1. Introduction
Seafood is a major part of the diet along the Gulf Coast of United States. It is a significant source of protein in the developing world. Its production generates significant biomass waste (60–70%), such as shrimp and crab crustacean shells. Increasing reliance on disposing such wastes in water bodies (inland, coastal, or international) and/or landfills are increasing pollution and off-gas emissions from landfills. The energy intensity of modern seafood harvesting, combined with transport and food processing, makes it a major contributor of global greenhouse gas emissions. However, its generation provides significant advantages as it is a separated waste in a central processing facility [
1]. To promote a sustainable environment while ensuring steady food supply, a circular approach is critical to deal with crustacean wastes. A way to minimize some of these climate change effects of seafood production is finding new uses for crustacean shells traditionally considered waste. Converting shell waste to valuable byproducts will establish sustainable processes [
2,
3].
The biomass waste to byproduct concept can be profitable by turning waste materials into products of high grade and low volume [
4,
5]. Our research group, as a waste to energy and biomass to product lab, is devoted to valorizing waste materials. We assess the potential of sustainable resource use to transition to a foreseeable carbon-neutral future. Harmful chemicals are typically used in fertilizer production; hence, alternative natural fertilizers are optimal in agriculture. Shrimp shell waste was investigated for hydrothermally enhanced mineral rich fertilizer that will be biodegradable and non-polluting. An elemental analysis of hydrothermally carbonized shrimp shell waste and untreated crab waste investigated the enhancement in nitrogen–phosphorus–potassium ratios (N:P:K) crucial for their use as a bioavailable fertilizer. Spray trials were completed with crustacean shell waste to evaluate their use as a biodegradable, non-hazardous, and readily available fertilizer that also acts as a long-term carbon fixer deep in the soil [
6].
Shrimp shell waste was studied to develop methods to convert them into valuable products, such as sprayable fertilizer and dry biochar fertilizer pellets that are inexpensive and environment friendly. The main focus of this work is on shrimp shells, while crab shells are a secondary material of interest. Saha et al. (2023) investigated the fuel properties of hydrothermally carbonized shrimp shells showing poor energy utility [
7]. We used processes such as hydrothermal carbonization (HTC) and pelletization to enhance the fertilizer potential of shrimp shell waste. HTC transforms biomass waste into a carbon-rich product that resembles coal called hydrochar using heat and pressure operations mimicking coalification processes in geological rocks [
8]. Our objective in using shell waste as a fertilizer was testing the ‘
sprayability’ of the synthesized mixture, aiming to find the optimal ratio of shrimp to water that gives the best surface area coverage and particle size range.
2. Materials and Methods
2.1. Shell Waste
Frozen shrimp shell waste (2 cm pieces, 25 kg) was received from Laitram (Harahan, LA, USA), a shrimp meat processing company in south Louisiana. Frozen crab shell waste (whole and 2 cm pieces, 5 kg) was received from Pontchartrain Blue Crab (Slidell, LA, USA), a crab meat processing company in south Louisiana.
2.2. Drying
The crustacean shell waste was thawed and dried overnight in an oven at 105 °C to expunge pathogens and to remove moisture. In addition, the autoclave drying process (Easyclave Model: TR250M) was tested to eliminate pathogens from crustacean shell waste at 0.1–0.15 MPa and 121 °C and to sterilize them. After autoclaving, the shell waste was dried in an oven at 105 °C for an hour to remove any remaining moisture.
2.3. Crushing
After drying, the shell waste was brought to room temperature for crushing. Dried shells were hand-crushed in a large Ziplock bag to break them into small pieces and reduce their size. They were then grinded in a tabletop coffee grinder on fine setting for three passes to ensure consistent grinding.
In addition, dried pieces of crustacean shell waste were ball milled in an Across International PQ-N04 Planetary Ball Mill (Livingston, NJ, USA), according to ASTM standards.
