Antibiotic Adsorption by Microplastics: Effect of Weathering, Polymer Type, Size, and Shape
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Artificial Weathering
2.3. Batch Adsorption Experiments
2.4. Analytical and Microscopy Techniques
2.5. Adsorption Isotherms
3. Results
3.1. Adsorption of Ceftazidime by Microplastics
3.2. The Effect of Weathering on Microplastic Surface Structure
3.3. The Effect of Weathering on Polymer Surface Chemistry
3.4. PET Fibre Microplastic Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miloloža, M.; Grgić, D.K.; Bolanča, T.; Ukić, Š.; Cvetnić, M.; Bulatović, V.O.; Dionysiou, D.D.; Kušić, H. Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review. Water 2021, 13, 56. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Naicker, N. Contribution of Wastewater to Antimicrobial Resistance: A Review Article. J. Glob. Antimicrob. Resist. 2023, 34, 23–29. [Google Scholar] [CrossRef]
- Tursi, A.; Baratta, M.; Easton, T.; Chatzisymeon, E.; Chidichimo, F.; De Biase, M.; De Filpo, G. Microplastics in Aquatic Systems, a Comprehensive Review: Origination, Accumulation, Impact, and Removal Technologies. RSC Adv. 2022, 12, 28318–28340. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M.; van Sebille, E. The Occurrence and Degradation of Aquatic Plastic Litter Based on Polymer Physicochemical Properties: A Review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Park, Y.K.; Kim, H.; Kwon, J.; Moon, H.M.; Lee, Y.; Watanabe, A.; Teramae, N.; Ohtani, H.; Kim, Y.M. Selective Solvent Extraction and Quantification of Synthetic Microfibers in Textile Laundry Wastewater Using Pyrolysis-Gas Chromatography/Mass Spectrometry. Chem. Eng. J. 2022, 434, 134653. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of Microplastic in Effluents of Waste Water Treatment Plants Using Focal Plane Array-Based Micro-Fourier-Transform Infrared Imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; de Alencastro, L.F. Plastic Pollution in Swiss Surface Waters: Nature and Concentrations, Interaction with Pollutants. (Special Issue: Microplastics in the Environment). Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. An Overview of Recent Advances in Micro/Nano Beads and Microfibers Research: Critical Assessment and Promoting the Less Known. Sci. Total Environ. 2020, 740, 139991. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gan, R.; Liu, J.; Xie, Y.; Xu, D.; Xiang, Y.; Su, J.; Teng, Z.; Hou, J. Adsorption and Desorption Behaviors of Antibiotics by Tire Wear Particles and Polyethylene Microplastics with or without Aging Processes. Sci. Total Environ. 