Do Waterborne Nanoplastics Affect the Shore Crab Carcinus maenas? A Case Study with Poly(methyl)methacrylate Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Nanoplastic Synthesis and Characterization
2.3. Experimental Design
2.4. Behavioral Assay
2.5. Biochemical Parameters
2.6. Statistical Analysis
3. Results
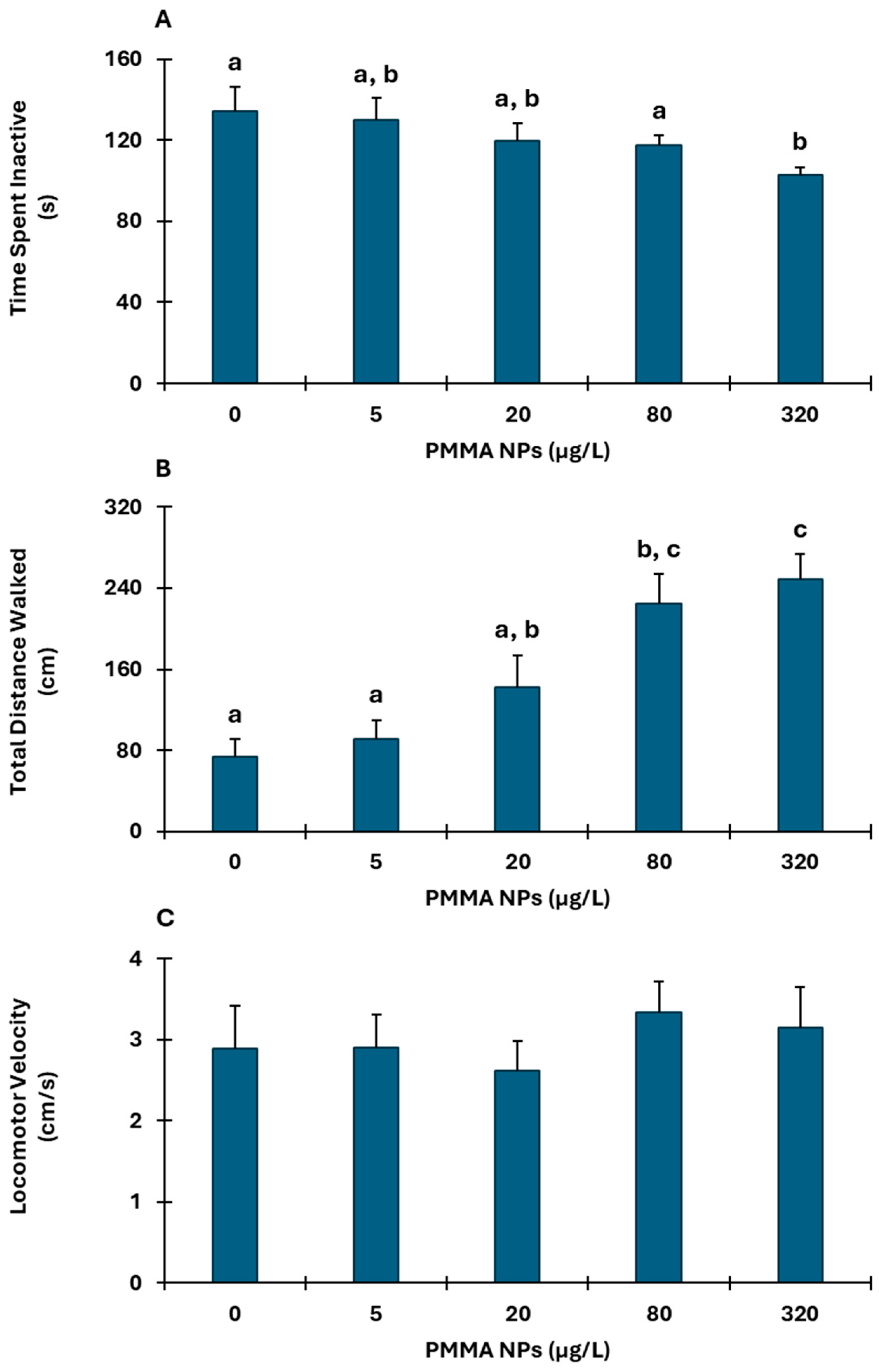
3.1. Behavioral Responses
3.2. Biochemical Responses
