Long-Term Monitoring of Arundo donax L. Range in Albufera Wetland (Spain): Management Challenges and Policy Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Work
2.3. Remote Sensing Analysis
2.4. Data Analysis
3. Results
3.1. Remote Sensing
3.2. Multivariate Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A









References
- Simberloff, D. Sustainability of biodiversity under global changes, with particular reference to biological invasions. In Sustainability Science: The Emerging Paradigm and the Urban Environment; Weinstein, M., Turner, E., Eds.; Springer: New York, NY, USA, 2012; pp. 139–157. Available online: https://link.springer.com/book/10.1007/978-1-4614-3188-6 (accessed on 4 April 2025).
- McGeoch, M.A.; Butchart, S.H.M.; Spear, D.; Marais, E.; Kleynhans, E.J.; Symes, A.; Chanson, J.; Hoffmann, M. Global indicators of biological invasion: Species, numbers, biodiversity impact and policy responses. Divers. Distrib. 2010, 16, 95–108. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Templeand, H.; Vineet, K. The Mediterranean: A Biodiversity Hotspot under Threat. In The 2008 Review of The IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2019; Available online: https://iucn.org/sites/default/files/import/downloads/the_mediterranean_a_biodiversity_hotspot_under_threat.pdf (accessed on 7 July 2025).
- Al Hassan, M.; Boscaiu, M.; Mayoral, O. Competition between halophytes and invasive species: Dittrichia viscosa and Limbarda crithmoides: A Study Case from the Valencian Salt Marshes. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer International Publishing: Cham, Switzerland, 2021; pp. 599–621. [Google Scholar] [CrossRef]
- Ley 11/1994, de 27 de Diciembre, de Espacios Naturales Protegidos de la Comunidad Valenciana. Available online: https://www.boe.es/eli/es-vc/l/1994/12/27/11 (accessed on 7 July 2025).
- Soria, J.M. Past, present and future of La Albufera of Valencia Natural Park. Limnetica 2006, 25, 135–142. [Google Scholar] [CrossRef]
- Bolòs, O.D.; Vigo, A. Flora dels Països Catalans, 2nd ed.; Editorial Barcino: Barcelona, España, 2001; Volume I (Monocotiledònies), Available online: https://publicacions.iec.cat/repository/pdf/00000300/00000020.pdf (accessed on 7 July 2025).
- Häfliger, E.; Scholz, H. Grass Weeds 2, Weeds of the Subfamilies Chloridoideae, Pooideae, Oryzoideae; Documenta Ciba-Geigy: Basel, Switzerland, 1981; pp. 32–45. [Google Scholar]
- Dudley, T.L. Arundo donax L. In Invasive Plants of California’s Wildlands; Bossard, C.C., Randall, J.M., Hoshovsky, M.C., Eds.; University of California Press: Berkeley, CA, USA, 2000; pp. 53–58. [Google Scholar]
- Bell, G.P. Biology and growth habits of giant reed (Arundo donax). In Arundo donax Workshop Proceedings; Jackson, N.E., Frandsen, P., Douthit, S., Eds.; California Exotic Pest Plant Council (CalEPPC): Pismo Beach, CA, USA, 1993; pp. 1–6. Available online: https://www.cal-ipc.org/docs/symposia/archive/pdf/Arundo_Proceedings_1993.pdf (accessed on 7 July 2025).