2.4. Microscale Sieving and Particles Size Characterization
After crushing to a fine particle size, the crustacean waste was sieved in a Gilson automatic sieve shaker (Gilson Company Inc., Lewis Center, OH, USA) into three fractions as follows: #20 sieve (retained on #20 sieve, >840 microns in size, cornflake like), #80 sieve (retained on #80 sieve, >180 microns, beach sand like), and #80 Pass Through (#80 sieve, <180 microns, flour-like, referred to as #80PT hereafter) in mesh sizes. Three fractions of dried shell waste were thus obtained, viz, particles larger than 840 µm in size, as retained on the #20 mesh, but smaller than 10 mm from visual observation (essentially, this fraction was in the 1–10 mm range), with particles smaller than 840 µm but larger than 180 µm in size as retained on the #80 mesh, and particles passing through the #80 mesh, which were smaller than 180 µm in size.
2.5. Hydrothermal Carbonization (HTC)
HTC treatment was carried out on shrimp shell waste only. Once sieved in appropriate sizes, hydrothermal carbonization (HTC) was carried out in a Parr reactor (4848 benchtop reactor, Parr Instrument Company, Moline, IL, USA). In the reactor, 100 g DI water for 10 g of dry shrimp shell waste was added (DI water, used as a solvent, to dissolve soluble contents of biomass at a ratio of 10:1). HTC was performed at either 180 °C or 230 °C for 5 min and then the solid shrimp shell waste rich in minerals along with the liquid fraction rich in chitosan were brought to room temperature through cold water rapid quenching. Once the HTC-treated products cooled down to room temperature, solid shells were separated from liquid using a nylon filter [
9].
2.6. Pelletization and Friability
Both treated and untreated shrimp shell waste along with untreated crab shell waste were compacted into pellets to assess how densification impacts their durability for transport through friability assessment [
9]. For sample preparation, 1 g of crustacean shell waste material was moistened with 0.2 g of DI water, i.e., a biomass-to-water ratio of 10:2 (i.e., 20% water by weight). The pellets were formed using a cylindrical steel die set 13 mm in inner diameter from Across International (model SDS13.H, Livingston, NJ, USA) heated to 140 °C. A crankshaft-driven hydraulic press (Model 50H, 50 ton capacity, Dake Corporation, Grand Haven, MI, USA) was used to press 5 tons of pressure for 30 s.
Friability was assessed on a BEXCO Tablet Digital Friability Tester (single drum) equipped with an i-therm KTM-443 timer (Bexco Exports, Ambala, HR, India and I-therm c/o Innovative Instruments & Controls, Va-sai, MH, India). Both dried untreated and HTC-treated shrimp shell waste pellets along with untreated crab shell waste pellets were placed in the clear acrylic drum, which rotated at a speed of 25 (±1 rpm). Pellets were rotated for 1 h and 24 h to simulate transportation test conditions. As the drum rotated, its arm lifted the pellets to a fixed height of 156 (±2 mm), from which they repeatedly dropped, simulating mechanical stress during handling and transport.
2.7. Bomb Calorimetry
The higher heating values (HHV) of both hydrothermally treated and untreated dried shrimp and untreated dried crab shell waste were collected using a model 1341EB Adiabatic Oxygen Bomb Calorimeter (Parr Instrument Company, Moline, IL, USA). It included a probe for continuous temperature tracking. Before testing, samples were oven dried at 105 °C for 24 h to ensure the results reflect dry weight measurements. For each test, 0.4–0.5 g of sample was placed on a metal crucible and approximately 10 cm of fuse wire was set on top of the shrimp or crab waste pellet. The combustion reactor was pressurized with oxygen to 3000 kPa (30 atm) and 1 L DI water was added to the calorimeter.
During calorimetry, various parameters were noted, including the starting and ending temperatures of the water, initial and post-combustion weights of the crucible and fuse wire, the mass of the biomass sample, and any residual ash. To estimate variability, the entire procedure was repeated three times for each waste and standard error HHV were calculated by running the procedure thrice on untreated respective waste.
HHV was calculated as follows:
where Cp
water = 1 cal/g·K, specific heat value of water;
Cpfuse = 1400 cal/g·K, specific heat value of fuse wire;
ΔT = change in temperature of water in the calorimeter bucket;
mwater = 1000 g (density of water = 1 for 1000 mL of DI water);
Heat of combustion, Q (MJ/g) = Q (cal/g) × 4.186 × 10−6;
Δmfuse = change in mass of fuse wire; and
Δmsample = change in mass of sample.
