Mofettes as Models for Basic Research on Soil and Rhizosphere Microbial Communities and Possible Applications of These Extreme Ecosystems
Abstract
:1. Introduction—Mofette Ecosystems
2. Methods
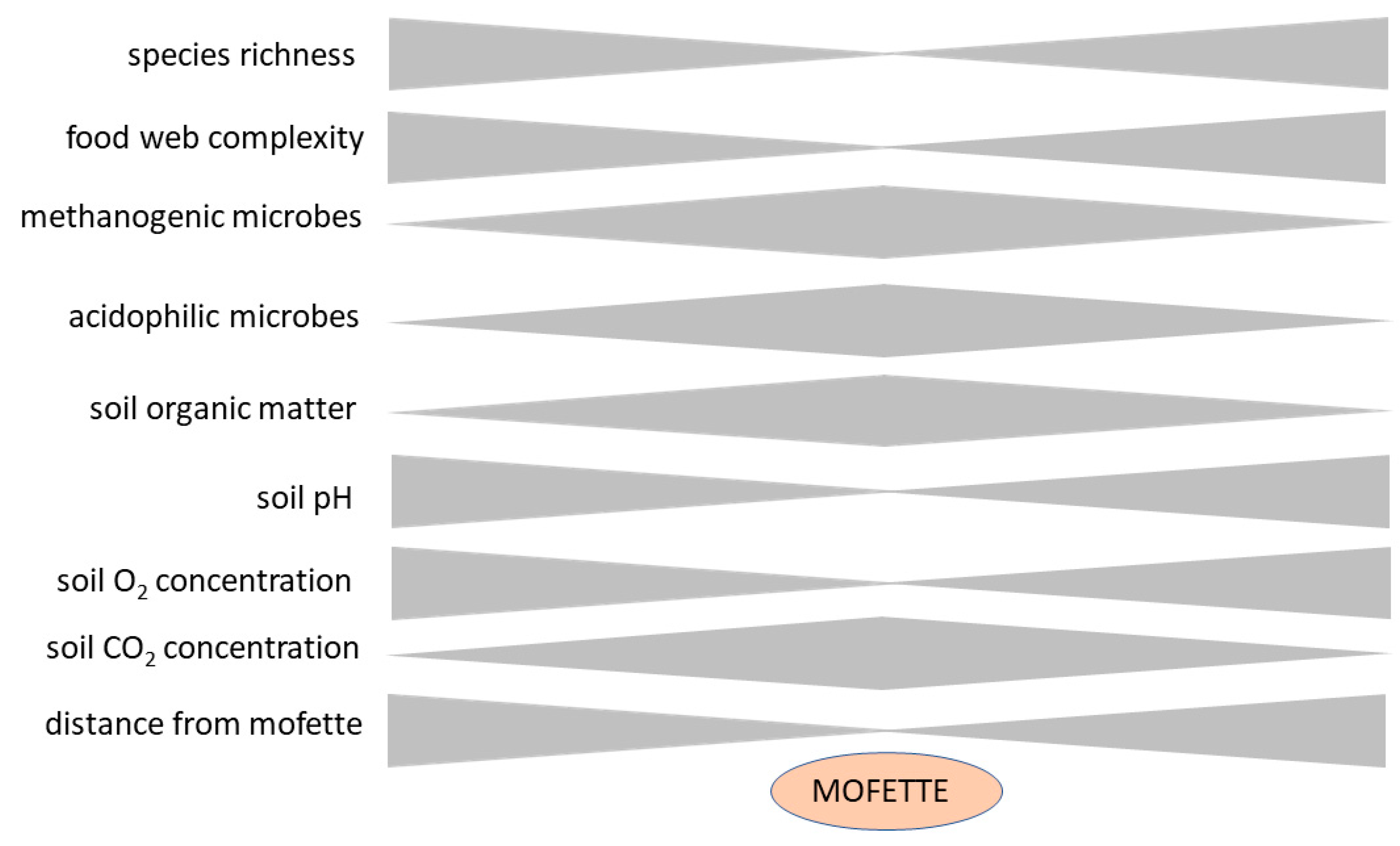
3. Results—Soil Ecology Studies in Mofettes
3.1. Rhizosphere and Plant Root Function in Mofette Soils
3.2. Microbial Diversity in Mofette Soils
3.2.1. Archaea and Bacteria
3.2.2. Fungi
3.2.3. Microalgae
3.2.4. Viruses
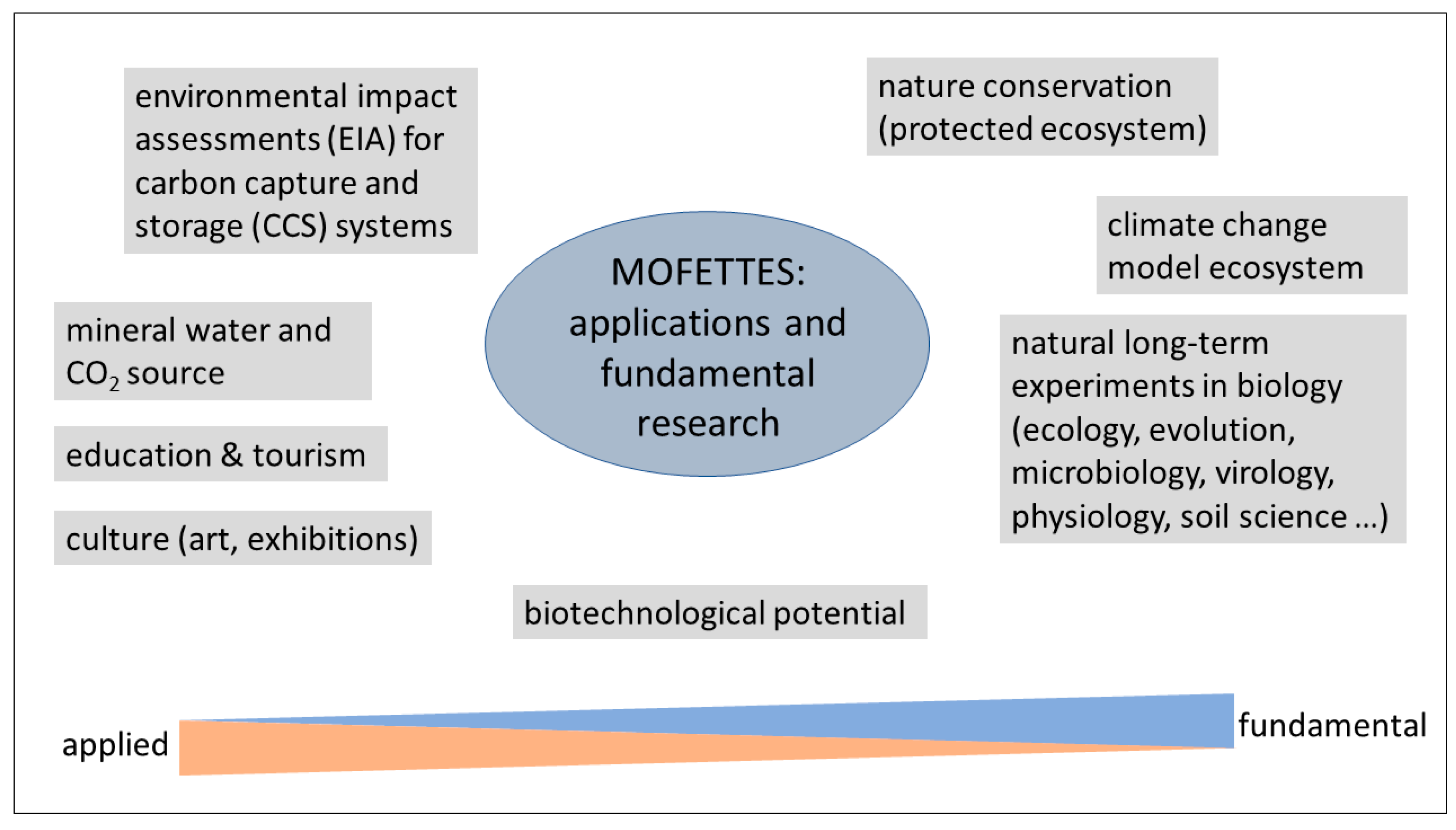
4. Potential Applications
4.1. Mofette Biotechnological Potential and CO2 Enrichment Model Systems
4.2. Mofettes as Model Ecosystems for Carbon Capture and Storage Assessments
4.3. Nature Conservation, Tourism, Culture and Education
5. Conclusions and Prospects
Funding
Data Availability Statement
Conflicts of Interest
References
- Pfanz, H.; Vodnik, D.D.D.; Wittmann, C.; Aschan, G.; Raschi, A. Plants and geothermal CO2 exhalations—Survival in and adaption to high CO2 environment. Prog. Bot. 2004, 65, 499–538. [Google Scholar]
- Maček, I.; Vodnik, D.; Pfanz, H.; Low-Décarie, E.; Dumbrell, A. Locally extreme environments as natural long-term experiments in ecology. In Advances in Ecological Research, Large Scale Ecology: Model Systems to Global Perspectives; Dumbrell, A.J., Kordas, R., Woodward, G., Eds.; Academic Press: Oxford, UK, 2016; Volume 55, pp. 283–323. [Google Scholar]
- Vodnik, D.; Kastelec, D.; Pfanz, H.; Maček, I.; Turk, B. Small-scale spatial variation in soil CO2 concentration in a natural carbon dioxide spring and some related plant responses. Geoderma 2006, 133, 309–319. [Google Scholar] [CrossRef]
- Vodnik, D.; Videmšek, U.; Pintar, M.; Maček, I.; Pfanz, H. The characteristics of soil CO2 fluxes at a site with natural CO2 enrichment. Geoderma 2009, 150, 32–37. [Google Scholar] [CrossRef]
- Kaligarič, M. Vegetation patterns and responses to elevated CO2 from natural CO2 springs at Strmec (Radenci, Slovenia). Acta Biol. Slov. 2001, 44, 31–38. [Google Scholar]
- Vodnik, D.; Pfanz, H.; Maček, I.; Kastelec, D.; Lojen, S.; Batič, F. Photosynthesis of cockspur (Echinochloa crus-galli (L.) Beauv.) at sites of naturally elevated CO2 concentration. Photosynthetica 2002, 40, 575–579. [Google Scholar] [CrossRef]
- Vodnik, D.; Pfanz, H.; Wittmann, C.; Maček, I.; Kastelec, D.; Turk, B.; Batič, F. FPhotosynthetic acclimation in plants growing near a carbon dioxide spring. Phyton 2002, 42, 239–244. [Google Scholar]
- Maček, I.; Pfanz, H.; Francetič, V.; Batič, F.; Vodnik, D. Root respiration response to high CO2 concentrations in plants from natural CO2 springs. Environ. Exp. Bot. 2005, 54, 90–99. [Google Scholar] [CrossRef]
