Abstract
The present study utilised a comprehensive, long-term dataset of toxic phytoplankton (2001–2022) to analyse the relationships between the abundance, distribution, and seasonal trends of toxic phytoplankton, as well as the influence of various sampling methods, physico-chemical parameters, nutrients, and meteo-climatic parameters. The data were obtained through institutional monitoring at four selected sites dedicated to shellfish farming in the Gulf of Trieste (Adriatic Sea, Italy). The results show significant gradients in the spatial distribution of toxic phytoplankton and clear seasonal patterns in regard to the physico-chemical parameters and nutrients. Toxic phytoplankton abundance peaked in late winter/early spring and early autumn, depending on the genus considered. Significant correlations suggest that rainfall influences the nutrient levels and the proliferation of toxic phytoplankton. The time series analysis highlighted significant increases in temperature, salinity, and nitrogen species, during the study period, and decreases in silicon and phosphorous, while chlorophyll a and the overall phytoplankton abundance remained relatively stable, except for a significant decrease in Lingulodinium from 2015 to 2022. This preliminary assessment provides a valuable basis for further approaches (e.g., continuous in situ measurements, modelling, machine learning) to investigate the potential impact of climate variability on toxic phytoplankton dynamics in the Gulf of Trieste and to support mussel-farming management from both health and environmental perspectives.
1. Introduction
Toxic phytoplankton are microalgae that are of particular concern because they can negatively impact human health. They include numerous species associated with food poisoning and that are responsible for various syndromes (Paralytic Shellfish Poisoning—PSP, diarrhetic shellfish poisoning—DSP, Azaspiracid Shellfish Poisoning—AZP, Amnesic Shellfish Poisoning—ASP, Neurotoxic Shellfish Poisoning—NSP, Ciguatera Fish Poisoning—CFP, and cyanotoxin poisoning) [1]. In this study we focused on the dinoflagellate genera, Alexandrium, Dinophysis, and Lingulodinium, and the diatom genus, Pseudo-nitzschia, which contain several potentially toxic species [1,2]. These genera produce saxitoxin (PSP), okadaic acid and yessotoxin (DSP), and domoic acid (ASP), respectively [3,4,5,6]. The presence of harmful algal blooms (HABs) caused by these genera, with the exception of Dinophysis, has been shown to harm marine ecosystems, natural marine resources, and aquaculture [7], by inducing hypoxia and anoxia, toxicity, and causing mechanical damage to organisms. Toxic phytoplankton also poses a significant risk to human health via ingestion and direct contact [8], and represents a substantial economic threat to the shellfish industry, particularly mussel farming.
The Gulf of Trieste (GoT) has a long tradition of shellfish farming, with numerous facilities located along its coastline. The most exploited species is the Mediterranean mussel, Mytilus galloprovincialis (Lamarck, 1819). M. galloprovincialis is a lamellibranch bivalve mollusc and filter feeder, capable of retaining part of the suspended particles (in the order of µm) floating in the water column. It feeds primarily on plankton and organic particles, with the filtration rate and assimilation efficiency influenced by environmental factors, such as temperature and the concentration of dissolved substances [9,10].
In 1973, the local production of M. galloprovincialis in the GoT peaked at 6000 t year−1 (200 employment units) [11]. Following an outbreak and subsequent recovery, production was restored in 1990 (7500 t year−1, 198 employment units) [11]. However, biotoxin events and competition from producers in Greece and Spain led to another decline in the late 1990s. Today, mussel farms cover approximately 300 ha along the coast (about 15 km) and are organised into 14 small cooperatives. Total production is in a serious constant decline (3249 to 1090 t year−1 in 2010 and 2023, respectively). According to Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 regarding official controls on products of animal origin intended for human consumption, nearly all GoT farms are classified as zone A [12], meaning live bivalves may be harvested for direct human consumption provided they meet the E. coli limits specified in Regulation (EC) No 853/2004 (80% of mollusc samples collected during the review period shall not exceed 230 E. coli per 100 g of flesh and intravalvular liquid and the remaining 20% of samples shall not exceed 700 E. coli per 100 g of flesh and intravalvular liquid) (EC, 2019/627) [13].
Solidoro et al. investigated the dietary preferences of M. galloprovincialis in the GoT and found selectivity of most genera of Bacillariophyceae (including Pseudo-nitzschia) and Dinophyceae (including Alexandrium, Dinophysis, and Lingulodinium), as well as protozoa, foraminifera, and the larvae of various organisms, while rejecting colonial or setaceous diatoms and dinoflagellates with elongated shapes and remarkable dimensions [12]. This poses a potential risk through the ingestion pathway, especially from September to November, coinciding with the presence of DSP-producing dinoflagellates [14].
As required by EU Regulation, water samples are collected periodically from sources close to the mussel farms to monitor changes in the composition of toxic phytoplankton and its geographical distribution, and live bivalve molluscs are tested weekly during harvesting (or more frequently if needed) for the presence of biotoxins. No triggers in regard to class, genus, or toxic species have been reported [13]. In the Friuli Venezia Giulia Region (the regional authority on the Italian side of the GoT), an early warning system on the possible toxicity of mussels is in operation, thus optimising prevention measures.
Phytoplankton dynamics are strongly influenced by the surrounding environment [15], yet the drivers of a toxic phytoplankton outburst remain poorly understood, although some hypotheses have been proposed (i.e., aquaculture expansion, climate change, ballast water discharge, the translocation of shellfish stocks to new sites, or excess nutrients) [16,17,18]. In addition, the enrichment of water with nutrients (primarily nitrogen, silicon, and phosphorus) can fuel algal biomass growth [19], a phenomenon recently investigated using a machine learning approach [20], and documented in different areas of the Mediterranean Sea [21,22].
Covering 2001–2022, this study examines toxic phytoplankton in terms of its spatial distribution, seasonal patterns, and temporal trends, within the framework of regulated mussel farm monitoring. We also analyse the physico-chemical parameters, nutrient concentrations, and precipitation to assess their influence on phytoplankton dynamics. These preliminary findings will be useful for subsequent modelling research and the potential application of machine learning in regard to the dynamics of toxic phytoplankton within mussel-farming areas under climate change scenarios.
2. Materials and Methods
2.1. Study Area
The GoT is a semi-enclosed basin, located in the northernmost part of the Adriatic Sea (Mediterranean Sea). It lies east of the coastline joining Grado (Italy) to Cape Savudrija (Republic of Croatia), covering approximately 600 km2 [23,24], with a maximum depth of 25 m; about 10% of the area is shallower than 10 m deep [25].
The Isonzo river (Soča in Slovenian), flowing from the Julian Alps into the northern Adriatic Sea (length = 135 km, basin area = 3430 km2), is the GoT’s primary freshwater source. The hydrological regime of the Isonzo River is characterised by a narrow flow range, with a mean discharge of 82 m3 s−1 (1998–2008 data) [26,27]. Secondary inputs are from smaller rivers in the Italian (Timavo and Rosandra) and Slovenian (Rižana, Badaševica, Drnica, and Dragonja) sectors [28,29]. The GoT’s hydrology is also influenced by temperature, wind, and precipitation [30,31]. In summer, the water column is predominantly stratified, while in winter, mixing prevails, aided by local winds. The Bora is notable, a cold northeasterly wind that blows especially in winter, which stirs the water column, cools surface waters, enhances oxygenation, and causes the sinking of water masses [32].
The anthropogenic pressures in the region include urban settlements (Trieste, Monfalcone, Koper) and the respective ports and industrial areas [24], as well as maritime traffic, fishing, aquaculture, and tourism [33]. Agricultural runoff delivers substantial nitrogen (N) and phosphorus (P) loads via the Isonzo river, supplemented by minor contributions from atmospheric deposition and urban/industrial wastewater discharge [34].
The GoT hosts several naturalistic areas of high ecological value and that are subject to special protection. At the mouth of the Isonzo river lies the Isonzo Nature Reserve, largely overlapping with the ZSC/ZPS Foce dell’Isonzo–Isola della Cona (IT3330005), which is categorised as a Natura 2000 site by the 92/43/EEC and 79/409/EEC European Directives (http://riservafoceisonzo.it/area-protetta/sito-natura-2000-zsc-zps/) (accessed on 10 February 2025). Moving eastwards, the Duino Cliffs Natural Reserve (https://www.falesiediduino.it/) (accessed on 10 February 2025) and the Miramare Marine Protected Area (SIC IT3340007; https://www.ampmiramare.it/) (accessed on 10 February 2025) are located on the Italian side, and are categorised according to the abovementioned Directives, while on the Slovenian side lies Punta Grossa Nature Park/Krajinski park Debeli Rtič (https://park-debelirtic.si/it/) (accessed on 10 February 2025), Strunjan Cliffs Park (https://parkstrunjan.si/it/) (accessed on 10 February 2025), and Sečovlje Salt Pans Park (https://www.kpss.si/si/intro) (accessed on 10 February 2025).
2.2. Sampling Strategy and Analysis
The phytoplankton were sampled between 2001 and 2022 in the vicinity of the mussel farms located in three distinct areas. From east to west, the first site is situated in the Bay of Muggia (area = 16 ha), followed by the largest site, extending from Grignano to the Bay of Sistiana (area = 150 ha), and, finally, the third site is located in the Gulf of Panzano, off the coast of Duino (area = 37 ha). As a first step, four macro-areas were identified on the basis of their geomorphological, physico-chemical, and meteorological differences: Muggia (A1), Grignano–Santa Croce (A2), Aurisina–Sistiana (A3), and Duino (A4). Specifically, A1 is located in the southernmost part of the Italian sector of the GoT and is generally only minimally influenced by freshwater inflow. Its characteristics, regarding sea currents, weather, climatic conditions, and water column structure, differ significantly from those of A4, which is situated near the mouth of the Isonzo river, a continuous source of freshwater that decreases the salinity and supplies the area with nutrients. A2 and A3 display intermediate characteristics between these two extremes [35].
Ancillary physico-chemical parameters, including temperature, salinity, and the concentration of chlorophyll a, as well as nutrient concentrations, were measured at three sampling sites, namely Duino, Grignano, and Muggia, corresponding to areas A4, A2 + A3, and A1, respectively. The physico-chemical parameters were measured in situ using a CTD multiparameter probe (Idronaut Ocean Seven 316). It is important to note that salinity was measured according to the Practical Salinity Scale, and, as required by the Joint Panel on Oceanographic Tables and Standards, is reported without units or symbols.
