Abstract
This study evaluated the individual and combined effects of different bio-inputs—traditional filter cake, filter cake composted with ash, and a microbial inoculant—on the growth and physiological performance of Mucuna pruriens cultivated in soil contaminated with the herbicide tebuthiuron. The experiment followed a completely randomized design with twelve treatments and five evaluation periods (7, 21, 35, 49, and 70 days after sowing). Morphophysiological variables such as plant height, root length, dry biomass, and chlorophyll content were assessed. The results showed that the addition of traditional filter cake promoted significant growth in tebuthiuron-contaminated soil, while, in uncontaminated conditions, both organic residues and the microbial inoculant enhanced plant development, particularly at later stages. Initial phytotoxicity was observed in treatments with organic residues (up to 67% of samples before 35 days), but these effects decreased over time. The microbial inoculant performed better in the absence of organic amendments, suggesting possible antagonistic interactions. Tebuthiuron reduced chlorophyll content by inhibiting photosystem II, but this effect was mitigated by the addition of filter cake. Overall, the findings highlight the potential of integrating Mucuna pruriens cultivation with organic residues and microbial inoculants as an effective phytomanagement strategy for tebuthiuron-affected soils. This approach provides a sustainable model for improving soil health, supporting legume-based rehabilitation, and advancing biological alternatives to conventional remediation practices.
1. Introduction
Tebuthiuron is a systemic and selective herbicide widely used for the pre-emergence control of broadleaf and grassy weeds in sugarcane cultivation. Its mechanism of action involves the inhibition of photosystem II, impairing the photosynthetic process of susceptible species []. Although effective in weed management, tebuthiuron poses serious environmental concerns due to its high-water solubility (2.50 g L−1 at 20 °C) [] and low soil sorption capacity (Kd = 0.1–0.7 mL g−1) [], which facilitate its mobility and leaching into both aquatic and terrestrial ecosystems []. Furthermore, its long residual activity (12 to 18 months) enhances weed control but also increases the risk of phytotoxicity in subsequent crops []. Tebuthiuron exhibits a half-life exceeding 450 days [] and has been reported as highly toxic to non-target organisms [], amplifying their environmental impact and persistence in agricultural systems.
To mitigate the adverse effects of such herbicides, phytoremediation represents an environmentally friendly alternative []. This technique uses plants to remove, stabilize, or degrade pollutants, contributing to soil and ecosystem recovery []. Before implementing phytoremediation in agricultural areas, however, it is essential to assess the growth performance of candidate species under contaminant stress []. At this initial stage, the aim is not yet contaminant removal, but rather the identification of plants with high tolerance and adaptive capacity. Typical assessment parameters include phytotoxicity diagnosis, morpho-agronomic traits, and biochemical indicators. Several leguminous and grass species commonly used in sugarcane field renovation have already undergone such screening for tebuthiuron remediation, including Arachis hypogaea [], Canavalia ensiformis [,,], Cajanus cajan [], Pennisetum glaucum [,], Mucuna aterrima [], Mucuna pruriens [,], Stizolobium aterrimum [], Lupinus albus [], and Sorghum bicolor [].
Among these species, Mucuna pruriens (Velvet bean) has emerged as a particularly promising candidate for soil recovery. Belonging to the Fabaceae family (subfamily Papilionoideae), it is a perennial climbing legume widely distributed across tropical and subtropical regions []. The species develops a deep taproot system capable of exploring soil layers efficiently, while its long, flexible stems exhibit a creeping and climbing growth habit that provides extensive ground coverage []. Through symbiotic associations with rhizobia, M. pruriens fixes atmospheric nitrogen, contributing to improved soil fertility and structure []. This legume also displays rapid biomass accumulation, thrives at temperatures between 25 and 30 °C, and performs best in well-drained soils under adequate light []. Together, these morphological and physiological traits confer resilience against herbicide stress, positioning M. pruriens as a key species for phytoremediation and sustainable soil management in sugarcane systems.
In parallel, microbial inoculants and microbial biostimulants have become essential components for sustainable organic production under biotic and abiotic stresses. These bioinputs, composed of beneficial microbial consortia, enhance plant performance through multiple mechanisms, including nitrogen fixation, phosphate solubilization, phytohormone production, and induction of systemic resistance [,]. Their application has been associated with improved growth and yield under stress conditions in several crops, as reported by [] for strawberries under reduced fertilization. In the context of tebuthiuron-contaminated soils, microbial inoculation has also been shown to enhance plant growth and promote herbicide dissipation [].
Furthermore, sugarcane processing generates nutrient-rich organic residues such as traditional filter cake and filter cake composted with ash. Their responsible reuse can improve soil fertility, stimulate microbial activity, and support pesticide biodegradation [,,]. Owing to their porous and siliceous structure, these materials may also adsorb and immobilize contaminants [], reducing their bioavailability and mitigating environmental impacts.
Despite the recognized benefits of microbial inoculants and sugarcane by-products when applied separately, the strategic combination of both for the biorestoration of tebuthiuron-contaminated soils has not yet been scientifically investigated. This represents an innovative approach for integrated phytomanagement and ecotoxicological modeling, addressing a critical gap in current bioremediation strategies. It is hypothesized that combining microbial bioinputs with sugarcane-derived organic residues can enhance plant tolerance, soil biological activity, and contaminant attenuation through synergistic interactions.
Therefore, this study aimed to evaluate the influence of microbial inoculants and sugarcane-based organic amendments, specifically traditional filter cake and filter cake composted with ash, on the growth and physiological responses of M. pruriens cultivated in tebuthiuron-contaminated soil. By elucidating these interactive effects, this research seeks to advance biotechnological approaches for the remediation of herbicide-polluted agricultural soils and to promote sustainable management practices within the sugar-energy sector.
2. Materials and Methods
The experiment was conducted between September–November 2023 in the greenhouse of Environmental Impact Action Group (GAIA), College of Agricultural and Technological Sciences—São Paulo State University (FCAT/Unesp)—Dracena Campus, São Paulo, Brazil (20°22′ S, 51°22′ W). Environmental variables, such as air temperature (°C) and relative humidity (%), were periodically monitored both in the greenhouse and on the farm (Figure S1—Supplementary Material). This monitoring ensured proper characterization of environmental conditions during the experimental period while allowing for the interpretation of results considering potential climatic influences on plant development and treatment performance.
2.1. Soil
The soil at this location is considered a dystrophic Red–Yellow Argisol with a sandy texture [], corresponding to a sandy Ultisol, according to soil taxonomy []. These properties influence nutrient and contaminant retention. Soil samples were collected, air-dried, sieved through a 2.0-mm mesh, and subjected to chemical characterization (Table 1). Granulometric (physical) analysis revealed a sandy loam texture, with 79.4% sand, 6.5% silt, and 14.1% clay. Additionally, the soil had no prior history of pesticide use, ensuring an uncontaminated baseline for studying tebuthiuron pollution and the proposed bioremediation interventions.

Table 1.
Chemical attributes of soil samples collected before and after acidity and fertility correction, Dracena, SP, Brazil, 2023.
2.2. Tebuthiuron Herbicide, Microbial Inoculant, and Plant Species
The herbicide tebuthiuron (TBT) was sourced from the commercial product Combine® 500SC (Batch 041-14-2000, Dow AgroSciences Industrial Ltda., São Paulo, Brazil). The microbial inoculant (MI) evaluated in this study was provided by AMTEC® Bioagricola Ltda. (Brazil; https://amtecbioagricola.com.br) as part of a collaborative research initiative with UNESP. Originally developed for agricultural use as a seed treatment, the formulation was repurposed in this work as a bioaugmentation agent under chemical stress conditions to assess its environmental potential. Although the detailed microbial composition remains proprietary, the inoculant is known to contain beneficial bacterial and fungal species capable of enhancing biological nitrogen fixation, improving nutrient dynamics, protecting plants against pests and pathogens, and increasing tolerance to abiotic stress. Inoculant survival (CFU g−1) and herbicide tolerance were not evaluated, as the study focused on plant and soil responses rather than microbial quantification. The plant species selected for this study was Mucuna pruriens, a legume commonly used as a green manure and cover crop in tropical and subtropical agricultural systems.
This species was chosen due to its vigorous growth, rapid biomass accumulation, symbiotic association with microorganisms, and proven potential for phytoremediation and soil restoration in areas contaminated with tebuthiuron and other herbicides [,]. Its deep root system and nitrogen-fixing ability make it suitable for improving soil fertility and microbial activity during sugarcane field renovation. Seeds (variety “M. pruriens var. utilis”) were obtained from Piraí Sementes® Ltda. (Piraí, Brazil).
2.3. Sugarcane By-Products
The sugarcane by-products were provided by Viterra Bioenergia S.A.—Rio Vermelho Unit (Junqueirópolis, Brazil), consisting of traditional filter cake (FC) and filter cake composted with ash (FA), with their characterization presented in Table 2.

Table 2.
Characteristics of sugarcane by-products used as soil amendments, including traditional filter cake (FC) and filter cake composted with ash (FA), Dracena, SP, Brazil, 2023.
Traditional filter cake (FC) is a by-product generated during the clarification stage of sugarcane juice processing []. It is formed by the precipitation of impurities during lime treatment and consists mainly of organic matter, calcium carbonate, residual sucrose, waxes, and fine soil particles derived from sugarcane washing []. This material was collected directly from the sugarcane mill, homogenised, and sieved through a 4 mm mesh before being incorporated into the soil.
