The Role of the Home Environment in Perinatal Depression: A Systematic Review and Meta-Analysis of Observational Epidemiological Studies
Abstract
1. Introduction
2. Methods
2.1. Literature Searches
2.2. Study Selection
2.3. Risk of Bias Assessment
2.4. Data Extraction and Synthesis
3. Results
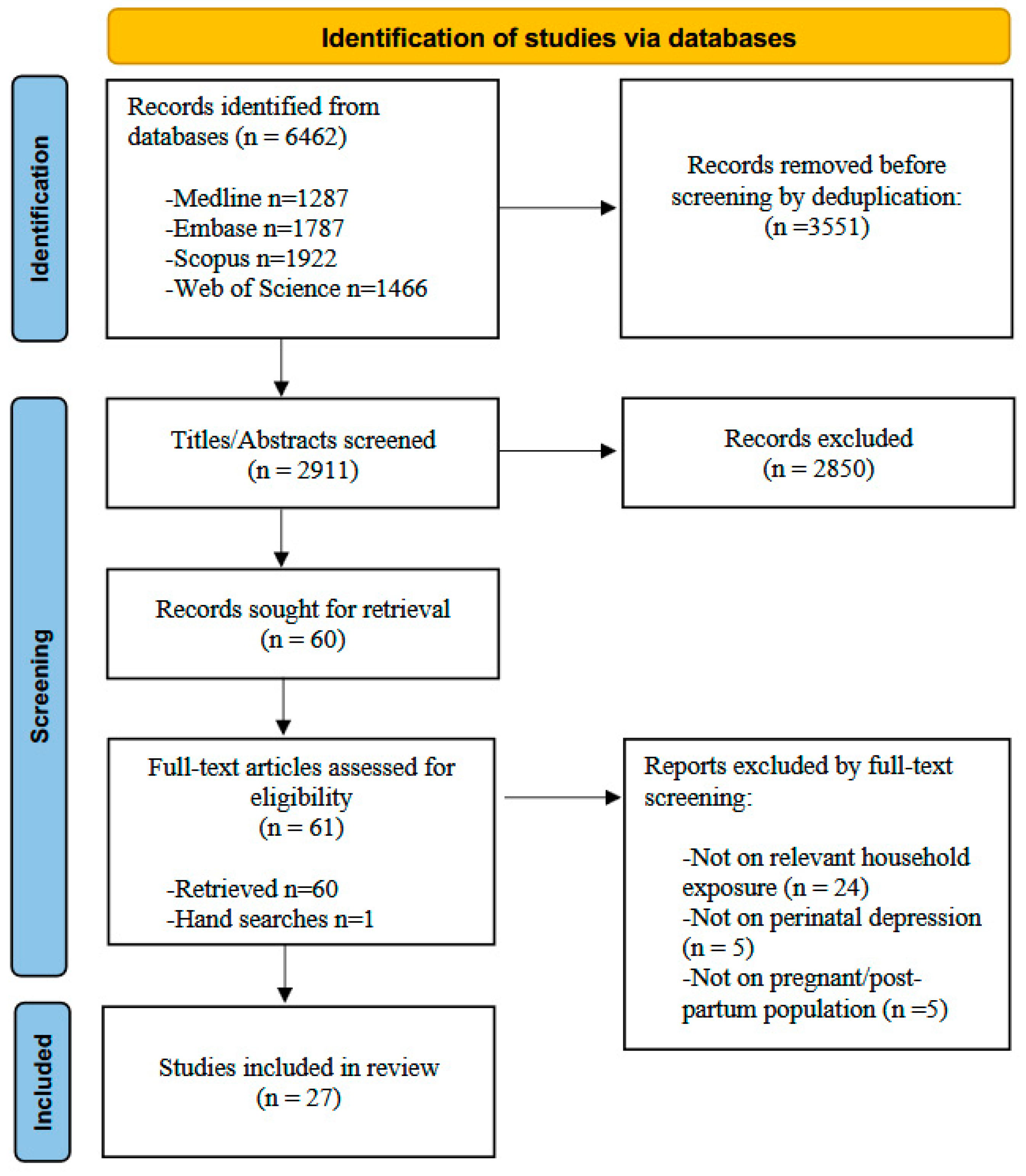
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. RoB Assessment
3.4. Summary of Findings
3.5. Housing Instability
3.6. Housing Quality
3.7. Indoor Air Quality
3.8. Household Chemicals
3.9. Biological Exposures
3.10. Noise
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riva, A.; Rebecchi, A.; Capolongo, S.; Gola, M. Can Homes Affect Well-Being? A Scoping Review among Housing Conditions, Indoor Environmental Quality, and Mental Health Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 15975. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.D.; Eisner, M.D.; Katz, P.P.; Yen, I.H.; Archea, C.; Earnest, G.; Janson, S.; Masharani, U.B.; Quinlan, P.J.; Hammond, S.K.; et al. Impact of the Home Indoor Environment on Adult Asthma and Rhinitis. J. Occup. Environ. Med. 2005, 47, 362. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.A.; Sharpe, R.A.; Taylor, T.; Morrissey, K. Indoor PM2.5, VOCs and asthma outcomes: A systematic review in adults and their home environments. Environ. Res. 2021, 202, 111631. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, A.; Gola, M.; Riva, A.; Capolongo, S. Can housing conditions and features affect well-being? A review through Indoor Environmental Quality aspects and Mental Health implications. Eur. J. Public Health 2023, 33 (Suppl. 2), ckad160.715. [Google Scholar] [CrossRef]
- Yi, L.; Xu, Y.; Eckel, S.P.; O’Connor, S.; Cabison, J.; Rosales, M.; Chu, D.; Chavez, T.A.; Johnson, M.; Mason, T.B.; et al. Time-activity and daily mobility patterns during pregnancy and early postpartum—Evidence from the MADRES cohort. Spat. Spatio-Temporal Epidemiol. 2022, 41, 100502. [Google Scholar] [CrossRef]
- Morganti, A.; Brambilla, A.; Aguglia, A.; Amerio, A.; Miletto, N.; Parodi, N.; Porcelli, C.; Odone, A.; Costanza, A.; Signorelli, C.; et al. Effect of Housing Quality on the Mental Health of University Students during the COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2022, 19, 2918. [Google Scholar] [CrossRef]