Water samples for the nutrient analysis were collected in pre-cleaned (HCl) polyethylene bottles (V = 100 mL). The dissolved inorganic species, including ammonium (N-NH4+), nitrite (N-NO2−), nitrate (N-NO3−), phosphorous (P-PO43−), and silicate (Si-SiO2), were analysed, following filtration (GF/F filters, porosity = 0.45 µM). The total nitrogen (TN) and total phosphorous (TP) were analysed, after persulphate oxidation [36]. Final determinations were carried out using a segmented flow autoanalyser (Autoanalyser II AAII and Analytical QuAAttro, SEAL), following the use of modified Grasshoff methods [37]. Method accuracy was verified for each analytical batch, using certified reference standards and sample spikes (Inorganic Ventures standard solutions and MOOS-2, NRC). Analytical performance was verified by proficiency tests (PTs) organised by the European network of PT providers (QUASIMEME programmes AQ1 and AQ2). The calculated limit of quantification (LOQ) was as follows: 0.025 μM for N-NO2−, 0.71 μM for N-NO3− and TN, 0.05 μM for N-NH4+, 0.01 μM for P-PO43−, and 0,75 µM for Si-SiO2.
Phytoplankton was collected at fortnightly intervals, except in the event of adverse weather conditions or legal restrictions on mussel harvesting, from ten sites belonging to the four study areas. Notably, three different sampling techniques were used. From 2001 to 2005, a Niskin bottle was used; from 2006 to 2016, sampling was conducted using a phytoplankton net; and, most recently, in compliance with European standards (UNI EN 15972:2012), a Lund tube was used to integrate the entire water column. In all cases, the water samples were collected in dark glass bottles (V = 500 mL), fixed with Lugol’s iodine solution (0.5–1.0%) [38], and stored in the dark at T = 4 °C, until the final determination, which was performed according to the Utermöhl method [39].
In this study, rainfall was considered a proxy variable influencing the drivers of primary production and, consequently, the presence of toxic phytoplankton. The rainfall data were obtained from OSMER FVG (Friuli–Venezia Giulia Regional Meteorological Observatory; https://www.osmer.fvg.it/clima.php?ln=) (accessed on 20 January 2025) and were expressed as millimetres of accumulated precipitation, calculated on a decadal basis. Given the high meteorological variability in the GoT, data from multiple rainfall stations located near the mussel farming areas were selected as reference points. Specifically, rainfall data from Fossalon were used for areas A2, A3, and A4, while data from the Trieste–Molo Fratelli Bandiera station were used for area A1.
A corresponding map of the sampling sites is presented in Figure 1.
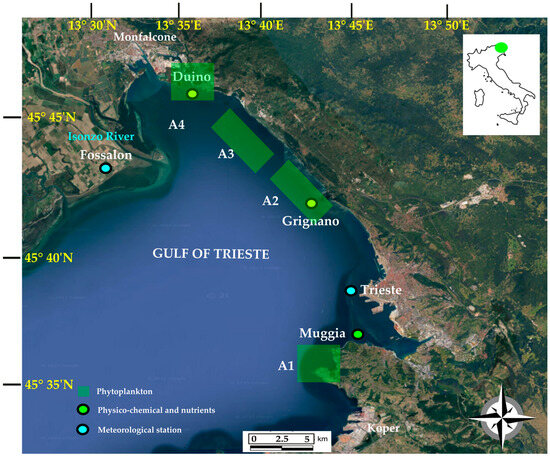
Figure 1.
Map of sampling sites in the Gulf of Trieste (modified from Google Earth®).
2.3. Database Setting and Statistical Analysis
The final dataset comprised approximately 10,900 measurements. Statistical analyses were performed using the following software packages: Excel TM®, PAST 2.17 (Paleontological STatistics) [40], ODV (Ocean Data View, 5.0.0) [41], and R 1.4 (https://www.r-project.org/) (accessed on 10 January 2025).
The spatial distribution and seasonal trends were illustrated using histograms (mean ± standard error). The Shapiro–Wilk test was applied to test data normality [42], while the non-parametric Kruskal–Wallis H test (K-W) [43] was used to evaluate significant differences in the median values. The presence of outliers was assessed using Grubbs’ test [44]. Spearman’s rank correlation coefficients (r) were calculated to examine the monotonic relationships between the variables [45], and the Mann–Kendall trend test (M–K) was employed to assess positive or negative temporal trends in the selected parameters [46]. The significance level for all the tests was set at p < 0.05.
The phytoplankton database was compiled from samples collected over a 20-year period, using different sampling techniques. As a result, preliminary assumptions were required prior to the statistical analysis. The selected phytoplankton species belong to four genera: Alexandrium, Dinophysis, Lingulodinium, and Pseudo-nitzschia. The first three genera are members of the class Dinophyceae, whereas Pseudo-nitzschia is classified within the class Bacillariophyceae [2]. In this study, only genus-level identification was performed during the initial sampling period; species-level identification of toxic taxa became a more prominent focus in subsequent years. Therefore, in order to ensure consistency across the full dataset, the analysis was conducted at the genus level. Considering the variability in sampling methodologies, particularly those involving the Niskin bottle, the values corresponding to different sampling depths were averaged, and phytoplankton counts below 120 cells L−1 were excluded. Moreover, each sampling method was statistically analysed separately to account for methodological differences.
3. Results
3.1. Physico-Chemical Parameters
The water temperature exhibited a wide range of values (3.6–28.6 °C). On average, the highest values were recorded in Duino (mean ± standard error = 16.7 ± 0.1 °C), while the lowest were observed in Grignano (16.0 ± 0.1 °C) (Figure 2). The salinity had an average value of 37.0 ± 0.01, with the lowest value measured in Duino (8.7) and the highest in Muggia (38.8). The chlorophyll a concentrations ranged from <0.20 to 7.36 μg L−1 (mean = 0.94 ± 0.01 μg L−1), with the highest concentration observed in Grignano. A significant difference in terms of the spatial distribution was observed for all the parameters (K-W test: p = 1.4 × 10−11 for temperature, 0 for salinity, and 6.5 × 10−243 for chlorophyll a). Notably, numerous positive outliers were detected for the chlorophyll a concentration, while negative outliers were observed for salinity.
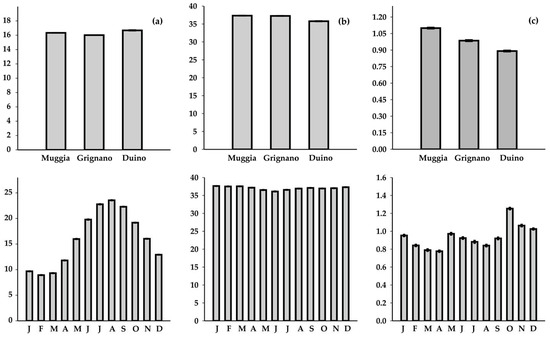
Figure 2.
Spatial variability and monthly trends of physico-chemical parameters (mean ± standard error): (a) water temperature (°C), (b) salinity, and (c) chlorophyll a concentration (µg L−1).
A pronounced and statistically significant monthly variability was observed for all the parameters (K-W: p = 0 for all the variables). On average, temperature exhibited marked seasonal variability, with the minimum value occurring in February (8.93 ± 0.03 °C) and the maximum in August (23.56 ± 0.05 °C). Salinity showed less variation, ranging from 36.11 ± 0.05 in June to 37.64 ± 0.02 in January, with negative outliers distributed homogeneously throughout the year. The chlorophyll a concentrations were characterised by enrichments in late spring (0.97 ± 0.01 µg L−1 in May) and early autumn (1.25 ± 0.01 µg L−1 in October).
The time series analysis revealed significant positive trends for both the temperature and salinity (Table S1). Specifically, the temperature increased between +0.06 °C year−1 and +0.07 °C year−1 depending on the site (average = +0.06 °C year−1) (p = 4 × 10−32). The salinity also exhibited a positive trend (average = +0.01 per year) (p = 2.5 × 10−8), and the highest value was found in Duino (+0.03 per year). In contrast, the chlorophyll a concentration remained quite stable (+0.00 µg L−1), except for a significant increase found in Duino (+0.01 µg L−1; p = 6.3 × 10−5) (Figure 3).
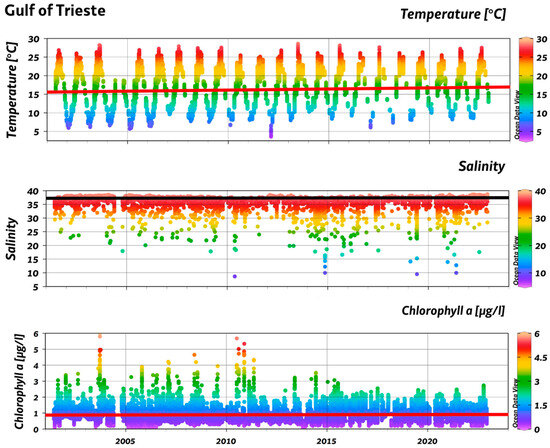
Figure 3.
Time series analysis of temperature, salinity, and chlorophyll a concentration.
3.2. Nutrients
The results of the spatial distribution and monthly variability of the nutrients are reported in Figure 4 and Figure 5. Concentrations of N-NO3− + N-NO2− reached up to 130.93 μM in Duino, which also exhibited the highest mean concentration (19.00 ± 1.16 μM), which is one order of magnitude higher than the mean values recorded in Muggia and Grignano. The ammonium (N-NH4+) concentrations ranged, on average, from 1.47 ± 0.08 in Duino to 1.05 ± 0.08 in Grignano. The spatial distribution of TN mirrored that of N-NO3− + N-NO2−, with an average concentration of 18.74 ± 0.62 µM. Several measurements of P-PO43− and TP were the LOQ (0.01 µM). Nonetheless, P-PO43− was most enriched in Duino (0.14 ± 0.01 µM), while the TP concentrations were fivefold higher, ranging from 0.53 ± 0.04 to 0.60 ± 0.05 in Grignano and Duino, respectively. The distribution of Si-SiO2 followed a similar pattern to the other nutrients, with the highest mean concentration recorded in Duino (17.21 ± 1.19 µM) and the lowest in Muggia (3.74 ± 0.21 µM). All the nutrients, with the exception of TP, exhibited significant spatial variability (K-W: p = 8.1 × 10−59, 3.8 × 10−13, 5.5 × 10−48, 6.1 × 10−7, 0.41, and 8.7 × 10−45 for N-NO3− + N-NO2−, N-NH4+, TN, P-PO43−, TP, and Si-SiO2, respectively), along with the presence of numerous positive outliers.