- Pfanz, H.; Vodnik, D.; Wittmann, C.; Aschan, G.; Batič, F.; Turk, B.; Maček, I. Photosynthetic performance (CO2-compensation point, carboxylation and net photosynthesis) of timothy grass (Phleum pratense L.) is affected by elevated carbon dioxide in post-volcanic mofette areas. Environ. Exp. Bot. 2007, 61, 41–48. [Google Scholar] [CrossRef]
- Saban, J.M.; Chapman, M.A.; Taylor, G. FACE facts hold for multiple generations; Evidence from natural CO2 springs. Glob. Change Biol. 2018, 25, 14437. [Google Scholar] [CrossRef]
- Paoletti, E.; Pfanz, H.; Raschi, A. Pros and cons of CO2 springs as experimental sites. In Plant Responses to Air Pollution and Global Change; Omasa, K., Nauchi, I., De Kok, L.J., Eds.; Springer: Tokyo, Japan, 2005; pp. 195–202. [Google Scholar]
- Maček, I.; Clark, D.R.; Šibanc, N.; Moser, G.; Vodnik, D.; Müller, C.; Dumbrell, A.J. Impacts of long-term elevated atmospheric CO2 concentrations on communities of arbuscular mycorrhizal fungi. Mol. Ecol. 2019, 28, 3445–3458. [Google Scholar] [CrossRef]
- Maček, I.; Dumbrell, A.J.; Nelson, M.; Fitter, A.H.; Vodnik, D.; Helgason, T. Local Adaptation to Soil Hypoxia Determines the Structure of an Arbuscular Mycorrhizal Fungal Community in Roots from Natural CO2 Springs. Appl. Environ. Microbiol. 2011, 77, 4770–4777. [Google Scholar] [CrossRef] [PubMed]
- Šibanc, N.; Dumbrell, A.J.; Mandić-Mulec, I.; Maček, I. Impacts of naturally elevated soil CO2 concentrations on communities of soil archaea and bacteria. Soil Biol. Biochem. 2014, 68, 348–356. [Google Scholar] [CrossRef]
- Šibanc, N.; Clark, D.R.; Helgason, T.; Dumbrell, A.J.; Maček, I. Extreme environments simplify reassembly of communities of arbuscular mycorrhizal fungi. mSystems 2024, 9, e0133123. [Google Scholar] [CrossRef]
- Maček, I.; Dumbrell, A.J. Long-Term Experiments in Natural Locally-Extreme High CO2 Environments: Roadmap for Future Research; David, A.B., Alex, J.D., Eds.; Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2023; Volume 68, pp. 35–49. [Google Scholar] [CrossRef]
- Videmšek, U.; Turk, B.; Vodnik, D. Root aerenchyma—Formation and function. Acta Agric. Slov. 2006, 87, 445–453. [Google Scholar] [CrossRef]
- Ibanc, N.; Zalar, P.; Schroers, H.; Zajc, J.; Pontes, A.; Sampaio, J.P. Occultifur mephitis f.a., sp. nov. and other yeast species from hypoxic and elevated CO2 mofette environments. Int. J. Syst. Evol. Microbiol. 2018, 68, 2285–2298. [Google Scholar] [CrossRef]
