1. Introduction
Climate change tends to intensify episodes of extreme heat and drought, increasing the vulnerability of agricultural systems, especially in arid and semiarid regions [
1]. These areas are characterized by low and irregular precipitation, high solar radiation, extreme temperatures and marked vapor pressure deficit (VPD), causing limitations in agricultural production and sustainability [
2]. Furthermore, reduced water availability, combined with rising average and maximum air temperatures, places additional pressures on cultivated plants, directly affecting essential physiological processes such as photosynthesis, transpiration, and energy balance [
3]. In this context, the adoption of species with high tolerance to water and heat stresses is an essential strategy to mitigate the effects of climate change and ensure the sustainability of agricultural production [
2].
Cacti, especially those of the genera
Opuntia and
Nopalea, are highly promising plants for cultivation in arid and semiarid regions [
3,
4]. In addition to their productive importance in these environments, these cacti have high potential for use in areas subject to environmental degradation, contributing to the recovery and sustainability of ecosystems [
3]. This adaptive capacity is associated with crassulacean acid metabolism (CAM), a photosynthetic pathway that is highly efficient in the use of water. In this mechanism, the initial fixation of carbon dioxide (CO
2) occurs predominantly at night, through the action of the enzyme phosphoenolpyruvate carboxylase (PEPC), reducing water losses through transpiration. During the day, CO
2 stored in the form of organic acids is released and reassimilated in the Calvin–Benson cycle, coupled with the photochemical reactions of photosynthesis [
5,
6]. The CAM pathway, associated with the succulence of cladodes and the storage of water in specialized cells, gives cacti high resilience to dry environments [
3]. This adaptation is reinforced by anatomical characteristics, such as a thick cuticle, mucilaginous cells, low spatial density and high stomatal regulation, in addition to succulent cladodes covered by epicuticular wax [
5,
7]. Together, these attributes make cacti important forage species for semiarid regions.
However, even in CAM plants, photosynthetic efficiency is not static, being modulated by environmental conditions throughout the year and varying according to the species’ tolerance to seasonal fluctuations. Jardim et al. [
8] found that photochemical responses vary significantly between different clones of forage cacti, with semiarid environmental conditions reducing the efficiency of electron use in carbon fixation, causing damage to of photosystem II (PSII), and increasing energy dissipation. However, the observations obtained in that study were specific, not allowing for a complete understanding of the metabolic responses of these clones to stress, since cacti are long-cycle species with high phenotypic plasticity. Additionally, Jardim et al. [
4] observed that, under conditions of greater water availability and lower VPD, plants of the genera
Opuntia and
Nopalea present similar evapotranspiration rates. However, during the dry period, characterized by higher VPD,
Opuntia species are able to maintain higher evapotranspiration rates, as a result of their greater water storage capacity, greater chlorophyll production and better physiological performance.
The mechanisms of CAM are closely related to the pattern of gas exchange, an area still little explored in the literature [
9]. Despite the recognized tolerance of these plants to stressful conditions, studies have shown that the highest rates of CO
2 assimilation occur in the rainy season, when there is greater water availability, milder temperatures and lower VPD values, favoring the activity of PEPC and the maintenance of photochemical integrity [
7,
9]. CAM plants have developed specific mechanisms to mitigate photoinhibition, highlighting the ability to reduce photochemical damage during drought and to maintain a basal metabolism (idle CAM) that preserves photosystems, enabling rapid recovery when environmental conditions become favorable [
10].
However, the transition periods between water extremes (rainy and dry seasons) remain little investigated, despite representing critical phases in which plants rapidly adjust their physiological and photochemical mechanisms. In these transitions, the interaction between water availability, solar radiation and thermal variations can intensify the combined stress, resulting in differentiated responses among forage cactus clones. Understanding these seasonal variations is essential for identifying genetic materials with greater functional stability and capable of sustaining photosynthetic performance in the face of stressful climatic conditions.
In this context, the integrated evaluation of gas exchange and chlorophyll fluorescence parameters in forage cactus clones constitutes a valuable tool for understanding the adaptive plasticity of these species and supporting selection programs for more tolerant genotypes. Thus, this study aimed to evaluate the modulation of physiological and photochemical responses of forage cactus clones (Nopalea and Opuntia) in a semiarid environment, considering seasonal variation and comparing performance in the rainy and dry seasons and in transition periods.
