Abstract
Magnetic Resonance (MR) technology is extensively used in academic and industrial research laboratories and represents one of the most significant methodologies in clinical radiology. Although MR does not use ionizing radiation, it cannot be considered risk-free due to the strong static magnetic fields and time-varying electromagnetic fields employed in the technology. To mitigate risks for MR operators, the European Community and ICNIRP have established safety limits based on the existing literature, primarily related to diagnostic MR. However, the literature on occupational exposure in non-clinical nuclear magnetic resonance (NMR) spectroscopy is limited. Due to their specificity, non-medical NMR environments present unique challenges from the point of view of operator exposure. NMR spectrometers are characterized by extremely high static magnetic fields, reaching up to 28 T in commercial systems; moreover, routine activities performed near the magnet, where field gradients are highest, increase operator exposure. Such environments are not typically perceived as hazardous and are frequented by various types of personnel, often without specific training. This study aims to highlight the critical issues in managing a preclinical MR laboratory equipped with a high-field NMR spectrometer, discussing operator safety challenges and presenting risk assessment data.
1. Introduction
Before its widespread adoption in medicine as a diagnostic technique, magnetic resonance (MR) was predominantly used in fundamental laboratory research, food quality assessment, and molecular studies through nuclear magnetic resonance (NMR) spectroscopy. NMR spectroscopy provides a comprehensive understanding of molecular structure, dynamics, reaction states, and chemical environments [1,2,3]. Concurrently, there has been a rapid proliferation of high-field magnetic resonance imaging (MRI) scanners in research settings (both academic and non-academic), particularly for preclinical studies involving animal models, especially rodents [4].
Diagnostic MRI and NMR spectrometers share the same operating principles, involving three phases: the polarization of nuclear spins in a static magnetic field (B0); the excitation of the sample with a radiofrequency (RF) magnetic field (B1) at the Larmor frequency; and the application of spatial magnetic field gradients for spin localization. NMR spectrometers generate extremely high magnetic fields (up to 28 T), which are typically well-confined near the instrument. Some risks associated with MR devices are documented in the literature and should not be underestimated, especially given the rapid development and dissemination of the technology [5,6].
In magnetic resonance environments, the primary exposure risks for workers stem from both static and spatially heterogeneous magnetic fields, as well as, in some cases, radiofrequency (RF) fields. Workers who move in close proximity to the magnet are additionally exposed to time-varying magnetic fields, which can induce electric currents within the body. If these currents reach sufficient strength, they may interact with the central nervous system (CNS), potentially leading to neurological effects, and can also stimulate peripheral nerves, causing sensations such as tingling or muscle contractions.
Recent research has documented various transient symptoms experienced by workers due to movement within these magnetic fields, including dizziness, nausea, visual disturbances, and a sensation of vertigo [7,8,9,10,11,12].
Recent studies have explored the neural impact of static magnetic fields and dynamic magnetic fields using animal models. Research on the influence of magnetic fields on cognitive functions such as learning and memory, as well as emotional behaviour, neuronal activity, and neurotransmitter dynamics, is steadily growing. However, due to variations in magnetic field parameters, experimental models, and study conditions, findings remain inconsistent [13,14].
As magnetic resonance technology advances and field strengths increase, further investigation into occupational safety measures and exposure limits is essential to mitigate potential health risks for personnel working in these environments.
To address these concerns, the European Union (EU) and the International Commission on Non-Ionizing Radiation Protection (ICNIRP) have established limits for the exposure of workers to the risks arising from physical agents (electromagnetic fields, EMF) [15,16,17,18]. Several studies evaluate health risks for workers in MR diagnostic environments [19,20,21,22,23]. Worker exposure to static magnetic fields and movements in fringe fields has been assessed and discussed for MRI scanners with field strengths from 0.25 T to 3.0 T [22,24,25,26,27,28].
However, there is limited research on occupational risk assessment for workers in NMR spectroscopy and, more broadly, in non-clinical magnetic resonance environments, such as preclinical or fundamental research laboratories. NMR spectrometers, operating at up to 28 T @ 1.2 GHz, are commonly found in universities and research centres. These instruments are also used by doctoral students and research fellows who are considered scientific users rather than workers. Although they may be proficient in the scientific applications of the technique, they often lack awareness of safety aspects and may mistakenly perceive NMR spectrometers as low-risk devices. In this context, it is crucial to educate workers on appropriate behaviour and movement control to prevent adverse events.
Given the nature of NMR equipment and considering that some operational tasks involve direct interaction with the devices, it is presumed that researchers working in spectrometry laboratories may experience daily exposure to relatively high levels of magnetic fields and/or EMF. However, these exposure levels and conditions have not yet been thoroughly analysed and require evaluation through targeted on-site measurements.
This study highlights the challenges of high-field NMR spectroscopy laboratories regarding magnetic field exposure and discusses risk assessment aspects for operators. Particular attention is given to workers at particular risk, such as operators with active implantable medical devices (AIMDs), a sensitive risk group requiring protection from electromagnetic field interference.
2. Materials and Methods
2.1. High-Field NMR Spectroscopy Laboratory
An NMR spectrometer (Bruker, Billerica, Massachusetts the manufacturer, city, and country) comprises a superconducting coil immersed in a liquid helium jacket surrounded by a liquid nitrogen jacket. At the centre of the spectrometer is an empty cylinder, known as the gantry, where the static magnetic field is generated. This bore accommodates the probe, a cylindrical container that holds the sample to be analysed. Depending on the experiment, different probes are used, which may be nucleus-specific or multinuclear, with variations in internal coil design determining specificity. Sample insertion operations are typically performed at the top of the equipment, accessible via ladders, or near the base of the magnet, requiring operators to crouch near the magnet.