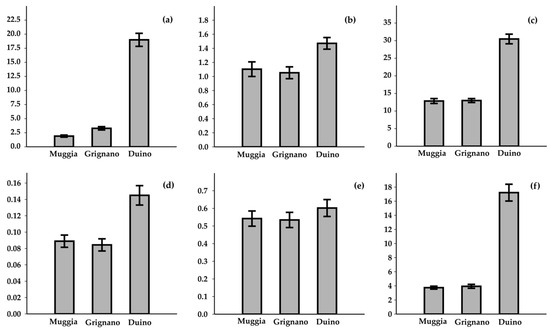
Figure 4.
Spatial variability of nutrient concentrations, expressed as µM l−1: (a) N-NO3− + N-NO2−, (b) N-NH4+, (c) TN, (d) P-PO43−, (e) TP, and (f) Si-SiO2.
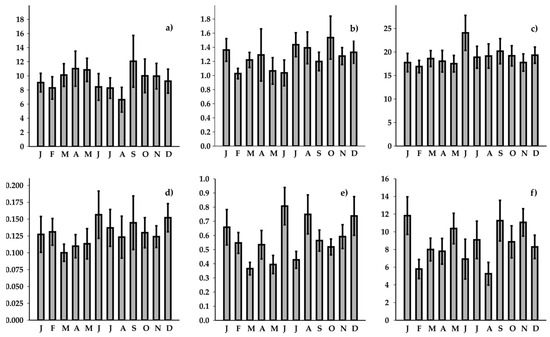
Figure 5.
Monthly trends in nutrient concentrations, expressed as µM: (a) N-NO3− + N-NO2−, (b) N-NH4+, (c) TN, (d) P-PO43−, (e) TP, and (f) Si-SiO2.
The monthly variability patterns revealed typical nutrient enrichments during late spring/early summer and early autumn, consistent across all the nutrients considered. However, no statistically significant differences were detected, with the exception of Si-SiO2 (K-W: p = 0.4377, 0.0821, 0.332, 0.4774, 0.0831, and 5.3 × 10−5, for N-NO3− + N-NO2−, N-NH4+, TN, P-PO43−, TP, and Si-SiO2, respectively). Overall, all the nutrient species exhibited numerous positive outliers.
The elevated concentrations of N compounds relative to P resulted in a marked deviation from the optimal nutrient ratio for phytoplankton growth, as defined by the Redfield ratio (DIN/PO43−), which is typically 16:1 [47]. This imbalance was observed in all of the investigated sites. The mean ratio was 227 (minimum value 1, maximum value 6385). The highest average ratio was recorded in Duino (418), and the lowest in Grignano (58).
The monthly time series analysis revealed both positive and negative trends for the nutrient species. Ammonium (N-NH4+) exhibited a significant increase in late summer and early autumn months. The nitrate (N-NO₃−), total nitrogen (TN), and silicate (Si-SiO2) concentration showed significant positive trends across all the months considered. In contrast, the patterns in terms of phosphorus species were quite different. Orthophosphate (P-PO₄3−) showed no significant trends, except for a positive trend observed in November. Total phosphorus (TP), on the other hand, exhibited a consistent negative trend throughout the year, with the exception of March, April, and December, during which no significant trends were detected (Table S2).
3.3. Toxic Phytoplankton
Among the toxic phytoplankton genera, Pseudo-nitzschia was the most frequently detected, being present in 68.1% of the collected samples, followed by Dinophysis (22.7%), while Lingulodinium was found in only 8.3% of the samples. On average, Pseudo-nitzschia also exhibited the highest abundance (9060 ± 673 cells L−1), with a cell density approximately two orders of magnitude higher than that of Lingulodinium (12 ± 2 cell L−1). The spatial distribution of the toxic genera at the investigated sites is shown in Figure 6.
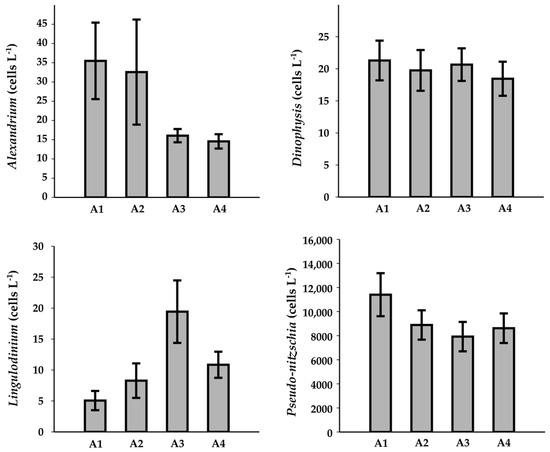
Figure 6.
Spatial variability of toxic phytoplankton in the four selected areas.
It is noteworthy that site A1 exhibited higher concentrations of all the genera compared to the other sites. In particular, Pseudo-nitzschia (mean = 11,403 ± 1787 cells L−1) was predominant, exceeding the abundance of Alexandrium (35 ± 10 cells L−1) and Dinophysis (21 ± 3 cells L−1). Conversely, Lingulodinium showed the highest abundance in site A3 (19 ± 5 cells L−1). Despite these spatial differences, the Kruskal–Wallis test did not indicate any statistically significant differences in the distribution of the genera among the four sites (p = 0.392, 0.064, 0.091, and 0.5302 for Alexandrium, Dinophysis, Lingulodinium, and Pseudo-nitzschia, respectively). The same result was obtained when considering the sampling methods independently (Table S3).
The analysis of monthly variability revealed that high abundances of the genera were primarily found in early spring and late summer/early autumn, a pattern consistent across all the genera. However, some specific differences were reported. Alexandrium exhibited a progressive increase in its monthly abundance from winter to spring, reaching a maximum of 110 ± 39 cells L−1, followed by a decline in summer and a secondary, less pronounced, peak in early autumn (8 ± 3 cells L−1). The monthly patterns of Dinophysis and Pseudo-nitzschia were notably similar, both displaying two distinct peaks: the first, in late winter/early spring (8 ± 3 and 14,114 ± 2736 cells L−1, respectively), and, the second, in early autumn (71 ± 10 and 24,454 ± 3770 cells L−1, respectively). Lingulodinium was almost absent in winter, with peak abundances occurring in late spring and late summer (13 ± 3 and 44 ± 13 cells L−1, respectively) (Figure 7).
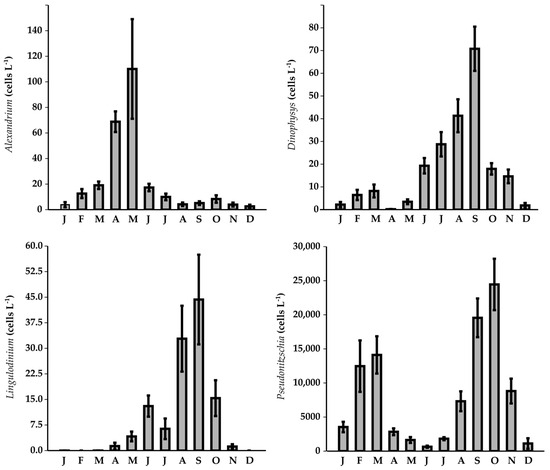
Figure 7.
Monthly abundance of toxic phytoplankton genera.
The analysis of the trends in cell abundance for the four microalgal genera in relation to the different sampling techniques revealed a significant decrease over time exclusively for the genus Lingulodinium when sampled using a Lund tube. In contrast, no significant increasing or decreasing trends were observed over the years for the other genera, regardless of the sampling method applied (Table S4).
3.4. Analysis of Precipitation
Precipitation analysis was conducted by considering the accumulated rainfall (mm), calculated as the sum of daily precipitation occurring during the ten days preceding each sampling event. The maximum value was recorded in October 2015 in Fossalon (177 mm), while in Trieste, the highest value was observed in November 2019 (150 mm). On average, Fossalon experienced greater rainfall impact, with a mean value of 28 ± 34 mm (median = 15 mm), compared to Trieste (22 ± 24 mm). A statistically significant difference between the two sites was identified (K-W: p = 0.031).
3.5. Relationships Between Variables
The occurrence of significant correlations among the investigated parameters, considering the entire study period and the different sampling methods, is presented in Figure 8. Temperature was generally positively correlated with the abundance of toxic phytoplankton at all sites, except for the genus Alexandrium in Muggia. Conversely, temperature showed a negative correlation with salinity, except in Duino. It is noteworthy that the different phytoplankton genera were generally positively correlated with one another. Regarding nutrients, a general positive correlation was observed at all the sites, except for TP, along with a consistent inverse correlation with salinity. The concentration of some nutrient species, particularly nitrogen and silicate, was positively associated with the presence of phytoplankton, whereas TP exhibited a negative correlation, except in the case of Pseudo-nitzschia. P-PO₄3− was positively correlated with the abundance of Lingulodinium in Muggia and, with Dinophysis, when considering the entire study area.
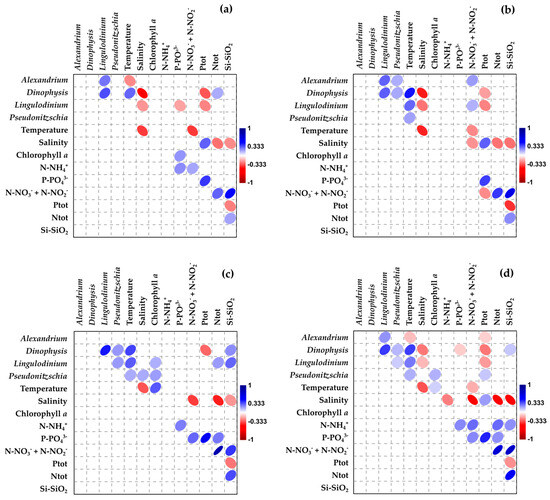
Figure 8.