3. Results
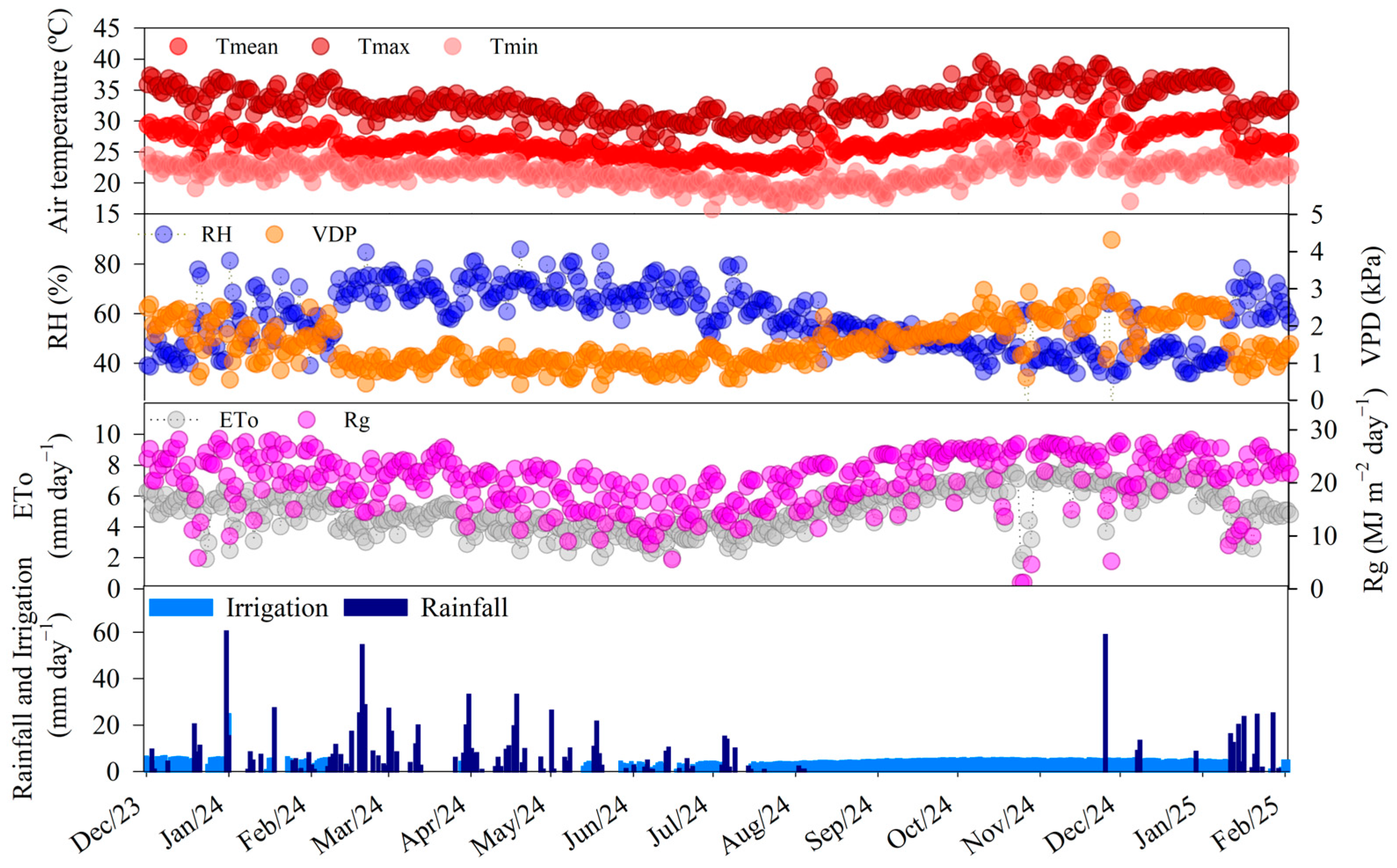
Our results showed that the rainy and rainy–dry seasons concentrated the largest number of rainfall events, with 103 and 11 occurrences, respectively, as well as accumulating the largest rainfall volumes, totaling 799.7 mm and 17.2 mm (
Table 2). At these stations, the lowest incidences of global solar radiation (19.5 and 18.2 MJ m
−2 day
−1) were also recorded, in addition to the highest values of relative air humidity (64.4% and 59.5%) and the lowest vapor pressure deficits (1.2 kPa) (
Table 2). During the rainy season, we observed temperatures ranging from 21.7 to 32.2 °C, with an average of 26 °C, while, in the rainy–dry season, temperatures ranged from 19.1 °C to 30.3 °C, with an average of 24 °C (
Table 2). In the dry season, only one rainfall event was recorded, with a volume of 0.2 mm (
Table 2). During this period, global solar radiation reached 22.2 MJ m
−2 day
−1, relative humidity was 48.5%, and VPD reached 1.8 kPa (
Table 2). Temperatures ranged from 21.3 °C to 33.8 °C, with an average of 26.8 °C (
Table 2). In the dry–rainy transition season, nine rainfall events occurred, with an accumulated volume of 139.8 mm (
Table 2). Global solar radiation was 22.6 MJ m
−2 day
−1, while relative humidity presented the lowest values of the cycle (45.6%) and VPD the highest value recorded (2.2 kPa) (
Table 2). Air temperature values ranged between 23.3 °C and 26.1 °C, with an average of 29.1 °C (
Table 2).