A high-field NMR spectroscopy laboratory is a specialized environment housing the NMR spectrometer and essential components for its operation, including the cryogenic cooling system for the superconducting magnet with liquid helium and nitrogen tanks and the refilling system, the console and electronic components, and safety systems such as ventilation systems, quench piping, and oxygen sensors. Additional auxiliary systems (e.g., sample containers, ladders, and sample insertion/removal structures) and at least one workstation are typically present.
The NMR spectroscopy laboratories can house spectrometers of various generations. Older installations, with lower magnetic fields, often neglect manufacturer safety recommendations. For example, older NMR equipment typically lacks safety systems found in modern superconducting magnets used in medical imaging, such as quench piping to direct helium outside the laboratory, auxiliary safety devices (e.g., oxygen sensors), or ventilation systems to expel helium gas released within the laboratory during a quench event. Conversely, newer spectrometers, operating at higher fields and frequencies (up to 1200 MHz), necessitate advanced safety systems like cryogenic gas evacuation pipes and oxygen monitoring sensors. As a case in point, new-generation solid-state NMR spectrometers utilize ultra-high field pulses and decoupling power [29]. This may result in potential hazards relative to high-power RF fields, which may interfere with electronics and sensors within the NMR environment. It is imperative to ensure adequate RF shielding to prevent unintended exposure and to position sensitive equipment safely outside of high-field and high-RF zones. Manufacturers of modern equipment include detailed safety system guidelines in instruction manuals, outlining risk assessments and management strategies, which, if implemented, represent a significant safety improvement.
2.2. Potential Hazards and Specific Critical Points
In the context of NMR applications, it is hypothesized that workers may be exposed on a daily basis to relatively high levels of magnetic and/or RF fields. The primary source of exposure is the static magnetic field; as reported by Berlana and Ubeda [30], no detectable emissions of RF field were found in the surrounding environment, indicating that the electromagnetic radiation generated by the NMR remained confined within the devices. The potential hazards associated with magnetic fields can be classified as either direct or indirect. Direct risks manifest in the human body through biological effects, which can be sensory or health-related. Indirect risks are associated with magnetic fields, such as the projectile effect (attraction of ferromagnetic materials), a significant torque applied to objects, and potential interference with implanted medical devices.
In contrast to clinical MRI sites, the primary users of the NMR equipment consist of students, postgraduates, scholarship holders, and contract workers. These individuals do not qualify as employees but rather as scientific users, sometimes even occasional, often highly competent in the scientific application of technology but generally not very sensitive to safety aspects because they are unaware of the associated risks, having most often not been properly trained and made aware of these important aspects. Those with a greater familiarity with the operational situation may erroneously assume that the utilization of NMR spectrometers in the present context entails reduced exposure for operators. For example, the sample loading typically occurs over a significantly shorter time interval compared to other MRI applications. However, in the majority of cases, this process is carried out by approaching the instrument, either at the top or by crouching down with the arms positioned underneath the structure and the head placed very close to the instrument. Moreover, it should be noted that for NMR investigations, an additional level of exposure exists that is not present in other cases and is related to the tuning and matching procedure. This procedure must be performed for each type of sample introduced unless the analysis is repeated on the same types of samples. This underscores the significance of the dwell time at high levels of static magnetic field as a crucial factor in ensuring protection.
The NMR laboratory is typically a single room where the highest-risk exposure area (near the magnet) is easily accessible and not always marked with appropriate signage (such as the 5 Gauss line).
This also means that, in many NMR laboratories, the operators’ workstation, the equipment controls area, and sometimes the chemical laboratory where the samples are prepared are situated within the same room as the NMR spectrometer or in close proximity. This configuration necessitates a comprehensive and detailed investigation into safety concerns.
2.3. Regulatory Framework and Current Challenges
Currently, there is a notable lack of a specific regulatory framework for NMR spectroscopy environments, resulting in limited awareness of safety requirements. In Europe, the primary regulatory reference is the Directive 2013/35/EU, which addresses worker exposure to electromagnetic fields, focusing on short-term effects while excluding long-term effects due to insufficient scientific evidence.
For general population protection, exposure limits are established by the European Recommendation 1999/519/EC, which sets a threshold of 40 mT for potential human risk (excluding indirect effects) [31].
According to current regulations, static magnetic fields are significant for operators when exposure exceeds 2 Tesla for sensory effects (transient sensory perception disturbances, nausea, vomiting, dizziness, and minor changes in brain functions) and 8 Tesla for health effects (thermal heating and/or the stimulation of nervous or muscular tissue).
It is important to note that these limits do not apply to individuals with specific contraindications to magnetic field exposure (e.g., wearers of active implantable devices), for whom the established threshold is significantly lower, at 0.5 mT (5 Gauss). With regard to passive implanted devices, current regulations do not set action values; therefore, an assessment must be conducted on a case-by-case basis. However, with regard to the projectile effect, European Recommendation 1999/519/EC establishes an action level of 3 mT (3 Gauss) [31].
