Assessing Pollution and Diatom-Based Bioindicators in the Arieș River, Romania
Abstract
1. Introduction
2. Materials and Methods
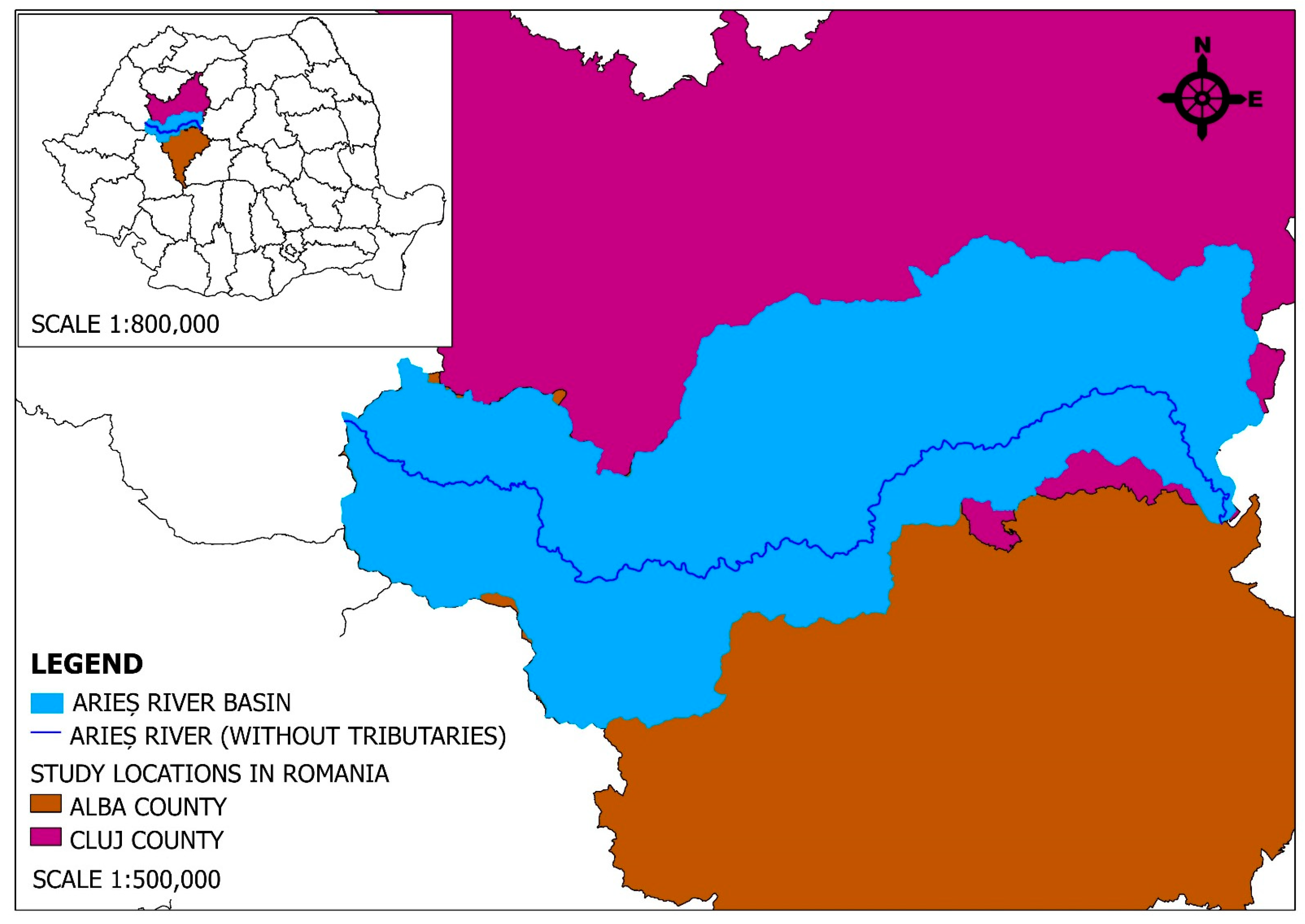
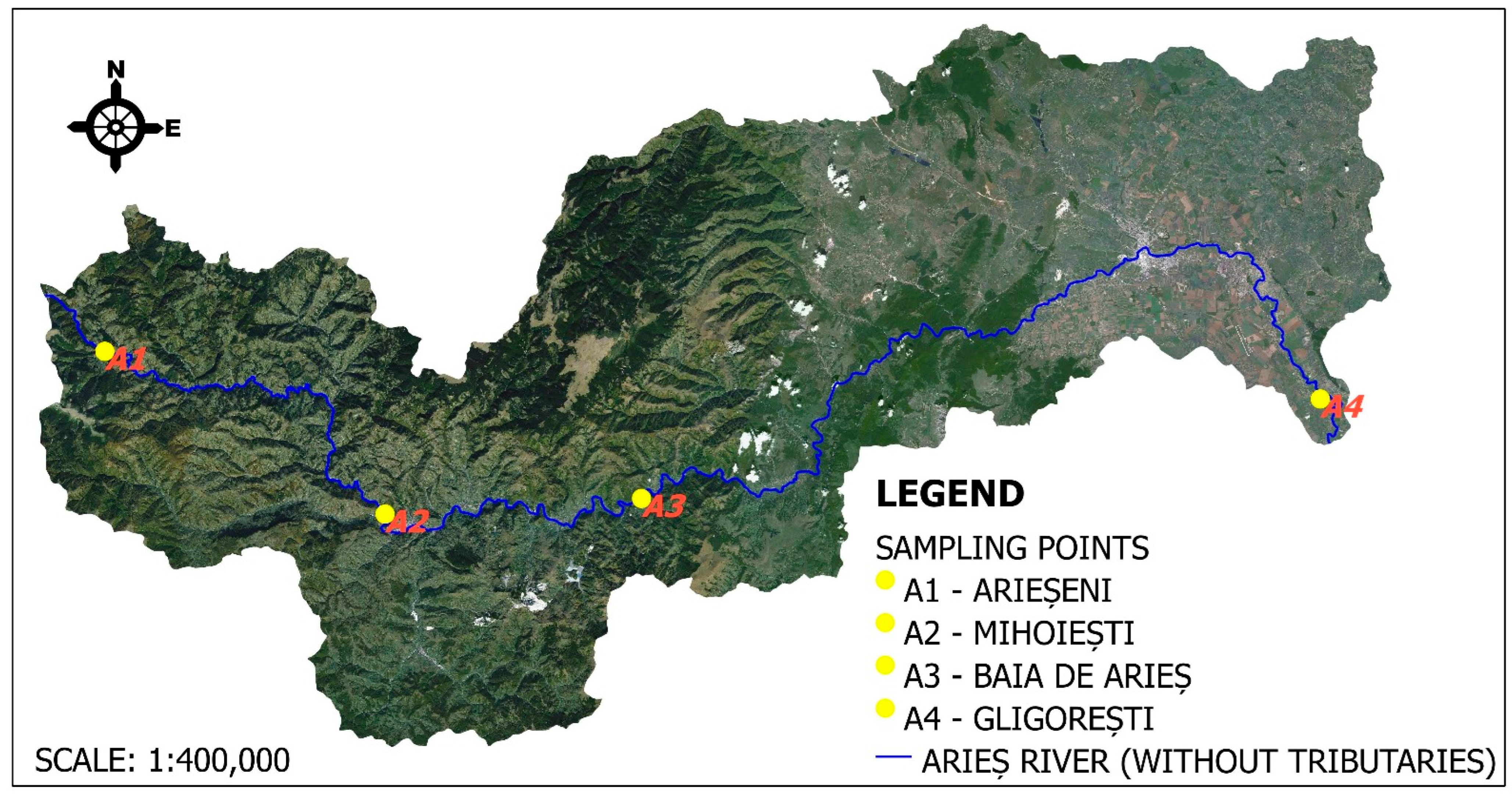
2.1. The Studied Region and Its Characteristics
2.2. Water Sample Collection
2.3. Physico-Chemical Analysis of River Water Samples
2.3.1. River Water Quality Parameters
2.3.2. River Water Salinity Parameters
2.3.3. Metal Analysis in River Water Samples [33,34,35,36,37]
2.4. Periphytic Biofilm Sampling and Interpretation
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Influence of Physico-Chemical Parameters on Water Quality
4.2. Diatom Communities of the Arieș River
4.3. Teratological Forms of Diatoms
4.4. Statistical Analysis [75]
4.4.1. Principal Component Analysis of Water Chemical Parameters
4.4.2. Canonical Correspondence Analysis of Heavy Metal Effects on Diatoms
4.4.3. Metal Trace Elements–Diatom Correlations (Pearson)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghimi Dehkordi, M.; Pournuroz Nodeh, Z.; Soleimani Dehkordi, K.; Salmanvandi, H.; Rasouli Khorjestan, R.; Ghaffarzadeh, M. Soil, air, and water pollution from mining and industrial activities: Sources of pollution, environmental impacts, and prevention and control methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Ejiohuo, O.; Onyeaka, H.; Akinsemolu, A.; Nwabor, O.F.; Siyanbola, K.F.; Tamasiga, P.; Al-Sharify, Z.T. Ensuring water purity: Mitigating environmental risks and safeguarding human health. Water Biol. Secur. 2025, 4, 100341. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Popa, M.; Dumitrel, G.-A.; Mirel, G.; Popa, D.-V. Anthropogenic Contamination of Water from Galda River—Alba County, Romania. Agric. Agric. Sci. Proc. 2015, 6, 446–452. [Google Scholar] [CrossRef][Green Version]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Tanaskovski, B.; Petrović, M.; Kljajić, Z.; Degetto, S.; Stanković, S. Analysis of major, minor and trace elements in surface sediments by X-ray fluorescence spectrometry for assessment of possible contamination of Boka Kotorska Bay, Montenegro. Maced. J. Chem. Chem. Eng. 2014, 33, 139–150. [Google Scholar] [CrossRef]
- Dumitrel, G.A.; Glevitzky, M.; Popa, M.; Vica, M.L. Studies regarding the heavy metals’ pollution of streams and rivers in Roșia Montană area, Romania. J. Environ. Prot. Ecol. 2015, 16, 850–860. [Google Scholar]
- Topan, M.V.; Moldovan, B.; Odagiu, A.; Burduhos, P.; Iederan, C.; Mălinaș, C.; Brașovean, I. Heavy Metals Pollution Risk in Roșia Montană Area. Note II: Copper, Zinc and Arsenic Soil Occurrence. ProEnvironment 2024, 17, 232–238. [Google Scholar]
- Battes, K.P.; Cîmpean, M.; Momeu, L.; Avram, A.; Battes, K.W.; Stoica, I.V. Multiple impact assessment and water quality based on diatom, benthic invertebrate and fish communities in the Arieș River catchment area (Transylvania, Romania). Stud. Univ. Babeș-Bolyai Biol. 2018, 63, 27–40. [Google Scholar] [CrossRef]
- Szekely-Andorko, J.; Peterfi, L.Ș.; Momeu, L. Benthic Diatoms Used as Bioindicators for Water Quality Evaluation in the Drainage Basin of the Arieș River (Transylvania, Romania). Contrib. Bot. 2011, 46, 107–115. [Google Scholar]
- Butiuc-Keul, A.; Momeu, L.; Craciunas, C.; Dobrota, C.; Cuna, S.; Balas, G. Physico-chemical and biological studies on water from Arieș River (Romania). J. Environ. Manag. 2012, 95 (Suppl.), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Olenici, A.; Blanco, S.; Jiménez-Gómez, F.; Borrego-Ramos, M.; Baciu, C. Effects of Water Pollution on Diatom Communities of Roșia Montană Mining Area, Romania. Sustainability 2025, 17, 4592. [Google Scholar] [CrossRef]
- Mamanazarova, K.; Alimjanova, K.; Barinova, S. Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan. Land 2024, 13, 1809. [Google Scholar] [CrossRef]
- Radu, V.M.; Vîjdea, A.M.; Ivanov, A.A.; Alexe, V.E.; Dincă, G.; Cetean, V.M.; Filiuță, A.E. Research on the Closure and Remediation Processes of Mining Areas in Romania and Approaches to the Strategy for Heavy Metal Pollution Remediation. Sustainability 2023, 15, 15293. [Google Scholar] [CrossRef]