2.8. Elemental Analysis
Shrimp (untreated dried and HTC-treated) and crab shell waste elemental composition including N:P:K ratios were collected to understand their fertilizer potential in an energy dispersive X-ray spectrometer (EDS) coupled with a field emission scanning electron microscope (FE-SEM) on a Hitachi S4800 FE-SEM (Hitachi Corp, Tokyo, Japan).
2.9. Spraying Conditions
- a.
Sprayable fertilizer mix
Shrimp or crab shell waste powder mixed with DI water in a 1:10 ratio (1 part biomass to 10 parts water) was chosen as a testable sprayable solution. Such mixtures must be refrigerated if out for long periods before spraying, otherwise they will develop pungent odors. The slurry may separate after 1 h or longer periods if not stirred.
- b.
Spray gun and compressed air set up
Sprayability tests were conducted with a Central Pneumatic Sand Blasting Gun (Camarillo, CA, USA). This sand blasting gun uses compressed air to blow out powders at high velocities. The average air consumption rate was 3.2 CFM, with 1 L tank capacity and a 6 mm nozzle aperture. The working pressure range was 87–145 psi. The compressor used for testing had a consistent air pressure of 95 psi. While operating the blast gun, pressure dropped to 85–90 psi. The spray nozzle was closely inspected for any signs of clogging before each spray trial.
- c.
Spray set up
The process involved filling the spray tank with a water–shrimp or –crab slurry, according to the test ratio, screwing on the handle, attaching the gun to compressed air line, and spraying perpendicularly on a 1 m
2 sheet of cardboard for 30 s at a 1 m distance. After spraying, a circle drawn around the area covered by the shrimp or crab spray before it dried indicated the surface area coverage (
Figure S4).
2.10. pH and Viscosity
The prepared spray mixes were pH tested at room temperature (25 °C) using an Orion Star A111 pH meter (Thermo Scientific, Waltham, MA, USA). The viscosities of the prepared spray mixes were measured at room temperature (25 °C) using a USS-DVT4 digital viscometer working on the shear force principle (US Solid, Cleveland, OH, USA).
2.11. Nursery Trials
Indicative nursery trials were conducted at Windmill Nursery (Franklinton, LA, USA) using a JD9-C High Pressure Spray from HD Hudson (Lowell, MI, USA) and Fogg-it handheld spray tank with 7.5–15 LPM spray rate.
4. Discussion
4.1. Drying Effects
Oven drying usually results in darker-colored dried shrimp/crab shells, as compared to autoclaving, but no distinct chemical effect was observed, as seen from the elemental analysis. Hence, there was no difference in these drying treatments. Oven drying is usually faster and more energy efficient, as compared to autoclaving at an industrial scale, so long as shrimp/crab shell waste is evenly distributed on a tray for drying.
4.2. HTC
Hydrothermally carbonizing shrimp shell waste formed mineral-rich solid fuel pellets that can be used as a fertilizer. HTC treatment was posited to separate and enhance the bioavailable mineral content from shrimp shell waste under the effects of high temperature and pressure. The ionic product effect from compressed liquid water, which indicates water’s dual role as both an acid and base at high temperature and pressure, was particularly noticeable for the HTC temperatures investigated here [
12]. Polymerization reactions may produce higher-molecular-weight products, typically occurring at >200 °C temperatures, usually in the liquid fraction, though not analyzed here [
13,
14].