The ash-composted filter cake (FA) was produced by mixing traditional filter cake with boiler ash resulting from the combustion of sugarcane bagasse and was subjected to several months of composting, with frequent manual turning and humidity maintained at around 60%. This process stabilised the organic matter, reduced the initial moisture content, and neutralised part of the acidity of the filter cake due to the alkaline nature of the ash []. After composting, the FA was air-dried and sieved (4 mm) before being applied to the soil.
Although no microbiological analysis was conducted on the filter cake composted with ash (FA), the material was produced under typical industrial composting conditions at the sugarcane mill. During this process, microbial colonisation and succession are naturally expected, as they drive organic matter stabilisation and nutrient transformation []. Thus, the presence of saprophytic and decomposer micro-organisms in the composted residue is inherent to its formation.
Furthermore, these wastes were analyzed using scanning electron microscopy (SEM). The samples were first mounted on double-sided conductive carbon tape to ensure their adhesion to the support. Subsequently, they were coated with a gold film approximately 10 nm thick to enhance the interaction between the material and the electron beam. This metallization process was carried out using a Quorum® sputter coater, model Q150T E (United Kingdom). Afterwards, the samples were examined in a Zeiss® SEM, model EVO LS15 (Germany). The images were acquired using a secondary electron detector under an accelerating voltage of 20 kV. This SEM-based morphological characterization was performed with the aim of identifying structural and surface features of the residues, such as porosity, texture, and particle shape.
The morphological differences observed in Figure 1, obtained through scanning electron microscopy, reflect and help explain the chemical characteristics presented in Table 2. The traditional filter cake (FC), with higher contents of organic carbon (28.74%) and total nitrogen (2.35%), exhibited a compact and cohesive microstructure, characterized by smooth, continuous surfaces and low visible porosity (Figure 1B). This morphology is consistent with a biomass that is still slightly decomposed and retains a high moisture content (75.74%), indicating the predominance of amorphous organic components that tend to fill interparticle spaces and limit pore formation. Such compactness suggests lower gas and water diffusion capacity, typical of less stabilized organic residues.
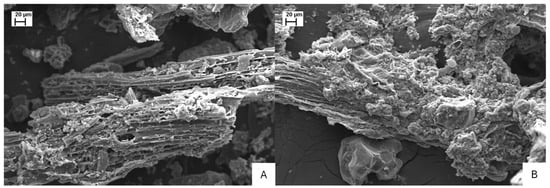
Figure 1.
Scanning electron microscopy at 500× magnification of sugarcane-derived residues used as soil amendments, Dracena, SP, Brazil, 2023: (A) filter cake composted with ash—FA, and (B) traditional filter cake—FC.
In contrast, the filter cake composted with ash (FA) presented a fragmented and porous morphology, characterized by granular aggregates, irregular surfaces, and visible interparticle voids (Figure 1A). This structure is consistent with its lower levels of organic carbon (8.11%) and moisture (31.13%), suggesting a higher degree of decomposition and stabilization of organic matter. The oxidative and thermochemical effects of composting, combined with the alkaline properties of the ashes, promoted organic matter mineralization, water loss, and the formation of micro- and mesopores. Additionally, the incorporation of ash contributed to the enrichment of cations such as calcium (3.32%), magnesium (1.13%), and potassium (0.93%), which favored particle aggregation and surface restructuring. These physicochemical transformations increased the specific surface area and cation exchange potential of FA, enhancing its ability to retain nutrients, improve soil structure, and provide favorable microsites for microbial colonization.
2.4. Experimental Setup
The experiments were conducted in a randomized design with 12 treatments and 30 replications. The experimental design included five evaluation periods, corresponding to days after sowing (DAS), with analyses performed at five time points: 14, 28, 42, 56, and 70 DAS. At each evaluation time, 72 pots were assessed, with 6 replications per treatment, ensuring that the data obtained were statistically robust. Thus, the experiment involved a total of 360 pots (72 pots per time × 5 times).
The interaction among the treatments included: tebuthiuron concentration (TBT—presence and absence), microbial inoculant (MI—presence and absence), and wastes from the sugarcane industry (absence, FC—traditional filter cake, FA—filter cake composted with ashes). It is noteworthy that M. pruriens was grown in all experimental units. Additionally, the control treatment (cultivation with M. pruriens only) was labeled as CTL. The table of treatments and evaluation periods is available in the Supplementary Material (Table S1).
Before sowing, the soil was prepared to adjust its acidity and fertility. For 1440 kg of soil, 525 g (950 kg ha−1) of limestone (with a PRNT of 90%) was applied to adjust the pH. Regarding fertility correction, the soil was divided into two halves: (1) application of synthetic fertilizers—125 g (451.40 kg ha−1) of single superphosphate and 26.50 g (95.70 kg ha−1) of potassium chloride were applied to 720 kg of soil; and (2) incorporation of filter cake manually according to the recommendation of Viterra Bioenergia S.A. for sugarcane cultivation—1.35 kg of FA (9.75 t ha−1) in 360 kg of soil, and 0.99 kg of FC (7.15 t ha−1) in 360 kg of soil. This difference between FA and FC is based on the composition of the residues and the crop’s nutritional requirements. After the addition of the amendments, the soil was thoroughly mixed and used to fill 4.0 L pots.
The herbicide tebuthiuron was applied at a rate of 2.0 L ha−1 of the commercial product (equivalent to 1000 g ha−1 do a.i.), using a sprayer equipped with flat-fan nozzles XR 11,002 (pressure: 2 bar; flow rate: 0.65 L min−1; speed: 5 km h−1), resulting in a spray volume of 156 L ha−1. According to [], this low spray volume was intentionally selected for three technical reasons: (1) adaptation to the controlled conditions of the greenhouse, where drift is minimized; (2) the systemic nature of tebuthiuron, which allows for good absorption even with reduced application volumes; and (3) compatibility with sandy soils, which require lower volumes to prevent leaching before absorption by the seed bank. Environmental conditions (27.2 °C; 63% of relative humidity) were monitored during application. The control treatment received an equivalent volume of water.
Seven days after herbicide application, five seeds of M. pruriens were sown in each experimental unit. However, prior to sowing, seeds intended for microbial inoculation were treated with the agricultural inoculant at a rate of 20 mL of the commercial product per kg of seeds, as recommended by the manufacturer.
It is noteworthy that the plants were grown in a greenhouse equipped with an automated irrigation system. To ensure optimal growth and development conditions, irrigation was performed daily, maintaining the soil at 60% of its field capacity. The automated system is equipped with a digital timer, programmed to perform up to four irrigation cycles per day, each lasting approximately 40 ± 10 min. The micro-sprinkler, located at the top of the greenhouse, has a flow rate ranging from 80 L h−1, operating under a pressure of 2.0 bar. The uniformity of water distribution across the area containing the pots was verified using the Christiansen’s Coefficient of Uniformity test [].
2.5. Plant Assessment
The assessment of M. pruriens vegetative development in the soil samples began with the daily recording of seedling emergence per treatment, starting from day 1 until 9 days after sowing (DAS) (see Table S2—Supplementary Material).
After this period, thinning was carried out in all experimental units, leaving only one plant per pot. Thus, biometric assessments were conducted every two weeks, at five evaluation periods throughout the experiment (14, 28, 42, 56, and 70 days after sowing—DAS). All analyses were based on six replicates per treatment and included: phytotoxicity (%) assessed using a visual rating scale []; plant height and root length measured with a tape measure (cm); total dry biomass (g); and relative leaf chlorophyll content, measured using a portable chlorophyll meter, model CFL 1030 (ChlorofiLOG®—Falker® Agricultural Automation, Brazil), with readings taken from the central third of the leaves.
The five sampling periods were defined as coinciding with the main developmental phases of M. pruriens under greenhouse conditions. Under tebuthiuron stress, the plants exhibited a moderate delay in these phenophases, particularly in leaf expansion and chlorophyll accumulation. However, the use of organic amendments, especially traditional filter cake, mitigated this delay and promoted an acceleration of vegetative recovery, aligning development more closely with the control plants.
Furthermore, nodules larger than 1.0 mm were manually counted on the plants at 70 days after sowing (DAS). This result showed a high coefficient of variation and is therefore presented in Table S3 (Supplementary Material).
Furthermore, tebuthiuron residues and their degradation products were not chemically quantified, as the objective of this study was to evaluate morphophysiological responses rather than chemical dissipation or biodegradation kinetics. Therefore, any references to herbicide “decomposition” should be interpreted as biological mitigation of toxic effects, rather than confirmed chemical transformation.
2.6. Data Analysis
All data was subjected to tests for homogeneity of variances and normality using the Bartlett and Shapiro–Wilk statistical methods. This analysis was performed using R software 4.5.2 and Microsoft Excel® 360.
The results for plant height, root length, and number of nodules at the final evaluation time (70 DAS) were analyzed using analysis of variance (ANOVA, p < 0.05) and mean comparison test (Scott-Knott, p < 0.05), conducted with the “AgroR” package. For phytotoxicity and total dry biomass, a decision tree analysis was performed using the “rpart” and “rpart.plot” packages. Regarding relative leaf chlorophyll content, data was analyzed using a second-order polynomial regression. Finally, a principal component analysis (PCA) was conducted for each treatment and its associated variables, using the “readr”, “ggplot2”, and “factoextra” packages.