- Forray, F.; Hallbauer, D. A study of the pollution of the Arieș River (Romania) using capillary electrophoresis as analytical technique. Environ. Geol. 2000, 39, 1372–1384. [Google Scholar] [CrossRef]
- Bătinaș, R.; Sorocovschi, V. The Hydrographic Network—Mobilisation Vector of Pollutants in Roșia Montană Area. In Roșia Montană in Universal History; Cluj University Press: Cluj-Napoca, Romania, 2012. [Google Scholar]
- Bătinaș, R.; Sorocovschi, V. Water Interferences in the Apuseni Mountains. Riscuri și Catastr. 2012, 11, XI. [Google Scholar]
- Taurozzi, D.; Cesarini, G.; Scalici, M. Diatoms as bioindicators for health assessments of ephemeral freshwater ecosystems: A comprehensive review. Ecol. Indic. 2024, 166, 112309. [Google Scholar] [CrossRef]
- Saxena, A.; Tiwari, A.; Kaushik, R.; Iqbal, H.M.N.; Parra-Saldívar, R. Diatoms recovery from wastewater: Overview from an ecological and economic perspective. J. Water Process Eng. 2021, 39, 101705. [Google Scholar] [CrossRef]
- Çelekli, A.; Lekesiz, H.; Yavuzatmaca, M. Bioassessment of water quality of surface waters using diatom metrics. Turk. J. Bot. 2021, 45, 379–396. [Google Scholar] [CrossRef]
- Lavoie, I.; Hamilton, P.B.; Morin, S.; Tiam, S.K.; Kahlert, M.; Gonçalves, S.; Falasco, E.; Fortin, C.; Gontero, B.; Heudre, D.; et al. Diatom teratologies as biomarkers of contamination: Are all deformities ecologically meaningful? Ecol. Indic. 2017, 82, 539–550. [Google Scholar] [CrossRef]
- SR EN 872:2005; Water Quality—Determination of Suspended Solids—Method by Filtration Through Glass Fibre Filters. BSI: London, UK, 2005. Available online: https://e-standard.eu/en/standard/109893 (accessed on 10 January 2022).
- SR EN 25813:2000; Water Quality—Determination of Dissolved Oxygen—Iodometric Method. BSI: London, UK, 2000. Available online: https://e-standard.eu/en/standard/25490 (accessed on 11 January 2022).
- SR EN 1899-2:2002; Water Quality—Determination of Biochemical Oxygen Demand After n Days (BODn)—Part 2: Method for Undiluted Samples (ISO 5815:1989, Modified). BSI: London, UK, 2002. Available online: https://e-standard.eu/en/standard/27527 (accessed on 12 January 2022).
- SR ISO 15705:2022; Water Quality—Determination of Chemical Oxygen Demand (COD-ST)—Small-Scale Closed-Tube Method. BSI: London, UK, 2022.
- SR ISO 7150-1:2001; Water Quality—Determination of Ammonium Content—Part 1: Manual Spectrometric Method. BSI: London, UK, 2001.
- SR EN 26777:2002; Water Quality—Determination of Nitrite Content—Method by Molecular Absorption Spectrometry. BSI: London, UK, 2002.
- SR ISO 7890-3:2000; Water Quality—Determination of Nitrate Content—Part 3: Spectrometric Method Using Sulfosalicylic Acid. BSI: London, UK, 2000.
- STAS 9187:1984; Surface, Underground and Waste Waters—Residuum Determination. ASRO: Bucharest, Romania, 1984. Available online: https://e-standard.eu/en/standard/16815 (accessed on 15 January 2022).
- STAS 6194/1-85; Water Quality—Determination of Bicarbonates (HCO3−) in Water. ASRO: Bucharest, Romania, 1985.
- SR ISO 9297:2001; Water Quality—Determination of Chloride Content—Titration with Silver Nitrate Using Chromate as Indicator (Mohr Method). BSI: London, UK, 2001.
- Method 4500-SO42−; Standard Methods for the Examination of Water and Wastewater. APHA: Washington, DC, USA, 1992. Available online: https://law.resource.org/pub/us/cfr/ibr/002/apha.method.4500-so42.1992.pdf (accessed on 9 January 2022).
- Bucurica, I.-A.; Dulama, I.-D.; Radulescu, C.; Banica, A.L. Surface Water Quality Assessment Using Electroanalytical Methods and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Rom. J. Phys. 2022, 67, 802. [Google Scholar]
- EN ISO 17294-1:2016; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines and Performance Criteria. International Organization for Standardization: Geneva, Switzerland, 2016.