In most NMR installations, the limits for direct effects—2 T for the body and 8 T for the limbs—remain confined within the equipment; consequently, workers are not directly exposed. Additionally, there are no exposures related to the radio frequencies emitted during the operation of the NMR. However, the 0.5 mT limit is probably exceeded in the vicinity of the NMR device. According to regulations, a colour-coded floor marking delineates the magnetic induction dispersion field, as outlined below:
- Red/white line for 0.5 mT;
- Yellow line for 0.4 mT;
- Green line for 0.3 mT.
The 0.5 mT limit can only be exceeded by authorized personnel, who are trained, informed, and certified as fit for the specific task by the occupational physicians.
The ICNIRP guidelines published in 2014 provide recommendations for limiting exposure to static magnetic fields and time-varying magnetic fields below 1 Hz [18]. The primary objective of these guidelines is to prevent peripheral nerve stimulation (PNS) and reduce the likelihood of transient sensory effects caused by electric fields induced in the human body due to movements within spatially heterogeneous static magnetic fields. These effects can include sensations such as tingling, dizziness, or even muscle contractions, which may pose safety concerns in occupational and clinical settings. To ensure controlled exposure conditions, the ICNIRP has established specific safety thresholds. According to ICNIRP guidelines, a basic restriction is set at 1.1 V/m for peak-induced electric fields within the body. Additionally, a reference level of 2.7 T/s is defined for the time derivative of magnetic flux density (dB/dt), aiming to limit excessive exposure to rapidly changing magnetic fields that could exacerbate neuromuscular stimulation. These guidelines serve as an essential framework for safeguarding individuals working in or exposed to strong magnetic environments, such as those found in MRI facilities [18]. The basic restriction and reference levels for uncontrolled exposures (protection against magnetophosphenes and peripheral nerve stimulation) change as a function of 1/f (up to 0.66 Hz), where f is the frequency of motion-induced fields.
2.4. Risk Assessment Procedure
A correct risk assessment, linked to the presence of the static magnetic field in the NMR environment, must necessarily consider the construction characteristics of the equipment and the dispersion of the field around it in order to identify the areas of risk, the attention levels to be kept, and the operating procedures to be followed to minimize exposure and the possibility of the occurrence of risk scenarios. First of all, the 0.5 mT line should be identified to delineate the area restricted for unauthorized access, which is to be made accessible exclusively to authorized personnel (listed in a dedicated register). This is initiated by the precise measurement of the fringe field surrounding the NMR spectrometer.
Then, regarding the occupational exposure to time-varying magnetic fields, an estimation of the reference parameters should be conducted. In this evaluation, worker exposure is assessed as part of a comprehensive risk analysis, focusing on the induced electric field (∣E∣) and the time derivative of the magnetic flux density (∣dB/dt∣) to establish safe operating procedures. Special attention is devoted to individuals with active implantable medical devices (AIMDs), who represent a particularly vulnerable group requiring stringent protection from the risks associated with electromagnetic field interference [32].
We present here the risk assessment for a research NMR laboratory including a 7T (@300 MHz) spectrometer. First of all, the static magnetic field was mapped by positioning a three-axis Hall magnetometer (THM 1176, Metrolab Instruments SA, Plan-les-Ouates, Switzerland) at five different heights above the ground plane (z = 0 cm, 40 cm, 80 cm, 120 cm, and 160 cm). Measurements were taken along a predefined theoretical path extending from the console to the spectrometer and back, with a spatial resolution of 10 cm, as previously described in [27]. Based on these measurements, the fringe field distribution in the vertical (xz) plane was determined. Subsequently, the magnitude of the induced electric field |E| was calculated for a trajectory representative of a worker’s movement during routine sample processing operations. This calculation was performed using the analytical model outlined in the ICNIRP guidelines, allowing for an assessment of potential exposure risks associated with workplace activities in the presence of spatially varying magnetic fields [18]. By way of example, we considered the typical movement of an operator who, starting from a standing position, kneels down next to the NMR spectrometer (Figure 1a). The movement speed was set at 1 m/s along the entire movement [33]. Furthermore, a 90° torso flexion was also considered, with a maximum velocity of 4 rad/s (Figure 1b). For both of these movements, a point on the head (shown in Figure 1 as a red point) and a point on the torso corresponding to the heart (shown in Figure 1 as a black star), were designated as the reference points.
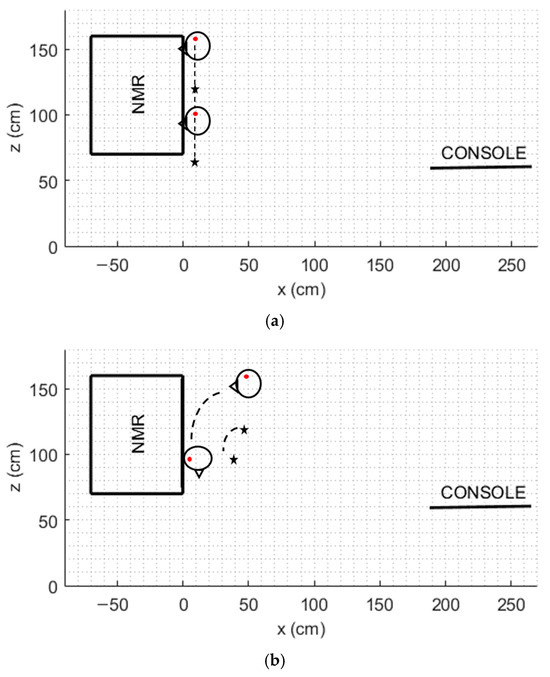
Figure 1.