- Ascone, L.; Mascherek, A.; Weber, S.; Fischer, D.; Augustin, J.; Cheng, B.; Thomalla, G.; Augustin, M.; Zyriax, B.-C.; Gallinat, J.; et al. Subjective evaluation of home environment and levels of self-reported depression in middle to old age: Results from the HCHS study. J. Clin. Psychol. 2024, 80, 1115–1129. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Han, B.; van der Kuijp, T.J.; Xia, Y.; Chen, K. Exposure and perception of PM2.5 pollution on the mental stress of pregnant women. Environ. Int. 2021, 156, 106686. [Google Scholar] [CrossRef]
- Aguilera, J.; Konvinse, K.; Lee, A.; Maecker, H.; Prunicki, M.; Mahalingaiah, S.; Sampath, V.; Utz, P.J.; Yang, E.; Nadeau, K.C. Air pollution and pregnancy. Semin. Perinatol. 2023, 47, 151838. [Google Scholar] [CrossRef]
- Sobotova, L.; Liu, Y.-H.; Burakoff, A.; Sevcikova, L.; Weitzman, M. Household exposure to secondhand smoke is associated with decreased physical and mental health of mothers in the USA. Matern. Child Health J. 2011, 15, 128. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Cao, L.; Chang, Q.; Zhao, Y. Associations between long term exposures to outdoor air pollution and indoor solid fuel use and depression in China. J. Environ. Manag. 2022, 302, 113982. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yan, Z. Depression in the house: The effects of household air pollution from solid fuel use among the middle-aged and older population in China. Sci. Total Environ. 2020, 703, 134706. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, H.; Liu, Y.; Liu, H.; Shi, J.; Zhao, C.; He, M. Association of using biomass fuel for cooking with depression and anxiety symptoms in older Chinese adults. Sci. Total Environ. 2022, 811, 152256. [Google Scholar] [CrossRef]
- Okun, M.L. Disturbed Sleep and Postpartum Depression. Curr. Psychiatry Rep. 2016, 18, 66. [Google Scholar] [CrossRef]
- He, S.; Smargiassi, A.; Low, N.; Bilodeau-Bertrand, M.; Ayoub, A.; Auger, N. Residential noise exposure and the longitudinal risk of hospitalization for depression after pregnancy: Postpartum and beyond. Environ. Res. 2019, 170, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Roddy Mitchell, A.; Gordon, H.; Lindquist, A.; Walker, S.P.; Homer, C.S.E.; Middleton, A.; Cluver, C.A.; Tong, S.; Hastie, R. Prevalence of Perinatal Depression in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry 2023, 80, 425–431. [Google Scholar] [CrossRef]
- Al-Abri, K.; Edge, D.; Armitage, C.J. Prevalence and correlates of perinatal depression. Soc. Psychiatry Psychiatr. Epidemiol. 2023, 58, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 18 November 2024).