- EN ISO 17294-2:2023; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Geneva, Switzerland, 2023.
- Glevitzky, M.; Bostan, R.; Vică, M.L.; Dumitrel, G.-A.; Corcheş, M.-T.; Popa, M.; Glevitzky, I.; Matei, H.-V. Environmental Contamination and Mining Impact: Physico-Chemical and Biological Characterization of Propolis as an Indicator of Pollution in the Roșia Montană Area, Romania. Plants 2025, 14, 866. [Google Scholar] [CrossRef]
- Method 6020A (SW-846); Inductively Coupled Plasma–Mass Spectrometry; Revision 1, 1998. United States Environmental Protection Agency: Washington, DC, USA, 1998.
- SR EN 13946:2014; Water Quality—Guidance on the Routine Sampling and Pretreatment of Benthic Diatoms from Rivers and Lakes. BSI: London, UK, 2014.
- Pantle, E.; Buck, H. Die Biologische Überwachung der Gewässer und die Darstellung der Ergebnisse (Biological monitoring of waterbodies and the presentation of results). Gas Wasserfach 1955, 96, 604–618. [Google Scholar]
- Romanian Ministry of Environment. Normative of 16 February 2006 on the Classification of Surface Water Quality for Establishing the Ecological Status of Water Bodies; Approved by Order No. 161/2006; Ministry of Environment: Bucharest, Romania, 2006.
- SR EN 14407:2014; Water Quality—Guidance for the Identification and Counting of Benthic Diatom Samples from Rivers and Lakes. BSI: London, UK, 2014.
- Chu, E.W.; Karr, J.R. Environmental Impact: Concept, Consequences, Measurement. Ref. Modul. Life Sci. 2017, 25, B978-0-12-809633-8.02380-3. [Google Scholar] [CrossRef]
- Heinrichs, M.E.; Mori, C.; Dlugosch, L. Complex Interactions Between Aquatic Organisms and Their Chemical Environment Elucidated from Different Perspectives. In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 277–290. [Google Scholar] [CrossRef]
- Lupea, A.X. Biochemistry; Politehnica Publishing House: Timișoara, Romania, 2003. (In Romanian) [Google Scholar]
- Botnariuc, N.; Vădineanu, A. Ecology; Didactic and Pedagogical Publishing House: Bucharest, Romania, 1982. (In Romanian) [Google Scholar]
- Nguyen, H.L.; Braun, M.; Szaloki, I.; Baeyens, W.; Van Grieken, R.; Leermakers, M. Tracing the Metal Pollution History of the Tisza River through the Analysis of a Sediment Depth Profile. Water Air Soil Pollut. 2008, 200, 119–132. [Google Scholar] [CrossRef]
- Rybicka, E.H.; Adamiec, E.; Aleksander-Kwaterczak, U. Distribution of Trace Metals in the Odra River System: Water–Suspended Matter–Sediments. Limnologica 2005, 35, 185–198. [Google Scholar] [CrossRef]
- Suchara, I.; Sucharová, J.; Holá, M. Contamination of Bank Sediments near Historic Glass Works in the Bohemian Forest, Czech Republic. Silva Gabreta 2022, 28, 17–47. [Google Scholar]
- Moldovan, A.; Török, A.I.; Kovacs, E.; Cadar, O.; Mirea, I.C.; Micle, V. Metal Contents and Pollution Indices Assessment of Surface Water, Soil, and Sediment from the Arieș River Basin Mining Area, Romania. Sustainability 2022, 14, 8024. [Google Scholar] [CrossRef]
- Ghidra, V. Ecotoxicology and Monitoring of Major Pollutants; Studia Publishing House: Bucharest, Romania, 2004. (In Romanian) [Google Scholar]
- Mazur, R.; Kowalewski, Z.; Głowienka, E.; Santos, L.; Jakubiak, M. Sustainability in aquatic ecosystem restoration: Combining classical and remote sensing methods for effective water quality management. Sustainability 2024, 16, 3716. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Sur, I.M.; Moldovan, A.; Micle, V.; Polyak, E.T. Assessment of surface water quality in the Baia Mare area, Romania. Water 2022, 14, 3118. [Google Scholar] [CrossRef]
- Zobrist, J.; Sima, M.; Dogaru, D.; Senila, M.; Yang, H.; Popescu, C.; Roman, C.; Bela, A.; Frei, L.; Dold, B.; et al. Environmental and socioeconomic assessment of impacts by mining activities—A case study in the Certej River catchment, Western Carpathians, Romania. Environ. Sci. Pollut. Res. Int. 2009, 16 (Suppl. S1), S14–S26. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Jain, V.K. Studies on effect of cadmium on the growth pattern of Phaseolus aurius varieties. Absi I. Bot. Conf. JIBS 1978, 532, 57–84. [Google Scholar]
- Balkis, N.; Aksu, A.; Okuş, E.; Apak, R. Heavy metal concentrations in water, suspended matter, and sediment from Gökova Bay, Turkey. Environ. Monit. Assess. 2010, 167, 359–370. [Google Scholar] [CrossRef]
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Assessment of Metal Pollution in Water and Surface Sediments of the Seyhan River, Turkey, Using Different Indexes. CLEAN Soil Air Water 2011, 39, 185–194. [Google Scholar] [CrossRef]
- Kar, D.; Sur, P.; Mandal, S.K.; Saha, T.; Kole, R.K. Assessment of heavy metal pollution in surface water. Int. J. Environ. Sci. Technol. 2008, 5, 119–124. [Google Scholar] [CrossRef]
- Bilgin, A.; Konanç, M.U. Evaluation of surface water quality and heavy metal pollution of Coruh River Basin (Turkey) by multivariate statistical methods. Environ. Earth Sci. 2016, 75, 280. [Google Scholar] [CrossRef]
- Wei, W.; Ma, R.; Sun, Z.; Zhou, A.; Bu, J.; Long, X.; Liu, Y. Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China. Int. J. Environ. Res. Public Health 2018, 15, 1987. [Google Scholar] [CrossRef] [PubMed]
- Popa, A. Gold Mining in the Apuseni Mountains; Infomin Publishing House: Deva, Romania, 2000. (In Romanian) [Google Scholar]
- Falasco, E.; Ector, L.; Wetzel, C.E.; Badino, G.; Bona, F. Looking Back, Looking Forward: A Review of the New Literature on Diatom Teratological Forms (2010–2020). Hydrobiologia 2021, 848, 1675–1753. [Google Scholar] [CrossRef]
- Morin, S.; Coste, M.; Hamilton, P.B. Scanning Electron Microscopy Observations of Deformities in Small Pennate Diatoms Exposed to High Cadmium Concentrations. J. Phycol. 2008, 44, 1512–1518. [Google Scholar] [CrossRef]