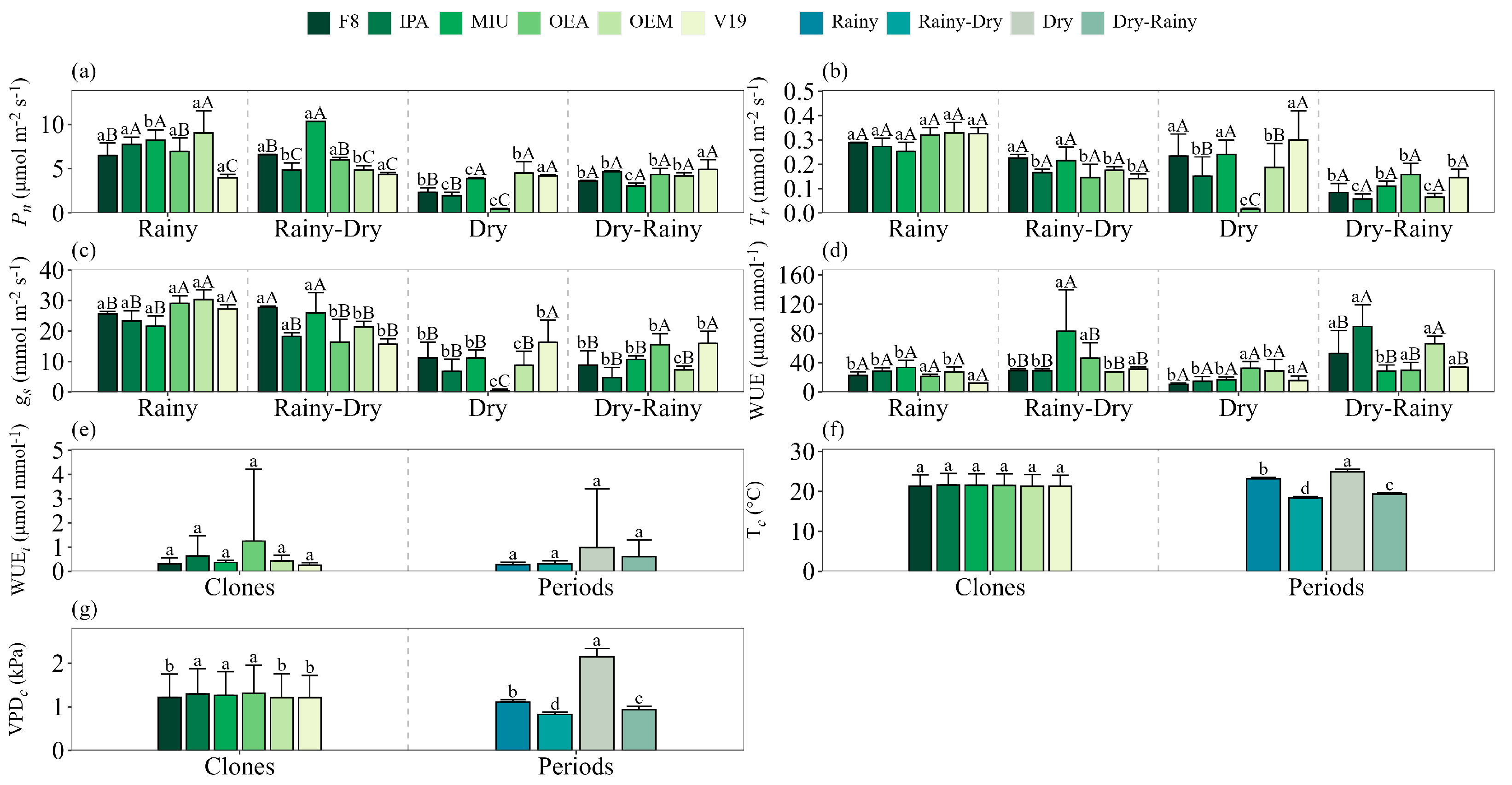
The net photosynthesis rate (
Pn) values varied among the forage cactus clones throughout the seasons (
Figure 3a). In general, the highest
Pn values were recorded during the rainy season, with emphasis on clones IPA, MIU and OEM (
Figure 3a). Clone V19 maintained a practically constant photosynthesis rate throughout all seasons (mean of 4.4 µmol m
−2 s
−1), while OEA and F8 maintained
Pn of about 6.5 µmol m
−2 s
−1, both in the rainy season and in the rainy–dry transition with a reduction of 93 and 64% in the dry season (
Figure 3a). The MIU and IPA clones also stood out in the rainy season but suffered a reduction of 53% and 75% respectively in
Pn during the dry season (
Figure 3a). In the rainy–dry transition, only the MIU clone maintained high levels of photosynthesis (10.3 µmol m
−2 s
−1), while, in the dry period, the highest values were observed in the clones MIU (3.9 µmol m
−2 s
−1), OEM (4.5 µmol m
−2 s
−1) and V19 (4.2 µmol m
−2 s
−1) (
Figure 3a). In the dry–rainy transition, there was no significant variation in
Pn values between clones (
Figure 3a).
The highest transpiration rate values (
Tr) were recorded in the rainy season, with an average of 0.3 mmol m
−2 s
−1, without significant differences between clones (
Figure 3b). In the rainy–dry transition, only clones F8 and MIU maintained high transpiration rates (
Figure 3b). In the dry season, the highest values were observed in the F8 clones (0.233 mmol m
−2 s
−1), MIU (0.252 mmol m
−2 s
−1) e V19 (0.3 mmol m
−2 s