Spearman’s correlation between physico-chemical parameters, nutrients, and the abundance of toxic phytoplankton: (a) Muggia, (b) Grignano, (c) Duino, and (d) entire study area. The colour of the shapes is proportional to the correlation coefficient (r).
With regard to phytoplankton abundance and their relationship with rainfall, a positive correlation was observed for the genera, Dinophysis and Lingulodinium. This association is graphically illustrated by superimposing the trends in the cell abundance and rainfall data, using the two-year period, 2015–2016, as an example. Notably, the seasonal peaks in these two genera substantially coincide with the periods of increased rainfall (Figure 9). In contrast, no correlation was found between rainfall and the genera, Alexandrium and Pseudo-nitzschia.
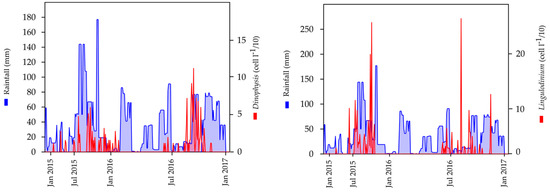
Figure 9.
Trends in rainfall and cell abundance for the genera Dinophysis and Lingulodinium during the 2015–2016 period.
4. Discussion
4.1. Physico-Chemical Parameters and Nutrients
The presence of toxic phytoplankton represents a well-known risk for mussel farming, due to its potential impact on human health [1] and the associated economic losses [48]. These species are a permanent component of the phytoplankton community in both the Mediterranean and Adriatic Sea [49,50,51,52]. However, a comprehensive, long-term investigation of the abundance of toxic phytoplankton and its relationship with physico-chemical and meteorological parameters in the Gulf of Trieste (GoT) has yet to be conducted. This is relevant given the variability and long-term trends observed in phytoplankton communities as a consequence of climate change [53,54,55,56]. For instance, this issue is exemplified by the significant increase in air temperature recorded in Europe (+0.9 °C from 1901 to 2005, with a trend of +0.4 °C decade−1) [57], as well as in the Mediterranean Sea [58].
In this study, the spatial and temporal variability typical of ecological systems [59] was confirmed by the occurrence of numerous anomalies across all the investigated parameters. The water column exhibited a significant increase in temperature of 0.06 °C per year over the period 2001–2022, which is comparable to the findings by Malačič et al. [35] for the GoT, where an increase of approximately 0.1 °C per year in the surface temperature was reported in spring and summer, during the period 1991–2003. A significant temperature gradient was observed, in contrast to the findings by Cossarini et al. [60], who reported that seasonal variability predominates over spatial variability in the northern Adriatic. This is likely attributable to the differing influence of freshwater inputs (e.g., Isonzo river, Karst springs, and minor Slovenian watercourses) across the GoT area [61], which also contributes to the salinity gradient and its year-round fluctuation. The observed increase in salinity was lower than that calculated by Malačič et al. [35], ranging from 0.1 to 0.3 per year, but it should be noted that their values refer to surface waters. The increase in salinity, particularly during the spring–summer period, is possibly related to a reduction in river discharge [62,63], as observed in Duino, which exhibited the lowest average salinity, but the highest increase during the study period.
The chlorophyll a levels indicate an oligotrophic condition, consistent with previous findings by Mozetič et al. [64], who reported similar results, based on field analysis and satellite data collected over the period 1970–2007. High chlorophyll a concentrations were rare, with the 90th percentile reaching only 1.57 µg L−1, as also observed by Lipizer et al. [65]. The trophic status was further supported by the application of the trophic index (TRIX) [66], which ranged from 2.09 to 5.92 (average 3.74 ± 0.62), corresponding to conditions classified from a high to a good state [67]. The time series analysis revealed no significant trends overall, except for a slight increase observed in Duino, similar to findings in the Venice Lagoon and the northern Adriatic, reported by Mozetič et al. and Bernard-Aubry et al. [64,68], respectively. Cozzi et al. [69] reported substantial fluctuations over a longer time series (1986–2018), partially overlapping with the present study. Notably, the highest chlorophyll a values were recorded before 1992, followed by a marked decrease from 1997 to 2009, and a subsequent shift toward oligotrophic conditions up to 2018, consistent with the levels observed in this investigation.
The processes influencing nutrient dynamics in the GoT have been extensively described in the literature, including riverine inputs [29,61,70,71], urban discharges [24,72,73,74], atmospheric deposition [75], and benthic fluxes [76]. In this study, the spatial distribution of nutrients, particularly nitrogen species and silicates, and, to a lesser extent, phosphorus, highlights the key role of the Isonzo river in transporting agricultural runoff from inland soils, both in dissolved and particulate forms. This is supported by the significant positive correlations observed among the nutrient concentrations, along with the negative correlation with salinity. Seasonal patterns showed the lowest nutrient levels during summer and a marked increase in autumn, consistent with trends reported in previous studies involving shorter time series (1999–2006; 1999–2010) [60,65]. Overall, the anomalies in terms of the nutrient concentrations are widespread as consequence of the forces acting in the GoT (i.e., dilution from fluvial inputs, mixing of water column, winds), which are common in coastal areas of the Mediterranean [32,77,78,79,80]. The Mediterranean Sea is recognised as a phosphorous-limited system [81,82,83] and this is also observed in regard to the GoT [65], as confirmed by this long-term survey. The regulatory reduction of polyphosphates in detergents (G.U. no. 178, 1983) has had significant effects since the early 2000s [84,85], a trend that persists and is reflected in the observed decrease in the total phosphorus (TP) concentrations. In contrast, nitrogen species have shown a substantial increase, supporting the findings reported by Giani et al. [34].
4.2. Toxic Phytoplankton
The genera Alexandrium, Dinophysis, Lingulodinium, and Pseudo-nitzschia are typical components of the phytoplankton community in the northern Adriatic Sea [86,87,88,89,90,91], and were periodically detected throughout the two decades assessed in this study, exhibiting genus-level differences consistent with previous findings [4,72,73,74]. Alexandrium typically occurred in late winter and spring, primarily represented by Alexandrium minutum in the southern sector of the GoT [92]. Dinophysis was generally observed between June and October; however, in recent years, its presence has extended into the late autumn–winter period. Francé and Mozetič [4] reported that Dinophysis sacculus is typical of the late spring/early summer period, while D. caudata and D. fortii are more prevalent in late summer and autumn. Similarly, Ninčević-Gladan et al. [93] described the seasonal occurrence of D. acuminata and D. sacculus in spring, D. caudata and D. fortii in summer, and, finally, D. tripos in winter in the central Adriatic. As previously noted [89], Lingulodinium was generally present at low abundances. In the present study, both net and tube sampling proved more effective in detecting this genus [38], allowing for the identification of abundance peaks in May and October, an observation not previously documented. Finally, Pseudo-nitzschia was more abundant in spring and autumn (net and tube collections), consistent with the findings from both the Lim Channel (Istria, Croatia) and the GoT [92,94].
The metabolism of dinoflagellates and diatoms differs markedly, mixotrophic versus obligate autotrophic, respectively, resulting in distinct environmental requirements. Diatoms, such as Pseudo-nitzschia, rely on specific light and nutrient conditions. In the GoT, population peaks are clearly observed when the photoperiod favours photosynthetic activity and nutrient availability is enhanced by rainfall, supporting bloom development [95,96]. In contrast, dinoflagellates exhibit the ability to survive and proliferate during summer, when nutrient concentrations are low, as well as in late autumn, despite the reduced occurrence of photoperiods [88,97,98,99,100,101]. Overall, a significant positive correlation was found among the abundances of the studied genera, suggesting the existence of shared environmental triggers promoting population growth.
No statistically significant increase or decrease in phytoplankton abundance was observed over the study period, with the sole exception of Lingulodinium, which showed a decreasing trend when sampled using the tube method. This is particularly noteworthy, as Lingulodinium is considered a rare genus. Our findings are consistent with those reported by Zingone et al. [101] for the Adriatic Sea, who found no specific trends in the abundance or frequency of toxic species. However, the same authors did observe a sustained increase in species richness, marked by the continuous emergence of other toxic species.
4.3. Correlation of Toxic Phytoplankton with Physico-Chemical Parameters, Nutrients, and Rainfall
The diversity and dynamics of phytoplankton populations are influenced by a wide range of complex processes, involving both abiotic and biotic factors. Among the biotic drivers, predation pressure on primary producers and viral infections have been identified as key contributors to the structuring of the phytoplankton community [90].
In general, the trophic state of a coastal system tends to favour the presence of toxic phytoplankton species, as nutrients promote their growth [102]. However, considerable variability exists depending on the site, time, and species, which can lead to deviations from this general pattern. As expected, temperature showed a positive effect on phytoplankton growth, whereas salinity was inversely correlated, reflecting the role of freshwater as the primary source of nutrients in the GoT. The abundance of Dinophysis was negatively correlated with TP, while TN and Si-SiO2 were positively associated. Due to its mixotrophic metabolism, it may be hypothesised that Dinophysis benefit indirectly from nutrient enrichment via the stimulation of its prey, rather than through the direct uptake of bioavailable nutrients [103,104,105].
Lingulodinium also exhibited a negative correlation with TP, consistent with the findings by Domingues and Lima [106], who reported an inverse relationship between the proliferation of Lingulodinium polyedra and the phosphorus concentration in the Ria Formosa Lagoon (Portugal). Conversely, positive correlations were observed between Lingulodinium and both nitrogen species and Si-SiO2.
Pseudo-nitzschia showed a positive correlation with TP, and several studies have also highlighted associations with both organic and inorganic nitrogen in various environments [107,108], but not with silicate, which is essential for frustule formation. However, no clear correlation was observed with silicate, despite its essential role in frustule formation. A lack of correlation between silicate concentrations and diatom abundance may occur when silicate is in excess and no longer a limiting factor, allowing other environmental variables to play a more influential role [109,110]. Additionally, in oligotrophic conditions, limited nutrient availability, particularly phosphorus, can constrain diatom growth, even when the amount of silicate is sufficient [111].
Finally, Alexandrium was positively correlated with dissolved nitrogen at the Grignano site only. These results support the hypothesis that toxic microalgae often exhibit low affinity for the direct uptake of inorganic nitrogen and phosphorus and frequently rely on mixotrophic strategies [96,110,112]. Some authors have also suggested that these microalgae are capable of adapting to limited phosphorus and nitrogen concentrations [113].