2021, 771, 145451. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Sabatino, R.; Sathicq, M.B.; Eckert, E.M.; Fontaneto, D.; Dalla Fontana, G.; Mossotti, R.; Corno, G.; Volta, P.; Di Cesare, A. Contribution of Microplastic Particles to the Spread of Resistances and Pathogenic Bacteria in Treated Wastewaters. Water Res. 2021, 201, 117368. [Google Scholar] [CrossRef]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of Microplastics Increases Their Adsorption Affinity towards Organic Contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption Behavior of Organic Pollutants and Metals on Micro/Nanoplastics in the Aquatic Environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Du, S.; Zhu, R.; Cai, Y.; Xu, N.; Yap, P.S.; Zhang, Y.; He, Y.; Zhang, Y. Environmental Fate and Impacts of Microplastics in Aquatic Ecosystems: A Review. RSC Adv. 2021, 11, 15762–15784. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G.; Pérez-Guevara, F. Microplastisphere Antibiotic Resistance Genes: A Bird’s-Eye View on the Plastic-Specific Diversity and Enrichment. Sci. Total Environ. 2024, 912, 169316. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2018; ISBN 2013206534. [Google Scholar]
- Gaşpar, C.M.; Cziszter, L.T.; Lăzărescu, C.F.; Ţibru, I.; Pentea, M.; Butnariu, M. Antibiotic Resistance among Escherichia Coli Isolates from Hospital Wastewater Compared to Community Wastewater. Water 2021, 13, 3449. [Google Scholar] [CrossRef]
- Ajala, O.J.; Tijani, J.O.; Salau, R.B.; Abdulkareem, A.S.; Aremu, O.S. A Review of Emerging Micro-Pollutants in Hospital Wastewater: Environmental Fate and Remediation Options. Results Eng. 2022, 16, 100671. [Google Scholar] [CrossRef]
- Tejedor-Junco, M.T.; Díaz, V.C.; González-Martín, M.; Tuya, F. Presence of Microplastics and Antimicrobial-Resistant Bacteria in Sea Cucumbers under Different Anthropogenic Influences in Gran Canaria (Canary Islands, Spain). Mar. Biol. Res. 2021, 17, 537–544. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X. Adsorption of Ceftazidime from Aqueous Solution by Multi-Walled Carbon Nanotubes. Polish J. Environ. Stud. 2015, 24, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Tumrani, S.H.; Soomro, R.A.; Zhang, X.; Bhutto, D.A.; Bux, N.; Ji, X. Coal Fly Ash Driven Zeolites for the Adsorptive Removal of the Ceftazidime Drug. RSC Adv. 2021, 11, 26110–26119. [Google Scholar] [CrossRef]