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connnell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 18 November 2024).
- Voaklander, B.; Rowe, S.; Sanni, O.; Campbell, S.; Eurich, D.; Ospina, M.B. Prevalence of diabetes in pregnancy among Indigenous women in Australia, Canada, New Zealand, and the USA: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e681–e698. [Google Scholar] [CrossRef]
- Amjad, S.; Tromburg, C.; Adesunkanmi, M.; Mawa, J.; Mahbub, N.; Campbell, S.; Chari, R.; Rowe, B.H.; Ospina, M.B. Social Determinants of Health and Pediatric Emergency Department Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Ann. Emerg. Med. 2024, 83, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Cerin, E.; Nathan, A.; van Cauwenberg, J.; Barnett, D.W.; Barnett, A.; on behalf of the Council on Environment and Physical Activity (CEPA)—Older Adults working group. The neighbourhood physical environment and active travel in older adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 15. [Google Scholar] [CrossRef]
- Chandrabose, M.; Rachele, J.N.; Gunn, L.; Kavanagh, A.; Owen, N.; Turrell, G.; Giles-Corti, B.; Sugiyama, T. Built environment and cardio-metabolic health: Systematic review and meta-analysis of longitudinal studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2019, 20, 41–54. [Google Scholar] [CrossRef]
- Stouffer, S.A.; Suchman, E.A.; Devinney, L.C.; Star, S.A.; Williams, R.M., Jr. The American Soldier: Adjustment During Army Life. (Studies in Social Psychology in World War II), Vol. 1; Princeton University Press: Oxford, UK, 1949; pp. xii, 599. [Google Scholar]
- Bland, M. An Introduction to Medical Statistics; Oxford University Press: Oxford, UK, 2015; 737p, ISBN 978-0-19-251839-2. [Google Scholar]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Inadera, H. Pet ownership during pregnancy and mothers’ mental health conditions up to 1 year postpartum: A nationwide birth cohort-the Japan Environment and Children’s Study. Soc. Sci. Med. 2022, 309, 115216. [Google Scholar] [CrossRef]
- Huang, J.; Wen, G.; Yang, W.; Yao, Z.; Wu, C.; Ye, X. The association between second-hand smoke exposure and depressive symptoms among pregnant women. Psychiatry Res. 2017, 256, 469–474. [Google Scholar] [CrossRef]
- Agostini, F.; Neri, E.; Salvatori, P.; Dellabartola, S.; Bozicevic, L.; Monti, F. Antenatal Depressive Symptoms Associated with Specific Life Events and Sources of Social Support Among Italian Women. Matern. Child Health J. 2014, 19, 1131–1141. [Google Scholar] [CrossRef]
- Aung, M.T.; Eick, S.M.; Padula, A.M.; Smith, S.; Park, J.-S.; DeMicco, E.; Woodruff, T.J.; Morello-Frosch, R. Maternal per- and poly-fluoroalkyl substances exposures associated with higher depressive symptom scores among immigrant women in the Chemicals in Our Bodies cohort in San Francisco. Environ. Int. 2023, 172, 107758. [Google Scholar] [CrossRef]
- Hu, J.; Wan, N.; Ma, Y.; Liu, Y.; Liu, B.; Li, L.; Liu, C.; Qiao, C.; Wen, D. Trimester-specific association of perceived indoor air quality with antenatal depression: China medical university birth cohort study. Indoor Air 2022, 32, e13167. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Mei, H.; Feng, H.; Huang, Y.; Cai, X.; Xiang, F.; Chen, L.; Xiao, H. Exposure to bisphenols, parabens and phthalates during pregnancy and postpartum anxiety and depression symptoms: Evidence from women with twin pregnancies. Environ. Res. 2023, 221, 115248. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Stein, C.R.; Liu, M.; Ackerman, M.G.; Blakemore, J.K.; Long, S.E.; Pinna, G.; Romay-Tallon, R.; Kannan, K.; Zhu, H.; et al. Prenatal Exposure to Bisphenols and Phthalates and Postpartum Depression: The Role of Neurosteroid Hormone Disruption. J. Clin. Endocrinol. Metab. 2021, 106, 1887–1899. [Google Scholar] [CrossRef]