3. Results and Discussion
3.1. Soil Characterization as a Function of Acidity and Fertility Correction
Analyses, based on Table 1 and Table 2, revealed that applying acidity and fertility correctives led to changes in soil chemistry. For instance, pH initially ranged from 7.5–7.7 in soil samples B, C, and D but decreased to 6.9–7.2 in samples E and F after cultivation. This reduction directly correlates with a decrease in calcium levels, which dropped from 24–28 mmol dm−3 before cultivation to 17.5–24 mmol dm−3 afterward. This decline might be due to M. pruriens consuming Ca2+, as this legume requires calcium for processes like nodulation and growth [].
However, sample G, which received treatments with filter cake composted with ash (FA), maintained its calcium content at 24 mmol dm−3. This outcome can be explained by three primary factors. First, the presence of ash in filter cake provides calcium oxide, which hydrolyzes in the soil to continuously release Ca2+, acting as both a direct calcium source and an acidity corrective []. Second, the system’s chemical equilibrium likely favored maintaining calcium in sample G’s soil. At a pH near 7.1, some Ca2+ tends to associate with anions like carbonate or phosphate, forming sparingly soluble compounds such as calcium carbonate []. Third, the soil’s cation exchange capacity in sample G (54 mmol dm−3) might also have promoted calcium retention in the exchangeable phase.
When comparing soil samples regarding phosphorus, traditional filter cake (sample F) showed the largest percentage reduction in this nutrient (50%), decreasing from 18 to 9 mg dm−3. In contrast, composted filter cake (sample G) experienced a smaller drop, from 22 to 16 mg dm−3 (27%). In soils with a pH between 6.9 and 7.2, as seen in post-cultivation samples, phosphorus tends to precipitate as calcium phosphates []. Conversely, the chemical fertilizer, even at a low dose, had a positive effect, increasing P content from 8 mg dm−3 (sample B) to 11 mg dm−3 (sample E).
Base saturation (V%) also decreased in all soil samples after cultivation. This reduction occurred because of the absorption of basic cations, which are essential elements for nodulation (calcium), magnesium, and potassium (photosynthesis) [].
Regarding organic matter, stability was observed throughout the experiment, remaining at 7 g dm−3 in most samples, except for a reduction in sample E (6.5 g dm−3). This observed stability can be attributed to the contribution of organic compounds present in the applied wastes []. For instance, traditional filter cake (FC) contains lignocellulosic fibers typical of sugarcane wastes, which exhibit slow degradation [], explained by its high C/N ratio (12.23%), as shown in Table 2. Conversely, the filter cake composted with ash (FA) may have promoted the formation of humic compounds, which also persist in the soil, reinforcing this stabilizing effect [].
Another attribute that remained stable was exchangeable aluminum (Al3+), which stayed at zero after the initial soil correction (samples B to G), in contrast to the initial value of 9 mmol dm−3 (sample A). This reflects the success of liming, which raised the soil pH from 4.3 to approximately 7.0, reducing Al3+ solubility and its toxicity. Micronutrients such as boron, copper, and zinc also maintained low and stable levels throughout the experiment.
In a detailed analysis of soil samples regarding magnesium, distinct behaviors of the element were noted depending on the type of input used before and during the cultivation of M. pruriens. In samples B:E, where chemical fertilizer was applied, a 9% reduction in Mg2+ levels was observed (from 17 to 15.5 mmol dm−3). However, in samples C:F, with traditional filter cake (FC) application, there was an 18% increase in Mg2+ (from 17 to 20 mmol dm−3). Part of this effect can be attributed to the initial soil correction and the cultivation period, which may have favored the gradual release of this nutrient. Conversely, in samples D:G, with filter cake composted with ash (FA), Mg2+ levels remained stable at 20 mmol dm−3, demonstrating a balance between the contributions from the ash (rich in Mg, at 1.13%) and plant absorption. The prior composting may have promoted an earlier release of some magnesium, while the remainder likely stayed as a reserve, contributing to the observed stability.
For sulfur, we saw a 61% increase from samples B to E, rising from 9 to 14.5 mg dm−3. This can be attributed to the type of fertilizer used, likely single superphosphate, which contains 10% to 12% sulfur. The corrected pH (around 6.9) also favors its retention in the soil solution. On the other hand, samples C:F, treated with traditional filter cake, showed a 47% reduction in sulfur content, dropping from 19 to 10 mg dm−3. However, in samples D:G, which used composted filter cake with ash, sulfur increased slightly from 9 to 10 mg dm−3 (+11%). This behavior indicates a more stable and predictable release of the nutrient, resulting from mineralization that began during the composting process []. The composted filter cake contains a higher proportion of this nutrient (0.20%) compared to FC (0.10%), and some of this sulfur is likely oxidized into forms readily available for plant uptake.
Finally, a 15.2% reduction in cation exchange capacity (CEC) was observed between soil samples B and E. This drop may be associated with gradual soil acidification []. For samples C:F, which received traditional filter cake, the CEC remained stable at 53 mmol dm−3, even after cultivation. Conversely, samples D:G, treated with filter cake composted with ash, showed a slight increase of 3.8% in CEC, rising from 52 to 54 mmol dm−3.
3.2. Root Length
Table 3 presents the data regarding the root length of plants subjected to different treatments. Although no statistically significant difference was observed between the treatments, plants grown in soil without tebuthiuron had an average root length of 36.9 cm, while those exposed to the herbicide had an average of 34.9 cm, corresponding to a reduction of 5.80%.

Table 3.
Root length (cm) of Mucuna pruriens cultivated under different treatments at 70 days after sowing, Dracena, SP, Brazil, 2023.
Despite the lack of statistical significance, this percentage difference highlights the negative impact of tebuthiuron presence. This trend may be related to the herbicide’s mode of action, which primarily affects photosystem II, mainly impacting the aerial parts of plants [,]. Thus, it is understandable that the most pronounced effects are observed in the aerial portion, while the root system remained less affected, at least during the evaluated period.
These results indicate that, under the conditions of the present study, the use of tebuthiuron does not pose a significant risk to the root development of M. pruriens. However, the analysis limited to root length may not be sufficient to capture more subtle or complex changes in the root system. Therefore, future investigations considering additional variables, such as root volume, secondary root density, and nutrient absorption efficiency, are recommended for a more comprehensive understanding of the herbicide’s effects, as well as its interaction with bioinputs.
3.3. Plant Height
The height of M. pruriens plants at 70 DAS varied significantly among treatments (Table 4), with the highest mean values recorded in FC and FA treatments without herbicide, followed by their combinations with the microbial inoculant (FC + MI and FA + MI), and notably in the TBT + FC treatment (128.0 cm). Quantitatively, plants grown under TBT + FC exhibited an increase of approximately 72% compared to TBT alone (74.4 cm) and 68% compared to the control (76.2 cm). These results indicate that organic amendments such as filter cake (FC) and filter cake composted ash (FA) promoted plant development under non-stressed conditions, while tebuthiuron exposure reduced shoot elongation.

Table 4.
Plant height (cm) of Mucuna pruriens at 70 days after sowing under different treatments, Dracena, SP, Brazil, 2023.
The superior performance of combined treatments suggests that the efficacy of these interventions was not determined by isolated bioinputs (MI, FC, FA, or TBT) but by their complementary effects, which improved soil structure, aeration, and nutrient availability []. Although not directly measured, it is biologically plausible that microorganisms naturally associated with organic residues may have contributed to growth stimulation through the production of phytohormones, known to enhance shoot elongation and leaf expansion []. This potential microbial–chemical synergy may explain the enhanced growth observed in treatments with organic amendments and represents an important aspect for future investigation.
In uncontaminated soil, the combination of microbial inoculant (MI) with traditional filter cake (FC) or filter cake composted ash (FA) promoted positive effects on plant height, in contrast to their standalone applications. Quantitatively, the combinations FC + MI and FA + MI resulted in 35–36% higher plant height than the control. This indicates that the combined action of these materials created an additive biotic system that enhanced plant growth, likely through increased nutrient availability and microbial activity. Traditional filter cake (FC) is rich in nitrogen (2.35%), phosphorus (5.36%), and organic carbon (28.74%), and it also contributes to an increase in cation exchange capacity, remaining stable at 53 mmol dm−3 even after cultivation. This enhances the retention and availability of essential nutrients such as Ca, Mg, and K. Filter cake composted ash (FA), in turn, proved effective in maintaining high calcium levels (24 mmol dm−3) and pH stability (7.1), in addition to containing significant concentrations of K, Mg, and S.
However, these benefits are not solely the result of nutrient enrichment but also of complex interactions between the residue matrix and the rhizospheric microbiota. The presence of organic carbon and mineral cations in the filter cake supports microbial colonization, enzymatic activation, and redox processes that favor root development and nutrient uptake. This multifactorial mechanism explains the superior growth observed in the combined treatments (FC + MI, FA + MI, and TBT + FC), where chemical and biological complementarities likely enhanced physiological recovery under herbicide stress.
On the other hand, in the isolated treatment with tebuthiuron (TBT—74.42 cm), plant growth was reduced compared to the synergistic treatments, although it did not statistically differ from the untreated control (CTL—76.20 cm). This suggests that M. pruriens may possess intrinsic mechanisms of tolerance to tebuthiuron, possibly involving metabolic detoxification, morphophysiological resilience, or antioxidant capacity—characteristics that position it as a potential candidate for phytoremediation of soils contaminated with photosystem II-inhibiting herbicides. Although this positive adaptation has been extensively discussed by [,], caution is required, as [] reported toxic effects that impair growth. These contradictions underscore the need for further studies to clarify the adaptive responses of species under varied conditions.