- Wang, G.; Sun, T.; Sun, Z.; Hu, X. Preparation of Copper Based Metal Organic Framework Materials and Its Effective Adsorptive Removal of Ceftazidime from Aqueous Solutions. Appl. Surf. Sci. 2020, 532, 147411. [Google Scholar] [CrossRef]
- Ashtikar, S.V.; Parkhi, A.D. Adsorption of Copper from Aqueous Solution Using Mango Seed Powder. J. Eng. Res. Appl. 2014, 4, 75–77. [Google Scholar]
- Fu, J.; Li, Y.; Peng, L.; Gao, W.; Wang, G. Distinct Chemical Adsorption Behaviors of Sulfanilamide as a Model Antibiotic onto Weathered Microplastics in Complex Systems. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129337. [Google Scholar] [CrossRef]
- Yang, C.; Guan, J.; Yang, Y.; Liu, Y.; Li, Y.; Fei, Y. Interface Behavior Changes of Weathered Polystyrene with Ciprofloxacin in Seawater Environment. Environ. Res. 2022, 212, 113132. [Google Scholar] [CrossRef]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the Adsorption and Desorption Behaviors of Antibiotics by Degradable MPs with or without UV Ageing Process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption Behavior and Mechanism of Hydrophilic Organic Chemicals to Virgin and Aged Microplastics in Freshwater and Seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. New Insights into Oxytetracycline (OTC) Adsorption Behavior on Polylactic Acid Microplastics Undergoing Microbial Adhesion and Degradation. Chem. Eng. J. 2021, 416, 129085. [Google Scholar] [CrossRef]
- Xue, X.D.; Fang, C.R.; Zhuang, H.F. Adsorption Behaviors of the Pristine and Aged Thermoplastic Polyurethane Microplastics in Cu(II)-OTC Coexisting System. J. Hazard. Mater. 2021, 407, 124835. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Yang, Y.; Shen, L.; Zhang, B.; Dong, Z.; Gao, C.; Xing, B. New Insights into Adsorption Mechanism of Pristine and Weathered Polyamide Microplastics towards Hydrophilic Organic Compounds. Environ. Pollut. 2023, 317, 120818. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Neema, P.; Andrews, S. Adsorption Behavior and Mechanisms of Trihalomethanes onto Virgin and Weathered Polyvinyl Chloride Microplastics. Toxics 2024, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qin, Q.; Zhang, X.; Ma, J. Characteristics and Adsorption Behavior of Typical Microplastics in Long-Term Accelerated Weathering Simulation. Environ. Sci. Process. Impacts 2024, 26, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced Adsorption of Oxytetracycline to Weathered Microplastic Polystyrene: Kinetics, Isotherms and Influencing Factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Shen, X.C.; Li, D.C.; Sima, X.F.; Cheng, H.Y.; Jiang, H. The Effects of Environmental Conditions on the Enrichment of Antibiotics on Microplastics in Simulated Natural Water Column. Environ. Res. 2018, 166, 377–383. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Chen, J.; Lei, Y.; Wen, J.; Zheng, Y.; Gan, X.; Liang, Q.; Huang, C.; Song, Y. The Neurodevelopmental Toxicity Induced by Combined Exposure of Nanoplastics and Penicillin in Embryonic Zebrafish: The Role of Aging Processes. Environ. Pollut. 2023, 335, 122281. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, J.; Li, L.; Wang, Y.; Dai, X.; Yu, F.; Ma, J. Interfacial Interaction between Micro/Nanoplastics and Typical PPCPs and Nanoplastics Removal via Electrosorption from an Aqueous Solution. Water Res. 2020, 184, 116100. [Google Scholar] [CrossRef]
- Ricardo, I.A.; Alberto, E.A.; Silva Júnior, A.H.; Macuvele, D.L.P.; Padoin, N.; Soares, C.; Gracher Riella, H.; Starling, M.C.V.M.; Trovó, A.G. A Critical Review on Microplastics, Interaction with Organic and Inorganic Pollutants, Impacts and Effectiveness of Advanced Oxidation Processes Applied for Their Removal from Aqueous Matrices. Chem. Eng. J. 2021, 424, 130282. [Google Scholar] [CrossRef]
- Liu, X.; Deng, Q.; Zheng, Y.; Wang, D.; Ni, B.J. Microplastics Aging in Wastewater Treatment Plants: Focusing on Physicochemical Characteristics Changes and Corresponding Environmental Risks. Water Res. 2022, 221, 118780. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, H.; Xu, H.M.; Liu, Z.; Kan, G.; Yu, K.; Jiang, J. Adsorption Behaviors of Triclosan by Non-Biodegradable and Biodegradable Microplastics: Kinetics and Mechanism. Sci. Total Environ. 2022, 842, 156832. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, J.; Zheng, L.; Zhu, W.; Xue, X.; Yu, Y.; Deng, Y.; Wang, H. Adsorption Behaviors and Mechanisms of Humic Acid on Virgin and Aging Microplastics. J. Mol. Liq. 2022, 363, 119819. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Y.; Yu, X.; Huang, D.; Guo, X.; Zhu, L. Aging Significantly Increases the Interaction between Polystyrene Nanoplastic and Minerals. Water Res. 2022, 219, 118544. [Google Scholar] [CrossRef] [PubMed]
- Costigan, E.; Collins, A.; Hatinoglu, M.D.; Bhagat, K.; MacRae, J.; Perreault, F.; Apul, O. Adsorption of Organic Pollutants by Microplastics: Overview of a Dissonant Literature. J. Hazard. Mater. Adv. 2022, 6, 100091. [Google Scholar] [CrossRef]