Schematization of the considered movements in the fringe field of a 7T NMR spectrometer: (a) linear movement along the z axis, (b) flexion movement of 90°. Red point: head reference point. Black star: torso reference point.
Finally, for both of the considered movements, we also calculated the maximum induced voltage (electromotive force, EMF) in an active device implanted in the torso (such as a pacemaker) by simplifying the Faraday–Neumann–Lenz law as reported in [32].
3. Results
Figure 2 shows the map of the fringe field on an xz plane |B|xz through the isocentre of the NMR spectrometer described previously, with the indication of the 0.5 mT line (dashed red line). Moreover, Figure 2 also shows the vector representation of the two-dimensional spatial gradient of the magnetic field |B|xz together with the contour plot of |B|xz around the NMR spectrometer.
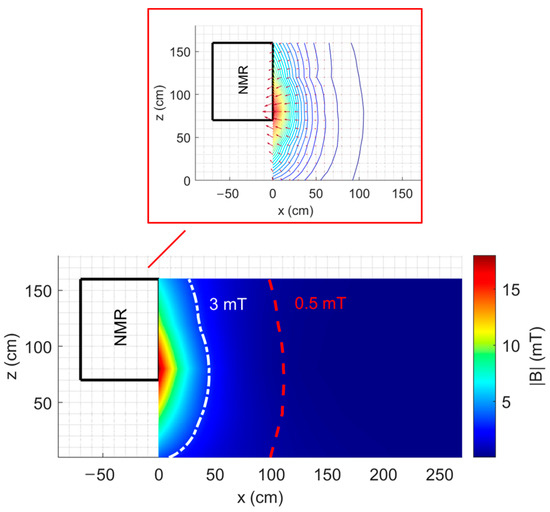
Figure 2.
Map of the fringe field |B|xz with the indication of the 0.5 mT line and the representation of the two-dimensional spatial gradient around a 7 T NMR spectrometer.
For the considered xz plane, the maximum value of |B| is 18.08 mT, the maximum value of |dB/dx| is 58.8 mT/m, and the maximum value of |dB/dz| is 19.35 mT/m.
Table 1 and Table 2 present the peak values of the estimated parameters for the risk assessment relative to the linear movement along the z-axis and the flexion movement of 90°, considering the head and the thorax–heart reference points.

Table 1.
Peak values of the estimated parameter for the risk assessment of a 7T NMR spectrometer: linear movement along the z-axis.

Table 2.
Estimated parameter for the risk assessment of a 7T NMR spectrometer: flexion movement of 90°.
4. Discussion
The occupational exposure literature in non-diagnostic NMR environments is currently sparse [30,34,35], particularly compared to the characterization of exposures for healthcare personnel working with MRI scanners [5,7,26,36,37,38,39]. However, well-established physical mechanisms describe interactions between electromagnetic fields and living tissues, responsible for acute and transient effects above exposure thresholds, leading to the establishment of exposure limits. For example, translational and rotational movements of operators within magnetic fields are the focus of numerous studies on occupational exposures in medical MRI environments, yielding insights into induced body currents and their effects on humans [7,40,41,42,43].
A classical NMR spectrometer generates a strong magnetic field that extends beyond the magnet itself, thereby creating a significant stray magnetic field around the instrument. This has the potential to interfere with nearby electronic devices and necessitates a dedicated, controlled environment to prevent unwanted interactions. The majority of new ultra-high field instruments (above 500 MHz) are ultra-shielded, with the design of these spectrometers being such that advanced shielding techniques are incorporated to minimize the stray magnetic field. In [30], the authors conducted a comparative analysis of the static magnetic field measurements obtained from both the classical and ultra-shielded NMR settings. It was established that all values were well below the exposure limits set by the European standard for workers’ protection. However, the study revealed that ultra-shield technology can achieve a 20–65-fold reduction in the field strength received by the operator. This reduction in external magnetic field strength enables the instrument to be situated in a wider range of environments with fewer spatial constraints. However, this fact gives rise to another potential hazard, since the stray field strength is observed to increase in a direct and significant manner with decreasing distance from the magnet, both directly above and below it. This increase in the attractive forces exerted on magnetic items is a consequence of this phenomenon.
Up until today, little is known about the long-term effects of chronic occupational exposure to static magnetic fields, as the available epidemiological and experimental evidence remains limited and inconclusive [12,40,44]. This knowledge gap makes it difficult to establish definitive conclusions regarding potential health risks. Regarding the direct effects of magnetic fields, particularly for long-term exposures, the current body of literature does not provide enough evidence to draw definitive conclusions about the biological and health effects of exposure to weak static magnetic fields (SMF). A lack of consistency in the studied biological systems, the examined endpoints, and scientific rigour in many reviewed studies has weakened the credibility of reported findings. While direct effects of weak magnetic fields have been reliably reported, including effects on melatonin bio-synthesis, locomotor activity, blood pressure regulation, brain enzyme activities, or neurotransmitter levels [44], they remain insufficiently understood to be incorporated into existing safety standards. Despite the paucity of long-term risks and potential benefits observed in the majority of animal and human studies conducted to date [45], further research is required to comprehensively assess the exact long-term biological effects of various SMFs on different physiological and pathological conditions. A recent report by the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) updated the potential health effects of exposure to EMF with regard to frequencies between 1 Hz and 100 kHz [46]. The SCHEER report identified a lack of recent systematic reviews or meta-analyses on low-frequency EMF exposure to update the previous (2015) Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) [40] assessment and concluded that there is no convincing evidence of a causal relationship between ELF magnetic field exposure and self-reported symptoms.