4.3. Pelletization
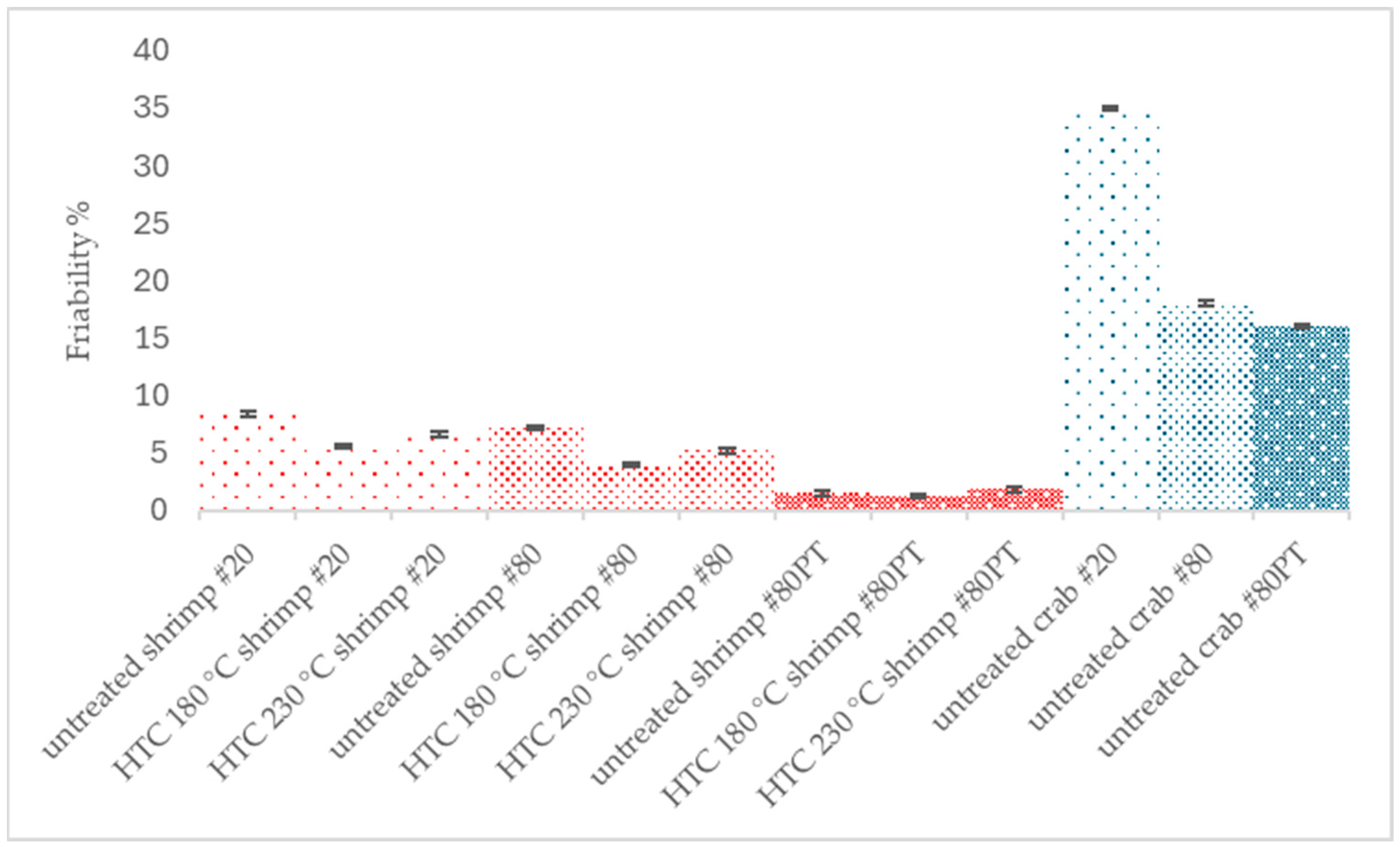
Sturdy pellets formed readily with the HTC-treated ground material, shrimp #80 PT, which would minimize transportation losses (2–3%) [
9]. Pelletization may replace powdered fertilizer losses, make their transportation compact, and ease material handling. Such pellets are not to be sprayed by themselves but dissolved in water before spraying. They could be utilized by direct dry biochar spreading in the fields for long-term carbon fixing and micronutrient release [
15]. If these pellets need to be used as sprayable fertilizer, they dissolve easily in water, as seen from indicative lab tests (
Figure S5) [
16]. Friability analysis allowed an understanding of the extent of pellet breakage that would contribute to transportation losses that can occur when solid fertilizer pellets are transported [
9,
17].
4.4. Bomb Calorimetry and Higher Heating Values (HHV)
The treatment with different HTC temperatures did not show any discernable difference in HHV for dried shrimp shells, HTC-treated shrimp shells at 180 °C, and HTC-treated shrimp shells at 230 °C. If a solid pellet is desired from HTC treatment on seafood wastes, a low temperature would be more desirable in production since that would require less processing energy.