- Lambert, A.M.; Dudley, T.L.; Saltonstall, K. Ecology and impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Sci. Manag. 2010, 3, 489–494. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Hardion, L.; Del Monte, J.P.; Vila, B.; Santín-Montanyá, M.I. Monographs on invasive plants in Europe N° 4: Arundo donax L. Bot. Lett. 2021, 168, 131–151. [Google Scholar] [CrossRef]
- Madsen, J.D.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Schoelynck, J.; De Groote, T.; Bal, K.; Vandenbruwaene, W.; Meire, P.; Temmerman, S. Self-organised patchiness and scale-dependent bio-geomorphic feedbacks in aquatic river vegetation. Ecography 2012, 35, 760–768. [Google Scholar] [CrossRef]
- Bal, K.D.; Bouma, T.J.; Buis, K.; Struyf, E.; Schoelynck, J.; Backx, H.; Meire, P. Trade-off between drag reduction and light interception of macrophytes: Comparing five aquatic plants with contrasting morphology. Funct. Ecol. 2011, 25, 1197–1205. [Google Scholar] [CrossRef]
- De Doncker, L.; Troch, P.; Verhoeven, R.; Bal, K.; Desmet, N.; Meire, P. Relation between resistance characteristics due to aquatic weed growth and the hydraulic capacity of the river Aa. River Res. Appl. 2009, 25, 1287–1303. [Google Scholar] [CrossRef]
- Tracy, J.L.; DeLoach, C.J. Biological control of saltcedar in the United States: Progress and projected ecological effects. In Arundo and Saltcedar: The Deadly Duo; Bell, C.E., Ed.; U.C. Cooperative Extension Service: Riverside, CA, USA, 1999; pp. 111–154. [Google Scholar]
- El Confidencial. Las Cañas Que Invaden Ríos y Barrancos son una Trampa Mortal. Available online: https://www.elconfidencial.com/medioambiente/agua/2024-12-19/canas-barrancos-riadas-dana-especies-invasoras_4025733/ (accessed on 7 April 2025).
- Boon, P.J.; Holmes, N.T.H.; Raven, P.J. Developing standard approaches for recording and assessing river hydromorphology: The role of the European Committee for Standardization (CEN). Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 55–61. [Google Scholar] [CrossRef]
- Schmutz, S.; Cowx, I.; Haidvogl, G.; Pont, D. Fish-based methods for assessing European running waters: A synthesis. Fish. Manag. Ecol. 2007, 14, 369–380. [Google Scholar] [CrossRef]
- Kettunen, M.; Genovesi, P.; Gollasch, S.; Pagad, S.; Starfinger, U.; ten Brink, P.; Shine, C. Technical Support to EU Strategy on Invasive Species (IAS)—Assessment of the Impacts of IAS in Europe and the EU (Final Module Report for the European Commission); Institute for European Environmental Policy (IEEP): Brussels, Belgium, 2008; Available online: https://www.actu-environnement.com/media/pdf/news-27629-ieep-etude.pdf (accessed on 7 July 2025).
- Cardoso, A.C.; Free, G. Incorporating invasive alien species into ecological assessment in the context of the Water Framework Directive. Aquat. Invasions 2008, 3, 361–366. [Google Scholar] [CrossRef]
- Carpenter, G.A.; Gopal, S.; Macomber, S.; Martens, S.; Woodcock, C.E. A Neural Network Method for Mixture Estimation for Vegetation Mapping. Remote Sens. Environ. 1999, 70, 139–152. [Google Scholar] [CrossRef]
- He, C.; Zhang, Q.; Li, Y.; Li, X.; Shi, P. Zoning grassland protection area using remote sensing and cellular automata modeling—A case study in Xilingol steppe grassland in northern China. J. Arid Environ. 2005, 63, 814–826. [Google Scholar] [CrossRef]
- Polunin, O.; Huxley, A. Flowers of the Mediterranean; Hogarth Press: London, UK, 1987; Available online: http://www.marelibri.com/topic/3290534-main/books/AUTHOR_AZ/14300?l=en (accessed on 7 July 2025).
- Zeven, A.C.; de Wet, J.M.J. Dictionary of Cultivated Plants and Their Centres of Diversity: Excluding Most Ornamentals, Forest Trees and Lower Plants; CAPD: Wageningen, The Netherlands, 1982; Available online: https://edepot.wur.nl/318076 (accessed on 7 July 2025).
- Ewel, K.; Cressa, C.; Kneib, R.; Levin, L.; Palmer, M.; Snelgrove, P.; Wall, D. Managing Critical Transition Zones. Ecosystems 2001, 4, 452–460. [Google Scholar] [CrossRef]
- Hood, W.G.; Naiman, R.J. Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecol. 2000, 148, 105–114. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- PlantNet. Arundo donax L. Available online: https://identify.plantnet.org/es/k-world-flora/species/Arundo%20donax%20L./data (accessed on 12 May 2025).
- PlantNet. Phragmites australis (Cav.) Trin. ex Steud. Available online: https://identify.plantnet.org/es/k-world-flora/species/Phragmites%20australis%20(Cav.)%20Trin.%20ex%20Steud./data#section-phenology (accessed on 8 May 2025).