Furthermore, standardized procedures for assessing occupational exposure in MR and NMR environments have yet to be developed. The lack of uniform assessment methods complicates efforts to evaluate and mitigate risks, particularly for highly specialized workers in industrial and research settings where exposure to extremely strong static magnetic fields—reaching up to 28 T in some NMR applications—is common. In view of the challenges identified, further studies are imperative to enhance our understanding of exposure conditions and establish safety guidelines that are tailored to workers in high-field environments. This is of crucial importance, as the basis for effective guidelines and best practices lies in a clear understanding of the physical parameters involved.
This is a significant consideration for risk assessment, as non-medical MR environments present unique challenges, including the presence of personnel often inadequately trained about occupational exposure, the high intensity of static magnetic fields and field gradients (active shielding), and the need to perform routine operations (such as tuning or cryogen refilling) in close proximity to the instrument, where magnetic field intensity is highest.
In this paper, we discuss a pathway for acquiring risk awareness for NMR laboratories. The pathway is based on a risk management model that is both concrete and applicable (Figure 3).
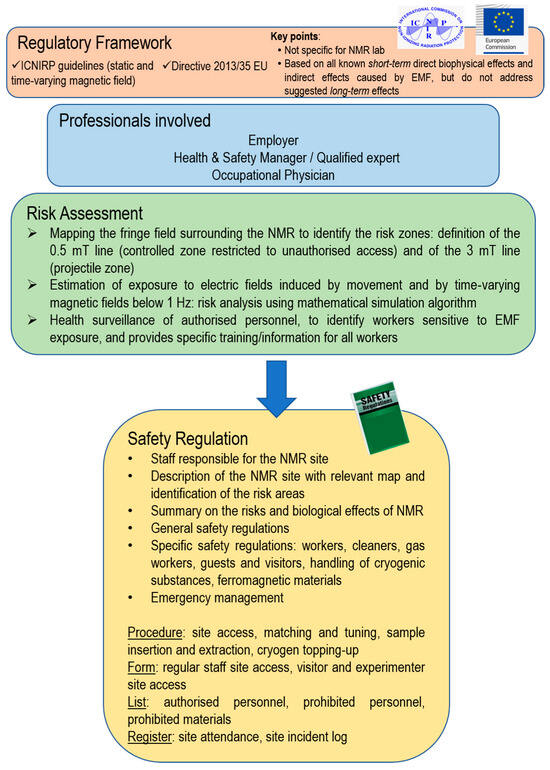
Figure 3.
General overview of NMR laboratory safety management.
As an example, a case study is presented in which mathematical models and algorithms are used to explore and characterize occupational exposure levels to magnetic fields generated by a 300 MHz (7 T) NMR spectrometer. The findings of this study indicate that the maximum magnetic field levels (|B|) and associated exposure levels were observed in the proximity of the spectrometer. The measurements of |B|, spatial distribution, and gradient indicate high-risk areas corresponding to zones with steep magnetic field gradients, where operators should exercise caution and move slowly.
Despite the selection of elevated values for motion velocity and areas exhibiting greater values for magnetic field spatial gradient (worst case), none of the calculated exposure parameters surpassed the relevant limits stipulated by the applicable guidelines [18], meaning that the considered exposure conditions remained in compliance with the stipulated restrictions. Nevertheless, it is not possible to generalize this conclusion due to the high variability of movements, particularly in terms of speed. This variability is contingent on the operator’s habits and body size, as well as the working conditions (whether routine or emergency). It is therefore important to have a simple yet reliable tool for risk assessment under different exposure conditions.
NMR environments can present significant risks for workers with certain implants, particularly AIMDs or other electronic medical equipment. Exposure to electromagnetic fields in these settings can interfere with the functionality of such devices, potentially leading to malfunctions, degraded performance, loss of functionality, or unintended physiological responses. Our findings indicate that workers may be exposed to magnetic fields exceeding 0.5 mT, especially during tasks that require close proximity to the equipment or at heights corresponding to the typical positioning of active medical implants, such as pacemakers and defibrillators. Additionally, movement within the fringe field can induce voltages in the leads of AIMDs, potentially triggering inappropriate device responses. These risks highlight the need for stringent safety measures and exposure assessments for workers with implanted medical devices in high-field NMR environments.
Mattei and colleagues [26] demonstrated that pacemaker (PM) performance degradation can occur during rotational movements at approximately 2 rad/s in an MRI environment. Similarly, for an implantable cardioverter-defibrillator (ICD), movements with a maximum speed of 6 rad/s may lead to a misclassification of ectopic beats as ventricular fibrillation. To the best of our knowledge, a comparable study has not yet been conducted in NMR laboratory settings.
Our simulation results indicate that the voltage induced by typical worker movements near an NMR spectrometer—at positions representative of those for individuals wearing a PM or an ICD—does not exceed the programmed sensitivity threshold of these devices (typically 2 mV) [47,48] in a unipolar configuration. However, in a bipolar configuration, the induced voltage can surpass the lower threshold of 0.3 mV. It is important to note that this threshold is defined for a standardized stimulus with higher frequency components than those of the low-frequency voltage signal induced by movement within the NMR magnetic field, which is approximately 1 Hz [24].