- Jigeer, G.; Tao, W.; Zhu, Q.; Xu, X.; Zhao, Y.; Kan, H.; Cai, J.; Xu, Z. Association of residential noise exposure with maternal anxiety and depression in late pregnancy. Environ. Int. 2022, 168, 107473. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Miyake, Y.; Tanaka, K.; Furukawa, S.; Arakawa, M. Smoking and secondhand smoke exposure and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. Tob. Induc. Dis. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Arif, A.A.; Laditka, J.N.; Racine, E.F. Prenatal exposure to secondhand smoke may increase the risk of postpartum depressive symptoms. J. Public Health 2015, 37, 406–411. [Google Scholar] [CrossRef][Green Version]
- Messer, L.C.; Maxson, P.; Miranda, M.L. The Urban Built Environment and Associations with Women’s Psychosocial Health. J. Urban Health 2012, 90, 857–871. [Google Scholar] [CrossRef]
- Mutic, A.D.; Barr, D.B.; Hertzberg, V.S.; Brennan, P.A.; Dunlop, A.L.; McCauley, L.A. Polybrominated Diphenyl Ether Serum Concentrations and Depressive Symptomatology in Pregnant African American Women. Int. J. Environ. Res. Public Health 2021, 18, 3614. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.R.; Fassett, M.J.; Arita, Y.; Chiu, V.Y.; Takhar, H.S.; Getahun, D. Exposure to polybrominated diphenyl ether-47 increases the risk of post-partum depression. J. Matern. Fetal Neonatal Med. 2021, 35, 8350–8354. [Google Scholar] [CrossRef]
- Sandel, M.; Sheward, R.; Ettinger de Cuba, S.; Coleman, S.M.; Frank, D.A.; Chilton, M.; Black, M.; Heeren, T.; Pasquariello, J.; Casey, P.; et al. Unstable Housing and Caregiver and Child Health in Renter Families. Pediatrics 2018, 141, e20172199. [Google Scholar] [CrossRef]
- Suglia, S.F.; Duarte, C.S.; Sandel, M.T. Housing Quality, Housing Instability, and Maternal Mental Health. J. Urban Health 2011, 88, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Courtney, L.P.; El-Mohandes, A.A.E.; Gantz, M.G.; Blake, S.M.; Thornberry, J.; El-Khorazaty, M.N.; Perry, D.; Kiely, M. Relationships Between Self-Reported Smoking, Household Environmental Tobacco Smoke Exposure and Depressive Symptoms in a Pregnant Minority Population. Matern. Child Health J. 2011, 15 (Suppl. S1), S65–S74. [Google Scholar] [CrossRef]
- Farrow, A.; Taylor, H.; Northstone, K.; Golding, J. Symptoms of Mothers and Infants Related to Total Volatile Organic Compounds in Household Products. Arch. Environ. Health Int. J. 2010, 58, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, K.; Kong, B.; Zhang, B. Exposure to third-hand smoke during pregnancy may increase the risk of postpartum depression in China. Tob. Induc. Dis. 2018, 16, 17. [Google Scholar] [CrossRef]
- Wang, H.; Ren, T.; Zhang, N.; Xia, W.; Xiang, M.; Ran, J.; Zhang, J. Poly- and perfluoroalkyl substances exposure during pregnancy and postpartum depression: Evidence from the Shanghai birth cohort. Chemosphere 2023, 318, 137941. [Google Scholar] [CrossRef]
- Wei, D.; Shen, S.; Lu, J.; Liu, W.; Chen, N.; Bong Hubert Lam, K.; Lun Au Yeung, S.; Xia, H.; Qiu, X. Association between incense burning and prenatal depressive symptoms: Evidence from the Born in Guangzhou Cohort Study. Environ. Sci. Pollut. Res. 2023, 30, 40860–40869. [Google Scholar] [CrossRef]
- Butler, S.; Williams, M.; Tukuitonga, C.; Paterson, J. Problems with damp and cold housing among Pacific families in New Zealand. N. Z. Med. J. 2003, 116, U494. [Google Scholar]