- Hu, E.; Yuan, H.; Du, Y.; Chen, X. LDPE and HDPE Microplastics Differently Affect the Transport of Tetracycline in Saturated Porous Media. Materials 2021, 14, 1757. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of Plastic Microbeads in Facial Scrubs and Their Estimated Emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Sarno, A.; Olafsen, K.; Kubowicz, S.; Karimov, F.; Sait, S.T.L.; Sørensen, L.; Booth, A.M. Accelerated Hydrolysis Method for Producing Partially Degraded Polyester Microplastic Fiber Reference Materials. Environ. Sci. Technol. Lett. 2021, 8, 250–255. [Google Scholar] [CrossRef]
- Easton, T.; Koutsos, V.; Chatzisymeon, E. Removal of Polyester Fibre Microplastics from Wastewater Using a UV/H2O2oxidation Process. J. Environ. Chem. Eng. 2023, 11, 109057. [Google Scholar] [CrossRef]
- Budhiraja, V.; Urh, A.; Horvat, P.; Krzan, A. Synergistic Adsorption of Organic Pollutants on Weathered Polyethylene Microplastics. Polymers 2022, 14, 2674. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible Light Photocatalytic Degradation of Microplastic Residues with Zinc Oxide Nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Lee, J.M.; Busquets, R.; Choi, I.C.; Lee, S.H.; Kim, J.K.; Campos, L.C. Photocatalytic Degradation of Polyamide 66: Evaluating the Feasibility of Photocatalysis as a Microfibre-Targeting Technology. Water 2020, 12, 3551. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. E-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Wu, J.; Xu, P.; Chen, Q.; Ma, D.; Ge, W.; Jiang, T.; Chai, C. Effects of Polymer Aging on Sorption of 2,2′,4,4′-Tetrabromodiphenyl Ether by Polystyrene Microplastics. Chemosphere 2020, 253, 126706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, Quantity and Sorptive Properties of Microplastics Extracted from Cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption Behavior of Organic Pollutants on Microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and Road Wear Particles (TRWP)—A Review of Generation, Properties, Emissions, Human Health Risk, Ecotoxicity, and Fate in the Environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, R.; Qu, H.; Zuo, Y.; Yu, Z.; Hu, G.; Li, Z.; Chen, H.; Lin, B.; Wang, B.; et al. Enhanced Adsorption of Tetrabromobisphenol a (TBBPA) on Cosmetic-Derived Plastic Microbeads and Combined Effects on Zebrafish. Chemosphere 2020, 248, 126067. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Y.; Huang, M.; Fu, S.; Hao, Y.; Hu, S.; Lai, D.; Zhao, L. Adsorption Behaviors and Mechanisms of Antibiotic Norfloxacin on Degradable and Nondegradable Microplastics. Sci. Total Environ. 2022, 807, 151042. [Google Scholar] [CrossRef]