Additionally, in soil contaminated with tebuthiuron, only the TBT + FC treatment (128.00 cm) resulted in significant growth gains, surpassing all other combinations. The application of traditional filter cake alone likely mitigated the herbicide’s toxicity due to its high organic matter and nutrient content, which enhances cation exchange capacity, soil structure, and nutrient availability. This creates a more favorable edaphic environment, even under chemical stress [,,].
This effect aligns with fertility data showing the maintenance of adequate levels of P, Ca, and Mg following FC amendment, as well as a moderate reduction in pH, which remained within the optimal range for nutrient availability. On the other hand, other combinations involving tebuthiuron with microbial inoculants and sugarcane by-products (TBT + FC + MI: 66.33 cm; TBT + FA + MI: 76.70 cm) did not improve plant height, suggesting that complex interactions in the soil may neutralize or antagonize the efficacy of the bioinputs.
The results emphasized that the effectiveness of organic and microbial depends not only on their presence but also on environmental compatibility, dosage, bioavailability, and biochemical synergy. For instance, the success of TBT + FC contrasts with the inefficacy of multiple-input combinations, highlighting the delicate balance required in contaminated systems. Therefore, sustainable management practices must consider edaphoclimatic conditions and the dynamic interaction between microorganisms, organic residues, and contaminants.
3.4. Phytotoxicity
Figure 2 presents a decision tree illustrating the combinations of experimental factors evaluated in terms of phytotoxicity in M. pruriens. These factors are organized into categories such as plant developmental stage, herbicide application (tebuthiuron), and microbial inoculant use (with or without). The numerical scale associated with each combination reflects the level of phytotoxicity, with lower values indicating greater damage to the plant. Colors follow a thermal gradient, where warm tones (red) represent high phytotoxicity and cool tones (green) indicate lower impact. The numerical values (e.g., 1.2; 6.9) correspond to mean phytotoxicity scores, while “n=” denotes the number of individuals analyzed. Percentages indicate the proportion of those samples relative to the total.
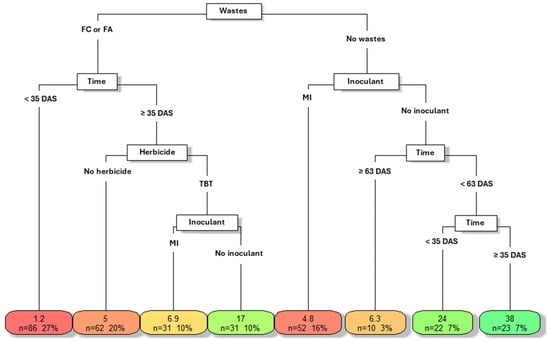
Figure 2.
Decision tree model describing the influence of tebuthiuron, microbial inoculant, and sugarcane residues on phytotoxicity of Mucuna pruriens, Dracena, SP, Brazil, 2023. Caption: TBT—tebuthiuron; MI—microbial inoculant; FC—traditional filter cake; FA—filter cake composted with ashes; DAS—days after sowing.
The first level of impact identified in the analysis was the presence of byproducts from the sugarcane industry (traditional filter cake—FC—or filter cake composted ash—FA). These wastes stand out due to their richness in organic and inorganic compounds, which can influence both nutrient availability and soil toxicity []. According to [], filter cake contains high levels of organic matter and macronutrients such as NPK (Table 2), which may contribute positively to soil fertility, although its initial effect could be detrimental depending on the incorporation time.
In the presence of these wastes, plant development proved to be a determining factor, categorized as either less than or greater than/equal to 35 DAS. In the period below 35 DAS, relatively high phytotoxicity was observed (1.2–27%), suggesting that immediate waste application may negatively impact M. pruriens development. This finding is agriculturally relevant, underscoring the need for careful planning when incorporating waste particularly given their high nutrient concentration [,]. Beyond this period (≥35 DAS), a significant reduction in phytotoxicity occurred (5.0–20%), indicating environmental stabilization and/or plant adaptation to the new edaphic conditions.
In this scenario, other factors such as the use of tebuthiuron and microbial inoculant emerged as secondary variables. In the absence of herbicide, phytotoxicity was 5.0 (20%), whereas its application resulted in lower levels of damage, suggesting a potential mitigation of toxic impact. Regarding the inoculant, its use led to a further reduction in phytotoxicity (6.9–10%), while in its absence, the value was 2.4 times higher (17.0–10%). Nevertheless, these factors showed a quantitatively smaller influence on the response variable when compared to the development time and, most notably, the presence of sugarcane industry wastes. This is evidenced by the fact that 67% of the samples analyzed were directly influenced by the addition of FC or FA.
On the right side of Figure 2, where no waste has been incorporated (“No wastes”), the primary factor influencing phytotoxicity was the use of a microbial inoculant. In this context, [] emphasized that inoculants can alter the physicochemical conditions of the soil and modulate the physiological response of plants. Additionally, different development times (35 and 63 DAS) also showed influence, ranking third and fourth in the hierarchy of impact. This finding reinforces the importance of exposure time to the edaphic environment as a key factor in the manifestation of phytotoxic symptoms, even in the absence of industrial residues.
Thus, the results highlighted the complexity of the interactions among the evaluated factors, underscoring the need for integrated management strategies that consider the type of waste, the timing of its application, and the use of bioproducts, with the aim of maximizing the benefits of agro-industrial byproduct recycling without compromising plant development.
Beyond the fertility contribution of these residues, their biochemical role in stress modulation deserves attention. Organic compounds from filter cake can stimulate antioxidant enzyme systems []. Although not directly measured, it is biologically plausible that microorganisms naturally associated with organic residues may promote cellular detoxification [] and mitigate oxidative damage caused by tebuthiuron []. Such mechanisms highlight that the recovery process is not merely nutritional but also physiological, involving both chemical buffering and metabolic adaptation [].
3.5. Relative Leaf Chlorophyll Content
The relative leaf chlorophyll content (RLCC) of M. pruriens was significantly influenced by the interaction between tebuthiuron, time, and organic amendments (p < 0.05). Its temporal evolution over 70 DAS was best described by second-degree polynomial regressions (Figure 3), which showed excellent goodness of fit across all treatments (R2 > 0.87) and residual homoscedasticity. The use of second-order models was justified by the parabolic pattern observed in chlorophyll dynamics, characterized by an initial increase during vegetative growth, a peak near pre-anthesis (42–56 DAS), and subsequent stabilization or slight decline toward the end of the period. This modeling approach allowed a more continuous and informative interpretation of photosynthetic behavior compared to discrete sampling-point comparisons, capturing the non-linear trajectory of plant recovery under tebuthiuron stress. Moreover, the regression coefficients made it possible to quantify both the magnitude of the chlorophyll peak and its temporal shift relative to the control, thereby elucidating how organic amendments modulated the timing and intensity of photosynthetic restoration.
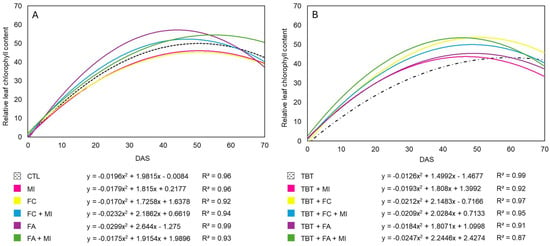
Figure 3.
Temporal evolution of relative leaf chlorophyll content in Mucuna pruriens grown (A) without and (B) with tebuthiuron, Dracena, SP, Brazil, 2023. Caption: CTL: cultivation with Mucuna pruriens only (Control); TBT: tebuthiuron; MI: microbial inoculant; FC: traditional filter cake; FA: filter cake composted with ash; DAS: days after sowing.
In the absence of the herbicide tebuthiuron (Figure 3A), plants grown in soil treated with filter cake composted with ash (FA) showed the highest RLCC values, especially when FA was not combined with the microbial inoculant. This trend can be explained by the improvement in soil fertility observed in the FA treatments, which maintained calcium (24 mmol dm−3), magnesium (20 mmol dm−3), and potassium (1.9 mmol dm−3) levels, in addition to stable pH (7.1) and increased cation exchange capacity (54 mmol dm−3). These factors enhance photosynthetic activity and chlorophyll synthesis. Furthermore, the FA provides a gradual release of nutrients such as sulfur (with an 11% increase) and a stable contribution of organic matter, promoting a more balanced environment for plant development.
In contrast, the isolated use of traditional filter cake (FC) or microbial inoculant (MI) resulted in RLCC values even lower than those of the control (CTL), possibly due to lower nutrient stability or the absence of synergy between factors. Although FC is rich in N (2.35%) and C (28.74%), it exhibited higher phosphorus losses after cultivation (a 50% reduction) and slight soil acidification, which may limit the uptake of elements essential for photosynthesis [].
When cultivated in tebuthiuron-treated soil (Figure 3B), the biological treatments also proved decisive for RLCC dynamics, though with an inversion in peak intensity between samples containing FC or FA. The control sample containing only herbicide (TBT) exhibited the lowest RLCC value, consistent with tebuthiuron’s mode of action, which inhibits photosynthesis by blocking electron flow in Photosystem II. This blockade reduces photochemical efficiency and impairs the synthesis of ATP and NADPH, both essential for the photosynthetic process []. Furthermore, herbicide induces oxidative stress through the generation of reactive oxygen species, damaging cellular structures such as membranes, proteins, and DNA, thereby compromising chloroplast functionality and reducing chlorophyll production []. Tebuthiuron’s interference with nutrient uptake and translocation—particularly magnesium—further exacerbates the decline in photosynthetic capacity []. These effects explain the delayed RLCC peak observed in herbicide-exposed plants.