- Foster, S.A.; Kile, M.L.; Hystad, P.; Diamond, M.L.; Jantunen, L.M.; Mandhane, P.J.; Moraes, T.J.; Navaranjan, G.; Scott, J.A.; Simons, E.; et al. Organophosphate ester flame retardants and plasticizers in house dust and mental health outcomes among Canadian mothers: A nested prospective cohort study in CHILD. Environ. Res. 2024, 240 Pt 1, 117451. [Google Scholar] [CrossRef] [PubMed]
- Chilukuri, P.; Patel, N.; Cockerham, C.; Su, L.; Stromberg, A.; O’Brien, J.; Parilla, B. Association of Food and Housing Insecurity on Outcomes in Pregnant Patients with Substance Use Disorder. Subst. Use Addict. J. 2024, 45, 645–652. [Google Scholar] [CrossRef]
- McGovern, M.E.; Rokicki, S.; Von Jaglinsky, A.; Reichman, N.E. Neighborhood-level housing affordability and maternal depression. SSM—Ment. Health 2023, 3, 100192. [Google Scholar] [CrossRef]
- Hu, L.; Mei, H.; Cai, X.; Song, L.; Xu, Q.; Gao, W.; Zhang, D.; Zhou, J.; Sun, C.; Li, Y.; et al. Prenatal exposure to poly- and perfluoroalkyl substances and postpartum depression in women with twin pregnancies. Int. J. Hyg. Environ. Health 2024, 256, 114324. [Google Scholar] [CrossRef]
- Hu, J.; Liu, B.; Cui, H.; Liu, Y.; Wan, N.; Li, L.; Zheng, L.; Wang, X.; Yang, Z.; Ma, Y.; et al. Dose-response associations of maternal prenatal noise exposure duration with antepartum depression status. BMC Pregnancy Childbirth 2024, 24, 7. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Hamra, G.B.; Monk, C.; Crum, R.M.; Upadhyaya, S.; Avalos, L.A.; Bastain, T.M.; Barrett, E.S.; Bush, N.R.; Dunlop, A.L.; et al. Prenatal Exposure to Nonpersistent Environmental Chemicals and Postpartum Depression. JAMA Psychiatry 2023, 81, 67. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Ghassabian, A.; Gore, A.C.; Trasande, L. Exposure to environmental chemicals and perinatal psychopathology. Biochem. Pharmacol. 2022, 195, 114835. [Google Scholar] [CrossRef]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef]
- Neven, K.Y.; Saenen, N.D.; Tarantini, L.; Janssen, B.G.; Lefebvre, W.; Vanpoucke, C.; Bollati, V.; Nawrot, T.S. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: An ENVIRONAGE cohort study. Lancet Planet. Health 2018, 2, e174–e183. [Google Scholar] [CrossRef] [PubMed]
- Hoisington, A.J.; Stearns-Yoder, K.A.; Stamper, C.E.; Holliday, R.; Brostow, D.P.; Penzenik, M.E.; Forster, J.E.; Postolache, T.T.; Lowry, C.A.; Brenner, L.A. Association of homelessness and diet on the gut microbiome: A United States-Veteran Microbiome Project (US-VMP) study. mSystems 2023, 9, e01021-23. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients?: Le microbiote Intestinal et la Santé Mentale: Que Devrions-Nous dire à nos Patients? Can. J. Psychiatry Rev. Can. Psychiatr. 2019, 64, 747–760. [Google Scholar] [CrossRef]
- Robinson, K.; Sherman, A.D.F.; Ogunwole, S.; Meggett, J.; Sharps, P. Social Determinant of Housing Instability and Adverse Pregnancy Outcomes: A Scoping Review. J. Perinat. Neonatal Nurs. 2022, 36, 118. [Google Scholar] [CrossRef]
- DiTosto, J.D.; Holder, K.; Soyemi, E.; Beestrum, M.; Yee, L.M. Housing instability and adverse perinatal outcomes: A systematic review. Am. J. Obstet. Gynecol. 2021, 3, 100477. [Google Scholar] [CrossRef]
- Kelly, L.; Martin-Kerry, J.; Prady, S. Postnatal Depression and Homelessness in Women Living in High-Income Countries: A Scoping Review. Psychol. Stud. 2023, 68, 489–501. [Google Scholar] [CrossRef]
- Liu, Y.; Njai, R.S.; Greenlund, K.J.; Chapman, D.P.; Croft, J.B. Relationships Between Housing and Food Insecurity, Frequent Mental Distress, and Insufficient Sleep Among Adults in 12 US States. 2009. Available online: https://stacks.cdc.gov/view/cdc/22186 (accessed on 18 November 2024).