A significant positive correlation between the toxic phytoplankton abundance and decadal cumulative rainfall was observed for the genera Dinophysis and Lingulodinium, whereas no such relationship was found for Alexandrium and Pseudo-nitzschia. Ninčević-Gladan et al. [93] reported that among the Dinophysis species generally associated with high temperatures, such as D. caudate, D. fortii, D. acuta, and D. tripos, they are instead linked to elevated precipitation. In the northern Adriatic, intense rainfall events combined with high temperatures have been shown to trigger the proliferation of D. fortii during summer and late summer [93], with a frequency greater than that observed in the southern Adriatic basin [114]. The same authors also reported a correlation between precipitation and the genus Pseudo-nitzschia, particularly during the colder months; however, this was not confirmed in the present study.
Similarly, Alexandrium has been linked to freshwater inputs and conditions involving high water column stability [93], which are typically associated with prolonged water residence times and elevated nutrient availability [115]. Dinoflagellates are well-adapted to relatively calm, stratified water column conditions. Their ability for active movement allows them to remain within the euphotic zone and to access deeper, nutrient-rich layers when surface nutrients are depleted [116]. Nevertheless, Weise et al. [117] observed a positive correlation between Alexandrium tamarense blooms and precipitation events, along with other environmental factors, such as temperature, salinity, river discharge, and wind. They concluded that bloom development depends largely on the persistence of favourable conditions.
Phytoplankton distribution is influenced by biological interactions, such as zooplankton grazing, and is further modulated by hydrodynamic factors, including local circulation patterns and prevailing currents. Previous studies conducted in the Gulf of Trieste (GoT) have generally reported a negligible impact of zooplankton grazing on nutrient-enriched phytoplankton assemblages [118,119]. Unfortunately, no supporting data were available in this study, such as measurements of the current intensity and prevailing current direction, thereby limiting a more detailed interpretation.
4.4. Toxic Phytoplankton and Sampling Techniques
The use of different sampling techniques posed a challenge in terms of the comparability of phytoplankton abundance across different time periods. To address this limitation, the data were initially treated separately. Nonetheless, this apparent constraint provided an opportunity to evaluate and compare the efficiency of the three sampling methods employed.
The Niskin bottle method presents limitations in regard to representing low-density phytoplankton species. Additionally, some species exhibit vertical migration within the water column, as reported by Francé and Mozetič, who demonstrated the ability of Dinophysis sacculus to migrate from surface layers to the thermocline. As a result, sampling with the Niskin bottle may underestimate the actual phytoplankton abundance.
Conversely, the Niskin method proved to be the most suitable for assessing correlations between phytoplankton abundance and the physico-chemical parameters and nutrients, whereas the net and tube methods were less effective in this context. The phytoplankton net offers the advantage of concentrating the samples, as evidenced by the successful detection of Lingulodinium, a genus typically found at very low densities. The tube method represents an improvement, as it integrates the entire water column, thereby enabling a more accurate qualitative and quantitative representation of the phytoplankton community.
5. Conclusions and Future Perspectives
The relationship between phytoplankton and the climate is a highly topical issue, as noted by Henson et al. [55], who emphasised that climate change may alter phytoplankton diversity by accelerating community turnover, thereby increasing instability and reducing ecosystem resilience. This presents a considerable challenge for the development of reliable long-term predictive models, particularly concerning the behaviour of potentially toxic dinoflagellates, such as Dinophysis. As a predominantly opportunistic genus, its environmental interactions during periods of widespread toxicity remain difficult to interpret [120].
One major concern relates to the unpredictability of mussel trade bans, which have varied significantly over time. For instance, in the Slovenian sector of the GoT, no clear annual pattern of trade bans was observed prior to 2012, with restrictions occurring throughout the year, although more frequently in summer and autumn. In contrast, from 2013 onwards, trade bans consistently occurred during late summer and autumn (1999–2019 period) [14]. The authors concluded that the observed interannual variability in toxicity and phytoplankton dynamics, potentially exacerbated by future climatic and anthropogenic stressors, underscores the need for more efficient and adaptive surveillance and monitoring strategies.
To date, following the issuance of EU Regulation (EU, 2019/627), in Article 61, namely “Sampling plans”, it is reported that: “Sampling plans to check for the presence of toxin-producing plankton in the water in classified production and relaying areas and for marine biotoxins in live bivalve molluscs shall take particular account of possible variations in the presence of plankton containing marine biotoxins”. The planned actions can be summarised as follows: periodic sampling to monitor changes in the composition and distribution of toxic phytoplankton, followed by intensified sampling and periodic toxicity tests on live bivalve molluscs. During harvesting periods, toxin analysis must be performed weekly, unless a risk assessment indicates a very low probability of toxic episodes. If the assessment suggests that weekly sampling is insufficient, the frequency must be increased. If the monitoring results indicate that health standards are not being met or that there is a risk to human health, the competent authorities must close the affected classified production or relaying area. Reopening is allowed only when toxin levels fall below regulatory thresholds and phytoplankton trends are considered.
This study suggests that rainfall and salinity are valuable proxies for developing early warning models to predict conditions affecting mussel farming. Incorporating nutrient data may improve model accuracy. Validating these relationships could support the integration of weather forecasts with trophic status and phytoplankton seasonality, enabling proactive monitoring. This approach aligns with the study by Boivin-Rioux et al. [121], which successfully used variations in salinity and temperature to predict the occurrence of the potentially toxic species, Alexandrium catenella. More recently, explainable machine learning techniques were applied to forecast mussel toxicity in the Slovenian sector of the GoT, using a 28-year dataset of toxic phytoplankton records from mussel-farming areas [122]. Salinity, river discharge, and precipitation emerged as the strongest predictors of diarrhetic shellfish poisoning (DSP) toxin levels exceeding regulatory thresholds.
Building on the findings in this study, future research should pursue more integrated and predictive approaches to better understand the dynamics of toxic phytoplankton in coastal environments. In particular, the following directions are recommended:
- -
- Implementing continuous, in situ measurements (e.g., automated sensors for monitoring the temperature, salinity, chlorophyll a concentration, and turbidity) would enable the real-time detection of environmental shifts that precede bloom events;
- -
- Developing and calibrating hydrodynamic models integrated with phytoplankton dynamics can improve forecasts on bloom formation, spread, and persistence. The adoption of machine learning algorithms, particularly explainable models, should be further explored to enhance transparency and stakeholder confidence in early warning systems;
- -
- Long-term, cross-border collaboration in regard to data collection and the harmonisation of sampling protocols will be critical to ensure comparability and broader applicability of predictive tools;
- -
- Interdisciplinary studies that include economic risk assessments, especially for the aquaculture sector, can support evidence-based decision making and adaptive management under future climate scenarios.
These perspectives aim to bridge scientific research with practical applications, offering tools to anticipate and mitigate the impacts of harmful algal blooms in the context of increasing environmental variability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12050152/s1, Table S1: Univariate statistics of physico-chemical parameters. Table S2: Monthly temporal trends in nutrient concentrations. Table S3: Statistical test of significant differences in phytoplankton abundance by sampling area and collection method. Table S4: Temporal trends in phytoplankton abundance by genus and collection method.
Author Contributions
Conceptualisation, L.T., O.B., N.B. and A.A.; methodology, L.T. and O.B.; software, L.T. and A.A.; validation, L.T., O.B., A.A. and M.C.; investigation, O.B. and A.A.; data curation, L.T., O.B., N.B., M.C. and A.A.; writing—original draft preparation, A.A.; writing—review and editing, L.T., O.B., N.B., M.C. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are partially openly available via the following website: https://www.dati.friuliveneziagiulia.it/Ambiente/Acqua-Acque-di-classificazione-Superficiali-marino/qcsf-bwk5/about_data, accessed on 3 March 2025. Further requests should be submitted to urp@arpa.fvg.it and to the corresponding author.