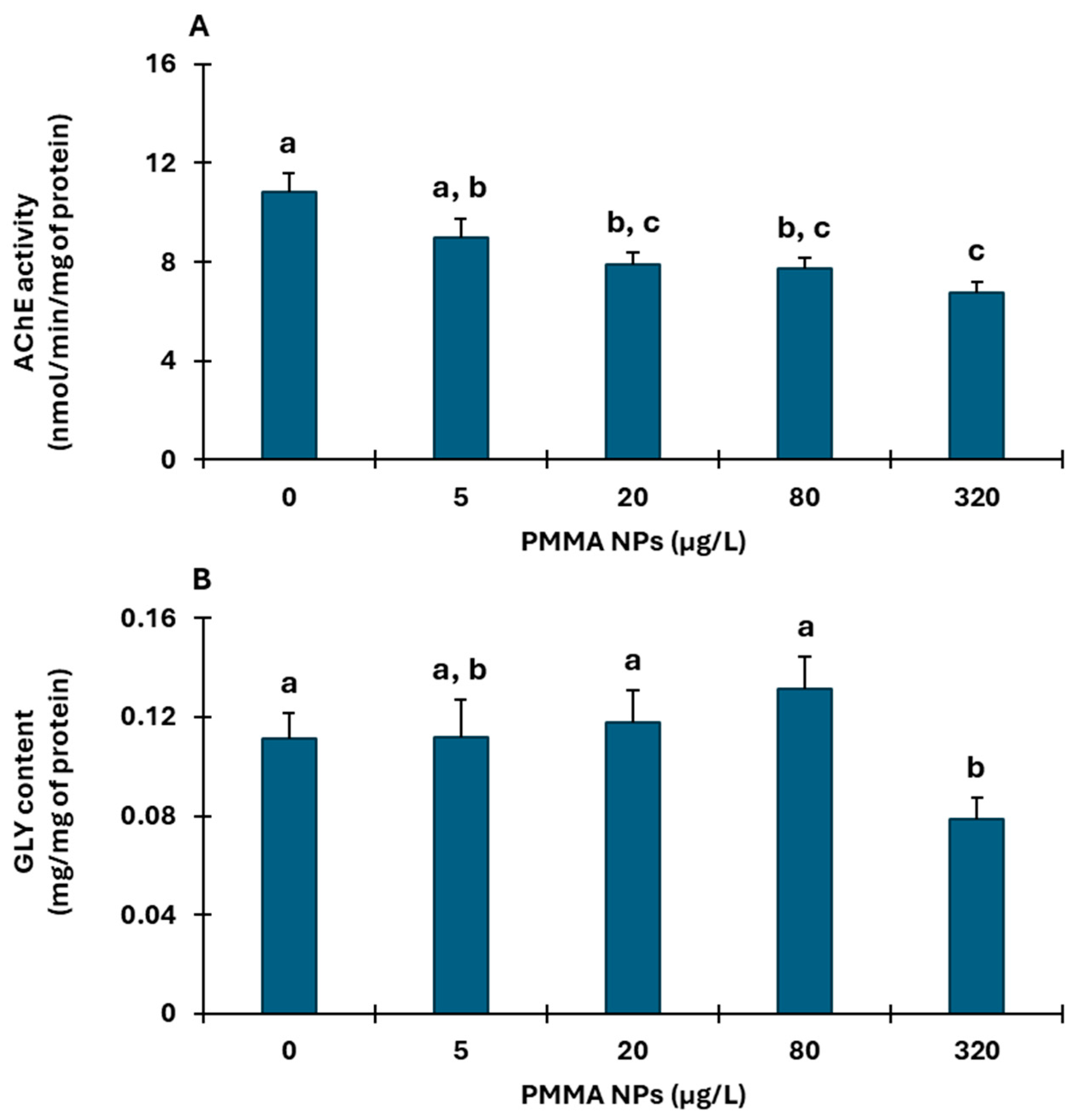
3.2.1. Muscle
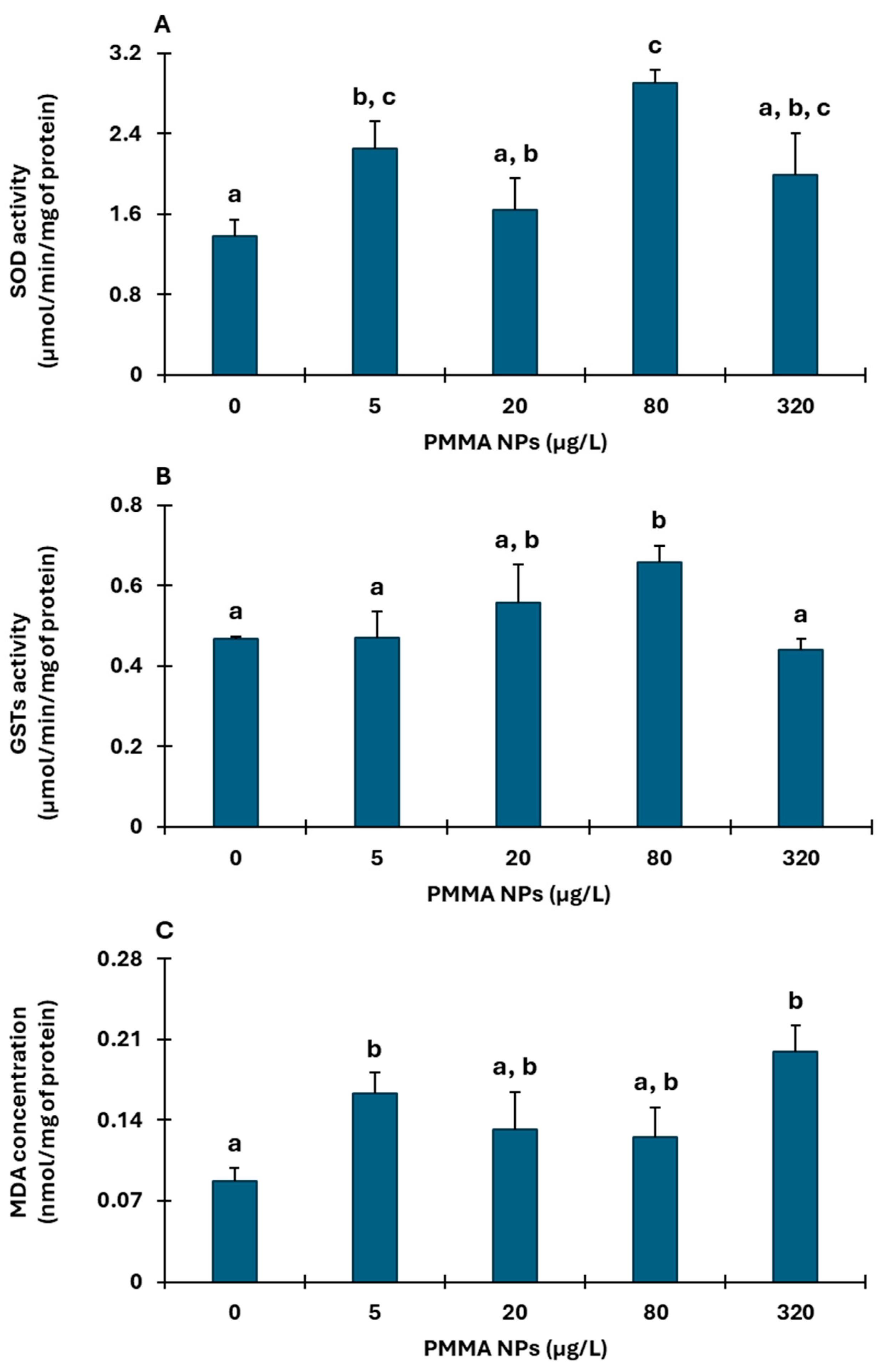
3.2.2. Gills
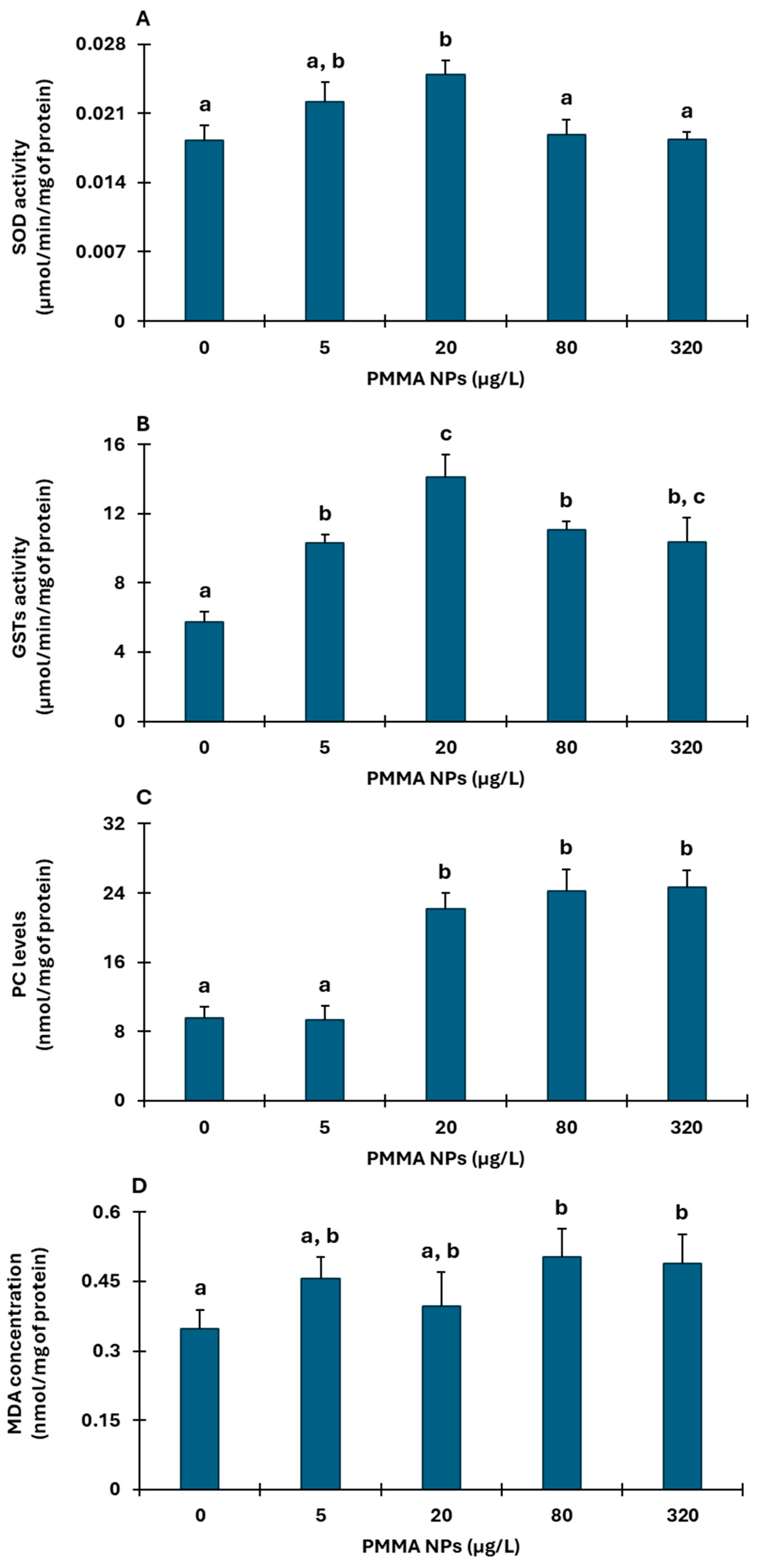
3.2.3. Hepatopancreas
3.2.4. Hemolymph
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.E.; McLeod, K.L.; Tallis, H.; Ruckelshaus, M.; Halpern, B.S.; Levin, P.S.; Chavez, F.P.; Pomeroy, C.; McCay, B.J.; Costello, C.; et al. Science in support of ecosystem-based management for the US West Coast and beyond. Biol. Conserv. 2010, 143, 576–587. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Wu, Y.; Guo, X. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. J. Hazard. Mater. 2020, 392, 122418. [Google Scholar] [CrossRef] [PubMed]
- Tun, T.Z.; Nurlatifah Htwe, A.T.; Than, N.N.; Khine, M.M.; Chavanich, S.; Viyakarn, V.; Isobe, A.; Nakata, H. Polymer types and additive concentrations in single-use plastic products collected from Indonesia, Japan, Myanmar, and Thailand. Sci. Total Environ. 2023, 889, 163983. [Google Scholar] [CrossRef]
- Wiesinger, H.; Wang, Z.; Hellweg, S. Deep Dive into Plastic Monomers, Additives, and Processing Aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef] [PubMed]
- Europe, P. Plastics the Fast Facts 2023. 2024. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 12 January 2025).