- Sienkiewicz, E.; Gąsiorowski, M.; Sekudewicz, I.; Kowalewska, U.; Matoušková, Š. Responses of Diatom Composition and Teratological Forms to Environmental Pollution in a Post-Mining Lake (SW Poland). Environ. Sci. Pollut. Res. 2023, 30, 110623–110638. [Google Scholar] [CrossRef]
- Costa, L.F.; Wetzel, C.E.; Maquardt, G.C.; Zanon, J.E.; Ector, L.; Bicudo, D.C. Taxonomy and Ecology of Achnanthidium (Bacillariophyta, Achnanthidiaceae) from Southeastern Brazil with the Description of Six New Species. Phytotaxa 2022, 575, 187–223. [Google Scholar] [CrossRef]
- Falasco, E.; Bona, F.; Ginepro, M.; Hlúbiková, D.; Hoffmann, L.; Ector, L. Morphological Abnormalities of Diatom Silica Walls in Relation to Heavy Metal Contamination and Artificial Growth Conditions. Water SA 2009, 35, 595–606. [Google Scholar] [CrossRef][Green Version]
- Duong, T.T.; Morin, S.; Herlory, O.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Seasonal Effects of Cadmium Accumulation in Periphytic Diatom Communities of Freshwater Biofilms. Aquat. Toxicol. 2008, 90, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, N.; Barranguet, C.; Jonker, M.; Kraak, M.H.S.; Admiraal, W. Metal-Induced Tolerance in the Freshwater Microbenthic Diatom Gomphonema parvulum. Environ. Pollut. 2002, 116, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Traudt, E.M.; Ranville, J.F.; Meyer, J.S. Acute Toxicity of Ternary Cd-Cu-Ni and Cd-Ni-Zn Mixtures to Daphnia magna: Dominant Metal Pairs Change along a Concentration Gradient. Environ. Sci. Technol. 2017, 51, 4471–4481. [Google Scholar] [CrossRef]
- Morin, S.; Coste, M.; Delmas, F. A Comparison of Specific Growth Rates of Periphytic Diatoms of Varying Cell Size under Laboratory and Field Conditions. Hydrobiologia 2008, 614, 285–297. [Google Scholar] [CrossRef][Green Version]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (Water Framework Directive). Off. J. Eur. Communities 2000, L327, 1–73. [Google Scholar][Green Version]
- Kim Tiam, S.; Lavoie, I.; Liu, F.; Hamilton, P.B.; Fortin, C. Diatom Deformities and Tolerance to Cadmium Contamination in Four Species. Environments 2019, 6, 102. [Google Scholar] [CrossRef]
- Pandey, L.K.; Bergey, E.A. Exploring the Status of Motility, Lipid Bodies, Deformities and Size Reduction in Periphytic Diatom Community from Chronically Metal (Cu, Zn) Polluted Waterbodies as a Biomonitoring Tool. Sci. Total Environ. 2016, 550, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Gluck, A. Mathematical Methods in the Chemical Industry; Tehnică Publishing House: Bucureşti, Romania, 1971; pp. 134–139. (In Romanian) [Google Scholar]



| Sample | Sampling Points Coding | Sampling Area | Site Characteristics/Pollution Sources |
|---|---|---|---|
| 1 | A1 | Upstream of Arieseni village | In the upstream sector of the two sampling points (A1 and A2), the Arieș River is predominantly influenced by diffuse pollution sources generated by tourism and household activities (inadequately collected and treated wastewater), nutrient and organic matter inputs from surface runoff related to livestock waste management, as well as possible natural contributions linked to erosion and sediment transport processes. There are no major industrial pollution sources in the area, but the aquatic ecosystem is highly vulnerable due to the relatively low flow and limited dilution capacity. |
| 2 | A2 | Downstream of the Mihoești reservoir and dam | |
| 3 | A3 | In the area of Baia de Arieș | In the Baia de Arieș area, the Arieș River is affected by pressures from both point and diffuse sources, mainly associated with inadequately collected and treated domestic wastewater, as well as stormwater that drains various discharges from historical mining waste deposits (acidic waters loaded with metal trace elements and suspended solids-TSS). Industrial wastewater from the only active mining site in the area (Cupru Min SA Abrud), which exploits the local copper deposit, also contributes to pollution. Additionally, diffuse inputs from agricultural and household activities, as well as livestock waste management, are noticeable in the area. |
| 4 | A4 | Upstream of the confluence with the Mureș River | In the section of the Arieș River located upstream of its confluence with the Mureș River, the water is influenced by cumulative pollution sources across the entire watershed. These include domestic and industrial discharges from urban areas (Turda, Câmpia Turzii), historical and ongoing inputs of mining pollutants (TSS, metal trace elements), diffuse runoff of nutrients and pesticides from agriculture, as well as impacts from livestock activities. These anthropogenic pressures result in an increased pollutant load in the river. |
| Parameter | pH | TSS, mg/L | DO, mgO/L | BOD5, mgO/L | COD-Cr, mgO/L | NH4+, mgN/L | NO2−, mgN/L | NO3−, mgN/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 7.5 | 10.8 | 9.87 | 0.83 | 1.13 | 0.002 | 0.005 | 0.167 |
| April | 7.7 | 13.4 | 10.01 | 0.87 | 1.74 | 0.003 | 0.006 | 0.214 | |
| July | 7.6 | 14.9 | 8.04 | 1.34 | 2.32 | 0.005 | 0.011 | 0.584 | |
| October | 7.5 | 9.8 | 10.81 | 0.81 | 1.81 | 0.004 | 0.007 | 0.298 | |
| 2023 | January | 7.8 | 10.3 | 9.60 | 0.91 | 1.56 | 0.001 | 0.006 | 0.170 |
| April | 7.6 | 13.4 | 9.91 | 0.91 | 1.71 | 0.002 | 0.008 | 0.199 | |
| July | 7.4 | 15.4 | 7.98 | 1.12 | 2.14 | 0.006 | 0.009 | 0.604 | |
| October | 7.5 | 9.1 | 10.47 | 0.94 | 1.75 | 0.003 | 0.008 | 0.304 | |
| 2024 | January | 7.6 | 12.3 | 9.74 | 0.82 | 1.38 | 0.002 | 0.005 | 0.186 |
| April | 7.7 | 14.1 | 10.24 | 0.86 | 1.69 | 0.004 | 0.007 | 0.228 | |