The addition of FC was shown to mitigate the negative effects of the herbicide tebuthiuron on plants. When considering all treatments, the maximum RLCC value ranged between approximately 40 and 63 units (Figure 3). Under non-contaminated conditions, plants with FA + MI reached approximately 40% higher RLCC than the control. Under tebuthiuron exposure, the TBT + FC treatment exhibited approximately 50% higher RLCC than TBT alone at the chlorophyll peak (around 42 DAS), followed by TBT + FA (approximately 38%) and TBT + FA + MI (approximately 25%). This marked increase indicates an effective mitigation of tebuthiuron-induced photosynthetic inhibition through organic residue application, demonstrating consistent chlorophyll recovery across bioinput treatments, with filter cake-based amendments showing the strongest response. The treatment combining TBT + FC exhibited higher chlorophyll content, likely due to the organic residue’s ability to enhance soil fertility. For instance, organic matter may have acted as an adsorbent, reducing the herbicide’s bioavailability. Furthermore, this treatment maintained stable cation exchange capacity (53 mmol dm−3) and increased levels of magnesium (20 mmol dm−3) and potassium (1.9 mmol dm−3), essential nutrients for chlorophyll synthesis. These findings support the observation that adding organic matter, such as composted filter cake, can mitigate tebuthiuron toxicity through adsorption []. Additionally, the presence of beneficial microorganisms may facilitate herbicide degradation while inducing physiological and biochemical responses in plants, such as increased activity of antioxidant enzymes that protect the photosynthetic system [].
Beyond general fertility effects, magnesium (Mg) likely played a central role in the observed recovery of RLCC because Mg is the central atom of the chlorophyll molecule, an activator of photosynthetic enzymes, and a key regulator of thylakoid charge balance and electron transport. Recent research has shown that Mg deficiency consistently reduces chlorophyll concentration, depresses PSI/PSII ratios and electron transport efficiency, and triggers oxidative stress and chlorosis, ultimately lowering photosynthetic performance [,,]. New experimental evidence also indicates that maintaining adequate Mg levels restores chlorophyll content and photosynthetic activity, with clear critical Mg thresholds below which biomass and chlorophyll synthesis decline sharply [,]. Therefore, treatments that preserved or increased available Mg, such as those involving filter cake or composted filter cake with ash, are mechanistically consistent with the higher RLCC values and earlier chlorophyll peaks observed under TBT + FC. Additionally, recent evaluations reaffirm a robust correlation between SPAD/RLCC readings and total chlorophyll, supporting their use as reliable indicators of photosynthetic recovery [,,,,].
Analysis of the polynomial equation coefficients reinforces the toxic effect of tebuthiuron. In the control treatment (CTL), the coefficient was −0.0196, with peak RLCC occurring around 35 DAS. In contrast, the TBT treatment showed a less pronounced coefficient (−0.0126) and a delayed peak at approximately 45 DAS, confirming the herbicide-induced delay in photosynthetic development. Although bioinputs such as the FA + MI combination were tested, the results did not demonstrate superior performance, suggesting that in contaminated soils, the interaction between herbicide, microbiota, and nutrients is complex and may offset the benefits of composting. The simultaneous presence of organic compounds and microorganisms can affect mineralization rates, alter pH, and interfere with nutrient dynamics, making the effects less predictable [].
It is evident that the use of bioinputs, such as filter cake, either composted or in natura, constitutes an effective strategy to mitigate the phytotoxic effects of tebuthiuron and accelerate soil recovery through the cultivation of legumes like M. pruriens. Their efficacy, however, relies on the interplay between soil chemical composition, organic amendment, microbial interactions, and the transformation dynamics of organic compounds. Within this integrated framework, bioinputs act through complementary mechanisms: they adsorb and complex the herbicide, reducing its bioavailability; stimulate enzymatic defense systems that protect the photosynthetic apparatus; and promote the gradual release of nutrients essential for chlorophyll synthesis. Together, these processes create a favorable biochemical and microbial environment that enhances nutrient cycling and stress resilience. Consequently, the observed recovery of relative leaf chlorophyll content (RLCC) reflects the synergistic action between chemical and biological factors, reinforcing the importance of coordinated bioinput-based management for sustainable soil restoration and plant physiological recovery under herbicide stress.
3.6. Total Dry Biomass
As illustrated in Figure 4, the decision tree model highlights the interaction of experimental factors influencing the total dry biomass of M. pruriens, including plant growth stage, herbicide application (tebuthiuron), and the use of microbial inoculant (MI). In this context, growth duration emerged as the most significant factor driving dry biomass accumulation. Plants harvested before 49 DAS showed the lowest biomass values (1.8–20%), particularly during the early growth stages (<21 DAS), when growth is inherently limited. In contrast, plants grown for ≥63 DAS achieved the highest biomass levels (28–21%), representing a 15-fold increase compared to the <21 DAS group. These results underscore the crucial role of extended growth periods in maximizing biomass production, likely due to prolonged nutrient uptake and enhanced photosynthetic activity.
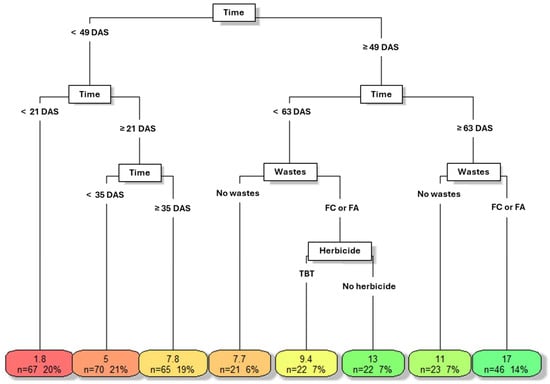
Figure 4.
Decision tree illustrating total dry biomass accumulation in Mucuna pruriens under varying treatments and growth stages, Dracena, SP, Brazil, 2023. Caption: TBT—tebuthiuron; FC—traditional filter cake; FA—filter cake composted with ash; DAS—days after sowing.
For plants grown ≥49 DAS, the incorporation of organic residues, traditional filter cake (FC) or composted with ash (FA) emerged as a secondary determinant of biomass. This finding aligns with the phytotoxicity decision tree (Figure 2), in which FC and FA reduced herbicide-induced damage and enhanced nutrient availability. These residues are likely to improve soil structure, cation exchange capacity, and microbial activity, thereby promoting plant development at advanced growth stages [,]. These results suggest that organic amendments act synergistically with time, enhancing biomass accumulation as plants mature.
Although playing a secondary role in the decision tree, tebuthiuron significantly reduced biomass (9.4–7% with herbicide vs. 13–7% without). Notably, its phytotoxic effects appeared to be exacerbated in soils with higher organic matter content, possibly due to altered herbicide bioavailability or prolonged retention in the soil []. This paradoxical interaction, in which organic matter mitigates phytotoxicity (via FC/FA) yet amplifies herbicide-induced damage, highlights the complexity of soil–herbicide dynamics.
The absence of microbial inoculant (MI) in the decision tree suggests its minimal impact on biomass accumulation compared to growth duration and organic wastes. This finding contrasts with previous studies that emphasize the role of MI in nutrient cycling and stress tolerance []. However, its negligible effect here may reflect competition with native soil microbiota, suboptimal timing of inoculation, or insufficient compatibility with environments contaminated by tebuthiuron.
The predominance of growth duration underscores the need for extended cultivation periods to maximize M. pruriens biomass, particularly in phytoremediation contexts. Although organic residues enhance growth at later stages, their interaction with herbicides such as tebuthiuron requires careful management to avoid unintended toxicity. The limited effectiveness of microbial inoculants (MI) in this system indicates that their performance is strongly conditioned by soil physicochemical properties and the contaminant profile.
The inconsistent performance of the MI suggests that part of the introduced microbial community may not have survived or adapted under tebuthiuron-induced stress. The AMTEC® formulation, originally developed to enhance agricultural productivity under conventional conditions, was repurposed here as a bioaugmentation agent in chemically contaminated soils. This exploratory environmental application likely faced ecological compatibility constraints, since M. pruriens naturally associates with specific rhizobia, whereas the tested inoculant consisted of a mixed microbial consortium with no prior symbiotic relationship to this legume. As a result, portions of the inoculated community may have been partially inactivated, limiting its contribution to phytoremediation. Nonetheless, the MI may have indirectly supported plant stress tolerance by enhancing rhizosphere resilience and nutrient turnover. These findings highlight that microbial activity in tebuthiuron-contaminated soils depends on the balance between degradation kinetics and the adaptive capacity of the inoculated strains, reinforcing the need for the development of site-specific inoculants with proven herbicide tolerance and functional persistence to optimize biodegradation and promote sustainable soil recovery.
3.7. Principal Component Analysis
Principal component analysis (PCA), as depicted in Figure 5, was employed as a multivariate tool to synthesize and visualize patterns in variables related to M. pruriens development and soil characteristics under different treatments. This approach allowed for the identification of the main sources of variation among treatments and revealed complex interactions between edaphic factors and the plant’s morphophysiological responses. The first two principal components (PC1 and PC2) jointly accounted for 56.19% of the total data variance (34.38% by PC1 and 21.81% by PC2), which is considered satisfactory in agroecosystems studies given the multiplicity of factors involved [].