- Van Emmerik, T.H.M.; González-Fernández, D.; Laufkötter, C.; Blettler, M.; Lusher, A.; Hurley, R.; Ryan, P.G. Focus on plastics from land to aquatic ecosystems. Environ. Res. Lett. 2023, 18, 040401. [Google Scholar] [CrossRef]
- Rashid, S.; Majeed, L.R.; Mehta, N.; Radu, T.; Martín-Fabiani, I.; Bhat, M.A. Microplastics in terrestrial ecosystems: Sources, transport, fate, mitigation, and remediation strategies. Euro-Mediterr. J. Environ. Integr. 2025. [Google Scholar] [CrossRef]
- Hechmi, S.; Bhat, M.A.; Kallel, A.; Khiari, O.; Louati, Z.; Khelil, M.N.; Zoghlami, R.I.; Cherni, Y.; Melki, S.; Trabelsi, I.; et al. Soil contamination with microplastics (MPs) from treated wastewater and sewage sludge: Risks and sustainable mitigation strategies. Discov. Environ. 2024, 2, 95. [Google Scholar] [CrossRef]
- Colton, J.B.; Knapp, F.D.; Burns, B.R. Plastic Particles in Surface Waters of the Northwestern Atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef]
- Brandts, I.; Barría, C.; Martins, M.A.; Franco-Martínez, L.; Barreto, A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. Hazard. Mater. 2021, 403, 123590. [Google Scholar] [CrossRef]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; Hadri, H.E.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gutow, L.; Klages, M. (Eds.) Marine Anthropogenic Litter; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Xu, J.; Feng, G.; Yan, Y. Effects of polystyrene nanoplastics and copper on gill tissue structure, metabolism, and immune function of the Chinese mitten crab (Eriocheir sinensis). Front. Mar. Sci. 2025, 12, 1538734. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a Persistent Marine Pollutant. Annu. Rev. Environ. Resour. 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Halle, A.T.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef] [PubMed]
- Kampouris, T.E.; Syranidou, E.; Seridou, P.; Gagoulis, K.; Batjakas, I.E.; Kalogerakis, N. MPs and NPs intake and heavy metals accumulation in tissues of Palinurus elephas (J.C. Fabricius, 1787), from NW Aegean sea, Greece. Environ. Pollut. 2023, 316, 120725. [Google Scholar] [CrossRef]
- Materić, D.; Holzinger, R.; Niemann, H. Nanoplastics and ultrafine microplastic in the Dutch Wadden Sea—The hidden plastics debris? Sci. Total Environ. 2022, 846, 157371. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, L.; Zhang, W.; Huang, R.; Lv, X.W.; Jing, X.; Yang, Z.; Wang, J.; Qiu, Y. Role of surface functionalities of nanoplastics on their transport in seawater-saturated sea sand. Environ. Pollut. 2019, 255, 113177. [Google Scholar] [CrossRef]
- Riveiro, A.; Chantada, A.; Soto, R.; Val, J.d.; Arias-González, F.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface texturing of thermoplastics to improve biological performance. In Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–56. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Hermsen, E.; Pompe, R.; Besseling, E.; Koelmans, A.A. Detection of low numbers of microplastics in North Sea fish using strict quality assurance criteria. Mar. Pollut. Bull. 2017, 122, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.L.; Martins, M.A.; Tort, L.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.; Oliveira, M.; Frazão, C.; Almeida, M.; Pinto, R.J.B.; Figueira, E.; Pires, A. The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA). Toxics 2024, 12, 352. [Google Scholar] [CrossRef]