−1), while the OEA clone had the lowest rate (0.015 mmol m
−2 s
−1) (
Figure 3b). During the dry–rainy transition, we observed that there were no significant variations between clones (
Figure 3b). In general, the MIU and F8 clones maintained high values of
Tr across the seasons, with reductions of approximately 51% and 67%, respectively, only in the dry–rainy transition (
Figure 3b). The IPA, OEM and OEA clones showed more significant reductions from the rainy season to the dry season, of the order of 45%, 95% and 43%, respectively (
Figure 3b). Clone V19 stood out with high transpiration rates in the rainy seasons (0.33 mmol m
−2 s
−1) and dry (0.3 mmol m
−2 s
−1), presenting a significant reduction of 54% in the transition periods (
Figure 3b).
In the present study, stomatal conductance (
gs) presented the highest values during the rainy season in OEM clones (30.3 mmol m
−2 s
−1), OEA (29.1 mmol m
−2 s
−1) and V19 (27.2 mmol m
−2 s
−1), which suffered reductions of 71%, 98% and 40%, respectively, in the dry and dry–rainy periods (
Figure 3c). Here, the clones F8 (26.7 mmol m
−2 s
−1), MIU (23.8 mmol m
−2 s
−1) and IPA (20.8 mmol m
−2 s
−1) did not present a significant difference between the rainy and rainy–dry transition periods, but also showed significant drops in the dry season, with reductions of 63%, 54% and 71%, respectively (
Figure 3c). In the dry season, the highest value of
gs was observed in clone V19 (16.27 mmol m
−2 s
−1), while the lowest values were recorded in clones OEA (0.49 mmol m
−2 s
−1) and OEM (8.75 mmol m
−2 s
−1) (
Figure 3c).
