health. In Microelements and Their Role in Human Health; Springer: Dordrecht, The Netherlands, 2018; pp. 317–374. [Google Scholar]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Bouis, H.; Foley, J.; Lividini, K.; Jumrani, J.; Reinke, R.; Van Der Straeten, D.; Zagado, R.; Boy, E.; Brown, L.R.; Mudyahoto, B.; et al. Biofortification: Future Challenges for Newly Emerging Technology to Improve Nutrition Security Sustainably. Curr. Dev. Nutr. 2024, 8, 104478. [Google Scholar] [CrossRef]
- Fassinou, H.; Sohindji, N.V.; Salaou, F.S.; Agbandou, M.A.B.; Azonhoumon, P.C.; Tchokponhoué, L.W.S.; Houdegbe, D.; Adjé, C.A.O.; Achigan-Dako, E.G. Agronomic biofortification of cereals and legumes with iron, zinc, calcium and magnesium for food and nutrition security: Available options for farmers in Sub-Saharan Africa. J. Agric. Food Res. 2024, 18, 101391. [Google Scholar] [CrossRef]
- Naik, B.; Kumar, V.; Rizwanuddin, S.; Mishra, S.; Kumar, V.; Saris, P.E.J.; Khanduri, N.; Kumar, A.; Pandey, P.; Gupta, A.K.; et al. Biofortification as a solution for addressing nutrient deficiencies and malnutrition. Heliyon 2024, 10, e30595. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during post-harvest ripening. J. Sci. Food Agric. 2019, 99, 2463–2472. [Google Scholar] [CrossRef]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Iodine biofortification of Swiss chard (Beta vulgaris ssp. vulgaris var. cicla) and its wild ancestor sea beet (Beta vulgaris ssp. maritima) grown hydroponically as baby leaves: Effects on leaf production and quality. J. Sci. Food Agric. 2023, 103, 7888–7895. [Google Scholar] [CrossRef] [PubMed]
- Shiriaev, A.; Pezzarossa, B.; Rosellini, I.; Malorgio, F.; Lampis, S.; Ippolito, A.; Tonutti, P. Efficacy and comparison of different strategies for selenium biofortification of tomatoes. Horticulturae 2022, 8, 800. [Google Scholar] [CrossRef]
- Kumari, V.; Banerjee, P.; Nath, R.; Sengupta, K.; Chandran, M.A.S.; Veni, V.G.; Hossain, A. Foliar Application of Zinc, Boron, and Iron Improved Seed Nutrients, Protein Content, and Yield in Late-Sown Stressed Lentil (Lens culinaris Medikus) Crop. Gesunde Pflanzen 2023, 75, 1133–1141. [Google Scholar] [CrossRef]
- Kumar, J.; Saini, D.K.; Kumar, A.; Kumari, S.; Gahlaut, V.; Rahim, M.S.; Pandey, A.K.; Garg, M.; Roy, J. Biofortification of Triticum species: A stepping stone to combat malnutrition. BMC Plant Biol. 2024, 24, 668. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smiths, E.A.H. Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Arroyo, I.; Pickering, I.J.; Yang, S.I.; Freeman, J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015, 166, 603–608. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Kang, Y.; Shi, M.; Yang, X.; Li, H.; Yu, H.; Wang, Y.; Qin, S. Application of different foliar iron fertilizers for improving the photosynthesis and tuber quality of potato (Solanum tuberosum L.) and enhancing iron biofortification. Chem. Biol. Technol. Agric. 2022, 9, 79. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Romano, I.; Zelikoff, J.T. Soil health is human health. Explore 2024, 20, 103047. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).