- Silva, M.S.S.; Pires, A.; Vethaak, A.D.; Martínez-Gómez, C.; Almeida, M.; Pinto, R.; Figueira, E.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on the polychaete Hediste diversicolor: Behavioural, regenerative, and biochemical responses. Aquat. Toxicol. 2023, 265, 106743. [Google Scholar] [CrossRef]
- Li, D.; Tang, X.; Xu, X.; Zhao, Y.; Li, L.; Zhang, B.; Zhao, Y. UV-B radiation alleviated detrimental effects of polymethyl methacrylate microplastics on marine diatom Thalassiosira pseudonana. Sci. Total Environ. 2023, 892, 164388. [Google Scholar] [CrossRef]
- Xu, T.-T.; Li, Z.-L.; Li, H.-X.; Lin, L.; Hou, R.; Liu, S.; Li, T.; Zeng, E.Y.; Yu, K.-F.; Xu, X.-R. Unraveling the toxicity mechanisms of nanoplastics with various surface modifications on Skeletonema costatum: Cellular and molecular perspectives. Sci. Total Environ. 2024, 953, 176164. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, P.; Jing, S.; Li, J.; Wanger, T.C.; Liu, W.; Liu, K.; Chen, X.; Li, L. Mass concentrations, compositions and burial fluxes of nano- and micro-plastics in a multi-species saltmarsh. Environ. Pollut. 2024, 363, 125181. [Google Scholar] [CrossRef]
- Cobelas, Á.; Rojo, C. Bridging community and metacommunity perspectives in benthic photosynthetic organisms (Serranía de Cuenca, Central Spain). Limnetica 2023, 42, 37–54. [Google Scholar] [CrossRef]
- Pourabadehei, M.; Mulligan, C.N. Resuspension of sediment, a new approach for remediation of contaminated sediment. Environ. Pollut. 2016, 213, 63–75. [Google Scholar] [CrossRef]
- Carlton, J.T.; Cohen, A.N. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A. Life history and population dynamics of green crabs (Carcinus maenas). Fishes 2020, 5, 4. [Google Scholar] [CrossRef]
- Simberloff, D.; Rejmanek, M. (Eds.) 100 of the World’s Worst Invasive Alien Species: A Selection from The Global Invasive Species Database. In Encyclopedia of Biological Invasions; University of California Press: Oakland, CA, USA, 2019; pp. 715–716. [Google Scholar] [CrossRef]
- Cruz, D.R.S. Caracterização da População de Carcinus maenas na Lagoa de Santo André e Potencial de Exploração. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Ghedira, J.; Jebali, J.; Banni, M.; Chouba, L.; Boussetta, H.; López-Barea, J.; Alhama, J. Use of oxidative stress biomarkers in Carcinus maenas to assess littoral zone contamination in tunisia. Aquat. Biol. 2011, 14, 87–98. [Google Scholar] [CrossRef]
- Jebali, J.; Ben-Khedher, S.; Ghedira, J.; Kamel, N.; Boussetta, H. Integrated assessment of biochemical responses in Mediterranean crab (Carcinus maenas) collected from Monastir Bay, Tunisia. J. Environ. Sci. 2011, 23, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.T.; Pardal, M.Â. The crab Carcinus maenas as a suitable experimental model in ecotoxicology. Environ. Int. 2014, 70, 158–182. [Google Scholar] [CrossRef]
- Elumalai, M.; Antunes, C.; Guilhermino, L. Effects of Single Metals and Their Mixture on Selected Enzymes of Carcinus maenas. Water Air Soil Pollut. 2002, 141, 273–280. [Google Scholar] [CrossRef]
- Elumalai, M.; Antunes, C.; Guilhermino, L. Enzymatic biomarkers in the crab Carcinus maenas from the Minho River estuary (NW Portugal) exposed to zinc and mercury. Chemosphere 2007, 66, 273–280. [Google Scholar] [CrossRef]
- Martín-Díaz, M.L.; Bamber, S.; Casado-Martínez, C.; Sales, D.; DelValls, T.A. Toxicokinetics of heavy metals from a mining spill using Carcinus maenas. Mar. Environ. Res. 2004, 58, 833–837. [Google Scholar] [CrossRef]