Acknowledgments
The authors kindly acknowledge Bruno Zanolin for his support in the collection of phytoplankton data, as well as our colleagues from the microbiology laboratory for their analysis of toxic phytoplankton. We also extend our sincere thanks to all the staff at ARPA FVG involved in the sampling operations. The valuable suggestions provided by the two anonymous reviewers are gratefully acknowledged and have significantly contributed to the improvement of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Lassus, P.; Chomérat, N.; Hess, P.; Nézan, E. Toxic and Harmful Microalgae of the World Ocean/Micro-Algues Toxiques et Nuisibles de l’océan Mondial; Manuals and guides/IOC; International Society for the Study of Harmful Algae: Copenhagen, Denmark, 2016; ISBN 978-87-990827-6-6. [Google Scholar]
- Hasle, G.R.; Syvertsen, E.E.; Steidinger, K.A.; Tangen, K.; Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Valbi, E.; Ricci, F.; Capellacci, S.; Casabianca, S.; Scardi, M.; Penna, A. A Model Predicting the PSP Toxic Dinoflagellate Alexandrium Minutum Occurrence in the Coastal Waters of the NW Adriatic Sea. Sci. Rep. 2019, 9, 4166. [Google Scholar] [CrossRef] [PubMed]
- France, J.; Mozetič, P. Ecological Characterization of Toxic Phytoplankton Species (Dinophysis Spp., Dinophyceae) in Slovenian Mariculture Areas (Gulf of Trieste, Adriatic Sea) and the Implications for Monitoring. Mar. Pollut. Bull. 2006, 52, 1504–1516. [Google Scholar] [CrossRef]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobó, P.; Franco, J.M.; Fernández, J.J. Yessotoxins, a Group of Marine Polyether Toxins: An Overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-Nitzschia, Nitzschia, and Domoic Acid: New Research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Pushparaj, S.S.C.; Muthu, M.; Gopal, J. Review of Harmful Algal Blooms (HABs) Causing Marine Fish Kills: Toxicity and Mitigation. Plants 2023, 12, 3936. [Google Scholar] [CrossRef]
- Alvarez, S.; Brown, C.E.; Garcia Diaz, M.; O’Leary, H.; Solís, D. Non-Linear Impacts of Harmful Algae Blooms on the Coastal Tourism Economy. J. Environ. Manag. 2024, 351, 119811. [Google Scholar] [CrossRef]
- Denis, L. Clearance Rate Responses of Mediterranean Mussels, Mytilus Galloprovincialis, to Variations in the Flow, Water Temperature, Food Quality and Quantity. Aquat. Living Resour. 1999, 12, 279–288. [Google Scholar] [CrossRef]
- Curpan, A.-S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Review: Mytilus Galloprovincialis: An Essential, Low-Cost Model Organism for the Impact of Xenobiotics on Oxidative Stress and Public Health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef]
- Melaku Canu, D.; Solidoro, C. Socio-Economic Analysis and Stakeholder Involvement: Mussel-Farming in the Gulf of Trieste. Mar. Policy 2014, 43, 55–62. [Google Scholar] [CrossRef]
- Solidoro, C.; Del Negro, P.; Mosetti, R.; De Walderstein, W.; Gomes Ferreira, J.; Zentilin, A.; Bricelj, A.; Beran, A.; Libralato, S.; Melaku Canu, D.; et al. Sostenibilità Della Mitilicoltura Triestina; Istituto Nazionale di Oceanografia e di Geofisica Sperimentale: Borgo Grotta Gigante, Italy, 2010. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls. Off. J. Eur. Union 2019, 131, 51–100. [Google Scholar]
- Henigman, U.; Mozetič, P.; Francé, J.; Knific, T.; Vadnjal, S.; Dolenc, J.; Kirbiš, A.; Biasizzo, M. Okadaic Acid as a Major Problem for the Seafood Safety (Mytilus Galloprovincialis) and the Dynamics of Toxic Phytoplankton in the Slovenian Coastal Sea (Gulf of Trieste, Adriatic Sea). Harmful Algae 2024, 135, 102632. [Google Scholar] [CrossRef] [PubMed]
- Karydis, M. Toxic Phytoplankton in Eutrophic Regional Seas: An Overview. Glob. NEST J. 2023, 25, 178–211. [Google Scholar] [CrossRef]
- Glibert, P.M. Harmful Algae at the Complex Nexus of Eutrophication and Climate Change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and Perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Heneash, A.M.M.; Tadrose, H.R.Z.; Hussein, M.M.A.; Hamdona, S.K.; Abdel-Aziz, N.; Gharib, S.M. Potential Effects of Abiotic Factors on the Abundance and Distribution of the Plankton in the Western Harbour, South-Eastern Mediterranean Sea, Egypt. Oceanologia 2015, 57, 61–70. [Google Scholar] [CrossRef]
- Drakulovic, D.; Gvozdenovic, S.; Joksimovic, D.; Mandic, M.; Pestoric, B. Toxic and Potentially Toxic Phytoplankton in the Mussel and Fish Farms in the Transitional Area of Montenegrin Coast (South-Eastern Adriatic Sea). Turk. J. Fish. Aquat. Sci. 2017, 17, 885–900. [Google Scholar] [CrossRef]
- Tamvakis, A.; Tsirtsis, G.; Karydis, M.; Patsidis, K.; Kokkoris, G.D. Drivers of Harmful Algal Blooms in Coastal Areas of Eastern Mediterranean: A Machine Learning Methodological Approach. Math. Biosci. Eng. 2021, 18, 6484–6505. [Google Scholar] [CrossRef]
- Tsikoti, C.; Genitsaris, S. Review of Harmful Algal Blooms in the Coastal Mediterranean Sea, with a Focus on Greek Waters. Diversity 2021, 13, 396. [Google Scholar] [CrossRef]
- Karydis, M.; Kitsiou, D. Eutrophication and Environmental Policy in the Mediterranean Sea: A Review. Environ. Monit. Assess. 2012, 184, 4931–4984. [Google Scholar] [CrossRef]
- Olivotti, R.; Faganeli, J.; Malej, A. Eutrophication of Coastal Waters–Gulf of Trieste. Water Sci. Technol. 1986, 18, 303–316. [Google Scholar] [CrossRef]
- Cozzi, S.; Mistaro, A.; Sparnocchia, S.; Colugnati, L.; Bajt, O.; Toniatti, L. Anthropogenic Loads and Biogeochemical Role of Urea in the Gulf of Trieste. Sci. Total Environ. 2014, 493, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Malej, A.; Malacic, V. Factors Affecting Bottom Layer Oxygen Depletion in the Gulf of Trieste(Adriatic Sea). Ann. An. Istrske Mediter. Stud. (Hist. Nat.) 1995, 6, 33–42. [Google Scholar]
- Comici, C.; Bussani, A. Analysis of the River Isonzo Discharge (1998–2005). Boll. Geofis. Teor. Appl. 2007, 48, 435–454. [Google Scholar]
- Cozzi, S.; Giani, M. River Water and Nutrient Discharges in the Northern Adriatic Sea: Current Importance and Long Term Changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Turk, V.; Mozetic, P.; Malej, A. Overview of Eutrophication-Related Events and Other Irregular Episodes in Slovenian Sea (Gulf of Trieste, Adriatic Sea). In Annales: Series Historia Naturalis; Scientific and Research Center of the Republic of Slovenia: Koper, Slovenia, 2007; Volume 17, p. 197. [Google Scholar]
- Malej, A.; Mozetic, P.; Malacic, V.; Terzic, S.; Ahel, M. Phytoplankton Responses to Freshwater Inputs in a Small Semi-Enclosed Gulf (Gulf of Trieste, Adriatic Sea). Mar. Ecol. Prog. Ser. 1995, 120, 111–121. [Google Scholar] [CrossRef]
- Stravisi, F. Some Characteristics of the Circulation in the Gulf of Trieste. Thalass. Jugosl. 1983, 19, 355–363. [Google Scholar]
- Stravisi, F. The Vertical Structure Annual Cycle of the Mass Field Parameters in the Gulf of Trieste. Boll. Oceanogr. Teor. Appl. 1983, 3, 239–250. [Google Scholar]
- Boldrin, A.; Carniel, S.; Giani, M.; Marini, M.; Bernardi Aubry, F.; Campanelli, A.; Grilli, F.; Russo, A. Effects of Bora Wind on Physical and Biogeochemical Properties of Stratified Waters in the Northern Adriatic. J. Geophys. Res. Ocean. 2009, 114, C08S92. [Google Scholar] [CrossRef]
- Pagano, M.; Fernetti, M.; Busetti, M.; Ghribi, M.; Camerlenghi, A. Multicriteria GIS-Based Analysis for the Evaluation of the Vulnerability of the Marine Environment in the Gulf of Trieste (North-Eastern Adriatic Sea) for Sustainable Blue Economy and Maritime Spatial Planning. People Nat. 2023, 5, 2006–2025. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent Changes in the Marine Ecosystems of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Malačič, V.; Celio, M.; Čermelj, B.; Bussani, A.; Comici, C. Interannual Evolution of Seasonal Thermohaline Properties in the Gulf of Trieste (Northern Adriatic) 1991–2003. J. Geophys. Res. 2006, 111, 2005JC003267. [Google Scholar] [CrossRef]
- Valderrama, J.C. The Simultaneous Analysis of Total Nitrogen and Total Phosphorus in Natural Waters. Mar. Chem. 1981, 10, 109–122. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Zingone, A.; Totti, C.; Sarno, D.; Cabrini, M.; Caroppo, C.; Giacobbe, M.; Lugliè, A.; Nuccio, C.; Socal, G. CAPITOLO 21. FITOPLANCTON: METODICHE DI ANALISI QUALI-QUAN-TITATIVA. In Metodologie di Studio del Plancton Marino; ISPRA: Rome, Italy, 2010. [Google Scholar]
- Uthermöhl, H. On the Perfecting of Quantitative Phytoplankton Method. Int. Assoc. Theor. Appl. Limnol. Commun. 1958, 9, 1–38. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Schlitzer, R.; Anderson, R.; Dodas, E.; Lohan, M.; Geibert, W.; Tagliabue, A.; Schlitzer, R.; Cullen, J.T.; Janssen, D.J.; Velazquez, S.; et al. UVicSPACE: Research & Learning Repository. Chem. Geol. 2018, 493, 210–223. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson Education India: Noida, India, 1999. [Google Scholar]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations; University of Michigan: Ann Arbor, MI, USA, 1949. [Google Scholar]