Due to the high degree of variability in each individual biomass shell waste component, their efficient application in energy production is limited [
7]. To estimate variability in bomb calorimetry, the procedure was repeated three times for each biomass sample for the shell waste and then standard error values for HHV were included. Even through scientific repeatability criteria in bomb calorimetry, we find poor calorific values for this class of biomass. Additionally, since these indicative tests did not find any significant energy content, we judged it best to not pursue energy enrichment of crustacean shell waste. Little research is available on this topic in widely published literature [
18].
4.5. Elemental Analysis
The pellets provide balanced N:P:K ratios as discussed. We observe significant carbon (40%) and oxygen (40%), with some calcium (10%) as principal elements (
Table S1). Other elements, such as sodium, magnesium, sulfur, and chlorine, were found in trace amounts with high errors, hence being considered insignificant. The crucial elements in evaluating a biomass’ fertilizer potential such as nitrogen, phosphorus, and potassium were noticed; however, phosphorus and potassium were insignificantly low [
19]. Nitrogen was 5% post mass normalization. The average N:P:K ratio was 65:15:1. The nitrogen in crustacean shells, usually bound in the exoskeleton, was composed of chitin and other proteins. This was present in a polymeric matrix and during hydrothermal carbonization, and the ionizing effects may have reduced them to their monomers or other smaller constituents of the polymer chain. This may explain the reduction in nitrogen abundance post HTC treatment. When HTC-treated shell waste was dissolved in water to be used as sprayable fertilizer, it became functionalized, releasing soluble nutrients at the root system of plants and providing excellent disease-resistant and yield-increasing effects on plants [
20]. Calcium-containing biochar improves salt resistance by promoting soil quality with antioxidant potential [
21]. This suggests that shrimp shells are unlikely to match the effectiveness of traditional chemical fertilizers as strong competitors economically. However, they may serve as valuable long-term contributors to soil health by acting as organic carbon fixers and enhancing oxygen bioavailability with trace minerals that support plant growth [
22].
The analyzed solid products of hydrothermally carbonized shrimp shell waste provided low N:P:K values for organic fertilizers without damaging the soil. Pulverizing shrimp shell waste produced a readily available biodegradable fertilizer that could function as a nonhazardous and long-term organic carbon stabilizer in the soil [
23]. Elemental analysis indicates that crab shells are not an advantageous fertilizer, but they may prove beneficial as carbon fixers in soil, with added oxygen bioavailability. Treated shrimp shells can enhance the availability of nutrients in the soil without concern about their concentration as they may promote the long-term, slow release of required nutrients [
24].
4.6. pH and Viscosity
Concentrated spray solutions had a higher pH (8–9 range) than neutral, indicating the basic nature of polysaccharide chitin found in large amounts in the shrimp/crab shells when they are dissolved in DI water. Such solutions were about 85 times more viscous than water at room temperature. A wider nozzle may be required for spraying effectively.
4.7. Sprayable Fertilizer
Environmentally harmful chemicals are typically used in fertilizer production, so alternative natural biofertilizers are optimal for food production via agriculture [
25]. The first step in testing the use of shrimp/crab shells as fertilizer was understanding the sprayability of the synthesized mixture. A liquid product spray was developed to investigate the fertilizer potential of shrimp/crab shell waste. Shrimp shells were found to be sprayable through spray properties, especially #80 PT after testing different sizes of shrimp shells (
Figure S6,
Table S2). This sprayable property may be due to special low microscopic peculiarities typically observed in the 1–10 µm range [
5,
19,
22,
23,
25]. These can be sprayed on the soil to act as a biodegradable natural fertilizer [
24]. The cardboard samples, once air-dried, stay covered with shrimp flakes for over 3 years under ambient conditions, i.e., they are stable long term. As mentioned, spray coverage was measured by drawing a circle around the area covered by the shrimp spray before it dried obtain an approximate diameter and coverage area (0.12 m
2 average for the smallest shrimp shell particles, 1–3 mm thick coat). The #80 PT shrimp had better sprayability and the highest spray coverage once spray gun clogging issues were mitigated. The goal of our study was to find the optimal ratio of shrimp particles to water that gives the best surface area coverage. It is suggested as a 1:10 ratio (biomass to water, by mass), which gives sprayability without issues.