Instantaneous water use efficiency (WUE) remained constant across seasons for clone OEA, averaging approximately 32.6 µmol mmol
−1 (
Figure 3e). Clone V19 presents higher WUE values in the rainy–dry and dry–rainy transition periods (32.6 µmol mmol
−1), compared to the rainy and dry seasons (13.9 µmol mmol
−1) (
Figure 3e). In the dry–rainy period, clones F8, IPA and OEM presented the highest WUE values, with an increase of 154%, 269% and 135%, respectively, in relation to the other periods (
Figure 3e). The MIU clone stood out in the rainy–dry transition, with a mean of 83.1 µmol mmol
−1 (
Figure 3e). In the wet and dry seasons, we did not observe significant differences in WUE among clones (
Figure 3e). However, in the rainy–dry transition, the highest values were recorded in the MIU clones (83.1 µmol mmol
−1) and OEA (46.5 µmol mmol
−1) (
Figure 3e). In the dry–rainy transition, the IPA clone showed the greatest efficiency (89.6 µmol mmol
−1), while MIU, OEA and V19 exhibited the lowest values, around 30.8 µmol mmol
−1 (
Figure 3e). Our results show that intrinsic water use efficiency (WUE
i) did not present significant interaction between the clone and season factors, with an overall average of 0.54 µmol mmol
−1 (
Figure 3e). Cladode temperature (T
c) did not vary significantly among the different forage cactus clones (
Figure 3f). The highest T
c values were observed during the dry season, with an average of 24.9 °C, followed by the rainy season, which averaged 23.3 °C (
Figure 3f). Regarding cladode vapor pressure deficit (VPD
c), the clones IPA, MIU, and OEA recorded the highest values, around 1.29 kPa. In general, VPD
c was higher in the dry season (2.14 kPa), decreasing in the rainy season (1.10 kPa) (
Figure 3g).
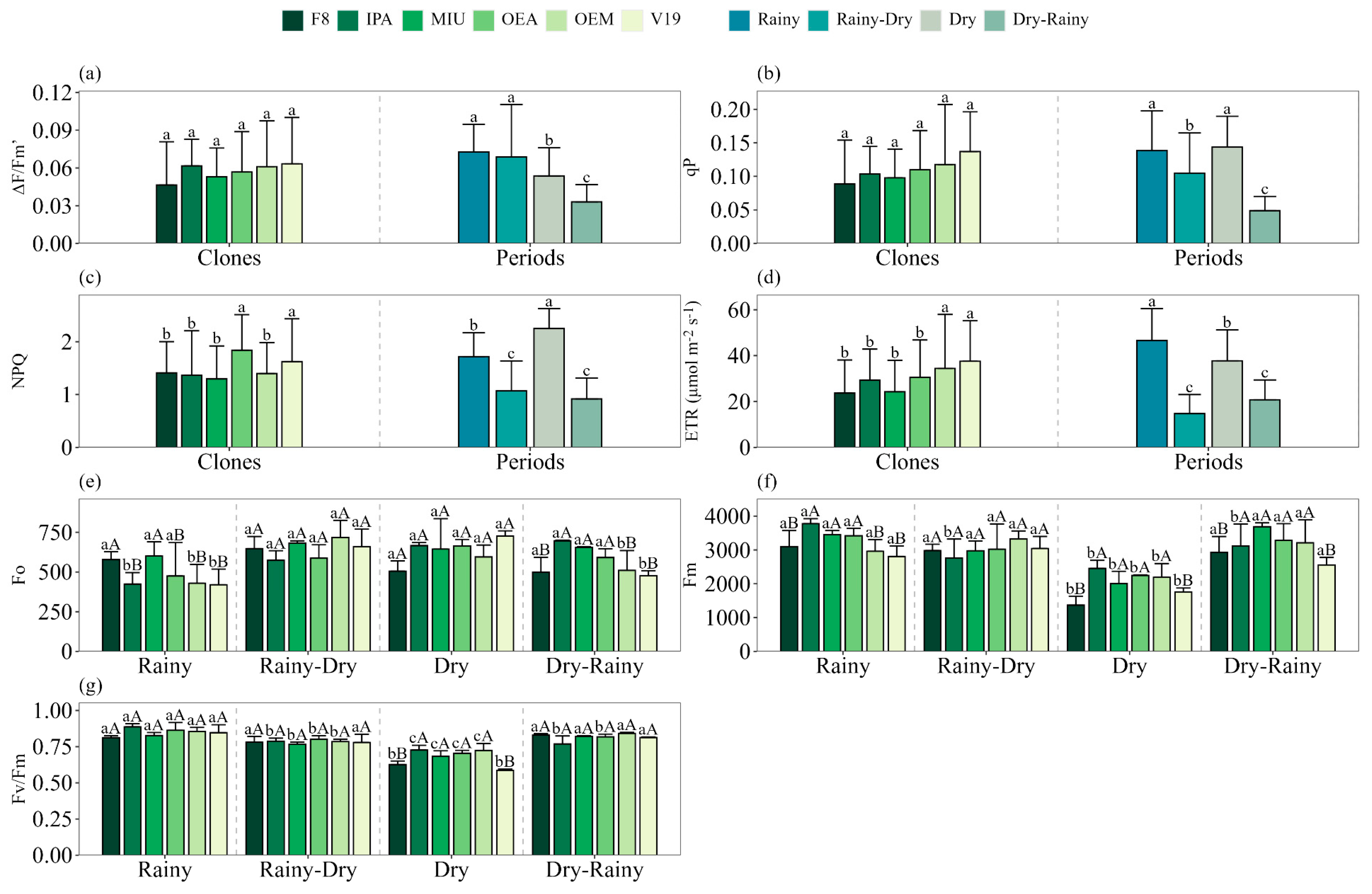
We did not find significant interaction between the clone and season factors for the variables effective quantum yield of photosystem II (ΔF/Fm′), photochemical quenching (qP), non-photochemical quenching (NPQ) and relative electron transport rate (ETR) (
Figure 4a–d). The variables ΔF/Fm′ and qP did not show variations among the forage cactus clones, but showed higher values during the rainy season and in the dry–rainy transition, with averages of 0.071 and 0.141, respectively (