- Press, W.; Teukolsky, S.; Vetterling, W.; Flannery, B. Numerical Recipes in C, 2nd ed.; Cambridge University Press: New York, NY, USA, 1992. [Google Scholar]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Redfield, A.C. The Biological Control of Chemical Factors in the Environment. Sci. Prog. 1960, 11, 150–170. [Google Scholar]
- Ragkos, A.; Skordos, D.; Koutouzidou, G.; Giantsis, I.A.; Delis, G.; Theodoridis, A. Socioeconomic Appraisal of an Early Prevention System against Toxic Conditions in Mussel Aquaculture. Animals 2022, 12, 2832. [Google Scholar] [CrossRef]
- Socal, G.; Boldrin, A.; Bianchi, F.; Civitarese, G.; De Lazzari, A.; Rabitti, S.; Totti, C.; Turchetto, M.M. Nutrient, Particulate Matter and Phytoplankton Variability in the Photic Layer of the Otranto Strait. J. Mar. Syst. 1999, 20, 381–398. [Google Scholar] [CrossRef]
- Orsini, L.; Sarno, D.; Procaccini, G.; Poletti, R.; Dahlmann, J.; Montresor, M. Toxic Pseudo-Nitzschia Multistriata (Bacillariophyceae) from the Gulf of Naples: Morphology, Toxin Analysis and Phylogenetic Relationships with Other Pseudo-Nitzschia Species. Eur. J. Phycol. 2002, 37, 247–257. [Google Scholar] [CrossRef]
- Quiroga, I. Pseudo-Nitzschia Blooms in the Bay of Banyuls-Sur-Mer, Northwestern Mediterranean Sea. Diatom Res. 2006, 21, 91–104. [Google Scholar] [CrossRef]
- Bosak, S.; Burić, Z.; Djakovac, T.; Viličić, D. Seasonal Distribution of Plankton Diatoms in Lim Bay, Northeastern Adriatic Sea. Acta Bot. Croat. 2009, 68, 351–365. [Google Scholar]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful Algal Blooms and Climate Change: Learning from the Past and Present to Forecast the Future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M. Toxicological Perspective on Climate Change: Aquatic Toxins. Chem. Res. Toxicol. 2016, 29, 619–625. [Google Scholar] [CrossRef]
- Henson, S.A.; Cael, B.B.; Allen, S.R.; Dutkiewicz, S. Future Phytoplankton Diversity in a Changing Climate. Nat. Commun. 2021, 12, 5372. [Google Scholar] [CrossRef]
- Edullantes, B.; Low-Decarie, E.; Steinke, M.; Cameron, T. Comparison of Thermal Traits between Non-Toxic and Potentially Toxic Marine Phytoplankton: Implications to Their Responses to Ocean Warming. J. Exp. Mar. Biol. Ecol. 2023, 562, 151883. [Google Scholar] [CrossRef]
- Alcamo, J.; Olesen, J.E. Life in Europe Under Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Marullo, S.; Artale, V.; Santoleri, R. The SST Multidecadal Variability in the Atlantic–Mediterranean Region and Its Relation to AMO. J. Clim. 2011, 24, 4385–4401. [Google Scholar] [CrossRef]
- Hastings, A. Timescales, Dynamics, and Ecological Understanding. Ecology 2010, 91, 3471–3480. [Google Scholar] [CrossRef]
- Cossarini, G.; Solidoro, C.; Umani, S.F. Dynamics of Biogeochemical Properties in Temperate Coastal Areas of Freshwater Influence: Lessons from the Northern Adriatic Sea (Gulf of Trieste). Estuar. Coast. Shelf Sci. 2012, 115, 63–74. [Google Scholar] [CrossRef]
- Cozzi, S.; Falconi, C.; Comici, C.; Čermelj, B.; Kovac, N.; Turk, V.; Giani, M. Recent Evolution of River Discharges in the Gulf of Trieste and Their Potential Response to Climate Changes and Anthropogenic Pressure. Estuar. Coast. Shelf Sci. 2012, 115, 14–24. [Google Scholar] [CrossRef]
- Zanchettin, D.; Traverso, P.; Tomasino, M. Po River Discharges: A Preliminary Analysis of a 200-Year Time Series. Clim. Change 2008, 89, 411–433. [Google Scholar] [CrossRef]
- Solidoro, C.; Bastianini, M.; Bandelj, V.; Codermatz, R.; Cossarini, G.; Melaku Canu, D.; Ravagnan, E.; Salon, S.; Trevisani, S. Current State, Scales of Variability, and Trends of Biogeochemical Properties in the Northern Adriatic Sea. J. Geophys. Res. Ocean. 2009, 114, C07S91. [Google Scholar] [CrossRef]
- Mozetič, P.; Solidoro, C.; Cossarini, G.; Socal, G.; Precali, R.; Francé, J.; Bianchi, F.; De Vittor, C.; Smodlaka, N.; Fonda Umani, S. Recent Trends Towards Oligotrophication of the Northern Adriatic: Evidence from Chlorophyll a Time Series. Estuaries Coasts 2010, 33, 362–375. [Google Scholar] [CrossRef]
- Lipizer, M.; De Vittor, C.; Falconi, C.; Comici, C.; Tamberlich, F.; Giani, M. Effects of Intense Physical and Biological Forcing Factors on CNP Pools in Coastal Waters (Gulf of Trieste, Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2012, 115, 40–50. [Google Scholar] [CrossRef]
- Vollenweider, R.; Giovanardi, F.; Montanari, G.; Rinaldi, A. Characterization of the Trophic Conditions of Marine Coastal Waters with Special Reference to the NW Adriatic Sea: Proposal for a Trophic Scale, Turbidity and Generalized Water Quality Index. Environmetrics Off. J. Int. Environmetrics Soc. 1998, 9, 329–357. [Google Scholar] [CrossRef]
- Pettine, M.; Casentini, B.; Fazi, S.; Giovanardi, F.; Pagnotta, R. A Revisitation of TRIX for Trophic Status Assessment in the Light of the European Water Framework Directive: Application to Italian Coastal Waters. Mar. Pollut. Bull. 2007, 54, 1413–1426. [Google Scholar] [CrossRef]
- Aubry, F.B.; Cossarini, G.; Acri, F.; Bastianini, M.; Bianchi, F.; Camatti, E.; De Lazzari, A.; Pugnetti, A.; Solidoro, C.; Socal, G. Plankton Communities in the Northern Adriatic Sea: Patterns and Changes over the Last 30 Years. Estuar. Coast. Shelf Sci. 2012, 115, 125–137. [Google Scholar] [CrossRef]
- Cozzi, S.; Cabrini, M.; Kralj, M.; De Vittor, C.; Celio, M.; Giani, M. Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea). Water 2020, 12, 2652. [Google Scholar] [CrossRef]
- Cantoni, C.; Cozzi, S.; Pecchiar, I.; Cabrini, M.; Mozetič, P.; Catalano, G.; Umani, S.F. Short-Term Variability of Primary Production and Inorganic Nitrogen Uptake Related to the Environmental Conditions in a Shallow Coastal Area (Gulf of Trieste, N Adriatic Sea). Oceanol. Acta 2003, 26, 565–575. [Google Scholar] [CrossRef]
- Mozetič, P.; Francé, J.; Kogovšek, T.; Talaber, I.; Malej, A. Plankton Trends and Community Changes in a Coastal Sea (Northern Adriatic): Bottom-up vs. Top-down Control in Relation to Environmental Drivers. Estuar. Coast. Shelf Sci. 2012, 115, 138–148. [Google Scholar] [CrossRef]
- Cozzi, S.; Reisenhofer, E.; Di Monte, L.; Cantoni, C.; Adami, G. Effect of Environmental Forcing on the Fate of Nutrients, Dissolved Organic Matter and Heavy Metals Released by a Coastal Wastewater Pipeline. Chem. Ecol. 2008, 24, 87–107. [Google Scholar] [CrossRef]
- Mozetič, P.; Malačič, V.; Turk, V. A Case Study of Sewage Discharge in the Shallow Coastal Area of the Northern Adriatic Sea (Gulf of Trieste). Mar. Ecol. 2008, 29, 483–494. [Google Scholar] [CrossRef]
- Scroccaro, I.; Ostoich, M.; Umgiesser, G.; De Pascalis, F.; Colugnati, L.; Mattassi, G.; Vazzoler, M.; Cuomo, M. Submarine Wastewater Discharges: Dispersion Modelling in the Northern Adriatic Sea. Environ. Sci. Pollut. Res. 2010, 17, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Malej, A.; MozetiČ, P.; MalaČČ, V.; Turk, V. Response of Summer Phytoplankton to Episodic Meteorological Events (Gulf of Trieste, Adriatic Sea). Mar. Ecol. 1997, 18, 273–288. [Google Scholar] [CrossRef]
- Faganeli, J.; Ogrinc, N. Oxic–Anoxic Transition of Benthic Fluxes from the Coastal Marine Environment (Gulf of Trieste, Northern Adriatic Sea). Mar. Freshw. Res. 2009, 60, 700–711. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agustı, S.; Kennedy, H.; Vaqué, D. The Mediterranean Climate as a Template for Mediterranean Marine Ecosystems: The Example of the Northeast Spanish Littoral. Prog. Oceanogr. 1999, 44, 245–270. [Google Scholar] [CrossRef]
- Gremare, A.; Amouroux, J.; Cauwet, G.; Charles, F.; Courties, C.; de Bovee, F.; Dinet, A.; Devenon, J.-L.; Durrieu de Madron, X.; Ferre, B.; et al. Effets d’une Forte Tempête Hivernale Sur Les Variables Physiques et Biologiques à Une Station Côtière Méditerranéenne. Oceanol. Acta 2003, 26, 407–419. [Google Scholar]
- De Vittor, C.; Paoli, A.; Umani, S.F. Dissolved Organic Carbon Variability in a Shallow Coastal Marine System (Gulf of Trieste, Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2008, 78, 280–290. [Google Scholar] [CrossRef]
- Guadayol, Ò.; Peters, F.; Marrasé, C.; Gasol, J.M.; Roldán, C.; Berdalet, E.; Massana, R.; Sabata, A. Episodic Meteorological and Nutrient-Load Events as Drivers of Coastal Planktonic Ecosystem Dynamics: A Time-Series Analysis. Mar. Ecol. Prog. Ser. 2009, 381, 139–155. [Google Scholar] [CrossRef]
- Lipizer, M.; Cozzi, S.; Catalano, G.; Falconi, C. Seasonal Fluctuations of DIN/DIP and DON/DOP Ratio in the Northern Adriatic Sea. Ann. Dell’istituto Super. Di Sanità 1999, 35, 383–388. [Google Scholar]
- Gismondi, M.; Giani, M.; Savelli, F.; Boldrin, A.; Rabitti, S. Particulate Organic Matter in the Northern and Central Adriatic. Chem. Ecol. 2002, 18, 27–38. [Google Scholar] [CrossRef]
- Lucea, A.; Duarte, C.M.; Agustí, S.; Kennedy, H. Nutrient Dynamics and Ecosystem Metabolism in the Bay of Blanes (NW Mediterranean). Biogeochemistry 2005, 73, 303–323. [Google Scholar] [CrossRef]
- Degobbis, D.; Precali, R.; Ivancic, I.; Smodlaka, N.; Fuks, D.; Kveder, S. Long-Term Changes in the Northern Adriatic Ecosystem Related to Anthropogenic Eutrophication. Int. J. Environ. Pollut. 2000, 13, 495–533. [Google Scholar] [CrossRef]