- An, D.; Na, J.; Song, J.; Jung, J. Size-Dependent Chronic Toxicity of Fragmented Polyethylene Microplastics to Daphnia Magna. Chemosphere 2021, 271, 129591. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.N.; Blázquez, M.; Brooks, S.J.; Thomas, K.V. Weathering Impacts the Uptake of Polyethylene Microparticles from Toothpaste in Mediterranean Mussels (M. Galloprovincialis). Sci. Total Environ. 2018, 626, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, J. Interaction between Antibiotics and Microplastics: Recent Advances and Perspective. Sci. Total Environ. 2023, 897, 165414. [Google Scholar] [CrossRef]
- Conradie, W.; Dorfling, C.; Chimphango, A.; Booth, A.M.; Sørensen, L.; Akdogan, G. Investigating the Physicochemical Property Changes of Plastic Packaging Exposed to UV Irradiation and Different Aqueous Environments. Microplastics 2022, 1, 456–476. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers V: Epoxies. Spectroscopy 2022, 37, 17–19. [Google Scholar] [CrossRef]
- Rathore, C.; Saha, M.; Gupta, P.; Kumar, M.; Naik, A.; de Boer, J. Standardization of Micro-FTIR Methods and Applicability for the Detection and Identification of Microplastics in Environmental Matrices. Sci. Total Environ. 2023, 888, 164157. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Wang, J. Sorption of Sulfamethoxazole onto Six Types of Microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.M.; Li, X.Y. The Partition Behavior of Perfluorooctanesulfonate (PFOS) and Perfluorooctanesulfonamide (FOSA) on Microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Wang, J. Sorption of Sulfamethazine onto Different Types of Microplastics: A Combined Experimental and Molecular Dynamics Simulation Study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar] [CrossRef]
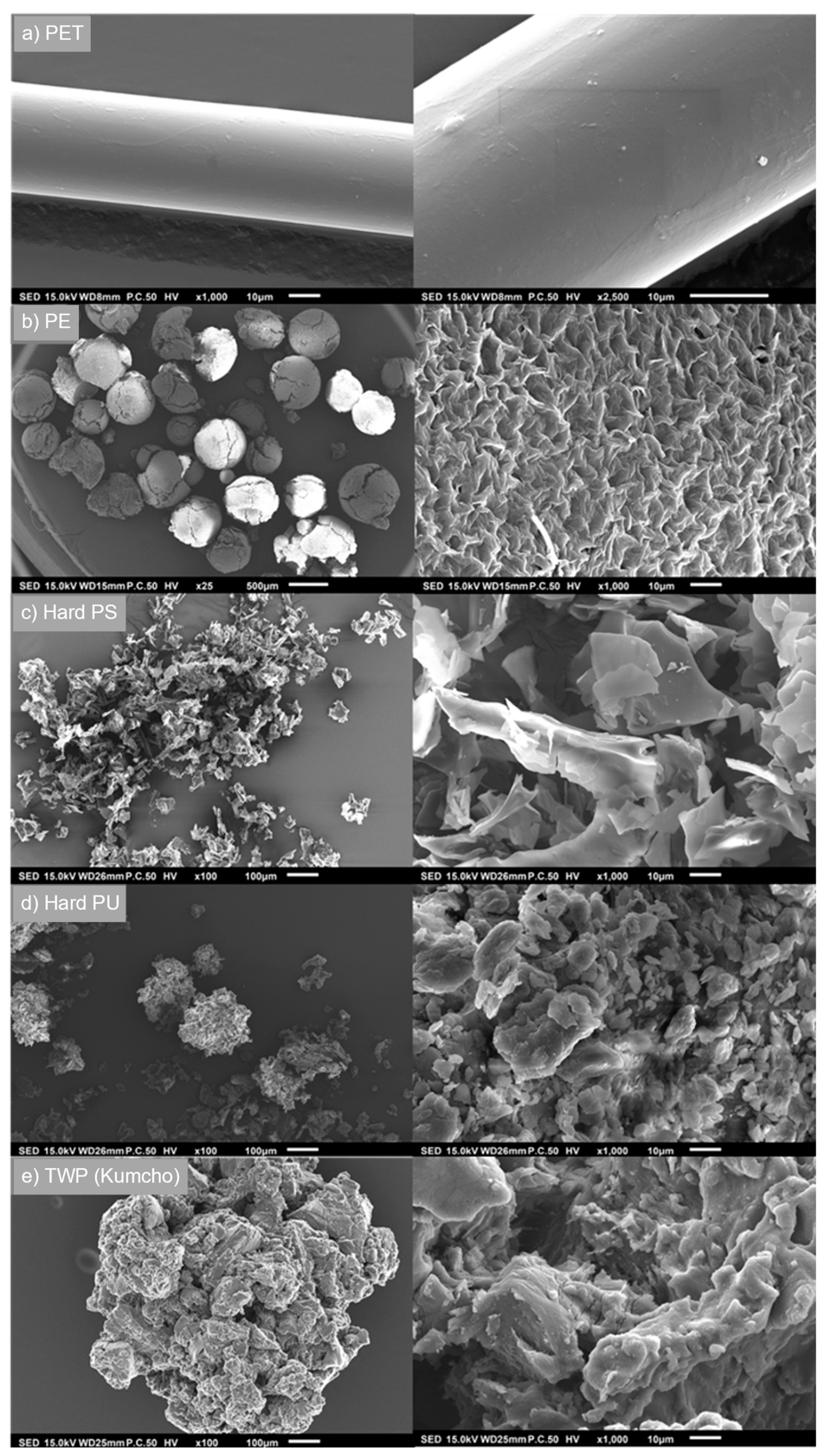

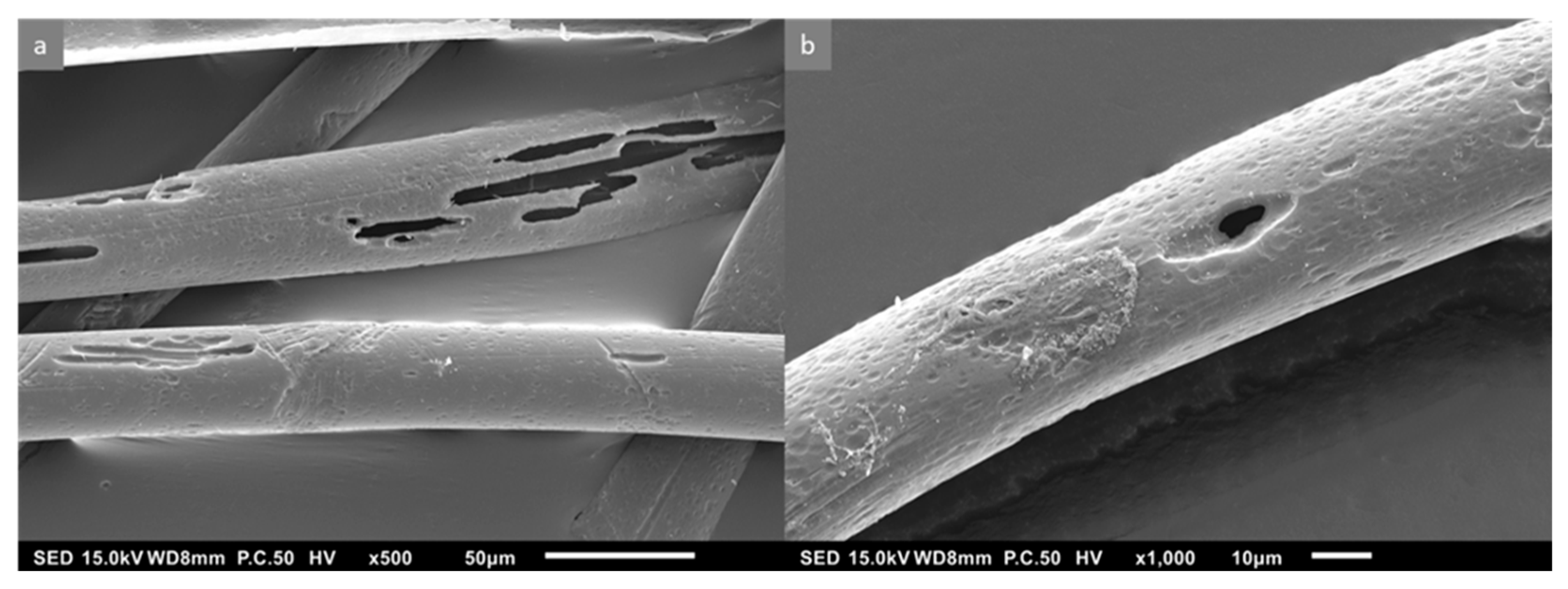
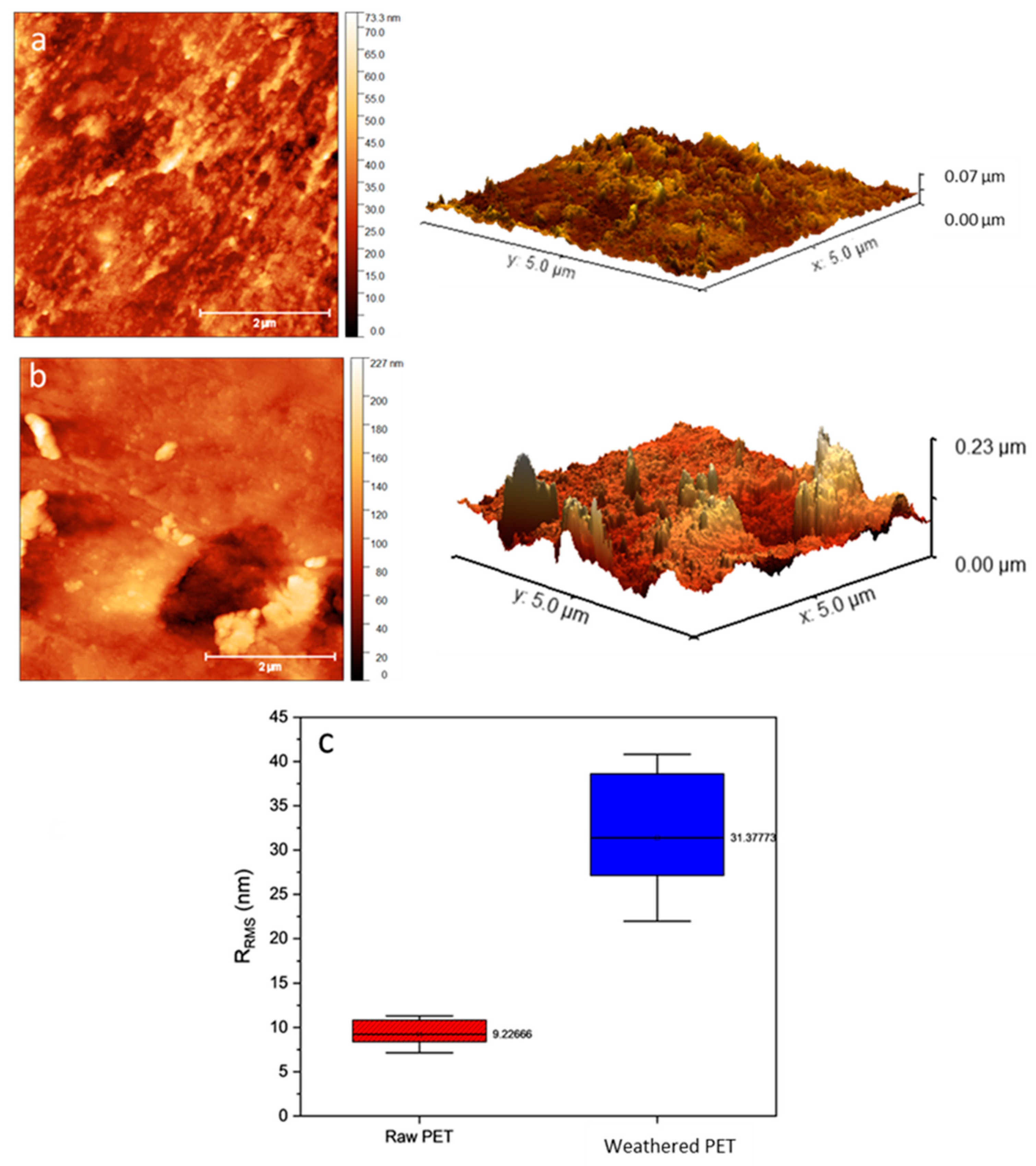

| Antibiotics | MPs Type | MPs Size | Adsorption Capacity | Weathering Method | Ref. |
|---|---|---|---|---|---|
| Sulphanilamide | Polyamide (PA), Polyvinyl chloride (PVC) and PET | 150 µm | 19.70 μg/g (W) 24.27 μg/g (W) 12.45 μg/g (W) | Ultraviolet (UV) irradiation and temperature fluctuation | [26] |
| Ciprofloxacin (CIP) | Polystyrene (PS) | - | 2.16 mg/g (P) 5.45 mg/g (W) | Fenton immersion method | [27] |