- Videmšek, U.; Hagn, A.; Suhadolc, M.; Radl, V.; Knicker, H.; Schloter, M.; Vodnik, D. Abundance and Diversity of CO2-fixing Bacteria in Grassland Soils Close to Natural Carbon Dioxide Springs. Microb. Ecol. 2008, 58, 1–9. [Google Scholar] [CrossRef]
- Frerichs, J.; Oppermann, B.I.; Gwosdz, S.; Möller, I.; Herrmann, M.; Krüger, M. Microbial community changes at a terrestrial volcanic CO2vent induced by soil acidification and anaerobic microhabitats within the soil column. FEMS Microbiol. Ecol. 2013, 84, 60–74. [Google Scholar] [CrossRef]
- Krüger, M.; West, J.; Frerichs, J.; Oppermann, B.; Dictor, M.-C.; Jouliand, C.; Jones, D.; Coombs, P.; Green, K.; Pearce, J.; et al. Ecosystem effects of elevated CO2 concentrations on microbial populations at a terrestrial CO2 vent at Laacher See, Germany. Energy Procedia 2009, 1, 1933–1939. [Google Scholar] [CrossRef]
- Krüger, M.; Jones, D.; Frerichs, J.; Oppermann, B.I.; West, J.; Coombs, P.; Green, K.; Barlow, T.; Lister, R.; Shaw, R.; et al. Effects of elevated CO2 concentrations on the vegetation and microbial populations at a terrestrial CO2 vent at Laacher See, Germany. Int. J. Greenh. Gas Control. 2011, 5, 1093–1098. [Google Scholar] [CrossRef]
- Oppermann, B.; Michaelis, W.; Blumenberg, M.; Frerichs, J.; Schulz, H.; Schippers, A.; Beaubien, S.; Krüger, M. Soil microbial community changes as a result of long-term exposure to a natural CO2 vent. Geochim. Cosmochim. Acta 2010, 74, 2697–2716. [Google Scholar] [CrossRef]
- Fazi, S.; Ungaro, F.; Venturi, S.; Vimercati, L.; Viggi, C.C.; Baronti, S.; Ugolini, F.; Calzolari, C.; Tassi, F.; Vaselli, O.; et al. Microbiomes in Soils Exposed to Naturally High Concentrations of CO2 (Bossoleto Mofette Tuscany, Italy). Front. Microbiol. 2019, 10, 2238. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Krauze, P.; Kämpf, H.; Horn, F.; Liu, Q.; Voropaev, A.; Wagner, D.; Alawi, M. Microbiological and Geochemical Survey of CO2-Dominated Mofette and Mineral Waters of the Cheb Basin, Czech Republic. Front. Microbiol. 2017, 8, 2446. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montiel, I.; Sidrach-Cardona, R.; Gabilondo, R.; Pedescoll, A.; Scheu, S.; Bécares, E. Soil communities are affected by CO2 belowground emissions at a natural vent in Spain. Soil Biol. Biochem. 2016, 97, 92–98. [Google Scholar] [CrossRef]
- Fernández-Montiel, I.; Pedescoll, A.; Bécares, E. Microbial communities in a range of carbon dioxide fluxes from a natural volcanic vent in Campo de Calatrava, Spain. Int. J. Greenh. Gas Control. 2016, 50, 70–79. [Google Scholar] [CrossRef]