- Soria, J.M.; Molner, J.V.; Pérez-González, R.; Alvado, B.; Vera-Herrera, L.; Romo, S. Monitoring the Extraordinary Ephemeral Emergence of Myriophyllum spicatum L. in the Coastal Lagoon Albufera of Valencia (Spain) and Assessing the Impact of Environmental Variables Using a Remote Sensing Approach. J. Mar. Sci. Eng. 2024, 12, 260. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Chen, J.M.; Black, T.A. Defining leaf-area index for non-flat leaves. Plant Cell Environ. 1992, 15, 421–429. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aguiar, F.C.; Silva, J.M.; Ferreira, M.T.; Pereira, J.M. Spectral discrimination of giant reed (Arundo donax L.): A seasonal study in riparian areas. ISPRS J. Photogramm. Remote Sens. 2013, 80, 80–90. [Google Scholar] [CrossRef]
- Berardi, U.; Iannace, G. Acoustic characterization of natural fibers for sound absorption applications. Build. Environ. 2015, 94, 840–852. [Google Scholar] [CrossRef]
- Boland, J.M. The importance of layering in the rapid spread of Arundo donax (giant reed). Madroño 2006, 53, 303–312. [Google Scholar] [CrossRef]
- Quinn, L.D.; Allen, D.J.; Stewart, J.R. Environmental Tolerance and Biomass Potential of Arundo donax in Subtropical Regions. Ind. Crops Prod. 2015, 76, 1000–1008. [Google Scholar] [CrossRef]
- Haslam, S.M. A Book of Reed: (Phragmites australis (Cav.) Trin. ex Steudel, Phragmites communis Trin.); Forrest Text; Cardigan: Wales, UK, 2010. [Google Scholar]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Thevs, N.; Zerbe, S.; Gahlert, F.; Mijit, M.; Succow, M. Productivity of reed (Phragmites australis Trin. ex. Staud.) in continental-arid NW China in relation to soil, groundwater, and land use. J. Appl. Bot. Food Qual. 2007, 81, 62–68. [Google Scholar]
- Martínez Gabarrón, A.; Flores Yepes, J.A.; Pastor Pérez, J.J.; Berná Serna, J.M.; Arnold, L.C.; Sánchez Medrano, F.J. Increase of the flexural strength of construction elements made with plaster (calcium sulfate dihydrate) and common reed (Arundo donax L.). Constr. Build. Mater. 2014, 66, 436–441. [Google Scholar] [CrossRef]
- Bołtryk, M.; Pawluczuk, E. Properties of a lightweight cement composite with an ecological organic filler. Constr. Build. Mater. 2014, 51, 97–105. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Jaeel, A.J. A novel use of undesirable wild giant reed biomass to replace aggregate in concrete. Constr. Build. Mater. 2014, 67, 68–73. [Google Scholar] [CrossRef]
- Khabaz, A. Construction and design requirements of green buildings’ roofs in Saudi Arabia depending on thermal conductivity principle. Constr. Build. Mater. 2018, 186, 1119–1131. [Google Scholar] [CrossRef]
- Sobhy, I.; Brakez, A.; Benhamou, B. Analysis for Thermal Behavior and Energy Savings of a Semi-Detached House with Different Insulation Strategies in a Hot Semi-Arid Climate. J. Green Build. 2017, 12, 78–106. [Google Scholar] [CrossRef]
- Yu, C.; Crump, D. A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ. 1998, 33, 357–374. [Google Scholar] [CrossRef]
- Barreca, F.; Martinez Gabarron, A.; Flores Yepes, J.A.; Pastor Pérez, J.J. Innovative Use of Giant Reed and Cork Residues for Panels of Buildings in Mediterranean Area. Resour. Conserv. Recycl. 2019, 140, 259–266. [Google Scholar] [CrossRef]
- Prusty, J.; Patro, S.; Basarkar, S. Concrete using agro-waste as fine aggregate for sustainable built environment—A review. Int. J. Sustain. Built Environ. 2016, 5, 312–333. [Google Scholar] [CrossRef]
- McCormick, K.; Kautto, N. The bioeconomy in Europe: An overview. Sustainability 2013, 5, 2589–2608. [Google Scholar] [CrossRef]
- Laborel-Préneron, A.; Aubert, J.; Magniont, C.; Tribout, C.; Bertron, A. Plant aggregates and fibers in earth construction materials: A review. Constr. Build. Mater. 2016, 111, 719–734. [Google Scholar] [CrossRef]
- Hawke, C.J.; Jose, P.V. Reedbed Management for Commercial and Wildlife Interests; The Royal Society for the Protection of Birds: Sandy, UK, 1996. [Google Scholar]
- Schmidt, M.H.; Lefebvre, G.; Poulin, B.; Tscharntke, T. Reed cutting affects arthropod communities, potentially reducing food for passerine birds. Biol. Conserv. 2005, 121, 157–166. [Google Scholar] [CrossRef]
- Papastergiadou, E.; Agami, M.; Waisel, Y. Restoration of aquatic vegetation in Mediterranean wetlands. In Restoration of Mediterranean Wetlands; MedWet: Arles, France, 2002; pp. 47–69. Available online: http://repository.biodiversity-info.gr/bitstream/11340/2061/1/251.pdf#page=202 (accessed on 7 July 2025).