Additionally, comparing the computed maximum induced electric field (EMFmax) with the immunity levels required for PM/ICD compliance with international market-entry standards presents challenges. These immunity levels vary depending on frequency and generally do not account for signals below 16.6 Hz, making direct comparisons difficult.
5. Conclusions
The objective of this study was to enhance awareness of the necessity for safety measures in environments where they have yet to be implemented. The overarching aim is to ensure the safety of NMR environment workers and all other individuals involved. This work constitutes a rigorous attempt to provide original insights that address the critical gap in the extant literature regarding the safety of NMR laboratories. It also offers valuable implications for both research and practical applications.
In conclusion, it is essential to consider that technological advancements in NMR are pushing towards increasingly higher static magnetic field intensities and operating frequencies, significantly increasing the risk level for workers. The risk assessment process in NMR environments must involve all relevant stakeholders. Employers play a crucial role by ensuring that workers undergo medical examinations to certify their health suitability for tasks involving exposure to the spectrometer’s magnetic fields. At the same time, workers must adhere to specific safety protocols and adopt appropriate behaviours, particularly when approaching the spectrometer, to minimize potential risks. Another critical aspect is the design and construction of NMR sites, which, in addition to incorporating necessary safety devices (e.g., quench pipes), must ensure adequate spaces for activities related to the spectrometer’s use, minimizing occupational risks for workers.
With regard to the potential effects of chronic exposure, it is advisable to await further comprehensive knowledge and to adopt technical and operational strategies for exposure minimization. In the absence of substantial data regarding the potential consequences associated with long-term exposure to EMF, the adequacy of health risk assessments pertaining to MR workers remains questionable, particularly with respect to exposure levels that fall below the thresholds associated with acute effects [9]. Therefore, we strongly encourage further, more systematic research in this area. Future studies should specifically investigate the effects of SMF exposure on human biological functioning to determine whether SMF pose any significant health risks.
Finally, updating regulations to ensure safety is essential, particularly for laboratories with outdated equipment lacking advanced safety systems that may pose significant risks. Additionally, raising operator awareness of safety aspects, through specific training programs, for example, and systematically assessing risks using innovative tools and methods, is imperative.
Author Contributions
Conceptualization, V.H. and G.A.; methodology, V.H. and A.F.; software, V.H.; validation, G.A., M.M. and M.A.D.; investigation A.F.; resources, A.F.; data curation, V.H. and G.A.; writing—original draft preparation, V.H. and A.F.; writing—review and editing, G.A., M.A.D. and M.M.; visualization, A.F.; supervision, V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by INAIL (National Institute for Insurance against Accidents at Work), grant number Bric 2022 CUP: J43C22001390005.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge the Multi-Modal Molecular Imaging (MMMI) Italian Node of the Eurobioimaging Research Infrastructure for supporting the research activity at the 7 T high-field MRI facility at the “G. Monasterio” Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wong, K.C. Review of NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry. J. Chem. Educ. 2014, 91, 1103–1104. [Google Scholar] [CrossRef]
- Keeler, J. Understanding NMR Spectroscopy; John Wiley and Sons: Hoboken, NJ, USA, 2010; ISBN 9781119964933. [Google Scholar]
- Acri, G.; Sansotta, C.; Ruello, E.V.; Denaro, L.; Salmeri, F.M.; Testagrossa, B. The Use of Time Domain NMR in Food Analysis: A Review. Curr. Nutr. Food Sci. 2020, 17, 558–565. [Google Scholar] [CrossRef]
- Moser, E.; Laistler, E.; Schmitt, F.; Kontaxis, G. Ultra-high field NMR and MRI-the role of magnet technology to increase sensitivity and specificity. Front. Phys. 2017, 5, 33. [Google Scholar] [CrossRef]
- Schaap, K.; Christopher-De Vries, Y.; Crozier, S.; De Vocht, F.; Kromhout, H. Exposure to static and time-varying magnetic fields from working in the static magnetic stray fields of MRI scanners: A comprehensive survey in the Netherlands. Ann. Occup. Hyg. 2014, 58, 1094–1110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.J.; Kim, K.A. Safety issues and updates under MR environments. Eur. J. Radiol. 2017, 89, 7–13. [Google Scholar] [CrossRef]