- Noble, R.R.; Stalker, L.; Wakelin, S.A.; Pejcic, B.; Leybourne, M.I.; Hortle, A.L.; Michael, K. Biological monitoring for carbon capture and storage—A review and potential future developments. Int. J. Greenh. Gas Control. 2012, 10, 520–535. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Rennert, T.; Pfanz, H. Geogenic CO2 affects stabilization of soil organic matter. Eur. J. Soil Sci. 2015, 66, 838–846. [Google Scholar] [CrossRef]
- Mcgrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Bown, A.W. CO2 and intracellular pH. Plant Cell Environ. 1985, 8, 459–465. [Google Scholar] [CrossRef]
- Pfanz, H.; Martinoia, E.; Lange, O.L.; Heber, U. Flux of sulfur dioxide into leaf cells and cellular acidification by sulfur dioxide. Plant Physiol. 1987, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; 672p. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Feddermann, N.; Finlay, R.; Boller, T.; Elfstrand, M. Functional diversity in arbuscular mycorrhiza—The role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Ecol. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- George, E.; Marschner, H.; Jakobsen, I. Role of Arbuscular Mycorrhizal Fungi in Uptake of Phosphorus and Nitrogen From Soil. Crit. Rev. Biotechnol. 1995, 15, 257–270. [Google Scholar] [CrossRef]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef]
- Pozo, M.J.; Verhage, A.; García-Andrade, J.; García, J.M.; Azcón-Aguilar, C. Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In Mycorrhizas-Functional Processes and Ecological Impact; Springer: Berlin/Heidelberg, Germany, 2009; pp. 123–135. [Google Scholar]
- Facelli, E.; Smith, S.E.; Facelli, J.M.; Christophersen, H.M.; Smith, F.A. Underground friends or enemies: Model plants help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytol. 2010, 185, 1050–1061. [Google Scholar] [CrossRef]
- Botha, A. Yeast in soil. In The Yeast Handbook Biodiversity and Ecophysiology of Yeasts; Rosa, C.A., Péter, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 221–240. [Google Scholar]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Beulig, F.; Urich, T.; Nowak, M.; Trumbore, S.E.; Gleixner, G.; Gilfillan, G.D.; Fjelland, K.E.; Küsel, K. Altered carbon turnover processes and microbiomes in soils under long-term extremely high CO2 exposure. Nat. Microbiol. 2016, 1, 1–9. [Google Scholar]
- Collins, S.; Bell, G. Evolution of natural algal populations at elevated CO2. Ecol. Lett. 2005, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Low-Décarie, E.; Jewell, M.D.; Fussmann, G.F.; Bell, G. Long-term culture at elevated atmospheric CO2 fails to evoke specific adaptation in seven freshwater phytoplankton species. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122598. [Google Scholar] [CrossRef]