Figure 4a,b). These values represented increases of approximately 115% (ΔF/Fm′) and 186% (qP) compared to the dry–rainy period, in which the lowest averages were observed: 0.033 for ΔF/Fm′ and 0.050 for qP (
Figure 4a,b). We found higher ETR and NPQ values in clones V19, OEM and OEA, with averages of 35.98 µmol m
−2 s
−1 for ETR and 1.73 for NPQ (
Figure 4c,d). During the dry season, the NPQ variable reached its peak, with an average of 2.25, representing an increase of approximately 126% in relation to the transition seasons (rainy–dry and dry–rainy), which presented the lowest values (
Figure 4c). On the other hand, the ETR was higher during the rainy season, with an average of 46.6 µmol m
−2 s
−1, a value approximately 163% higher than that recorded in the transition periods, whose average was 17.8 µmol m
−2 s
−1 (
Figure 4d).
It was found that the initial fluorescence (Fo) values did not vary throughout the seasons for clones F8, MIU and OEA, with averages of 557.3, 645.3 and 579.2, respectively (
Figure 4e). On the other hand, clones IPA, OEM and V19 showed a reduction in Fo values during the rainy season, with decreases of 36%, 28% and 42%, respectively, compared to the dry season (
Figure 4e). In the dry and rainy–dry transition seasons, no significant differences were observed between the clones evaluated (
Figure 4e). During the rainy season, the highest Fo values were recorded in clones F8 (578) and MIU (600), while in the dry–rainy transition, the highest values were observed in clones IPA (696), MIU (655) and OEA (591) (
Figure 4e).
Maximum fluorescence (Fm) values showed a reduction during the dry period in clones F8 (54%), MIU (41%), OEA (31%), OEM (31%) and V19 (37%) compared to the rainy and transition periods (rainy–dry and dry–rainy) (
Figure 4f). On the other hand, clone IPA presented the highest Fm value during the rainy season, with an average of 3776.3 (
Figure 4f). During the rainy–dry transition, no significant variations in Fm values were observed among the different clones. The lowest Fm values during the dry period and in the dry–rainy transition were recorded in clones F8 (2149) and V19 (2158) (
Figure 4f). In the rainy season, we found lower Fm values in clones F8 (3097), MIU (3453) and V19 (2806) (
Figure 4f).