- Mesquita, S.R.; Guilhermino, L.; Guimarães, L. Biochemical and locomotor responses of Carcinus maenas exposed to the serotonin reuptake inhibitor fluoxetine. Chemosphere 2011, 85, 967–976. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Oliveira, P.C.; Guilhermino, L.; Guimarães, L. Effects of salinity stress on neurotransmission, energy metabolism, and anti-oxidant biomarkers of Carcinus maenas from two estuaries of the NW Iberian Peninsula. Mar. Biol. 2012, 159, 2061–2074. [Google Scholar] [CrossRef]
- Damasceno, J.M.; Rato, L.D.; Simões, T.; Morão, I.F.C.; Meireles, G.; Novais, S.C.; Lemos, M.F.L. Exposure to the insecticide sulfoxaflor affects behaviour and biomarkers responses of Carcinus maenas (Crustacea: Decapoda). Biology 2021, 10, 1234. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Goodhead, R.; Moger, J.; Lewis, C.; Galloway, T.S. Effect of Microplastic on the Gills of the Shore Crab Carcinus maenas. Environ. Sci. Technol. 2016, 50, 5364–5369. [Google Scholar] [CrossRef] [PubMed]
- Manuel, P.; Almeida, M.; Martins, M.; Oliveira, M. Effects of nanoplastics on zebrafish embryo-larval stages: A case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res. 2022, 213, 113584. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of nanoplastics in the aquatic environment and possible impacts on aquatic organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef] [PubMed]
- Barría, C.; Brandts, I.; Tort, L.; Oliveira, M.; Teles, M. Effect of nanoplastics on fish health and performance: A review. Mar. Pollut. Bull. 2020, 151, 110791. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Pathirana, I.; Rajapakse, R.P.V.J.; Pathirana, E. Evaluation of Efficacy of Selected Anesthetic Agents on Blood-Spotted Crab (Portunus sanguinolentus). J. Shellfish Res. 2016, 35, 237–240. [Google Scholar] [CrossRef]
- Oliveira, M.; Cardoso, D.N.; Soares, A.M.V.M.; Loureiro, S. Effects of short-term exposure to fluoxetine and carbamazepine to the collembolan Folsomia candida. Chemosphere 2015, 120, 86–91. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G. The Biuret Reaction in the Determination of Serum Proteins I. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Acetylcholinesterase Activity in Juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Walop, J.N.; Boot, L.M. Studies on cholinesterase in Carcinus maenas. Biochim. Biophys. Acta 1950, 4, 566–571. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels1. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 218. [Google Scholar]
- Crump, A.; Mullens, C.; Bethell, E.J.; Cunningham, E.M.; Arnott, G. Microplastics disrupt hermit crab shell selection. Biol. Lett. 2020, 16, 20200030. [Google Scholar] [CrossRef]
- Crump, A.; Aiken, C.; Cunningham, E.M.; Arnott, G. Short-Term Microplastic Exposure Impairs Cognition in Hermit Crabs. Animals 2023, 13, 1055. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Mundye, A.; Kregting, L.; Dick, J.T.A.; Crump, A.; Riddell, G.; Arnott, G. Animal contests and microplastics: Evidence of disrupted behaviour in hermit crabs Pagurus bernhardus. R. Soc. Open Sci. 2021, 8, 211089. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Taylor, A.C.; Huntingford, F.A. Metabolic consequences of agonistic behaviour: Crab fights in declining oxygen tensions. Anim. Behav. 1999, 57, 353–363. [Google Scholar] [CrossRef][Green Version]