- Lee, C.Y.; Zhao, X.; Reesor-Oyer, L.; Cepni, A.B.; Hernandez, D.C. Bidirectional Relationship Between Food Insecurity and Housing Instability. J. Acad. Nutr. Diet. 2021, 121, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Priester, M.A.; Foster, K.A.; Shaw, T.C. Are Discrimination and Social Capital Related to Housing Instability? Hous. Policy Debate 2017, 27, 120–136. [Google Scholar] [CrossRef]
- Groot, J.; Keller, A.; Pedersen, M.; Sigsgaard, T.; Loft, S.; Nybo Andersen, A.-M. Indoor home environments of Danish children and the socioeconomic position and health of their parents: A descriptive study. Environ. Int. 2022, 160, 107059. [Google Scholar] [CrossRef]
- Muchomba, F.M.; Teitler, J.; Reichman, N.E. Association Between Housing Affordability and Severe Maternal Morbidity. JAMA Netw. Open 2022, 5, e2243225. [Google Scholar] [CrossRef]
- Singh, A.; Daniel, L.; Baker, E.; Bentley, R. Housing disadvantage and poor mental health: A systematic review. Am. J. Prev. Med. 2019, 57, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.M.; Hill, T.D.; Hale, L. Household Disrepair and the Mental Health of Low-Income Urban Women. J. Urban Health Bull. N. Y. Acad. Med. 2011, 88, 142–153. [Google Scholar] [CrossRef]
- Míguez, M.C.; Pereira, B. Effects of active and/or passive smoking during pregnancy and the postpartum period. An. Pediatría Engl. Ed. 2021, 95, 222–232. [Google Scholar] [CrossRef]
- Hoyt, A.T.; Canfield, M.A.; Romitti, P.A.; Botto, L.D.; Anderka, M.T.; Krikov, S.V.; Feldkamp, M.L. Does Maternal Exposure to Secondhand Tobacco Smoke During Pregnancy Increase the Risk for Preterm or Small-for-Gestational Age Birth? Matern. Child Health J. 2018, 22, 1418–1429. [Google Scholar] [CrossRef]
- Frazer, K.; Fitzpatrick, P.; Brosnan, M.; Dromey, A.M.; Kelly, S.; Murphy, M.; O’Brien, D.; Kelleher, C.C.; McAuliffe, F.M. Smoking Prevalence and Secondhand Smoke Exposure during Pregnancy and Postpartum—Establishing Risks to Health and Human Rights before Developing a Tailored Programme for Smoking Cessation. Int. J. Environ. Res. Public Health 2020, 17, 1838. [Google Scholar] [CrossRef]
- Nwosu, C.; Angus, K.; Cheeseman, H.; Semple, S. Reducing Secondhand Smoke Exposure Among Nonsmoking Pregnant Women: A Systematic Review. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2020, 22, 2127–2133. [Google Scholar] [CrossRef]
- Kerr, L.K.; Len, D.; Kerr, J. Screening tools for depression in primary care: The effects of culture, gender, and somatic symptoms on the detection of depression. West. J. Med. 2001, 175, 349. [Google Scholar] [CrossRef] [PubMed]
| Study, Year | Country | Study Design | Study Population | Sample Size (N) | Housing Factors Examined (Method of Assessment) | Perinatal Depression (Screening Tool Used, Timepoint(s)) |
|---|---|---|---|---|---|---|
| Agostini, 2015 [33] | Italy | Prospective Cohort |
| 404 |
| Edinburgh Postnatal Depression Scale (EPDS); third trimester |
| Aung, 2023 [34] | USA | Retrospective Cohort |
| 517 | Per- and poly-fluoroalkyl substances (PFAS) (serum samples collected in the second trimester) | Center for Epidemiological Studies Depression Scale (CES-D); second trimester |
| Butler, 2003 [51] | New Zealand | Cross-Sectional |
| 1376 |
| EPDS; six weeks after birth |
| Chilukuri, 2024 [53] | USA | Prospective Cohort |
| 494 | Housing Insecurity (self-reported) | EPDS; timepoint not reported (NR) |
| Farrow, 2003 [47] | UK | Prospective Cohort |
| 13,971 |
| EPDS; 9 months postpartum |
| Foster, 2024 [52] | USA | Prospective Cohort |
| 718 | Organophosphates (house dust collected at 3–4 months postpartum) | CES-D; 18- and 36-weeks’ gestation, 6 and 12 months postpartum |
| Hu, 2024 [55] | China | Prospective Cohort |