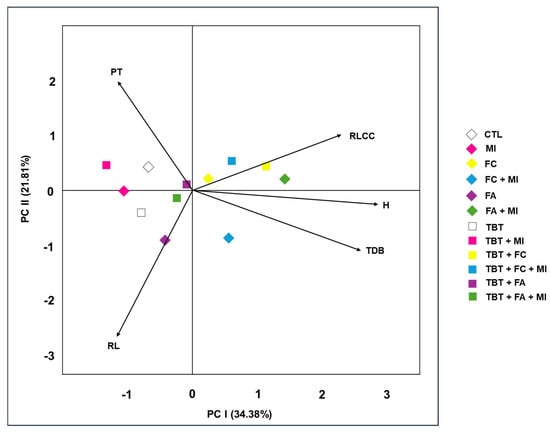
Figure 5.
Principal component analysis biplot summarizing relationships among morphophysiological and soil variables of Mucuna pruriens at 70 days after sowing, Dracena, SP, Brazil, 2023. Caption: CTL: cultivation with Mucuna pruriens only (Control); TBT: tebuthiuron; MI: microbial inoculant; FC: traditional filter cake; FA: filter cake composted with ash; PT: phytotoxicity; RLCC: relative leaf chlorophyll content; TDB: total dry biomass; H: height; RL: root length.
Principal component 1 (PC1) demonstrated a stronger positive association with the variables total dry biomass (TDB) and plant height (H). This indicates that this axis primarily reflects the vegetative growth and productive performance of M. pruriens. Consequently, the horizontal distribution of samples within the PCA biplot highlights the influence of treatments on promoting plant development. Treatments positioned to the right of the plot tend to exhibit better growth indices, while those on the left are associated with reduced vigor.
Principal component 2 (PC2), on the other hand, was strongly influenced by the variable phytotoxicity (PT), making it the primary indicator of chemical stress caused by tebuthiuron. The PT vector, oriented towards the upper part of the graph, indicates that high levels of this variable are associated with treatments having a lower capacity for herbicide mitigation. These treatments are characterized by visible symptoms such as chlorosis and foliar necrosis. This result demonstrates M. pruriens’ sensitivity to soil contamination and underscores the importance of environmental remediation strategies.
This inverse positioning of the phytotoxicity (PT) vector relates to plant growth indicators such as height (H), total dry biomass (TDB), and relative leaf chlorophyll content (RLCC) does not indicate a data inconsistency but rather a biologically coherent antagonistic relationship. Since phytotoxicity quantifies visible injury in comparison to the control, higher PT values inherently correspond to reduced growth and photosynthetic efficiency. Consequently, the negative correlation observed in the PCA, with the PT vector pointing in the opposite direction, is an expected outcome that reflects the trade-off between stress intensity and plant performance. Phytotoxicity values were calculated according to the official methodology of the Brazilian Society of Weed Science [], confirming the reliability of the procedure.
The relative leaf chlorophyll content (RLCC) showed a significant correlation with both PC1 and PC2, highlighting its dual nature. On one hand, high RLCC values directly correlated with improved plant photosynthetic and nutritional performance (PC1). On the other hand, reductions in RLCC were associated with increased phytotoxicity (PC2), suggesting that this variable can also serve as a stress bioindicator. Its diagonal position in the biplot indicates that the relative leaf chlorophyll content integrates information about both dimensions: healthy growth and tolerance to chemical stress.
Conversely, root length (RL) exhibited a negative correlation with both principal components, indicating that its increase does not always reflect improved agronomic performance. In contaminated soils, root elongation can be interpreted as an adaptive strategy by the plant to escape more toxic zones. However, this behavior does not necessarily translate into greater biomass accumulation or improved nutrition, which explains its inverse contribution in the PCA vectors. Treatments with elevated RL were frequently those subjected to tebuthiuron (TBT) or filter cake composted with ash (FA), corroborating the hypothesis of an adaptive stress response.
Among the evaluated treatments, FA + MI, TBT + FC, and TBT + FC + MI most positively influenced plant growth and physiology, as evidenced by the vectors for H and RLCC. These findings align with the data presented in Table 4 and Figure 3, where these treatments stood out for promoting greater plant development and lower stress levels.
The FA + MI treatment seems to have fostered conditions conducive to the soil microbiota, which in turn supported nutrient mineralization and herbicide degradation. Similarly, treatments incorporating filter cake enhanced soil fertility, stimulating microbial activity and lessening tebuthiuron’s toxic effects.
Regarding total dry biomass (TDB), it was observed that the absence of tebuthiuron generally resulted in higher values, particularly when combined with organic wastes (FC or FA). These results indicate that the addition of organic matter rich in carbon and nutrients promotes plant growth in uncontaminated soils. Nevertheless, the use of the microbial inoculant (MI) alone did not lead to significant biomass gains, suggesting a limited impact under the experimental conditions evaluated.
The treatments associated with the highest phytotoxicity were the control (CTL) and the exclusive use of the microbial inoculant (MI), as illustrated in Figure 2. Although CTL exhibited low phytotoxicity, plant growth was impaired due to the absence of nutritional and microbiological supplementation. In turn, MI—despite its recognized potential as bioinput—was insufficient to mitigate the effects of tebuthiuron, resulting in stress symptoms in M. pruriens. In contrast, treatments combining organic wastes and/or microbial inoculants stood out for their positive synergy, promoting greater biomass, increased nodule formation, and a reduction in phytotoxic symptoms.
Finally, it was found that the FA treatment alone showed limited effects on the evaluated morphophysiological parameters. Although it contributed positively to some variables, its effects appeared constrained, possibly due to the slow release of nutrients or changes in soil pH. Similarly, the microbial inoculant (MI), when applied alone, did not lead to significant differences, indicating that its effectiveness depends on specific substrate conditions. The combination of TBT + FA + MI, while promising, did not result in substantial gains, suggesting that the interaction among bioinputs still requires optimization to enhance performance in contaminated soils.
Similar multivariate trends have been reported for other phytoremediation species and microbial inoculants, supporting the generality of our findings. For instance, PCA studies with P. glaucum and A. hypogaea grown under tebuthiuron exposure also revealed positive associations among total biomass, chlorophyll content, and plant height, inversely correlated with phytotoxicity [,]. Comparable configurations were described for C. ensiformis and C. cajan, indicating that the co-variation among growth and physiological indicators under herbicide stress is consistent across different legumes []. These parallels confirm that the multivariate structure observed here for M. pruriens reflects broader mechanisms of physiological resilience and microbially mediated mitigation of herbicide toxicity.
These findings underscore the importance of integrated management strategies that balance the addition of organic residues and the use of bioinputs, aiming to restore contaminated areas and enhance agricultural sustainability.
4. Conclusions
This study demonstrated that Mucuna pruriens exhibits notable tolerance and adaptive capacity when cultivated in tebuthiuron-contaminated soil, confirming its potential as a bioindicator and phytomanagement species. The incorporation of sugarcane by-products influenced soil–plant interactions differently depending on their composition.
Traditional filter cake (FC) markedly improved plant height, chlorophyll content, and total biomass, indicating its strong contribution to physiological recovery and stress mitigation. In contrast, the composted filter cake with ash (FA) primarily enhanced soil chemical stability, particularly pH buffering and Ca–Mg equilibrium, rather than promoting direct biomass gains. These complementary effects underline the importance of residue type in defining the biostimulant response.
The microbial inoculant (MI) exhibited variable influence, suggesting that its performance depends on ecological compatibility and the physicochemical conditions of the substrate.
Overall, these findings demonstrate that integrating M. pruriens cultivation with organic soil amendments produces complementary improvements in plant performance and represents a promising strategy for the partial recovery of tebuthiuron-affected soils. However, the present findings should be interpreted as a controlled-laboratory assessment, and further investigations, including quantitative analyses of herbicide residues, soil ecotoxicity toward sensitive species, and long-term biodegradation tests, are necessary to validate the environmental remediation potential of this approach.
5. Future Directions
Future research should integrate complementary microbiota analyses to elucidate the dynamics of soil microbial communities influenced by tebuthiuron, either applied alone or combined with organic residues and bioinputs. Quantitative determination of tebuthiuron residues and their degradation products in soil is also essential to verify whether the observed reductions in toxicity correspond to actual chemical degradation or merely to reduced bioavailability. Chromatographic or spectrometric methods could be employed to track dissipation kinetics under different biological treatments, while metagenomic or enzymatic analyses would help identify functional genes and metabolic pathways associated with herbicide transformation.
Moreover, a broader assessment of plant physiological aspects, such as oxidative stress markers, enzymatic activity, and water uptake, could improve understanding of tebuthiuron’s effects on subsequent crops and clarify the potential of bioinputs to mitigate such impacts. Ecotoxicological bioassays using non-target organisms, including microorganisms, invertebrates, vertebrates, and plants, would allow for a more holistic evaluation of soil recovery and the effectiveness of remediation strategies. The application of isotopic labeling (e.g., 14C-tebuthiuron) could further confirm mineralization pathways and residual persistence.
Finally, large-scale field studies remain essential but are inherently complex due to multiple interacting variables. Establishing partnerships with the sugarcane–energy sector and incorporating artificial intelligence models would facilitate the prediction of optimal treatment combinations, reduce experimental costs, and enhance the real-world applicability and scalability of phytomanagement strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12110431/s1, Figure S1: Temporal variation in temperature and relative humidity in the agricultural greenhouse during the cultivation of Mucuna pruriens, Dracena, SP, Brazil, 2023; Table S1: Experimental scheme for treatments combining herbicide, residues, and microbial inoculant, Dracena, SP, Brazil, 2023.; Table S2. Mean emergence of Mucuna pruriens seedlings under different treatments over time, Dracena, SP, Brazil, 2023; Table S3. Number of root nodules of Mucuna pruriens in soil samples at 70 days after sowing, Dracena, SP, Brazil, 2023.