| July | 7.6 | 13.6 | 8.45 | 1.27 | 2.09 | 0.008 | 0.008 | 0.527 | |
| October | 7.5 | 9.8 | 10.57 | 0.89 | 1.94 | 0.001 | 0.005 | 0.356 | |
| Quality class [40] | I | 6.5– 8.5 | - | 6.2, not less than 80% oxygen saturation | 6 | 10 | 0.4 | 0.01 | 1 |
| II | 25 | 0.8 | 0.03 | 3 | |||||
| III | 50 | 1.2 | 0.06 | 5.6 | |||||
| IV | 125 | 3.2 | 0.3 | 11.2 | |||||
| V | >125 | >3.2 | >0.3 | >11.2 |
| Parameter | EC, μS/cm | FR, mg/L | Ca, mg/L | Mg, mg/L | Na, mg/L | Bicarbonates, mg/L | Chlorides, mg/L | Sulfates, mg/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 104.42 | 71.4 | 13.7 | 1.9 | 5.8 | 52.4 | 0.8 | 32.4 |
| April | 127.42 | 116.4 | 19.6 | 2.5 | 4.6 | 76.8 | 1.6 | 41.6 | |
| July | 211.79 | 147.4 | 41.5 | 4.7 | 7.1 | 125.7 | 4.1 | 58.0 | |
| October | 163.09 | 124.7 | 35.0 | 1.8 | 4.2 | 48.6 | 1.5 | 21.4 | |
| 2023 | January | 124.93 | 83.6 | 17.4 | 1.4 | 4.7 | 55.2 | 0.1 | 28.2 |
| April | 131.14 | 125.1 | 24.2 | 1.9 | 4.4 | 84.7 | 1.8 | 45.5 | |
| July | 198.72 | 138.1 | 34.8 | 3.8 | 6.3 | 113.0 | 3.5 | 51.7 | |
| October | 173.47 | 121.0 | 30.1 | 2.4 | 3.7 | 51.8 | 2.1 | 18.6 | |
| 2024 | January | 142.72 | 79.2 | 13.6 | 1.6 | 5.5 | 64.1 | 1.7 | 31.3 |
| April | 139.71 | 132.7 | 29.1 | 2.1 | 5.1 | 75.7 | 2.1 | 38.2 | |
| July | 199.67 | 152.4 | 38.3 | 4.1 | 6.6 | 132.4 | 3.8 | 54.8 | |
| October | 156.77 | 118.7 | 29.9 | 2.6 | 3.9 | 53.1 | 2.5 | 21.1 | |
| Quality class [40] | I | - | 500 | 50 | 12 | 25 | - | 25 | 60 |
| II | 750 | 100 | 50 | 50 | 50 | 120 | |||
| III | 1000 | 200 | 100 | 100 | 250 | 250 | |||
| IV | 1300 | 300 | 200 | 200 | 300 | 300 | |||
| V | >1300 | >300 | >200 | >200 | >300 | >300 |
| Parameter | As, μg/L | Cd, μg/L | Co, μg/L | Cr, μg/L | Cu, μg/L | Fe, mg/L | Mn, mg/L | Ni, μg/L | Zn, μg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | ||||||||||
| 2022 | January | 0.29 | 0.23 | 0.21 | 1.18 | 9.37 | 0.07 | 0.001 | 0.69 | 6.74 |
| April | 0.30 | 0.29 | 0.35 | 2.59 | 10.97 | 0.09 | 0.002 | 0.54 | 14.54 | |
| July | 0.32 | 0.44 | 1.74 | 8.41 | 13.13 | 0.11 | 0.005 | 1.44 | 31.84 | |
| October | 0.28 | 0.20 | 0.13 | 1.93 | 12.08 | 0.08 | 0.004 | 0.41 | 0.11 | |
| 2023 | January | 0.31 | 0.19 | 0.19 | 1.24 | 7.62 | 0.05 | 0.001 | 0.73 | 7.78 |
| April | 0.29 | 0.23 | 0.33 | 3.08 | 9.07 | 0.06 | 0.002 | 0.47 | 20.14 | |
| July | 0.35 | 0.51 | 1.56 | 7.96 | 12.24 | 0.09 | 0.006 | 1.54 | 39.14 | |
| October | 0.27 | 0.28 | 0.27 | 2.06 | 8.30 | 0.04 | 0.003 | 0.58 | 0.24 | |
| 2024 | January | 0.35 | 0.25 | 0.13 | 1.45 | 9.51 | 0.07 | 0.001 | 0.98 | 9.16 |
| April | 0.29 | 0.31 | 0.38 | 2.77 | 11.64 | 0.08 | 0.004 | 3.41 | 18.1 | |
| July | 0.37 | 0.53 | 1.62 | 8.25 | 12.47 | 0.10 | 0.007 | 1.47 | 31.71 | |
| October | 0.33 | 0.22 | 0.19 | 2.17 | 9.14 | 0.03 | 0.006 | 0.69 | 0.19 | |
| Quality class [40] | I | 10 | 0.5 | 10 | 25 | 20 | 0.3 | 0.05 | 10 | 100 |
| II | 20 | 1 | 20 | 50 | 30 | 0.5 | 0.1 | 25 | 200 | |
| III | 50 | 2 | 50 | 100 | 50 | 1.0 | 0.3 | 50 | 500 | |
| IV | 100 | 5 | 100 | 250 | 100 | 2 | 1 | 100 | 1000 | |
| V | >100 | >5 | >100 | >250 | >100 | >2 | >1 | >100 | >1000 |
| Parameter | pH | TSS, mg/L | DO, mgO/L | BOD5, mgO/L | COD-Cr, mgO/L | NH4+, mgN/L | NO2−, mgN/L | NO3−, mgN/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 7.6 | 18.4 | 10.53 | 0.97 | 1.21 | 0.004 | 0.003 | 0.327 |
| April | 7.8 | 42.9 | 11.18 | 0.79 | 1.80 | 0.014 | 0.005 | 0.381 | |
| July | 7.7 | 21.4 | 8.14 | 1.84 | 3.34 | 0.021 | 0.010 | 0.847 | |
| October | 7.8 | 31.7 | 10.93 | 1.21 | 2.47 | 0.019 | 0.008 | 0.401 | |
| 2023 | January | 7.9 | 12.4 | 10.21 | 1.04 | 1.06 | 0.008 | 0.001 | 0.352 |
| April | 7.8 | 51.3 | 10.84 | 0.86 | 1.62 | 0.009 | 0.005 | 0.436 | |
| July | 7.6 | 31.1 | 8.59 | 1.89 | 3.21 | 0.014 | 0.009 | 0.773 | |
| October | 7.6 | 28.2 | 10.58 | 1.12 | 2.23 | 0.011 | 0.007 | 0.485 | |
| 2024 | January | 7.7 | 23.8 | 11.23 | 0.93 | 1.17 | 0.003 | 0.004 | 0.318 |
| April | 7.8 | 35.5 | 11.04 | 1.13 | 2.32 | 0.007 | 0.006 | 0.388 | |
| July | 7.6 | 12.2 | 8.04 | 1.97 | 3.07 | 0.028 | 0.011 | 0.686 | |
| October | 7.7 | 24.6 | 10.82 | 1.34 | 1.94 | 0.014 | 0.010 | 0.425 |
| Parameter | EC, μS/cm | FR, mg/L | Ca, mg/L | Mg, mg/l | Na, mg/l | Bicarbonates, mg/L | Chlorides, mg/L | Sulfates, mg/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 134.47 | 84.7 | 19.1 | 3.4 | 5.1 | 91.9 | 0.8 | 3.4 |
| April | 145.31 | 123.3 | 19.6 | 6.6 | 5.8 | 101.2 | 2.9 | 15.7 | |
| July | 184.02 | 124.5 | 22.3 | 12.1 | 6.2 | 105.1 | 7.2 | 17.6 | |
| October | 169.33 | 124.7 | 21.5 | 9.8 | 4.4 | 84.8 | 4.6 | 13.7 | |
| 2023 | January | 144.21 | 91.2 | 18.6 | 2.7 | 5.3 | 87.4 | 0.7 | 4.1 |
| April | 157.09 | 116.3 | 19.0 | 1.9 | 5.5 | 84.7 | 3.8 | 16.3 | |
| July | 169.23 | 118.9 | 21.6 | 7.2 | 5.9 | 98.5 | 6.4 | 14.6 | |
| October | 171.17 | 121.0 | 20.7 | 10.1 | 4.1 | 91.1 | 4.9 | 18.1 | |
| 2024 | January | 128.14 | 88.5 | 20.1 | 4.1 | 4.8 | 94.3 | 0.5 | 4.8 |
| April | 145.44 | 105.8 | 21.3 | 8.3 | 5.7 | 75.6 | 4.4 | 13.3 | |
| July | 177.08 | 122.2 | 22.7 | 11.7 | 6.0 | 98.7 | 6.8 | 16.8 | |
| October | 167.49 | 118.7 | 21.8 | 10.5 | 4.5 | 79.4 | 5.1 | 10.8 |
| Parameter | As, μg/L | Cd, μg/L | Co, μg/L | Cr, μg/L | Cu, μg/L | Fe, mg/L | Mn, mg/L | Ni, μg/L | Zn, μg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | ||||||||||
| 2022 | January | 0.30 | 0.53 | 0.86 | 3.53 | 5.32 | 0.03 | 0.006 | 0.69 | 2.24 |
| April | 0.35 | 0.66 | 1.71 | 6.38 | 11.89 | 0.11 | 0.012 | 1.48 | 11.17 | |
| July | 0.41 | 0.98 | 3.17 | 9.14 | 12.87 | 0.18 | 0.022 | 1.91 | 39.18 | |
| October | 0.39 | 0.41 | 0.62 | 6.14 | 11.42 | 0.06 | 0.018 | 1.09 | 1.31 | |
| 2023 | January | 0.33 | 0.46 | 0.60 | 4.07 | 6.08 | 0.04 | 0.011 | 0.73 | 7.78 |