No significant variations were observed in the Fv/Fm values among the different forage cactus clones during the rainy (0.848), rainy–dry (0.816) and dry–rainy (0.784) seasons (
Figure 4g). However, in the dry season, the lowest values were recorded in clones F8 (0.627) and V19 (0.587) (
Figure 4g). During the dry season, Fv/Fm values were reduced in all forage cactus clones (
Figure 4g). The averages recorded were 0.627 for clone F8, with a reduction of 24.5%; 0.727 for clone IPA (reduction of 18.1%); 0.683 for clone MIU (reduction of 16.9%); 0.705 for clone OEA (reduction of 6.5%); 0.724 for clone OEM (reduction of 14%); and 0.587 for clone V19, with the largest percentage drop (30.1%), compared to the values obtained in the rainy season (
Figure 4g).
The principal component analysis (PCA) explained 63.43% of the total data variation, distributed into two principal components, PC1 (43.05%) and PC2 (20.38%) (
Figure 5). The OEM, MIU, OEA, F8, V19 and IPA clones showed a positive correlation with the rainy season and with the variables
Pn,
gs,
Tr, RH, ΔF/Fm′ and rainfall, and negative correlation with Tair, Tmax, VPD and Rg (
Figure 5). The dry–rainy season showed a positive correlation with the same clones and with the variables Tair, Tmax, VPD, Rg and WUE. During the dry season, the clones IPA, OEM, V19 and F8 correlated positively with the variables Fo and NPQ and negatively with Fv/Fm and Fm (
Figure 5).
4. Discussion
The adaptive characteristics of cacti of the genera
Opuntia and
Nopalea favor their agronomic success in arid and semiarid environments [
8]. The crassulacean acid metabolism (CAM) allows the nocturnal assimilation of CO
2, significantly reducing water loss through transpiration. Furthermore, the succulence of the cladodes, typical of these species, acts as an important water reservoir, contributing to the maintenance of the turgidity of the photosynthetic tissues during periods of water deficit [
7].
In this study, we observed a seasonal variation in the net photosynthetic rate (
Pn) of the different clones of forage cactus, with higher rates of assimilation of CO
2 recorded during the rainy season and a sharp reduction in the dry season (
Figure 3a). Similar results were reported by Pimienta-Barrios et al. [
7], when investigating the seasonal variation of CO
2 assimilation in cacti, such as
Opuntia ficus-indica and
Stenocereus queretaroensis in a semiarid environment. Furthermore, the authors observed that the highest instantaneous rates and the highest daily net inflow of CO
2 occurred at the end of the rainy season, a period characterized by moderate daytime and nighttime temperatures (29/15 °C) and an increase in photosynthetically active radiation due to decreased cloud cover. In contrast, during the dry season, with higher temperatures (36/23 °C) and soil water content below 5%, CO
2 assimilation showed negative values.
The net photosynthetic rate (
Pn) values in plants with the CAM pathway present wide variation, depending on the species and environmental conditions. Pimienta-Barrios et al. [
7] recorded during the rainy season, rates of approximately 12 µmol m
−2 s
−1 for
Opuntia ficus-indica and 8 µmol m
−2 s
−1 for
Stenocereus queretaroensis. In contrast, during the dry season, rates dropped to about −10 µmol m
−2 s
−1 in both species. Nobel and La Barrera [
14], when studying the culture of white dragon fruit (
Hylocereus undatus), observed a
Pn of 8 µmol m
−2 s
−1 under humid conditions (soil water potential of −0.2 MPa), which reduced to 2.5 µmol m
−2 s
−1 after 10 days of drought (potential of −3.2 MPa). Under rainfed conditions, Araújo et al. [
9] reported that the MIU clone presented
Pn of 3.98 µmol m
−2 s
−1 during the rainy season and 2.05 µmol m
−2 s
−1 in the dry season. Already in a controlled environment, with irrigation, Silva et al. [
15] observed higher values, reaching 10.7 and 18.83 µmol m
−2 s
−1 for OEM and MIU clones, respectively. In this study, the highest values of
Pn were observed for the MIU clone, with 9.24 µmol m
−2 s
−1 in the rainy season and 10.34 µmol m
−2 s
−1 in the rainy–dry season, followed by OEM clones (9.03 µmol m
−2 s
−1) and IPA (7.74 µmol m
−2 s
−1) during the rainy season. The lowest value was recorded for the OEA clone (0.46 µmol m
−2 s
−1) in the dry season.