- Poulin, B.; Lefebvre, G. Effect of winter cutting on the passerine breeding assemblage in French Mediterranean reedbeds. Biodivers. Conserv. 2002, 11, 1567–1581. [Google Scholar] [CrossRef]
- Cowie, N.R.; Sutherland, W.J.; Ditlhogo, M.K.M.; James, R. The effects of conservation management of reed beds II. The flora and litter disappearance. J. Appl. Ecol. 1992, 29, 277–284. [Google Scholar] [CrossRef]
- Saltonstall, K.; Lambert, A.; Meyerson, L.A. Genetics and Reproduction of Common (Phragmites australis) and Giant Reed (Arundo donax). Invasive Plant Sci. Manag. 2010, 3, 495–505. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and Consequences of Invasive Plants in Wetlands: Opportunities, Opportunists, and Outcomes. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Lite, S.J.; Marler, R.; Paradzick, C.; Shafroth, P.B.; Shorrock, D.; White, J.M.; White, M.S. Altered stream-flow regimes and invasive plant species: The Tamarix case. Global Ecol. Biogeogr. 2007, 16, 381–393. [Google Scholar] [CrossRef]
- Lambert, A.M.; Dudley, T.L.; Robbins, J. Nutrient enrichment and soil conditions drive productivity in the large-statured invasive grass Arundo donax. Aquat. Bot. 2014, 112, 16–22. [Google Scholar] [CrossRef]
- Boland, J.M. The roles of floods and bulldozers in the break-up and dispersal of Arundo donax (giant reed). Madroño 2008, 55, 216–222. [Google Scholar] [CrossRef]
- Shaw, R.H. Weed biological control regulation in Europe: Boring but important. In Proceedings of the XII International Symposium on Biological Control of Weeds, La Grande-Motte, France, 22–27 April 2007. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- CHJ. La Confederación Hidrográfica del Júcar, O.A., Realiza una Inversión Histórica en Conservación, Mantenimiento y Restauración de los Cauces de la Demarcación. Available online: https://www.chj.es/es-es/ciudadano/salaprensa/Paginas/SerieDeActuacionesConservacionCauces2022.aspx (accessed on 8 April 2025). (In Spanish).
- Deltoro Torró, V.; Jiménez Ruiz, J.; Vilán Fragueiro, X.M. Bases Para el Manejo y Control de Arundo donax L. (Caña común), 1st ed.; Conselleria d’Infraestructures, Territori i Medi Ambient, Generalitat Valenciana: Valencia, Spain, 2012; Available online: https://www.miteco.gob.es/content/dam/miteco/es/ceneam/grupos-de-trabajo-y-seminarios/red-parques-nacionales/Bases%20para%20el%20manejo%20y%20control%20de%20Arundo%20donax_tcm30-169319.pdf (accessed on 8 April 2025).
- Generalitat Valenciana. Memoria de Gestión del Parc Natural de L’Albufera. 2012. Available online: https://parquesnaturales.gva.es/documents/80302883/161584555/05.Memoria+de+Gesti%C3%B3n+2012.pdf/806e2468-d7fc-432b-90b2-6f362eb6286f?t=1610550296628 (accessed on 11 March 2025). (In Spanish).