Author Contributions
Conceptualization: V.H.C. and P.R.M.L.; Methodology: V.H.C. and P.R.M.L.; Investigation: V.H.C., D.M.P., T.L.d.O., Y.A.F. and T.S.V.; Formal analysis: V.H.C. and P.R.M.L.; Data curation: V.H.C.; Writing—original draft preparation: V.H.C.; Writing—review & editing: Y.A.F., V.d.N., J.P.M. and P.R.M.L.; Funding acquisition, project administration, resources, supervision and validation: P.R.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordination for Improvement of Higher Education Personnel (CAPES-Brazil, Financing Code: 001); the National Council for Scientific and Technological Development (CNPq-Brazil, Financing Codes: 313530/2021-1 and 302567/2025-9); and the Agrisus Foundation (Brazil, Financing Code: PA 3740/24).
Institutional Review Board Statement
Authors confirm that the manuscript has not been submitted to journal for simultaneous consideration and has not been previously published. Results collection, selection, and processing were performed personally.
Informed Consent Statement
This study did not involve human or animal subjects.
Data Availability Statement
The datasets generated and analyzed during this study are available in the UNESP Institutional Repository or from the corresponding author upon reasonable request.
Acknowledgments
We acknowledge the Brazilian development agencies for financial assistance (CAPES-Brazil, CNPq-Brazil, Fundação Agrisus-Brazil); and also the NETA group (FCAT/Unesp-Brazil) for its collaboration in pesticide application and the company AMTec Bioagrícola® for scientific collaboration and donation of bioinputs.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; O’Brien, J.; Flores, F.; Negri, A.P. Degradation of herbicides in the tropical marine environment: Influence of light and sediment. PLoS ONE 2016, 11, e0165890. [Google Scholar] [CrossRef]
- Matallo, M.B.; Spadotto, C.A.; Luchini, L.C.; Gomes, M.A. Sorption, degradation, and leaching of tebuthiuron and diuron in soil columns. J. Environ. Sci. Health B 2005, 40, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Institute of Environment and Renewable Natural Resources (IBAMA). Environmental Profile: Tebuthiuron. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/arquivos/perfis-ambientais/2020/2020-12-03-Ibama-Perfil-Ambiental-Tebutiuron.pdf (accessed on 6 June 2025).
- Mendes, K.F. Pesticides in agriculture and environment. Pestic. Agric. Environ. 2021, 1, 1. [Google Scholar]
- du Toit, J.C.O.; Sekwadi, K.P. Tebuthiuron residues remain active in soil for at least eight years in a semi-arid grassland, South Africa. Afr. J. Range Forage Sci. 2012, 29, 85–90. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Vieira, L.C.; Dreossi, S.C.; Dorta, D.J.; Gravato, C.; Silva Ferreira, M.E.; Oliveira, D.P. Integrating morphological, biochemical, behavioural, and molecular approaches to investigate developmental toxicity triggered by tebuthiuron in zebrafish (Danio rerio). Chemosphere 2023, 340, 139894. [Google Scholar] [CrossRef]
- Brown, D.M.; Okoro, S.; van Gils, J.; van Spanning, R.; Bonte, M.; Hutchings, T.; Linden, O.; Egbuche, U.; Bruun, K.B.; Smith, J.W.N. Comparison of landfarming amendments to improve bioremediation of petroleum hydrocarbons in Niger Delta soils. Sci. Total Environ. 2017, 596, 284–292. [Google Scholar] [CrossRef]
- Garcia, C.; Teixeira, M.A.; Ribeiro, R.A.F. Fitorremediação: Uma alternativa sustentável para a remediação de solos contaminados. In Proceedings of the 29th Brazilian Congress of Sanitary and Environmental Engineering, São Paulo, Brazil, 25–27 June 2025; ABES: Rio de Janeiro, Brazil, 2018; pp. 1–8. [Google Scholar]
- Juwarkar, A.A.; Jambhulkar, H.P. Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Bioresour. Technol. 2008, 99, 4732–4741. [Google Scholar] [CrossRef]
- Conciani, P.A.; Mendes, K.F.; Sousa, R.N.; Ribeiro, A.P.; Pimpinato, R.F.; Tornisielo, V.L. Peanut and sorghum are excellent phytoremediators of 14C-tebuthiuron in herbicide-contaminated soil. Adv. Weed Sci. 2023, 41, 9. [Google Scholar] [CrossRef]
- Cruz, V.H.; Moreira, B.R.A.; Valério, T.S.; Frias, Y.A.; Silva, V.L.; Morais, E.B.; Vasconcelos, L.G.; Tropaldi, L.; Prado, E.P.; Montagnolli, R.N.; et al. Leguminous plants and microbial inoculation: An approach for biocatalytic phytoremediation of tebuthiuron in agricultural soil. Agronomy 2024, 14, 2805. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Moreira, B.R.A.; Montagnolli, R.N.; Prado, E.P.; Viana, R.S.; Tomaz, R.S.; Cruz, J.M.; Bidoia, E.D.; Frias, Y.A.; Lopes, P.R.M. Green manure species for phytoremediation of soil with tebuthiuron and vinasse. Front. Bioeng. Biotechnol. 2021, 8, 600185. [Google Scholar] [CrossRef]
- Mendes, K.F.; Maset, B.A.; Mielke, K.C.; Sousa, R.N.; Martins, B.A.B.; Tornisielo, V.L. Phytoremediation of quinclorac and tebuthiuron-polluted soil by green manure plants. Int. J. Phytoremediat. 2021, 23, 474–481. [Google Scholar] [CrossRef]
- Frias, Y.A.; Valério, T.S.; Moreira, B.R.A.; Cruz, V.H.; Santos, A.L.; Andriolli, V.; Pincerato, G.M.T.; Lopes, P.R.M. Pennisetum glaucum in reducing ecotoxicity in soil with tebuthiuron, thiamethoxam and vinasse. J. Agric. Food Res. 2024, 18, 101470. [Google Scholar] [CrossRef]
- Lima, L.A.R.; Souza, R.C.; Cunha, J.L.X.L.; França, P.H.B.; Rodrigues, G.N.; Bulhões, L.E.L. Fitorremediação de tebuthiuron por espécies de plantas tolerantes a herbicidas. Rev. Agronegócio Meio Ambiente 2024, 17, e11451. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Santi, A.L.; Eitelwein, M.T.; Weirich, S.W.; da Rosa, C.M.; Piazzetta, H.V.L. Guia Prático de Plantas de Cobertura: Aspectos Fitotécnicos e Impactos Sobre a Saúde Do Solo; ESALQ-USP: Piracicaba, Brazil, 2022; 126p. [Google Scholar]
- Lima Filho, O.F.; Fontana, A.; Silva, J.F.; Alves, B.J.R.; Santos, H.P. (Eds.) Adubação Verde e Plantas de Cobertura no Brasil: Fundamentos e Prática, 2nd ed.; Embrapa: Brasília, Brazil, 2023; Volume 1, 586p. [Google Scholar]
- Schiebelbein, P.; Zanon, A.J.; Comin, J.J.; Silva, R.F.; Lovato, P.E.; Lima, J.E.F. Plantas de Cobertura e as Inter-Relações Com a Saúde Do Solo; SolloAgro/ESALQ/USP: Piracicaba, Brazil, 2022; 44p. [Google Scholar]
- Wildner, L.P.; Morales, R.G.F.; Justen, J.G.K.; Krunvald, L. Plantas Para Adubação Verde e Cobertura Do Solo: Caracterização das Espécies e Informações Para Cultivo no Estado de Santa Catarina; Epagri: Florianópolis, Brazil, 2023; 140p. [Google Scholar]
- Mahawar, S. Bioinoculants: An ecofriendly approach towards artificial fertilizers in sustainable agriculture. Futur. Trends Biotechnol. 2024, 3, 282–290. [Google Scholar] [CrossRef]
- Štyriaková, D.; Hajnal-Jafari, T.; Žunić, V.; Šuba, J.; Prekopová, M.; Yetik, A.K.; Štyriaková, I. Influence of biostimulant applications on vegetative growth and yield of strawberry under full and reduced fertilization. DYSONA Appl. Sci. 2026, 7, 41–49. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduţ, V.; Biriş, S. Sustainable valorization of waste and by-products from sugarcane processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Assad, L. Aproveitamento de resíduos do setor sucroalcooleiro desafia empresas e pesquisadores. Cienc. Cult. 2017, 69, 13–16. [Google Scholar] [CrossRef]
- Faria, M.A.; Lopes, P.R.M.; Bidoia, E.D.; Ferreira, L.C.; Viana, R.S.; Prado, E.P.; Bonini, C.S.B.B.; Tomaz, R.S. Vinasse and tebuthiuron application to sugarcane soil and its effects on bacterial community and ecotoxicity after natural attenuation. Int. J. Dev. Res. 2019, 9, 28898–28904. [Google Scholar]
- Behrami, A.; Llach, L.; Pereira, M.J.; Kafei, M.; Rodrigues, E.M.G. Porous and siliceous materials for the removal of emerging contaminants from water and wastewater: A review. Chemosphere 2022, 287, 132223. [Google Scholar]
- Santos, H.G.D.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS: Washington, DC, USA, 2014. [Google Scholar]
- Soares, A.A.V.L.; Prado, R.M.; Bertani, R.M.A.; da Silva, A.P.R.; Deus, A.C.F.; Kano, C.; Furlaneto, F.P.B. Contribution of using filter cake and vinasse as a source of nutrients for sustainable agriculture—A review. Sustainability 2024, 16, 5411. [Google Scholar] [CrossRef]