| Chlortetracycline (CTC) and Amoxicillin (AMX) | TWP and PE | ≤74 μm (TWP) | CTC: 0.90 mg/g (P); 2.09 mg/g (W) AMX: 2.25 mg/g (P); 2.91 mg/g (W) CTC: 0.72 mg/g (P); 1.57 mg/g (W) AMX: 1.65 mg/g (P); 2.72 mg/g (W) | Heat-activated potassium persulfate | [12] |
| Tetracycline (TC) and CIP | Polylactic acid (PLA) and PVC | 250–550 μm 75–150 μm | TC: 0.96 mg/g (P); 1.57 mg/g (W) CIP: 0.67 mg/g (P); 0.85 mg/g (W) TC: 2.51 mg/g (P) to 5.49 mg/g (W) CIP: 3.19 mg/g (P); 3.77 mg/g (W) | UV irradiation | [28] |
| CIP | PS PVC | ∼75 μm | Kinetic model at 10 mg/L (PS < PVC < Aged PVC < Aged PS) | UV-accelerated aging | [29] |
| Oxytetracycline (OTC) | PLA | 75–150 μm | 581 μg/g (P); 1193 μg/g (W); 728 μg/g (Biofilm) | Incubated in sewage influents | [30] |
| Cu(II) and OTC | Thermoplastic Polyurethane (TPU) | 50–100 μm | Cu(II): 0.613 mg/g (P); 0.576 mg/g (W) OTC: 0.980 mg/g (P); 1.506 mg/g (W) | UV irradiation | [31] |
| Benzoic acid (BA), Sulfamethoxazole (MX), Sulphamerazine (MR) and CIP | PA | 75–150 μm | BA (0.75 mg/g) > SMX (0.54 mg/g) > SMR (0.11 mg/g) > CIP (0.08 mg/g)) | Xenon lamp irradiation, H2O2, and heat-activated K2S2O8 weathering | [32] |
| Trihalomethanes | PVC | 125–300 µm | 10–20 µg/g (P) Reduced by 10% (W) | Metal halide lamp | [33] |
| TC, Levofloxacin (OFL), CIP | PE, PS, Poly(butylene adipate-co-terephthalate) (PBAT) | 30–500 μm (PE), 75 μm (PS), and 150 μm (PBAT) | PS for TC, CIP increased by 58% (0.69–1.09 mg g−1), 138% (1.72–4.09 mg g−1); PBAT for CIP increased from 0.62 mg g−1 to 6.22 mg g−1 PE for OFL is 0.35 mg g−1 | UV weathering | [34] |
| OTC | PS | 0.45–1 mm | Freundlich Kf value of 894 ± 84 ((mg kg−1) (mg L−1)1/n) for beached foam | Plastic debris from coastal beaches | [35] |
| TC | PE | 150–250 µm | 120.5 μg/g (P) 91.7 μg/g (W) at pH = 7, 20 °C; 111.1 μg/g (W) at pH = 4; 114.9 μg/g (W) at pH = 10; 120.5 μg/g (W) at 2 mg/L HA; 48.1 μg/g (W) at 10 mg/L HA | Aged at different pH, temperature, ionic strength, ageing time, and humic acid | [36] |
| CIP | PS and High-density polyethylene | 50.4 ± 11.9 μm 45.5 ± 12.9 μm | 71.86 mg/g (P); 251.59 mg/g (W) for K2S2O8; 189.52 mg/g (W) for Fenton treatment. 51.61 mg/g (P); 167.19 mg/g (W) for K2S2O8; 126.21 mg/g (W) for Fenton treatment | Heat-activated K2S2O8 and Fenton treatments | [37] |
| Penicillin (PNC) | PS | 80 nm | Percentages of PNC adsorption on 73.0 ± 1.3 (PS); 80.8 ± 4.3 (UV), and 69.1 ± 6.4 (Ozonation) | UV irradiation and ozonation | [38] |