- Generalitat Valenciana. Memoria de Gestión del Parque Natural de L’Albufera. 2021. Available online: https://parquesnaturales.gva.es/documents/80302883/161584555/14.+Memoria+de+Gesti%C3%B3n+2021.pdf/342296bb-d96c-f725-e027-96fdfad79cb2?t=1678972124109 (accessed on 11 March 2025). (In Spanish).
- Molner, J.V.; Pérez-González, R.; Sòria-Perpinyà, X.; Soria, J. Climatic Influence on the Carotenoids Concentration in a Mediterranean Coastal Lagoon Through Remote Sensing. Remote Sens. 2024, 16, 4067. [Google Scholar] [CrossRef]
- Bruno, D.; Zapata, V.; Guareschi, S.; Picazo, F.; Dettori, E.; Carbonell, J.A.; Robledano, F. Short-Term Responses of Aquatic and Terrestrial Biodiversity to Riparian Restoration Measures Designed to Control the Invasive Arundo donax L. Water 2019, 11, 2551. [Google Scholar] [CrossRef]
- Nepf, H.M. Flow and transport in regions with aquatic vegetation. Annu. Rev. Fluid Mech. 2012, 44, 123–142. [Google Scholar] [CrossRef]


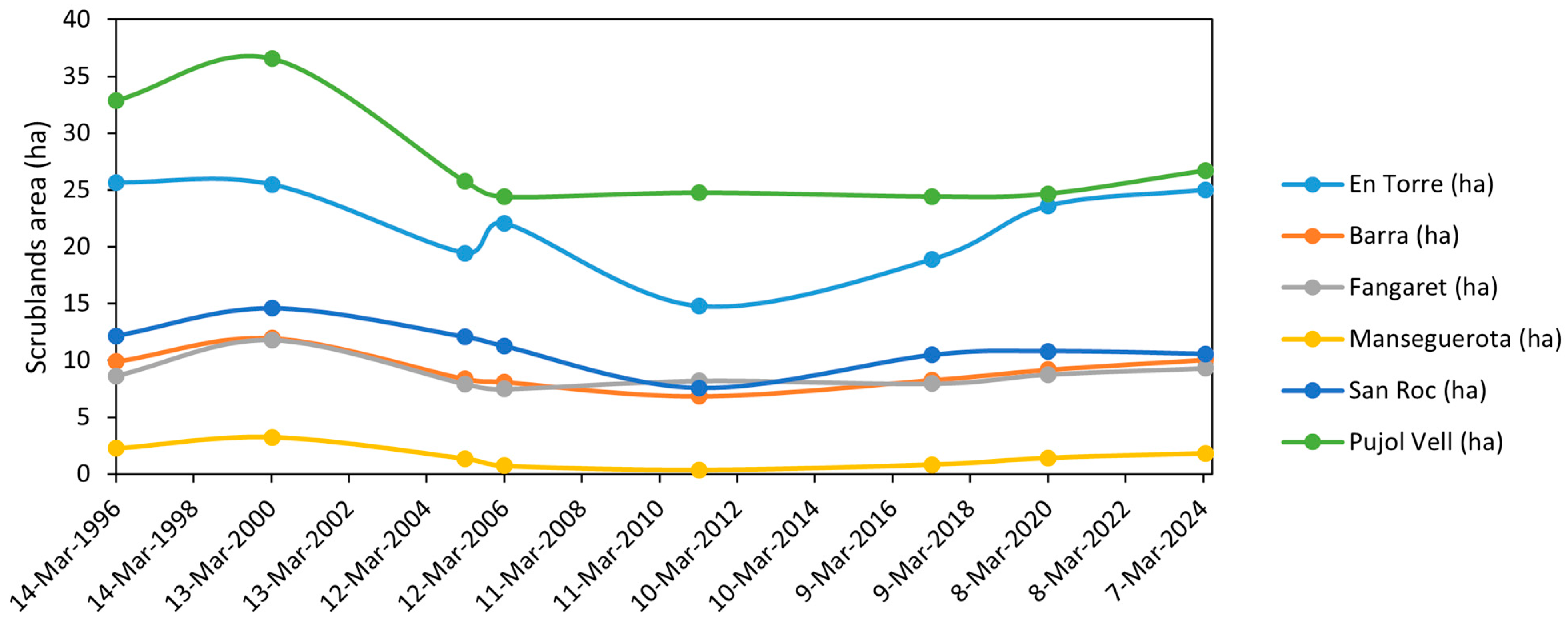
| Date | Satellite | Spatial Resolution (m) | En Torre (ha) | Barra (ha) | Fangaret (ha) | Manseguerota (ha) | San Roc (ha) | Pujol Vell (ha) | Total (ha) |
|---|---|---|---|---|---|---|---|---|---|
| 14 March 1996 | L5 | 30 | 25.65 | 9.90 | 8.64 | 2.25 | 12.15 | 32.85 | 91.44 |
| 18 March 2000 | L5 | 30 | 25.47 | 11.97 | 11.79 | 3.24 | 14.58 | 36.54 | 103.59 |
| 7 March 2005 | L5 | 30 | 19.44 | 8.37 | 7.92 | 1.35 | 12.06 | 25.74 | 74.88 |
| 10 March 2006 | L5 | 30 | 22.05 | 8.10 | 7.47 | 0.72 | 11.25 | 24.39 | 73.98 |
| 17 March 2011 | L5 | 30 | 14.76 | 6.84 | 8.19 | 0.36 | 7.56 | 24.75 | 62.46 |
| 17 March 2017 | S2A | 10 | 18.88 | 8.27 | 7.94 | 0.83 | 10.47 | 24.41 | 70.80 |
| 11 March 2020 | S2A | 10 | 23.59 | 9.18 | 8.75 | 1.42 | 10.81 | 24.66 | 78.41 |
| 30 March 2024 | S2A | 10 | 25.00 | 10.06 | 9.30 | 1.83 | 10.57 | 26.71 | 83.47 |
| Sites | En Torre | Barra | Fangaret | Manseguerota | San Roc | Pujol Vell | Total |