| 150 | Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) (maternal whole blood samples collected at each of three clinic visits) | Chinese CES-D; early pregnancy and followed-up either 1 or 6 months postpartum |
| Hu, 2024 [56] | China | Prospective Cohort |
| 2166 | Noise exposure (self-reported) | EPDS; one of three trimesters |
| Hu, 2022 [35] | China | Prospective Cohort |
| 2166 | Perceived indoor air quality (PIAQ) (self-reported) | EPDS; each trimester |
| Hu, 2023 [36] | China | Prospective Cohort |
| 432 |
| Chinese version of EPDS; early pregnancy, 1 and 6 months postpartum |
| Huang, 2017 [32] | China | Cross-Sectional |
| 2176 |
| CES-D; during pregnancy |
| Jacobson, 2021 [37] | USA | Prospective Cohort |
| 139 |
| Patient Health Questionnaire (PHQ-9) and EPDS; each trimester of pregnancy and 4 months postpartum |
| Jacobson, 2023 [57] | USA | Prospective Cohort |
| 2174 |
| EPDS and CES-D; 2 weeks and 12 months postpartum |
| Jigeer, 2022 [38] | China | Cross-sectional |
| 2018 | Noise (land use regression model based on participant’s residence) | Chinese version of CES-D; late pregnancy |
| Kawasaki, 2017 [39] | Japan | Retrospective Cohort |
| 1745 |
| Japanese version of CES-D; between the 5th and 39th weeks of pregnancy |
| Khan, 2015 [40] | USA | Retrospective Cohort |
| 6884 | Second-hand smoke (self-reported) | Two questions from the Pregnancy Risk Assessment Monitoring System; between 2nd and 6th months postpartum |
| Matsumura, 2022 [31] | Japan | Prospective Cohort |
| 80,814 | Pets (self-reported) | EPDS and Kessler Psychological Distress Scale; first trimester, second/third trimester, 1 month, 6 months and 1 year postpartum |
| Mcgovern, 2023 [54] | USA | Prospective Cohort |
| 4898 | Neighborhood rent burden (administrative data) | Composite International Diagnostic Interview Short Form (CIDI-SF); after giving birth, one-year post-partum |
| Messer, 2012 [41] | USA | Prospective Cohort |
| 723 | Housing damage (self-reported) | CES-D; timepoint NR |
| Mutic, 2021 [42] | USA | Retrospective Cohort |
| 193 | Polybrominated diphenyl ethers (PBDEs)
| EPDS; 8–14 weeks gestation |
| Peltier, 2022 [43] | USA | Case–control |
| 367 | PBDE 47 (plasma samples) | International Classification of Diseases (ICD-10 codes), year after delivery |
| Sandel, 2018 [44] | USA | Retrospective Cohort |
| 22,324 |
| 3-item screening test developed for maternal depression, NR |
| Suglia, 2011 [45] | USA | Retrospective Cohort |
| 2104 |
| CIDI-SF; 12- and 36-month post-partum |
| Tan, 2011 [46] | USA | Cross-Sectional |
| 929 | Household environmental tobacco smoke exposure (self-reported) | Back Depression Inventory (BDI) Fast Screen; second or third trimester |
| Wang, 2018 [48] | China | Cross-sectional |
| 973 | Third-hand smoke exposure (self-reported) | EPDS; post-partum |
| Wang, 2023 [49] | China | Retrospective Cohort |
| 2741 | PFAS (blood samples) | EPDS; 6 weeks postpartum |
| Wei, 2023 [50] | China | Retrospective Cohort |
| 21,306 | Incense burning (self-reported) | Self-Rating Depression Scale (SDS); early and late pregnancy |
| Design: Cohort * | |||||||
| Author, Year | Selection | Comparability | Outcome Assessment | ||||
| Representativeness of Study Sample | Ascertainment of Exposure | Outcomes Not Present at Outset | Study Controls for Confounders | Assessment of Outcome | Follow-Up Adequacy | RoB Rating ** | |
| Agostini, 2015 [33] | ? | ? | ? | Unclear | |||
| Aung, 2023 [34] | ? | ? | ? | Unclear | |||
| Chilukuri, 2024 [53] | ? | ⊕ | ⊕ | Unclear | |||
| Farrow, 2003 [47] | ? | ? | ⊕ | Unclear | |||
| Foster, 2024 [52] | ? | ⊕ | ⊕ | Unclear | |||
| Hu, 2022 [35] | ⊕ | ? | ⊕ | Low | |||
| Hu, 2023 [36] | ⊕ | ? | ⊕ | Low | |||
| Hu, 2024 [55] | ⊕ | ? | ⊕ | Low | |||
| Hu, 2024 [56] | ⊕ | ? | ⊕ | Low | |||