Viscosity is highly important to sprayability and coverage. More sprayability tests with higher water concentration may be needed to confirm if #20 and #80 shrimp are also sprayable with wide coverage. Wider nozzles on blast guns with higher aperture sizes may yield better sprayability for larger size particles. Additional measurements of pH and viscosity could analyze how these parameters affect fertilization capabilities as an avenue of investigation. One study using a mixture of water and cornstarch used a spray canister that continuously stirred the mixture to prevent settling [
26]. This study also used paint brushes to simulate spray conditions, which we suspect did not capture spray plume characteristics. Other studies have used paint brushes to simulate spray conditions, but such a method was not attempted here [
26]. Consequently, for crab shells, these particles may be denser than shrimp shell particles of the same size to begin with, as evident from poor spraying. A detailed particle size analysis study to conclude whether crab shells are not sprayable due to density differences would be required. Further, microscopy studies of these samples may be an area of investigation for future work [
23]. Due to the limitations in the scope of the present study timeframe and funding availability, we could only perform limited nursery field trials that indicated no harm to their plants under dilute spraying recommendation. Our research program as an applied engineering research group does not permit such a field trial. We propose further investigation through field trials of such bioavailable fertilizers [
27]. Unpleasant odors were the hardest aspects of the spray experiments.
4.8. Environment Friendly Waste Crab Shell Disposal
The best recommendation for using dehydrated crushed crab shells is as chicken feed [
28]. Of these, what is not eaten by poultry will just be combined with litter and can be composted to make biofertilizer or natural gas in a biocomposter [
29,
30,
31]. Such feeding will also help crush the shells into smaller pieces through feeding and trampling processes so they can be used as dry fertilizer easily. Further, 1 kg of crab meat results in approximately 4 kg of guts and shells, making a lot of feed available, with the excess repurposed after composting for fertilizer.
Whole crab shells may be saved and preserved for display and education purposes. Such preservation techniques require harsh chemicals and safer alternatives were explored. We recommend using 70% ethanol as a preservative. It is relatively cheap and does not degrade the structure of crab shells.
The crab processing facility relied on bleach and dial soap to clean and disinfect surfaces. Two suggested alternatives to bleach are hydrogen peroxide or isopropyl alcohol. Based on the cost and effectiveness, we recommended using 34% hydrogen peroxide sanitizing solution as an alternative to bleach, which would limit chlorine off gas harm.
5. Conclusions
Considering increased seafood production and consumption around the world, there will be a rise in biomass waste. Alternative solutions are needed to counter rising landfill waste and resulting off-gas emissions. This bio-investigation study aligns with the principles of the circular economy goals of sustainability and provides practical, scalable solutions for waste management in the seafood processing industry.
Hydrothermal carbonization has not been previously applied to shrimp shell waste. We made pellets from dried shrimp shells, crab shells, and hydrothermally carbonized shrimp shells to investigate the differences in their binding properties. Friability tests for the brittleness of pellets were conducted. We made durable pellets from HTC-treated biomass waste, as seen from the friability tests. This type of study on crustacean shells is not typically found in the literature. This process helps to compact the biochar to make it easy to transport and spray spread them. Such pellets were found to easily dissolve in water.
We investigated the spray properties of different sizes of shrimp shells and found that the smallest particles, i.e., finer than 180 microns, work best because of their low microscopic range characteristics. After spraying was complete, a circle drawn around the shrimp spray-covered area before it dried up indicated the approximate surface area coverage and proved sprayability. These crustacean shells can be sprayed on soil to act as biodegradable natural fertilizer, which also acts as long-term carbon fixer in the soil once degraded. They do not harm the root system of the plants, as seen from indicative nursery trials. This method may be used in agriculture as a seed treatment that helps plants fight off fungal infections. Therefore, as a by-product of seafood processing that is centrally aggregated, HTC-treated shrimp shell waste is a plausible biofertilizer resource.