- Maria, V.L.; Santos, M.A.; Bebianno, M.J. Contaminant effects in shore crabs (Carcinus maenas) from Ria Formosa Lagoon. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Hagiwara, A.; Park, H.G.; Lee, J.-S. The glutathione S-transferase genes in marine rotifers and copepods: Identification of GSTs and applications for ecotoxicological studies. Mar. Pollut. Bull. 2020, 156, 111080. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhu, X.; Duan, Y.; Huang, J.; Nan, Y.; Zhang, J. Toxic effects of nitrite and microplastics stress on histology, oxidative stress, and metabolic function in the gills of Pacific white shrimp, Litopenaeus vannamei. Mar. Pollut. Bull. 2023, 187, 114531. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Dzul-Caamal, R.; Rodríguez-Cab, E.M.; Borges-Ramírez, M.M.; Osten, J.R.; Beltran, K.; Pichardo-Casales, B.; Ramírez-Olivares, A.I.; Vargas-Abúndez, J.A.; Thurman, C.L.; et al. Synergistic effects of microplastic and lead trigger physiological and biochemical impairment in a mangrove crab. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109809. [Google Scholar] [CrossRef]
- Consigna, M.J.S.; Tseng, L.-C.; Chou, C.; Huang, C.-W.; Shao, Y.-T.; Hwang, J.-S. Pathological and biochemical effects of polyethylene microplastic exposure in hydrothermal vent crab, Xenograpsus testudinatus. Mar. Pollut. Bull. 2025, 212, 117546. [Google Scholar] [CrossRef]
- La Torre, K.B.; Vargas-Abúndez, J.A.; Dzul-Caamal, R.; Maraschi, A.C.; Capparelli, M.V. Warming-induced microplastic accumulation and physiological toxicity in fiddler crabs. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 290, 110142. [Google Scholar] [CrossRef]
- Arif, I.; Shang, Y.; Zhang, C.; Khan, F.U.; Tan, K.A.; Waiho, K.; Wang, Y.; Kwan, K.Y.; Hu, M. Combined effects of nanoplastics and heavy metal on antioxidant parameters of juvenile tri-spine horseshoe crabs. Front. Mar. Sci. 2022, 9, 1005820. [Google Scholar] [CrossRef]
- Fredrick, W.S.; Ravichandran, S. Hemolymph proteins in marine crustaceans. Asian Pac. J. Trop. Biomed. 2012, 2, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Eberini, I.; Palazzolo, L.; Miller, I. Hemolymph proteins: An overview across marine arthropods and molluscs. J. Proteom. 2021, 245, 104294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Poirier, D.G.; Helm, P.A.; Bayoumi, M.; Rochman, C.M. No evidence of spherical microplastics (10–300 μm) translocation in adult rainbow trout (Oncorhynchus mykiss) after a two-week dietary exposure. PLoS ONE 2020, 15, e0239128. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, B.; Oliveira, M.; Frazão, C.; Almeida, M.; Pinto, R.J.B.; Figueira, E.; Pires, A. Do Waterborne Nanoplastics Affect the Shore Crab Carcinus maenas? A Case Study with Poly(methyl)methacrylate Particles. Environments 2025, 12, 169. https://doi.org/10.3390/environments12050169
Neves B, Oliveira M, Frazão C, Almeida M, Pinto RJB, Figueira E, Pires A. Do Waterborne Nanoplastics Affect the Shore Crab Carcinus maenas? A Case Study with Poly(methyl)methacrylate Particles. Environments. 2025; 12(5):169. https://doi.org/10.3390/environments12050169
Chicago/Turabian StyleNeves, Beatriz, Miguel Oliveira, Carolina Frazão, Mónica Almeida, Ricardo J. B. Pinto, Etelvina Figueira, and Adília Pires. 2025. "Do Waterborne Nanoplastics Affect the Shore Crab Carcinus maenas? A Case Study with Poly(methyl)methacrylate Particles" Environments 12, no. 5: 169. https://doi.org/10.3390/environments12050169
APA StyleNeves, B., Oliveira, M., Frazão, C., Almeida, M., Pinto, R. J. B., Figueira, E., & Pires, A. (2025). Do Waterborne Nanoplastics Affect the Shore Crab Carcinus maenas? A Case Study with Poly(methyl)methacrylate Particles. Environments, 12(5), 169. https://doi.org/10.3390/environments12050169