- De Vocht, F.; Batistatou, E.; Mölter, A.; Kromhout, H.; Schaap, K.; van Tongeren, M.; Crozier, S.; Gowland, P.; Keevil, S. Transient health symptoms of MRI staff working with 1.5 and 3.0 Tesla scanners in the UK. Eur. Radiol. 2015, 25, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Inferrera, P.; Denaro, L.; Sansotta, C.; Ruello, E.; Anfuso, C.; Salmeri, F.M.; Garreffa, G.; Vermiglio, G.; Testagrossa, B. dB/dt evaluation in MRI sites: Is ICNIRP threshold limit (for workers) exceeded? Int. J. Environ. Res. Public Health 2018, 15, 1298. [Google Scholar] [CrossRef]
- Hartwig, V.; Virgili, G.; Mattei, F.E.; Biagini, C.; Romeo, S.; Zeni, O.; Scarfì, M.R.; Massa, R.; Campanella, F.; Landini, L.; et al. Occupational exposure to electromagnetic fields in magnetic resonance environment: An update on regulation, exposure assessment techniques, health risk evaluation, and surveillance. Med. Biol. Eng. Comput. 2021, 1, 3. [Google Scholar] [CrossRef]
- Bravo, G.; Modenese, A.; Arcangeli, G.; Bertoldi, C.; Camisa, V.; Corona, G.; Giglioli, S.; Ligabue, G.; Moccaldi, R.; Mucci, N.; et al. Subjective Symptoms in Magnetic Resonance Imaging Personnel: A Multi-Center Study in Italy. Front. Public Health 2021, 9, 699675. [Google Scholar] [CrossRef]
- Bouisset, N.; Nissi, J.; Laakso, I.; Reynolds, R.F.; Legros, A. Is activation of the vestibular system by electromagnetic induction a possibility in an MRI context? Bioelectromagnetics 2024, 45, 171–183. [Google Scholar] [CrossRef] [PubMed]
- König, A.M.; Pöschke, A.; Mahnken, A.H. Health risks for medical personnel due to magnetic fields in magnetic resonance imaging. RöFo—Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgeb. Verfahr. 2024, 197, 135–143. [Google Scholar] [CrossRef]
- Glans, A.; Wilén, J.; Lindgren, L.; Björkman-Burtscher, I.M.; Hansson, B. Health effects related to exposure of static magnetic fields and acoustic noise—Comparison between MR and CT radiographers. Eur. Radiol. 2022, 32, 7896–7909. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Y.; Zuo, H.; Li, Y. Neurobiological effects and mechanisms of magnetic fields: A review from 2000 to 2023. BMC Public Health 2024, 24, 3094. [Google Scholar] [CrossRef] [PubMed]
- ICNIRP. Guidelines on Limits of Exposure To Static Magnetic Fields. Health Phys. 2009, 96, 504–514. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Directive 2013/35/EC on the minimum health and safety requirements regarding the exposure of workers to the risks arising from physical agents (electromagnetic fields). Off. J. Eur. Union 2013, L179/1-21. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32013L0035 (accessed on 27 March 2025).
- ICNIRP. Guidelines for limiting exposure to electric fields induced by and by time-varying magnetic fields below 1 Hz. Health Phys. 2014, 106, 418–425. [Google Scholar] [CrossRef]
- Laakso, I.; Kännälä, S.; Jokela, K. Computational dosimetry of induced electric fields during realistic movements in the vicinity of a 3 T MRI scanner. Phys. Med. Biol. 2013, 58, 2625–2640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zilberti, L.; Bottauscio, O.; Chiampi, M. Assessment of exposure to MRI motion-induced fields based on the International Commission on Non-Ionizing Radiation Protection (ICNIRP) guidelines. Magn. Reson. Med. 2016, 76, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Gurrera, D.; Gallias, K.K.; Spanò, M.; Abbate, B.F.; D’Alia, F.; Iacoviello, G.; Caputo, V. Moving across the static magnetic field of a 1.5 T MRI scanner: Analysing compliance with Directive 2013/35/EU. Phys. Medica 2019, 57, 238–244. [Google Scholar] [CrossRef]
- Gurrera, D.; Leardini, A.; Ortolani, M.; Durante, S.; Caputo, V.; Gallias, K.K.; Abbate, B.F.; Rinaldi, C.; Iacoviello, G.; Acri, G.; et al. Experimental and Modeling Analyses of Human Motion Across the Static Magnetic Field of an MRI Scanner. Front. Bioeng. Biotechnol. 2021, 1, 613616. [Google Scholar] [CrossRef]
- Roemer, P.B.; Wade, T.; Alejski, A.; Ertan, K.; McKenzie, C.A.; Rutt, B.K.; Lutz, F. Electric field calculation and peripheral nerve stimulation prediction for head and body gradient coils. Magn. Reson. Med. 2021, 86, 2301–2315. [Google Scholar] [PubMed]
- Acri, G.; Testagrossa, B.; Causa, F.; Tripepi, M.G.; Vermiglio, G.; Novario, R.; Pozzi, L.; Quadrelli, G. Evaluation of occupational exposure in magnetic resonance sites. Radiol. Medica 2014, 119, 208–213. [Google Scholar] [CrossRef]
- Sannino, A.; Romeo, S.; Scarfì, M.R.; Massa, R.; d’Angelo, R.; Petrillo, A.; Cerciello, V.; Fusco, R.; Zeni, O. Exposure Assessment and Biomonitoring of Workers in Magnetic Resonance Environment: An Exploratory Study. Front. Public Health 2017, 5, 344. [Google Scholar] [CrossRef]
- Hartwig, V.; Virgili, G.; Ferrante Vero, L.F.; De Marchi, D.; Landini, L.; Giovannetti, G. Towards a Personalised and Interactive Assessment of Occupational Exposure To Magnetic Field During Daily Routine in Magnetic Resonance. Radiat. Prot. Dosim. 2018, 182, 546–554. [Google Scholar] [CrossRef]