- Teixeira, F.S.; Costa, P.T.; Vidigal, S.S.; Pintado, M.; Pimentel, L.L.; Rodríguez-Alcalá, L.M. Toward sustainable wax extraction from the Saccharum officinarum L. filter cake byproduct: Process optimization, physicochemical characterization, and antioxidant performance. ACS Sustain. Chem. Eng. 2023, 11, 13415–13428. [Google Scholar] [CrossRef]
- Kiruba, N.J.M.; Saeid, A. An insight into microbial inoculants for bioconversion of waste biomass into sustainable “bio-organic” fertilizers: A bibliometric analysis and systematic literature review. Int. J. Mol. Sci. 2022, 23, 13049. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.D.; de Paula, D.F.; Mendes, K.F.; de Sousa, R.N.; Araújo, G.R.; Inoue, M.H.; Tornisielo, V.L. Can soil type interfere in sorption-desorption, mobility, leaching, degradation, and microbial activity of the 14C-tebuthiuron herbicide? J. Hazard. Mater. Adv. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Maroufpoor, E.; Faryabi, A.; Ghamarnia, H.; Moshrefi, G. Evaluation of uniformity coefficients for sprinkler irrigation systems under different field conditions in Kurdistan province (Northwest of Iran). Soil Water Res. 2010, 5, 139–145. [Google Scholar] [CrossRef]
- Sociedade Brasileira de Ciência de Plantas Daninhas (SBCPD). Procedimentos Para Instalação, Avaliação e Análise de Experimentos Com Herbicidas; SBCPD: Londrina, Brazil, 1995; 42p. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- van Raij, B. Fertilidade do Solo e Manejo de Nutrientes; International Plant Nutrition Institute: Piracicaba, Brazil, 2011; 420p. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson Education: New York, NY, USA, 2014; 528p. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Lima, J.R.S.; Silva, L.F.; Souza, C.L.; Ferreira, V.M.; Santos, F.A.; Souza, M.F.; Lima, E.S.; Lima, G.S. Chemical and biochemical properties of sugarcane straw and its potential for bioenergy production. Biomass Bioenergy 2018, 116, 45–53. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J. Biogeochemistry of soil organic matter: Current knowledge and future research directions. Eur. J. Soil Sci. 2021, 72, 8–32. [Google Scholar] [CrossRef]
- Busato, J.; Paiva Leão, T.; Baldotto, M.; Canellas, L.P. Organic matter quality and dynamics in tropical soils amended with sugar industry residue. Rev. Bras. Cienc. Solo 2012, 36, 795–806. [Google Scholar] [CrossRef]
- Lopes, C.M.; Silva, A.M.M.; Estrada-Bonilla, G.A.; Ferraz-Almeida, R.; Vieira, J.L.V.; Otto, R.; Vitti, G.C.; Cardoso, E.J.B.N. Improving the fertilizer value of sugarcane wastes through phosphate rock amendment and phosphate-solubilizing bacteria inoculation. J. Clean. Prod. 2021, 298, 126821. [Google Scholar] [CrossRef]
- Girotto, M.; Araldi, R.; Velini, E.D.; Gomes, G.L.G.C.; Carbonari, C.A.; Jasper, S.P.; Trindade, L.M.B. Eficiência fotossintética da cana-de-açúcar submetida à aplicação de atrazine e tebuthiuron em pré-emergência. Rev. Bras. Herbic. 2011, 10, 134–142. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Aguiar, G.R.; Silva, J.M.; Silva, K.T.M.; Siebeneichler, S.C.; Júnior, O.J.F.; Lima, C.S.L.; Bastos, I.M.A.S.; Sousa, A.S.; Oliveira, M. Photosystem II inhibitor herbicides. Obs. Econ. Lat. 2024, 22, e5856. [Google Scholar] [CrossRef]
- Rezapour, S.; Nouri, A.; Asadzadeh, F.; Barin, M.; Erpul, G.; Jagadamma, S.; Qin, R. Combining chemical and organic treatments enhances remediation performance and soil health in saline-sodic soils. Commun. Earth Environ. 2023, 4, 285. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil microorganisms: Their role in enhancing crop nutrition and health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Frias, Y.A.; Lima, E.W.; Aragão, M.B.; Nantes, L.S.; Moreira, B.R.A.; Cruz, V.H.; Tomaz, R.S.; Lopes, P.R.M. Mucuna pruriens cannot develop phytoremediation of tebuthiuron in agricultural soil with vinasse: A morphometrical and ecotoxicological analysis. Front. Bioeng. Biotechnol. 2023, 11, 1156751. [Google Scholar] [CrossRef] [PubMed]
- Crusciol, C.A.C.; McCray, J.M.; de Campos, M.; do Nascimento, C.A.C.; Rossato, O.B.; Adorna, J.C.; Mellis, E.V. Filter cake as a long-standing source of micronutrients for sugarcane. J. Soil Sci. Plant Nutr. 2021, 21, 813–823. [Google Scholar] [CrossRef]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic matter-microorganism-plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Vasconcelos, R.L.; Cremasco, C.P.; de Almeida, H.J.; Garcia, A.; Neto, A.B.; Mauad, M.; Filho, L.R.A.G. Multivariate behavior of irrigated sugarcane with phosphate fertilizer and filter cake management: Nutritional state, biometry, and agroindustrial performance. J. Soil Sci. Plant Nutr. 2020, 20, 1625–1636. [Google Scholar] [CrossRef]
- Singh, N.K.; Sachan, K.; Ranjitha, G.; Chandana, S.; Manoj, B.P.; Panotra, N.; Katiyar, D. Building soil health and fertility through organic amendments and practices: A review. Asian J. Soil Sci. Plant Nutr. 2024, 10, 175–197. [Google Scholar] [CrossRef]
- Santos, R.M.; Kandasamy, S.; Rigobelo, E.C. Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. MicrobiologyOpen 2018, 7, e00617. [Google Scholar] [CrossRef]
- Irin, I.J.; Hasanuzzaman, M. Organic amendments: Enhancing plant tolerance to salinity and metal stress for improved agricultural productivity. Stresses 2024, 4, 185–209. [Google Scholar] [CrossRef]
- Zaghloul, E.A.; Awad, E.A.; Mohamed, I.R.; Abd El-Hameed, A.M.; Feng, D.; Desoky, E.S.; Algopishi, U.B.; Al Masoudi, L.M.; Elrys, A.S.; Mathew, B.T.; et al. Co-application of organic amendments and natural biostimulants on plants enhances wheat production and defense system under salt-alkali stress. Sci. Rep. 2024, 14, 29742. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. What can reactive oxygen species (ROS) tell us about the action mechanism of herbicides and other phytotoxins? Free Radic. Biol. Med. 2024, 220, 92–110. [Google Scholar] [CrossRef]
- Ferreira, J.H.S.; Melo, C.A.D.; Moraes, M.F.; Silva, I.P.F.E.; Chioderoli, C.A. Residual activity and sorption of tebuthiuron in different soils. Rev. Bras. Eng. Agric. Ambient 2023, 28, e275280. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Richard-Nwachukwu, N. Microbe-assisted remediation of pesticide residues from soil and water. In Eco-Restoration of Polluted Environment; CRC Press: Boca Raton, FL, USA, 2024; pp. 215–232. [Google Scholar]
- Meng, X.; Bai, S.; Wang, S.; Pan, Y.; Chen, K.; Xie, K.; Wang, M.; Guo, S. The sensitivity of photosynthesis to magnesium deficiency differs between rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1164866. [Google Scholar] [CrossRef]
- Pohland, A.C.; Bernát, G.; Geimer, S.; Schneider, D. Mg2+ limitation leads to a decrease in chlorophyll, resulting in an unbalanced photosynthetic apparatus in the cyanobacterium Synechocystis sp. PCC6803. Photosynth. Res. 2024, 162, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Xie, K.; Pan, Y.; Meng, X.; Wang, M.; Guo, S. Critical leaf magnesium thresholds for growth, chlorophyll, leaf area, and photosynthesis in rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Agronomy 2024, 14, 1508. [Google Scholar] [CrossRef]
- Lv, X.; Kong, H.; Luo, Y.; Dong, D.; Liu, W.; Wu, D.; Ye, Z.; Ma, J.; Liu, D. The impact of magnesium on the growth, physiology and quality of tea (Camellia sinensis L.) plants under acid stress. Agronomy 2024, 14, 767. [Google Scholar] [CrossRef]
- Visconti, D.; Ventorino, V.; Fagnano, M.; Woo, S.L.; Pepe, O.; Adamo, P.; Caporale, A.G.; Carrino, L.; Fiorentino, N. Compost and microbial biostimulant applications improve plant growth and soil biological fertility of a grass-based phytostabilization system. Environ. Geochem. Health 2023, 45, 787–807. [Google Scholar] [CrossRef]
- Teixeira, M.F.F.; Silva, A.A.; Nascimento, M.A.; Vieira, L.S.; Teixeira, T.P.M.; Souza, M.F. Effects of adding organic matter to a Red-Yellow Latosol in the sorption and desorption of tebuthiuron. Planta Daninha 2018, 36, e018168639. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; 944p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).