| April | 0.36 | 0.57 | 2.07 | 5.17 | 10.43 | 0.09 | 0.013 | 1.37 | 10.53 | |
| July | 0.37 | 0.81 | 3.22 | 8.22 | 11.45 | 0.17 | 0.018 | 1.72 | 34.8 | |
| October | 0.25 | 0.39 | 0.55 | 4.49 | 9.96 | 0.07 | 0.015 | 1.24 | 1.29 | |
| 2024 | January | 0.35 | 0.54 | 0.94 | 4.58 | 4.97 | 0.01 | 0.008 | 0.98 | 1.31 |
| April | 0.42 | 0.62 | 1.22 | 6.18 | 9.74 | 0.12 | 0.009 | 1.56 | 5.67 | |
| July | 0.48 | 0.79 | 2.85 | 7.73 | 13.08 | 0.13 | 0.013 | 1.83 | 43.01 | |
| October | 0.31 | 0.44 | 0.51 | 5.16 | 10.22 | 0.05 | 0.011 | 1.22 | 0.91 |
| Parameter | pH | TSS, mg/L | DO, mgO/L | BOD5, mgO/L | COD-Cr, mgO/L | NH4+, mgN/L | NO2−, mgN/L | NO3−, mgN/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 7.6 | 48.7 | 10.53 | 0.97 | 1.46 | 0.017 | 0.002 | 0.157 |
| April | 7.8 | 143.5 | 11.27 | 0.69 | 2.04 | 0.024 | 0.006 | 0.287 | |
| July | 5.7 | 75.8 | 8.48 | 1.84 | 4.17 | 0.078 | 0.009 | 0.612 | |
| October | 6.9 | 118.3 | 9.42 | 1.41 | 3.26 | 0.063 | 0.007 | 0.398 | |
| 2023 | January | 7.9 | 33.56 | 10.21 | 1.04 | 1.13 | 0.011 | 0.003 | 0.189 |
| April | 7.4 | 113.7 | 11.09 | 0.76 | 1.96 | 0.029 | 0.007 | 0.335 | |
| July | 5.9 | 47.3 | 9.14 | 1.89 | 3.97 | 0.081 | 0.008 | 0.585 | |
| October | 7.2 | 96.3 | 10.17 | 1.17 | 2.45 | 0.047 | 0.006 | 0.512 | |
| 2024 | January | 7.7 | 49.7 | 11.23 | 0.93 | 1.21 | 0.008 | 0.004 | 0.201 |
| April | 7.3 | 163.9 | 11.41 | 1.23 | 2.04 | 0.031 | 0.005 | 0.314 | |
| July | 6.1 | 78.5 | 8.39 | 1.97 | 3.68 | 0.092 | 0.010 | 0.537 | |
| October | 7.1 | 104.6 | 10.13 | 1.47 | 2.27 | 0.054 | 0.009 | 0.476 |
| Parameter | EC, μS/cm | FR, mg/L | Ca, mg/L | Mg, mg/L | Na, mg/L | Bicarbonates, mg/L | Chlorides, mg/L | Sulfates, mg/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 169.17 | 122.4 | 53.5 | 4.2 | 4.2 | 41.8 | 6.2 | 118.7 |
| April | 238.36 | 175.48 | 61.9 | 4.9 | 5.1 | 52.7 | 7.9 | 129.3 | |
| July | 367.12 | 201.7 | 72.7 | 7.5 | 7.2 | 101.5 | 11.2 | 198.4 | |
| October | 244.37 | 124.7 | 59.4 | 6.7 | 5.9 | 77.3 | 9.1 | 175.4 | |
| 2023 | January | 174.20 | 114.3 | 51.7 | 3.1 | 3.9 | 38.4 | 5.8 | 121.0 |
| April | 198.47 | 154.7 | 69.6 | 4.6 | 4.4 | 44.1 | 7.1 | 141.7 | |
| July | 417.13 | 196.4 | 81.4 | 11.2 | 6.9 | 94.5 | 10.8 | 201.5 | |
| October | 315.81 | 121.0 | 75.3 | 9.3 | 6.2 | 82.2 | 8.3 | 168.9 | |
| 2024 | January | 168.49 | 128.3 | 48.3 | 2.3 | 5.1 | 54.4 | 6.8 | 116.8 |
| April | 211.38 | 199.6 | 71.2 | 4.3 | 6.6 | 75.6 | 7.1 | 135.8 | |
| July | 354.25 | 234.7 | 78.0 | 11.7 | 8.1 | 63.8 | 9.8 | 187.2 | |
| October | 298.74 | 118.7 | 68.2 | 7.4 | 7.1 | 66.7 | 8.2 | 174.6 |
| Parameter | As, μg/L | Cd, μg/L | Co, μg/L | Cr, μg/L | Cu, μg/L | Fe, mg/L | Mn, mg/L | Ni, μg/L | Zn, μg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | ||||||||||
| 2022 | January | 0.27 | 0.32 | 0.78 | 2.42 | 18.18 | 0.09 | 0.038 | 1.23 | 74.85 |
| April | 0.31 | 2.14 | 3.26 | 10.04 | 47.33 | 0.36 | 0.096 | 1.84 | 125.39 | |
| July | 0.49 | 4.18 | 4.74 | 15.7 | 237.27 | 0.72 | 0.541 | 8.52 | 327.14 | |
| October | 0.35 | 1.27 | 2.22 | 8.82 | 101.71 | 0.19 | 0.384 | 3.16 | 165.28 | |
| 2023 | January | 0.35 | 0.45 | 1.13 | 3.61 | 9.23 | 0.28 | 0.053 | 1.35 | 84.61 |
| April | 0.37 | 3.07 | 2.39 | 9.28 | 39.52 | 0.52 | 0.137 | 2.22 | 120.84 | |
| July | 0.42 | 3.99 | 5.17 | 19.23 | 142.08 | 0.84 | 0.422 | 7.27 | 214.71 | |
| October | 0.40 | 1.52 | 1.56 | 6.74 | 58.32 | 0.43 | 0.226 | 3.69 | 156.71 | |
| 2024 | January | 0.41 | 0.53 | 1.54 | 5.27 | 13.78 | 0.24 | 0.031 | 1.08 | 96.74 |
| April | 0.42 | 2.25 | 2.34 | 11.29 | 23.27 | 0.41 | 0.127 | 4.26 | 122.6 | |
| July | 0.45 | 4.11 | 3.85 | 20.05 | 98.70 | 0.67 | 0.315 | 6.25 | 284.57 | |
| October | 0.44 | 1.19 | 1.89 | 7.69 | 44.28 | 0.33 | 0.285 | 5.60 | 165.38 |
| Parameter | pH | TSS, mg/L | DO, mgO/L | BOD5, mgO/L | COD-Cr, mgO/L | NH4+, mgN/L | NO2−, mgN/L | NO3−, mgN/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 7.9 | 38.5 | 10.69 | 0.83 | 1.27 | 0.021 | 0.003 | 0.164 |
| April | 8.2 | 112.7 | 11.03 | 0.57 | 1.67 | 0.027 | 0.007 | 0.294 | |
| July | 6.7 | 68.1 | 8.98 | 1.58 | 3.74 | 0.084 | 0.011 | 0.617 | |
| October | 7.2 | 105.2 | 9.67 | 1.27 | 2.67 | 0.071 | 0.008 | 0.427 | |
| 2023 | January | 8.1 | 30.0 | 10.61 | 0.89 | 1.04 | 0.019 | 0.004 | 0.217 |
| April | 7.9 | 109.6 | 10.87 | 0.57 | 1.72 | 0.034 | 0.009 | 0.367 | |
| July | 6.4 | 43.8 | 9.63 | 1.36 | 3.30 | 0.091 | 0.011 | 0.597 | |
| October | 7.7 | 91.9 | 10.45 | 1.07 | 2.17 | 0.053 | 0.009 | 0.542 | |
| 2024 | January | 8.1 | 39.3 | 10.87 | 0.84 | 1.07 | 0.015 | 0.007 | 0.231 |
| April | 7.9 | 154.9 | 11.23 | 1.18 | 1.79 | 0.042 | 0.008 | 0.333 | |
| July | 6.3 | 69.4 | 8.25 | 1.84 | 3.34 | 0.107 | 0.013 | 0.550 | |
| October | 7.8 | 98.3 | 10.05 | 1.36 | 2.11 | 0.068 | 0.011 | 0.497 |
| Parameter | EC, μS/cm | FR, mg/L | Ca, mg/L | Mg, mg/L | Na, mg/L | Bicarbonates, mg/L | Chlorides, mg/L | Sulfates, mg/L | |
|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | |||||||||
| 2022 | January | 178.23 | 135.4 | 64.0 | 5.8 | 5.2 | 52.3 | 6.2 | 18.7 |
| April | 247.37 | 181.2 | 61.3 | 6.7 | 6.4 | 63.1 | 7.9 | 29.3 | |
| July | 388.28 | 212.6 | 74.5 | 12.8 | 8.3 | 96.5 | 11.2 | 98.4 | |
| October | 269.07 | 135.0 | 48.2 | 9.4 | 7.7 | 78.7 | 9.1 | 75.4 | |
| 2023 | January | 184.69 | 123.1 | 54.7 | 6.9 | 4.5 | 61.4 | 5.8 | 21.0 |
| April | 217.01 | 161.9 | 55.1 | 8.1 | 5.6 | 69.9 | 7.1 | 41.7 | |
| July | 432.07 | 201.3 | 75.0 | 14.4 | 9.1 | 102.8 | 10.8 | 101.5 | |
| October | 356.61 | 142.5 | 62.1 | 9.9 | 7.0 | 94.4 | 8.3 | 68.9 | |
| 2024 | January | 187.10 | 133.8 | 48.2 | 5.3 | 5.4 | 52.3 | 6.8 | 16.8 |
| April | 232.24 | 212.4 | 55.3 | 7.6 | 5.6 | 63.8 | 7.1 | 35.8 | |