| CIP and bisphenol-A (BPA) | PS | ∼40 nm | 0.15 mg/g (P); 4.07 mg/g (W) for CIP 4.92 mg/g (P); 8.71 mg/g (W) for BPA | UV irradiation ageing | [39] |
| Microplastic Type | Size Range (µm) | Shape | Artificial Weathering Process |
|---|---|---|---|
| Polyester (PET) | 40 (diameter) 70–2117 (length) | Fibre | Alkaline hydrolysis (10% NaOH. 3 h) |
| Polyethylene (PE) | 250–850 | Beads | UVC-activated H2O2 (500 mg L−1, 9 h) |
| Hard polystyrene (Hard PS) | 50–80 | Foam particle | Xenon lamp (600 h) |
| Soft polystyrene (Soft PS) | 50–80 | Foam particle | Xenon lamp (600 h) |
| Hard polyurethane (Hard PU) | 10–80 | Foam particle | Xenon lamp (600 h) |
| Soft polyurethane (Soft PU) | 10–80 | Foam particle | Xenon lamp (600 h) |
| Bridgestone Tyre | 300–1000 | Rubber fragment | - |
| Kumho Tyre | 300–1000 | Rubber fragment | - |
| Michelin Tyre | 300–1000 | Rubber fragment | - |
| Goodyear Tyre | 300–1000 | Rubber fragment | - |
| Carbonyl Index (CI) | ||
|---|---|---|
| Pristine | Weathered | |
| PET | 6.7 | 5.5 |
| Hard PS | 0.31 | 2.35 |
| Soft PS | 0.37 | 3.51 |
| Hard PU | 2.26 | 2.39 |
| Soft PU | 0.74 | 1.70 |
| PET Fibers | Linear | Langmuir | |||
|---|---|---|---|---|---|
| Kd (L g−1) | r2 | Qmax (mg g−1) | KL (L mg−1) | r2 | |
| Pristine | 0.023 ± 0.002 | 0.983 | 1.10 ± 0.09 | 0.596 | 0.987 |
| Weathered | 0.045 ± 0.006 | 0.980 | 1.27 ± 0.09 | 0.753 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Easton, T.; Budhiraja, V.; He, Y.; Zhang, Q.; Arora, A.; Koutsos, V.; Chatzisymeon, E. Antibiotic Adsorption by Microplastics: Effect of Weathering, Polymer Type, Size, and Shape. Environments 2025, 12, 120. https://doi.org/10.3390/environments12040120
Easton T, Budhiraja V, He Y, Zhang Q, Arora A, Koutsos V, Chatzisymeon E. Antibiotic Adsorption by Microplastics: Effect of Weathering, Polymer Type, Size, and Shape. Environments. 2025; 12(4):120. https://doi.org/10.3390/environments12040120
Chicago/Turabian StyleEaston, Thomas, Vaibhav Budhiraja, Yuanzhe He, Qi Zhang, Ayushi Arora, Vasileios Koutsos, and Efthalia Chatzisymeon. 2025. "Antibiotic Adsorption by Microplastics: Effect of Weathering, Polymer Type, Size, and Shape" Environments 12, no. 4: 120. https://doi.org/10.3390/environments12040120
APA StyleEaston, T., Budhiraja, V., He, Y., Zhang, Q., Arora, A., Koutsos, V., & Chatzisymeon, E. (2025). Antibiotic Adsorption by Microplastics: Effect of Weathering, Polymer Type, Size, and Shape. Environments, 12(4), 120. https://doi.org/10.3390/environments12040120