| Jacobson, 2021 [37] | ⊕ | ? | ⊕ | Low | |||
| Jacobson, 2023 [57] | ⊕ | ? | ⊕ | Low | |||
| Kawasaki, 2017 [39] | ? | ⊕ | ? | Unclear | |||
| Khan, 2015 [40] | ? | ? | ⊕ | Unclear | |||
| Matsumura, 2022 [31] | ? | ⊕ | ⊕ | Unclear | |||
| Mcgovern, 2023 [54] | ⊕ | ⊕ | ⊕ | Low | |||
| Messer, 2012 [41] | ⊕ | ? | ? | Unclear | |||
| Mutic, 2021 [42] | ? | ? | ? | Unclear | |||
| Peltier, 2022 [43] | ⊕ | ? | ? | Unclear | |||
| Sandel, 2018 [44] | ? | ? | ? | Unclear | |||
| Suglia, 2011 [45] | ⊕ | ? | ⊕ | Low | |||
| Wang, 2023 [49] | ⊕ | ? | ⊕ | Low | |||
| Wei, 2023 [50] | ⊕ | ? | ⊕ | Low | |||
| Design: Cross-sectional + | |||||||
| Author, Year | External Validity | Internal Validity | RoB Rating ++ | ||||
| Butler, 2003 [51] | ⊕⊕⊕⊕ | ⊕⊕⊕⊕⊕⊗ | Low | ||||
| Huang, 2017 [32] | ⊕⊕⊕⊕ | ⊕⊕⊕⊕⊗⊕ | Low | ||||
| Jigeer, 2022 [38] | ⊕⊕⊗⊕ | ⊕⊕⊕⊕⊕⊗ | Low | ||||
| Tan, 2011 [46] | ⊕⊕⊕⊕ | ⊕⊗⊕⊕⊕⊕ | Low | ||||
| Wang, 2018 [48] | ⊕⊕⊕⊗ | ⊕⊕⊕⊕⊕⊕ | Low | ||||
| Home Environment Domains | Sample Size (Number of Studies) | Direction of Effect * | Overall Risk of Bias (RoB) | Weighted Z-Score (p-Value) ** | Strength of Evidence + |
|---|---|---|---|---|---|
| General Housing Characteristics | |||||
| 30,224 (5) | ↑ (3/5 studies) | Unclear with concerns in study selection and confounding | 2.8 (p = 0.003) | Strong |
| 4203 (3) | ≅ (2/3 studies) | Low | 1.2 (p = 0.218) | Null evidence |
| Chemical Contaminants | |||||
| 50,162 (8/8) | ↑ (8/8 studies) | Low | 5.5 (p < 0.001) | Very Strong |
| 12,719 (5) | ↑ (5/5 studies) | Low | 4.3 (p < 0.001) | Very Strong |
| 4536 (5) | ↑ (3/5 studies) | Unclear with concerns in study selection, exposure assessment and confounding bias | 2.2 (p = 0.025) | Weak |
| 3258 (2) | ≅ (1/2 studies) | Unclear with concerns in representativeness and confounding | 1.2 (p = 0.231) | Null evidence |
| 560 (2) | ↑ (2/2 studies) | Unclear with concerns in participant selection and confounding bias | 2.8 (p = 0.005) | Weak (downgraded because of small number of studies) |
| 2745 (3) | ≅ (2/3 studies) | Low | 1.1 (p = 0.277) | Null evidence |
| Biological Exposure | |||||
| 80,814 (1) | ↑ (1/1 studies) | Unclear with concerns in comparability and outcome assessment | - | - |
| 80,814 (1) | ↓ (1/1 studies) | Unclear with concerns in comparability and outcome assessment | - | - |
| Noise | 4184 (2) | ↑ (2/2 studies) | Low | 2.7 (p = 0.005) | Weak (downgraded because of small number of studies) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amjad, S.; Verghese, M.; Bohlouli, S.; Dennett, L.; Chandra, S.; Ospina, M.B.; Kozyrskyj, A. The Role of the Home Environment in Perinatal Depression: A Systematic Review and Meta-Analysis of Observational Epidemiological Studies. Environments 2025, 12, 112. https://doi.org/10.3390/environments12040112
Amjad S, Verghese M, Bohlouli S, Dennett L, Chandra S, Ospina MB, Kozyrskyj A. The Role of the Home Environment in Perinatal Depression: A Systematic Review and Meta-Analysis of Observational Epidemiological Studies. Environments. 2025; 12(4):112. https://doi.org/10.3390/environments12040112
Chicago/Turabian StyleAmjad, Sana, Myah Verghese, Solmaz Bohlouli, Liz Dennett, Sue Chandra, Maria B. Ospina, and Anita Kozyrskyj. 2025. "The Role of the Home Environment in Perinatal Depression: A Systematic Review and Meta-Analysis of Observational Epidemiological Studies" Environments 12, no. 4: 112. https://doi.org/10.3390/environments12040112
APA StyleAmjad, S., Verghese, M., Bohlouli, S., Dennett, L., Chandra, S., Ospina, M. B., & Kozyrskyj, A. (2025). The Role of the Home Environment in Perinatal Depression: A Systematic Review and Meta-Analysis of Observational Epidemiological Studies. Environments, 12(4), 112. https://doi.org/10.3390/environments12040112