| July | 369.41 | 245.3 | 67.8 | 12.5 | 7.9 | 89.6 | 9.8 | 87.2 | |
| October | 301.17 | 137.7 | 74.9 | 8.8 | 7.7 | 78.5 | 8.2 | 74.6 |
| Parameter | As, μg/L | Cd, μg/L | Co, μg/L | Cr, μg/L | Cu, μg/L | Fe, mg/L | Mn, mg/L | Ni, μg/L | Zn, μg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Time, Year/Month | ||||||||||
| 2022 | January | 0.31 | 0.11 | 1.12 | 3.41 | 12.27 | 0.09 | 0.096 | 1.19 | 8.85 |
| April | 0.32 | 1.29 | 2.98 | 8.96 | 35.71 | 0.10 | 0.124 | 1.74 | 42.56 | |
| July | 0.41 | 2.01 | 3.45 | 25.41 | 187.13 | 0.12 | 0.285 | 8.52 | 101.42 | |
| October | 0.39 | 1.43 | 2.01 | 7.62 | 74.36 | 0.11 | 0.181 | 2.97 | 71.38 | |
| 2023 | January | 0.34 | 0.09 | 1.23 | 2.94 | 10.21 | 0.04 | 0.108 | 1.20 | 14.61 |
| April | 0.37 | 1.42 | 2.11 | 6.52 | 23.84 | 0.06 | 0.143 | 2.74 | 37.62 | |
| July | 0.39 | 2.27 | 4.06 | 19.69 | 122.17 | 0.09 | 0.199 | 6.88 | 94.08 | |
| October | 0.40 | 1.19 | 1.79 | 9.96 | 63.86 | 0.07 | 0.162 | 4.19 | 75.36 | |
| 2024 | January | 0.28 | 0.18 | 1.42 | 4.18 | 13.78 | 0.03 | 0.088 | 1.08 | 19.97 |
| April | 0.31 | 1.36 | 1.84 | 12.49 | 28.73 | 0.12 | 0.117 | 1.23 | 32.86 | |
| July | 0.36 | 1.94 | 3.97 | 30.17 | 98.69 | 0.14 | 0.214 | 6.25 | 87.63 | |
| October | 0.37 | 0.85 | 1.49 | 7.12 | 54.28 | 0.08 | 0.249 | 3.48 | 44.20 |
| Species Name | Number of Individuals | Saprobic Zone | Saprobic Value |
|---|---|---|---|
| Planothidium rostratoholarcticum Lange-Bertalot & Bak | 1 | β | 2 |
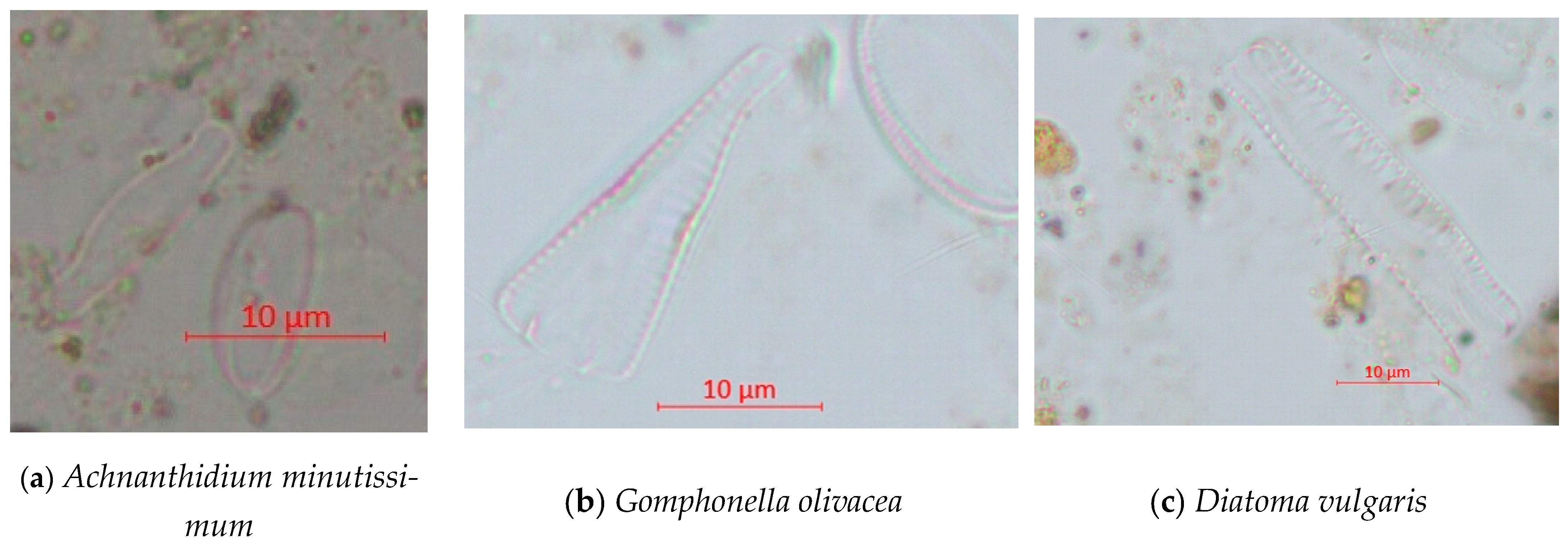
| Achnanthidium minutissimum (Kützing) Czarnecki | 188 | o-β | 1.5 |
| Achnanthidium minutissimum (Kützing) Czarnecki-teratological form | 11 | - | - |
| Cymbella affinis Kützing | 1 | o-β | 1.5 |
| Cymbella ventricosa Agardh | 3 | o-β | 1.5 |
| Gomphonella olivacea (Hornemann) Rabenhorst | 156 | β | 2 |
| Gomphonella olivacea (Hornemann) Rabenhorst-teratological form | 1 | - | - |
| Gomphonema pumilum (Grunow) E.Reichardt & Lange-Bertalot | 50 | o | 1 |
| Total | 411 | - | - |
| SI value | 1.40 | - | - |
| Species Name | Number of Individuals | Saprobic Zone | Saprobic Value |
|---|---|---|---|
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 2 | β | 2 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 339 | o-β | 1.5 |
| Achnanthidium minutissimum Kützing) Czarnecki-teratological form | 24 | - | - |
| Amphora pediculus (Kützing) Grunow | 13 | β | 2 |
| Hannaea arcus (Ehrenberg) R.M.Patrick | 2 | o | 1 |
| Cocconeis placentula Ehrenberg | 1 | β | 2 |
| Cymbella gracilis (Ehrenberg) Kützing | 3 | o | 1 |
| Cymbella ventricosa Agardh | 35 | o-β | 1.5 |
| Odontidium mesodon (Ehrenberg) Kützing | 1 | o | 1 |
| Diatoma vulgaris Bory | 50 | β-α | 2.5 |
| Diatoma vulgaris Bory-teratological form | 1 | - | - |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 5 | o | 1 |
| Fragilaria capucina Desmazières | 9 | β | 2 |
| Fragilaria capucina Desmazières-teretological form | 4 | - | - |
| Gomphonema pumilum (Grunow) E.Reichardt & Lange-Bertalot | 5 | o | 1 |
| Navicula cryptotenella Lange-Bertalot | 4 | β | 2 |
| Nitzschia dissipata (Kützing) Rabenhorst | 2 | o-β | 1.5 |
| Ulnaria ulna (Nitzsch) Compère | 2 | β | 2 |
| Total | 502 | - | - |
| SI value | 1.39 | - | - |
| Species Name | Number of Individuals | Saprobic Zone | Saprobic Value |
|---|---|---|---|
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 5 | β | 2 |
| Planothidium rostratoholarcticum Lange-Bertalot & Bak | 1 | β | 2 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 193 | o-β | 1.5 |
| Achnanthidium minutissimum Kützing) Czarnecki-teratological form | 34 | - | - |
| Amphora pediculus (Kützing) Grunow | 1 | β | 2 |
| Hannaea arcus (Ehrenberg) R.M.Patrick | 1 | o | 1 |
| Discostella stelligera (Cleve & Grunow) Houk & Klee | 3 | - | - |
| Cymbella ventricosa Agardh | 9 | o-β | 1.5 |
| Fragilaria capucina Desmazières | 13 | β | 2 |
| Fragilaria capucina Desmazières-teratological form | 3 | - | - |
| Fragilaria vaucheriae (Kützing) J.B.Petersen | 13 | β | 2 |
| Fragilaria vaucheriae (Kützing) J.B.Petersen-teratological form | 3 | - | - |
| Gomphonema parvulum Kützing | 3 | β | 2 |
| Gomphonema pumilum (Grunow) E.Reichardt & Lange-Bertalot | 1 | o | 1 |
| Navicula cryptotenella Lange-Bertalot | 4 | β | 2 |
| Navicula gregaria Donkin | 8 | β | 2 |
| Navicula cryptocephala Kützing | 2 | α | 3 |
| Navicula viridula (Kützing) Ehrenberg | 2 | α | 3 |
| Nitzschia dissipata (Kützing) Rabenhorst | 41 | o-β | 1.5 |
| Nitzschia gracilis Hantzsch | 7 | β | 2 |
| Nitzschia palea (Kützing) W.Smith | 13 | α | 3 |
| Surirella angusta Kützing | 3 | β | 2 |
| Ulnaria ulna (Nitzsch) Compère | 1 | β | 2 |
| Total | 364 | - | - |
| SI value | 1.65 | - | - |
| Species Name | Number of Individuals | Saprobic Zone | Saprobic Value |
|---|---|---|---|
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 2 | β | 2 |
| Amphora ovalis (Kützing) Kützing | 3 | α | 3 |
| Amphora pediculus (Kützing) Grunow | 2 | β | 2 |
| Cocconeis pediculus Ehrenberg | 4 | β | 2 |
| Cymbella affinis Kützing | 19 | o-β | 1.5 |
| Encyonema cespitosum Kützing | 66 | β | 2 |
| Cymbella tumida (Brébisson) Van Heurck | 19 | β-α | 2.5 |
| Cymbella ventricosa Agardh | 3 | o-β | 1.5 |
| Diatoma vulgaris Bory | 3 | β-α | 2.5 |
| Fragilaria capucina Desmazières | 35 | β | 2 |
| Fragilaria capucina Desmazières-teretological form | 5 | - | - |
| Gomphonella olivacea (Hornemann) Rabenhorst | 33 | β | 2 |
| Gomphonema parvulum Kützing | 23 | β | 2 |
| Luticola mutica (Kützing) D.G. Mann | 7 | α | 3 |
| Melosira varians Agardh | 34 | β | 2 |
| Navicula cincta (Ehrenberg) Ralfs | 93 | β-α | 2.5 |
| Navicula cryptocephala Kützing | 9 | α | 3 |
| Navicula cryptotenella Lange-Bertalot | 15 | β | 2 |