- Degobbis, D.; Precali, R.; Ferrari, C.R.; Djakovac, T.; Rinaldi, A.; Ivančić, I.; Gismondi, M.; Smodlaka, N. Changes in Nutrient Concentrations and Ratios during Mucilage Events in the Period 1999–2002. Sci. Total Environ. 2005, 353, 103–114. [Google Scholar] [CrossRef]
- Mozetic, P.; Umani, S.F.; Kamburska, L. Plankton Variability in the Gulf of Trieste (Northern Adriatic). Archo Oceanogr. Limnol. 2002, 23, 7–19. [Google Scholar]
- Viličić, D.; Marasović, I.; Mioković, D. Checklist of Phytoplankton in the Eastern Adriatic Sea. Acta Bot. Croat. 2002, 61, 57–91. [Google Scholar]
- Cabrini, M.; Fornasaro, D.; Cossarini, G.; Lipizer, M.; Virgilio, D. Phytoplankton Temporal Changes in a Coastal Northern Adriatic Site during the Last 25 Years. Estuar. Coast. Shelf Sci. 2012, 115, 113–124. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin Levels and Profiles in Microalgae from the North-Western Adriatic Sea—15 Years of Studies on Cultured Species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef]
- Cerino, F.; Fornasaro, D.; Kralj, M.; Giani, M.; Cabrini, M. Phytoplankton Temporal Dynamics in the Coastal Waters of the North-Eastern Adriatic Sea (Mediterranean Sea) from 2010 to 2017. Nat. Conserv. 2019, 34, 343–372. [Google Scholar] [CrossRef]
- Totti, C.; Romagnoli, T.; Accoroni, S.; Coluccelli, A.; Pellegrini, M.; Campanelli, A.; Grilli, F.; Marini, M. Phytoplankton Communities in the Northwestern Adriatic Sea: Interdecadal Variability over a 30-Years Period (1988–2016) and Relationships with Meteoclimatic Drivers. J. Mar. Syst. 2019, 193, 137–153. [Google Scholar] [CrossRef]
- Vascotto, I.; Mozetič, P.; Francé, J. Phytoplankton Time-Series in a LTER Site of the Adriatic Sea: Methodological Approach to Decipher Community Structure and Indicative Taxa. Water 2021, 13, 2045. [Google Scholar] [CrossRef]
- Gladan, Ž.N.; Matić, F.; Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Straka, M.; Dekneudt, Q.; Grbec, B.; Garber, R.; et al. The Relationship between Toxic Phytoplankton Species Occurrence and Environmental and Meteorological Factors along the Eastern Adriatic Coast. Harmful Algae 2020, 92, 101745. [Google Scholar] [CrossRef] [PubMed]
- Ljubešić, Z.; Bosak, S.; Viličić, D.; Borojević, K.K.; Marić, D.; Godrijan, J.; Ujević, I.; Peharec, P.; Đakovac, T. Ecology and Taxonomy of Potentially Toxic Pseudo-Nitzschia Species in Lim Bay (North-Eastern Adriatic Sea). Harmful Algae 2011, 10, 713–722. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Burkholder, J.M.; Glibert, P.M.; Skelton, H.M. Mixotrophy, a Major Mode of Nutrition for Harmful Algal Species in Eutrophic Waters. Harmful Algae 2008, 8, 77–93. [Google Scholar] [CrossRef]
- Fonda, S.; Franco, P.; Ghirardelli, E.; Malej, A. Outline of Oceanography and the Plankton of the Adriatic Sea. In Marine Eutrophication and Population Dynamics, Proceedings of the 25th EMBS, İstanbul, Turkey, 18–20 August 1992; Colombo, G., Ferrari, I., Ceccarelli, V.U., Rossi, R., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1992. [Google Scholar]
- Godrijan, J.; Marić, D.; Tomažić, I.; Precali, R.; Pfannkuchen, M. Seasonal Phytoplankton Dynamics in the Coastal Waters of the North-Eastern Adriatic Sea. J. Sea Res. 2013, 77, 32–44. [Google Scholar] [CrossRef]
- Brush, M.J.; Mozetič, P.; Francé, J.; Aubry, F.B.; Djakovac, T.; Faganeli, J.; Harris, L.A.; Niesen, M. Phytoplankton Dynamics in a Changing Environment. In Coastal Ecosystems in Transition: A Comparative Analysis of the Northern Adriatic and Chesapeake Bay; American Geophysical Union: Washington, DC, USA, 2020; pp. 49–74. [Google Scholar]
- Cabrini, M.; Cok, S.; Pecchiar, I. Dinamica e Struttura Del Fitoplancton Nella Fascia Costiera Del Golfo Di Trieste. Biol. Mar. Mediterr. 2000, 7, 850–853. [Google Scholar]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic Marine Microalgae and Noxious Blooms in the Mediterranean Sea: A Contribution to the Global HAB Status Report. Harmful Algae 2021, 102, 101843. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful Algal Blooms and Eutrophication: Nutrient Sources, Composition, and Consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Mittlesdorf, H.; Goleski, J.A.; Wang, Z.; Haynes, B.; Morton, S.L.; Gobler, C.J. Nitrogenous Nutrients Promote the Growth and Toxicity of Dinophysis Acuminata during Estuarine Bloom Events. PLoS ONE 2015, 10, e0124148. [Google Scholar] [CrossRef]
- Tong, M.; Smith, J.L.; Kulis, D.M.; Anderson, D.M. Role of Dissolved Nitrate and Phosphate in Isolates of Mesodinium Rubrum and Toxin-Producing Dinophysis Acuminata. Aquat. Microb. Ecol. 2015, 75, 169–185. [Google Scholar] [CrossRef]
- García-Portela, M.; Reguera, B.; Gago, J.; Le Gac, M.; Rodríguez, F. Uptake of Inorganic and Organic Nitrogen Sources by Dinophysis Acuminata and D. Acuta. Microorganisms 2020, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.B.; Lima, M.J. Unusual Red Tide of the Dinoflagellate Lingulodinium Polyedra during an Upwelling Event off the Algarve Coast (SW Iberia). Reg. Stud. Mar. Sci. 2023, 63, 102998. [Google Scholar] [CrossRef]
- Caroppo, C.; Congestri, R.; Bracchini, L.; Albertano, P. On the Presence of Pseudo-Nitzschia Calliantha Lundholm, Moestrup et Hasle and Pseudo-Nitzschia Delicatissima (Cleve) Heiden in the Southern Adriatic Sea (Mediterranean Sea, Italy). J. Plankton Res. 2005, 27, 763–774. [Google Scholar] [CrossRef]
- Sahraoui, I.; Grami, B.; Bates, S.S.; Bouchouicha, D.; Chikhaoui, M.A.; Mabrouk, H.H.; Hlaili, A.S. Response of Potentially Toxic Pseudo-Nitzschia (Bacillariophyceae) Populations and Domoic Acid to Environmental Conditions in a Eutrophied, SW Mediterranean Coastal Lagoon (Tunisia). Estuar. Coast. Shelf Sci. 2012, 102, 95–104. [Google Scholar] [CrossRef]
- Brzezinski, M.A. The Si: C: N Ratio of Marine Diatoms: Interspecific Variability and the Effect of Some Environmental Variables 1. J. Phycol. 1985, 21, 347–357. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful Algal Blooms: Their Ecophysiology and General Relevance to Phytoplankton Blooms in the Sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Krom, M.; Herut, B.; Mantoura, R. Nutrient Budget for the Eastern Mediterranean: Implications for Phosphorus Limitation. Limnol. Oceanogr. 2004, 49, 1582–1592. [Google Scholar] [CrossRef]
- Smayda, T.J. Turbulence, Watermass Stratification and Harmful Algal Blooms: An Alternative View and Frontal Zones as “Pelagic Seed Banks”. Harmful Algae 2002, 1, 95–112. [Google Scholar] [CrossRef]
- Klemm, K.; Cembella, A.; Clarke, D.; Cusack, C.; Arneborg, L.; Karlson, B.; Liu, Y.; Naustvoll, L.; Siano, R.; Gran-Stadniczeñko, S.; et al. Apparent Biogeographical Trends in Alexandrium Blooms for Northern Europe: Identifying Links to Climate Change and Effective Adaptive Actions. Harmful Algae 2022, 119, 102335. [Google Scholar] [CrossRef]
- Ninčević-Gladan, Ž.; Skejić, S.; Bužančić, M.; Marasović, I.; Arapov, J.; Ujević, I.; Bojanić, N.; Grbec, B.; Kušpilić, G.; Vidjak, O. Seasonal Variability in Dinophysis Spp. Abundances and Diarrhetic Shellfish Poisoning Outbreaks along the Eastern Adriatic Coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar] [CrossRef]
- Spatharis, S.; Danielidis, D.B.; Tsirtsis, G. Recurrent Pseudo-Nitzschia Calliantha (Bacillariophyceae) and Alexandrium Insuetum (Dinophyceae) Winter Blooms Induced by Agricultural Runoff. Harmful Algae 2007, 6, 811–822. [Google Scholar] [CrossRef]
- Margalef, R. Life-Forms of Phytoplankton as Survival Alternatives in an Unstable Environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Weise, A.M.; Levasseur, M.; Saucier, F.J.; Senneville, S.; Bonneau, E.; Roy, S.; Sauvé, G.; Michaud, S.; Fauchot, J. The Link between Precipitation, River Runoff, and Blooms of the Toxic Dinoflagellate Alexandrium Tamarense in the St. Lawrence. Can. J. Fish. Aquat. Sci. 2002, 59, 464–473. [Google Scholar] [CrossRef]
- Turner, J.T.; Tester, P.A.; Lincoln, J.A.; Carlsson, P.; Granéli, E. Effects of N: P: Si Ratios and Zooplankton Grazing on Phytoplankton Communities in the Northern Adriatic Sea. III. Zooplankton Populations and Grazing. Aquat. Microb. Ecol. 1999, 18, 67–75. [Google Scholar] [CrossRef]
- Mozetic, P.; Lipej, L. Phytoplankton-zooplankton Trophic Interactions along the Salinity Gradient (Gulf of Trieste). Rapp. De La Comm. Int. Pour L’exploration Sci. De La Mer Méditerranée 1998, 35, 468–469. [Google Scholar]
- Bill, B.D.; Moore, S.K.; Hay, L.R.; Anderson, D.M.; Trainer, V.L. Effects of Temperature and Salinity on the Growth of Alexandrium (Dinophyceae) Isolates from the Salish Sea. J. Phycol. 2016, 52, 230–238. [Google Scholar] [CrossRef]
- Boivin-Rioux, A.; Starr, M.; Chassé, J.; Scarratt, M.; Perrie, W.; Long, Z. Predicting the Effects of Climate Change on the Occurrence of the Toxic Dinoflagellate Alexandrium Catenella Along Canada’s East Coast. Front. Mar. Sci. 2021, 7, 608021. [Google Scholar] [CrossRef]
- Marzidovšek, M.; Francé, J.; Podpečan, V.; Vadnjal, S.; Dolenc, J.; Mozetič, P. Explainable Machine Learning for Predicting Diarrhetic Shellfish Poisoning Events in the Adriatic Sea Using Long-Term Monitoring Data. Harmful Algae 2024, 139, 102728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).