- Hartwig, V.; Biagini, C.; De Marchi, D.; Flori, A.; Gabellieri, C.; Virgili, G.; Ferrante Vero, L.F.L.F.; Landini, L.; Vanello, N.; Giovannetti, G. The Procedure for Quantitative Characterization and Analysis of Magnetic Fields in Magnetic Resonance Sites for Protection of Workers: A Pilot Study. Ann. Work Expo. Health 2019, 63, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, V.; Biagini, C.; De Marchi, D.; Flori, A.; Gabellieri, C.; Virgili, G.; Fabiano, L.; Vero, F.; Landini, L.; Vanello, N.; et al. Analysis, comparison and representation of occupational exposure to a static magnetic field in a 3-T MRI site. Int. J. Occup. Saf. Ergon. 2022, 28, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Reif, B.; Ashbrook, S.E.; Emsley, L.; Hong, M. Solid-state NMR spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 2. [Google Scholar] [CrossRef]
- Berlana, T.; Úbeda, A. Occupational exposure of NMR spectrometrists to static and radiofrequency fields. Radiat. Prot. Dosim. 2017, 177, 397–406. [Google Scholar] [CrossRef]
- Council of the European Communities. CELEX1, 1999/519/EC: Council Recommendation of 12 July 1999 on the Limitation of Exposure of the General Public to Electromagnetic Fields (0 Hz to 300 GHz); Publications Office of the European Union: Luxembourg, 1999. [Google Scholar]
- Mattei, E.; Censi, F.; Calcagnini, G.; Falsaperla, R.; Genovese, E.; Napolitano, A.; Cannatà, V. Pacemaker and ICD oversensing induced by movements near the MRI scanner bore. Med. Phys. 2016, 43, 6621–6631. [Google Scholar] [CrossRef]
- Shimizu, H.; Tsujita, K. Control strategy of human the sit-to-stand movement. In Proceedings of the 2021 60th Annual Conference of the Society of Instrument and Control Engineers of Japan (SICE), Tokyo, Japan, 8–10 September 2021; pp. 144–149. [Google Scholar]
- Decat, G. Occupational Exposure Assessment of the Static Magnetic Flux Density Generated by Nuclear Magnetic Resonance Spectroscopy for Biochemical Purposes. PIERS Online 2007, 3, 513–516. [Google Scholar] [CrossRef]
- Hartwig, V.; Sansotta, C.; Morelli, M.S.; Testagrossa, B.; Acri, G. Occupational Exposure Assessment of the Static Magnetic Field Generated by Nuclear Magnetic Resonance Spectroscopy: A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 7674. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, F.; Liket, L.; De Vocht, A.; Mistry, T.; Glover, P.; Gowland, P.; Kromhout, H. Exposure to alternating electromagnetic fields and effects on the visual and visuomotor systems. Br. J. Radiol. 2007, 80, 822–828. [Google Scholar] [CrossRef] [PubMed]
- McRobbie, D.W. Occupational exposure in MRI. Br. J. Radiol. 2012, 85, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Testagrossa, B.; Vermiglio, G. Personal Time-Varying Magnetic Fields Evaluation During Activities in MRI Sites. In Proceedings of the IFMBE Proceedings, Toronto, ON, Canada, 7–12 June 2015; Volume 51, pp. 741–744. [Google Scholar]
- Hartwig, V.; Romeo, S.; Zeni, O. Occupational exposure to electromagnetic fields in magnetic resonance environment: Basic aspects and review of exposure assessment approaches. Med. Biol. Eng. Comput. 2018, 56, 531–545. [Google Scholar] [CrossRef] [PubMed]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Potential Health Effects of Exposure to Electromagnetic Fields (EMF); European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Heinrich, A.; Szostek, A.; Nees, F.; Meyer, P.; Semmler, W.; Flor, H. Effects of static magnetic fields on cognition, vital signs, and sensory perception: A meta-analysis. J. Magn. Reson. Imaging 2011, 34, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Schaap, K.; Portengen, L.; Kromhout, H. Exposure to MRI-related magnetic fields and vertigo in MRI workers. Occup. Environ. Med. 2016, 73, 161–166. [Google Scholar]
- Walker, M.; Fultz, A.; Davies, C.; Brockopp, D. Symptoms Experienced by MR Technologists Exposed to Static Magnetic Fields. Radiol. Technol. 2020, 91, 316–323. [Google Scholar]
- Driessen, S.; Bodewein, L.; Dechent, D.; Graefrath, D.; Schmiedchen, K.; Stunder, D.; Kraus, T.; Petri, A.K. Biological and health-related effects of weak static magnetic fields (<1 mT) in humans and vertebrates: A systematic review. PLoS ONE 2020, 15, e0230038. [Google Scholar] [CrossRef]
- Zhang, X. Biological Effects of Static Magnetic Fields, 2nd ed.; Springer: Singapore, 2023; ISBN 9789811988691. [Google Scholar]
- SCHEER (Scientific Committee on Health Environmental and Emerging Risks). Potential Health Effects of Exposure to Electromagnetic Fields (EMF): Update with Regard to Frequencies Between 1 Hz and 100 kHz—Preliminary Opinion; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- UNI EN 45502-2-1:2005; Active Implantable Medical Devices—Part 2-1: Particular Requirements for Active Implantable Medical Devices Intended to Treat Bradyarrhythmia (Cardiac Pacemakers). UNI: Roma, Italia, 2005.
- UNI EN 45502-2-2:2008; Active implantable Medical Devices—Part 2-2: Particular Requirements for Active Implantable Medical Devices Intended to Treat Tachyarrhythmia (Includes Implantable Defibrillators). UNI: Roma, Italia, 2008.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).