| Navicula recens (H.Lange-Bertalot) H.Lange-Bertalot | 2 | α | 3 |
| Nitzschia dissipata (Kützing) Rabenhorst | 20 | o-β | 1.5 |
| Nitzschia inconspicua Grunow | 21 | α | 3 |
| Nitzschia inconspicua Grunow-teratological form | 3 | - | - |
| Rhoicosphenia curvata (Kützing) Grunow | 1 | β | 2 |
| Ulnaria ulna (Nitzsch) Compère | 8 | β | 2 |
| Ulnaria ulna (Nitzsch) Compère-teratological form | 10 | - | - |
| Total | 440 | - | - |
| SI value | 1.93 | - | - |
| Stations | Total Number of Individuals | Dominant Species | SI Value | Water Quality Interpretation | Observations Regarding the Influence of Mining Activities |
|---|---|---|---|---|---|
| A1–Arieșeni | 411 | A. minutissimum, G. olivacea | 1.40 | Class I–clean water | Typical community of oligosaprobic waters; low anthropogenic influence |
| A2–Mihoești | 502 | A. minutissimum, D. vulgaris | 1.39 | Class I–clean water | Water maintains oligotrophic characteristics; minimal anthropogenic influences |
| A3–Baia de Arieș | 364 | A. minutissimum, N. dissipata | 1.65 | Class I–II–slight organic pollution | Increase in α-mesosaprobic species indicating more pronounced anthropogenic influences, likely from wastewater and mining activities |
| A4–Gligorești | 440 | N. cincta, E. cespitosum, F. capucina | 1.93 | Class I–II–slight organic pollution | More diverse community, with α and β–α species, indicating the impact of mining activities and moderate pollution; slightly increased organic load |
| Sampling Point | Shannon (H’) | Simpson (1-D) | Richness (Number of Species) | Total Cells |
|---|---|---|---|---|
| A1 | 0.85 | 0.46 | 6 | 224 |
| A2 | 1.90 | 0.78 | 14 | 139 |
| A3 | 1.55 | 0.59 | 20 | 364 |
| A4 | 2.61 | 0.91 | 21 | 347 |
| Diatoms vs. Metal Trace Elements | As | Cd | Cr | Cu | Fe | Ni | Zn |
|---|---|---|---|---|---|---|---|
| Planothidium rostratoholarcticu | −0.083 | 0.194 | −0.294 | −0.112 | 0.472 | 0.967 | 0.556 |
| Achnanthidium minutissimum | 0.547 | 0.840 | 0.422 | 0.339 | 0.985 | 0.785 | 0.976 |
| Cymbella affinis | −0.522 | 0.142 | 0.602 | 0.743 | −0.301 | −0.625 | −0.171 |
| Cymbella ventricosa | 0.714 | −0.247 | −0.219 | −0.587 | −0.091 | −0.358 | −0.323 |
| Gomphonella olivacea | −0.809 | −0.631 | −0.698 | −0.347 | −0.531 | 0.242 | −0.386 |
| Gomphonema pumilum | −0.623 | −0.684 | −0.848 | −0.564 | −0.472 | 0.343 | −0.379 |
| Planothidium lanceolatum | 0.742 | 0.935 | 0.719 | 0.498 | 0.939 | 0.371 | 0.874 |
| Amphora pediculus | 0.584 | −0.327 | −0.174 | −0.518 | −0.256 | −0.561 | −0.472 |
| Hannaea arcus | 0.898 | 0.034 | −0.069 | −0.467 | 0.233 | −0.111 | −0.003 |
| Cocconeis placentula | 0.573 | −0.397 | −0.295 | −0.627 | −0.273 | −0.483 | −0.492 |
| Cymbella gracilis | 0.573 | −0.397 | −0.295 | −0.627 | −0.273 | −0.483 | −0.492 |
| Odontidium mesodon | 0.573 | −0.397 | −0.295 | −0.627 | −0.273 | −0.483 | −0.492 |
| Diatoma vulgaris | 0.555 | −0.394 | −0.262 | −0.593 | −0.294 | −0.530 | −0.510 |
| Didymosphenia geminata | 0.573 | −0.397 | −0.295 | −0.627 | −0.273 | −0.483 | −0.492 |
| Fragilaria capucina | −0.074 | 0.466 | 0.865 | 0.835 | 0.043 | −0.587 | 0.097 |
| Navicula cryptotenella Lange-Be | −0.200 | 0.345 | 0.788 | 0.797 | −0.094 | −0.653 | −0.024 |
| Nitzschia dissipata | 0.396 | 0.994 | 0.808 | 0.749 | 0.909 | 0.451 | 0.946 |
| Ulnaria ulna | −0.428 | 0.134 | 0.621 | 0.709 | −0.313 | −0.710 | −0.211 |
| Discostella stelligera | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Fragilaria vaucheriae | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Gomphonema parvulum | −0.418 | 0.296 | 0.719 | 0.833 | −0.148 | −0.555 | −0.023 |
| Navicula gregaria | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Navicula cryptocephala Kützing | −0.369 | 0.384 | 0.775 | 0.881 | −0.055 | −0.491 | 0.071 |
| Navicula viridula | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Nitzschia gracilis | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Nitzschia palea | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Surirella angusta | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Ulnaria ulna_1 | 0.573 | 0.860 | 0.457 | 0.359 | 0.992 | 0.753 | 0.978 |
| Amphora ovalis | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Cocconeis pediculus | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Encyonema cespitosum | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Cymbella tumida | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Luticola mutica | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Melosira varians | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Navicula recens | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Nitzschia inconspicua | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
| Rhoicosphenia curvata | −0.478 | 0.174 | 0.634 | 0.757 | −0.273 | −0.634 | −0.150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glevitzky, M.; Corcheş, M.T.; Popa, D.M. Assessing Pollution and Diatom-Based Bioindicators in the Arieș River, Romania. Environments 2025, 12, 398. https://doi.org/10.3390/environments12110398
Glevitzky M, Corcheş MT, Popa DM. Assessing Pollution and Diatom-Based Bioindicators in the Arieș River, Romania. Environments. 2025; 12(11):398. https://doi.org/10.3390/environments12110398
Chicago/Turabian StyleGlevitzky, Mirel, Mihai Teopent Corcheş, and Doriana Maria Popa. 2025. "Assessing Pollution and Diatom-Based Bioindicators in the Arieș River, Romania" Environments 12, no. 11: 398. https://doi.org/10.3390/environments12110398
APA StyleGlevitzky, M., Corcheş, M. T., & Popa, D. M. (2025). Assessing Pollution and Diatom-Based Bioindicators in the Arieș River, Romania. Environments, 12(11), 398. https://doi.org/10.3390/environments12110398