- Williamson, K.E.; Fuhrmann, J.J.; Wommack, K.E.; Radosevich, M. Viruses in Soil Ecosystems: An Unknown Quantity Within an Unexplored Territory. Annu. Rev. Virol. 2017, 4, 201–219. [Google Scholar] [CrossRef]
- Pfanz, H.; Saßmannshausen, F.; Wittmann, C.; Pfanz, B.; Thomalla, A. Mofette Vegetation as an Indicator for Geogenic CO2 Emission: A Case Study on the Banks of the Laacher See Volcano, Vulkaneifel, Germany. Geofluids 2019, 2019, 9589306. [Google Scholar] [CrossRef]
- Hohberg, K.; Schulz, H.-J.; Balkenhol, B.; Pilz, M.; Thomalla, A.; Russell, D.J.; Pfanz, H. Soil faunal communities from mofette fields: Effects of high geogenic carbon dioxide concentration. Soil Biol. Biochem. 2015, 88, 420–429. [Google Scholar] [CrossRef]
- Pilz, M.; Hohberg, K.; Pfanz, H.; Wittmann, C.; Xylander, W.E. Respiratory adaptations to a combination of oxygen deprivation and extreme carbon dioxide concentration in nematodes. Respir. Physiol. Neurobiol. 2017, 239, 34–40. [Google Scholar] [CrossRef]
- Balkenhol, B.; Hohberg, K.; Pfanz, H. Spiders in mofette fields—Survival of the toughest in natural carbon dioxide springs? Ecol. Indic. 2016, 69, 749–757. [Google Scholar] [CrossRef]
- Holloway, S.; Pearce, J.; Hards, V.; Ohsumi, T.; Gale, J. Natural emissions of CO2 from the geosphere and their bearing on the geological storage of carbon dioxide. Energy 2007, 32, 1194–1201. [Google Scholar] [CrossRef]
- Jones, D.; Beaubien, S.; Blackford, J.; Foekema, E.; Lions, J.; De Vittor, C.; West, J.; Widdicombe, S.; Hauton, C.; Queirós, A. Developments since 2005 in understanding potential environmental impacts of CO2 leakage from geological storage. Int. J. Greenh. Gas Control. 2015, 40, 350–377. [Google Scholar] [CrossRef]
- Roberts, J.J.; Stalker, L. What have we learnt about CO2 leakage from CO2 release field experiments, and what are the gaps for the future? Earth-Sci. Rev. 2020, 209, 102939. [Google Scholar] [CrossRef]
- von Heilborn, D.H.; Reinmüller, J.; Yurkov, A.; Stehle, P.; Moeller, R.; Lipski, A. Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov. J. Fungi 2023, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Dass, R.S.; Dhinakar, J.E.; Tirkey, A.; Ghose, M.; Suresh, A.J. Thermophilic fungi: Habitats and morpho-molecular adaptations. In Extremophilic Fungi; Sahay, S., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Tsiknia, M.; Skiada, V.; Ipsilantis, I.; Vasileiadis, S.; Kavroulakis, N.; Genitsaris, S.; Papadopoulou, K.K.; Hart, M.; Klironomos, J.; Karpouzas, D.G.; et al. Strong host-specific selection and over-dominance characterize arbuscular mycorrhizal fungal root colonizers of coastal sand dune plants of the Mediterranean region. FEMS Microbiol. Ecol. 2021, 97, fiab109. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, D.; Chu, C.; Zhao, Z.; Zhou, J.; Wu, S. Responses of arbuscular mycorrhizal fungi to rice–upland crop rotations in an 8-year paddy ecosystem. Soil Sci. Soc. Am. J. 2022, 87, 516–527. [Google Scholar] [CrossRef]
- Chialva, M.; Ghignone, S.; Cozzi, P.; Lazzari, B.; Bonfante, P.; Abbruscato, P.; Lumini, E. Water management and phenology influence the root-associated rice field microbiota. FEMS Microbiol. Ecol. 2020, 96, fiaa146. [Google Scholar] [CrossRef]
- Jing, X.; Classen, A.T.; Li, D.; Lin, L.; Lu, M.; Sanders, N.J.; Wang, Y.; Feng, W. Unraveling microbial community structure–function relationships in the horizontal and vertical spatial dimensions in extreme environments. Ecography 2024, 2024, e07118. [Google Scholar] [CrossRef]
- Xiang, N.; Liu, Z.; Tian, X.; Wang, D.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S.; Zou, Y.-N. Improved Waterlogging Tolerance in Roots of Cucumber Plants after Inoculation with Arbuscular Mycorrhizal Fungi. Horticulturae 2024, 10, 478. [Google Scholar] [CrossRef]
- Remelli, S.; Danise, T.; Galli, L.; Menta, C. Soil arthropods in bioindication and ecotoxicological approach: The case of the extreme environment Mefite (Ansanto Valley, Southern Italy). Heliyon 2024, 10, e36342. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maček, I. Mofettes as Models for Basic Research on Soil and Rhizosphere Microbial Communities and Possible Applications of These Extreme Ecosystems. Environments 2025, 12, 166. https://doi.org/10.3390/environments12050166
Maček I. Mofettes as Models for Basic Research on Soil and Rhizosphere Microbial Communities and Possible Applications of These Extreme Ecosystems. Environments. 2025; 12(5):166. https://doi.org/10.3390/environments12050166
Chicago/Turabian StyleMaček, Irena. 2025. "Mofettes as Models for Basic Research on Soil and Rhizosphere Microbial Communities and Possible Applications of These Extreme Ecosystems" Environments 12, no. 5: 166. https://doi.org/10.3390/environments12050166
APA StyleMaček, I. (2025). Mofettes as Models for Basic Research on Soil and Rhizosphere Microbial Communities and Possible Applications of These Extreme Ecosystems. Environments, 12(5), 166. https://doi.org/10.3390/environments12050166