|---|---|---|---|---|---|---|---|
| N | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Shapiro–Wilk W | 0.899 | 0.960 | 0.806 | 0.951 | 0.936 | 0.731 | 0.957 |
| p (normal) | 0.281 | 0.808 | 0.033 (n.s.) | 0.721 | 0.574 | 0.005 (n.s.) | 0.785 |
| Sites | 1996–2024 | 1996–2011 | 2011–2024 | |||
|---|---|---|---|---|---|---|
| S | p | S | p | S | p | |
| En Torre | −6 | 0.274 n.s. | −8 | 0.042 | 6 | 0.042 |
| Barra | −2 | 0.452 n.s. | −8 | 0.042 | 6 | 0.042 |
| Fangaret | 4 | 0.360 n.s. | −4 | 0.242 n.s. | 4 | 0.167 n.s. |
| Manseguerota | −4 | 0.360 n.s. | −8 | 0.042 | 6 | 0.042 |
| San Roc | −16 | 0.031 | −8 | 0.042 | 4 | 0.167 |
| Pujol Vell | −8 | 0.199 n.s. | −6 | 0.117 n.s. | 2 | 0.375 n.s. |
| Total | −6 | 0.274 | −8 | 0.042 | 6 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molner, J.V.; Campillo-Tamarit, N.; Jover-Cerdá, M.; Soria, J.M. Long-Term Monitoring of Arundo donax L. Range in Albufera Wetland (Spain): Management Challenges and Policy Implications. Environments 2025, 12, 432. https://doi.org/10.3390/environments12110432
Molner JV, Campillo-Tamarit N, Jover-Cerdá M, Soria JM. Long-Term Monitoring of Arundo donax L. Range in Albufera Wetland (Spain): Management Challenges and Policy Implications. Environments. 2025; 12(11):432. https://doi.org/10.3390/environments12110432
Chicago/Turabian StyleMolner, Juan Víctor, Noelia Campillo-Tamarit, Miguel Jover-Cerdá, and Juan M. Soria. 2025. "Long-Term Monitoring of Arundo donax L. Range in Albufera Wetland (Spain): Management Challenges and Policy Implications" Environments 12, no. 11: 432. https://doi.org/10.3390/environments12110432
APA StyleMolner, J. V., Campillo-Tamarit, N., Jover-Cerdá, M., & Soria, J. M. (2025). Long-Term Monitoring of Arundo donax L. Range in Albufera Wetland (Spain): Management Challenges and Policy Implications. Environments, 12(11), 432. https://doi.org/10.3390/environments12110432