In our study, during the rainy season, temperatures ranged from 21 to 32 °C, values higher than those reported by Pimienta-Barrios et al. [
7]. These results reinforce the remarkable acclimatization capacity of cacti to wide thermal variations [
8]. However, this thermal plasticity can vary depending on the species and growing conditions [
8]. Under low water availability, the optimal temperatures for net water uptake CO
2 tend to reduce. Winter et al. [
16], when investigating the plasticity of photosynthesis in
Agave angustifolia under different environmental conditions (i.e., light, temperature, CO
2, nutrition and irrigation), they found that, in well-irrigated plants, the main limiting factors for the assimilation of CO
2 were light availability and nutritional status. These findings indicate that, although tolerance to heat stress is an important characteristic in arid environments, the availability of water and nutrients still plays a determining role in the photosynthetic efficiency of CAM plants.
During the dry–rainy transition period, average temperatures reached 29.1 °C and VPD reached 2.2 kPa, an increase of 2.3 °C and 22% compared to the dry season, indicating more stressful environmental conditions. Still, the OEA and IPA clones showed an increase in assimilation rates CO
2 (
Pn), maintaining lower transpiration rates, which may be related to rapid physiological modulation, associated with the increase in precipitation volume, which went from just 0.2 mm in the dry season to 139.8 mm in the dry–rainy season. Consequently, these clones showed higher instantaneous water use efficiency (WUE). The higher photosynthetic rates during the rainy season may be related to the greater activity of the enzyme PEPC, responsible for the initial fixation of CO
2 in the CAM, whose efficiency is favored by moderate nighttime temperatures [
14].
During the rainy season, we observe the highest transpiration rates (
Tr) and stomatal conductance (
gs) in all clones evaluated. However, with reduced water availability during the dry period, clones MIU, F8, IPA, OEM and V19 were able to maintain high transpiration rates. This behavior may be associated with the ability of these genotypes to accumulate water in the parenchyma during the rainy season and subsequently use it to sustain physiological processes under water deficit conditions [
9]. In contrast, the OEA clone showed marked reductions in photosynthetic rate values, transpiration and stomatal conductance both in the dry and dry–rainy periods, suggesting less tolerance to typical semiarid conditions, such as high temperatures, high vapor pressure deficit, low relative humidity and water scarcity. During the dry–rainy transition period, the IPA and OEA clones showed an increase in the net photosynthetic rate, although with lower transpiration rates compared to the dry season. This indicates greater flexibility in the regulation of transpiration in response to increased water availability, demonstrating that increases in the assimilation of CO
2 are not always associated with greater water losses through transpiration [
9].
In plants with CAM, photochemical reactions and the Calvin–Benson cycle are temporally dissociated from CO
2 fixation by the enzyme PEPC. However, the amount of CO
2 released by decarboxylation during the day, added to the CO
2 absorbed in gas exchange, directly influences the energy demand of the photochemical reactions necessary for the full functioning of carboxylation in the Calvin–Benson cycle [
10]. Consequently, stressful environmental conditions, such as excessive heat and drought, can compromise this process, negatively affecting the performance of photosystems and, therefore, photosynthetic efficiency [
8,
10].
During the dry period, we found an increase in the initial fluorescence (Fo) values for clones IPA, MIU, and V19 and a reduction in maximum fluorescence (Fm) and maximum quantum efficiency of photosystem II (Fv/Fm) for all clones evaluated. The increase in Fo under light and heat stress conditions is a common indication of disturbances in the structure of PSII, and may be related to the dissociation of light-harvesting complexes, the inactivation of photochemical reactions, or the inhibition of electron transport, especially due to the reduced transfer of plastoquinone QA to QB [
17,
18]. In turn, the decrease in Fm values may reflect structural damage to the antenna complexes, such as the denaturation of proteins associated with light capture [
18]. In the present study, the increase in Fo was accompanied by a reduction in the electron transport rate (ETR) during the dry season, suggesting an impairment of PSII activity, possibly resulting from the functional loss of reaction centers and the instability of light-harvesting complexes [
19].
The Fv/Fm value is one of the main indicators of the occurrence of stress in plants, reflecting the maximum quantum efficiency of PSII. Under ideal conditions, these values range from 0.80 to 0.83; however, reductions in this ratio indicate damage to the photosynthetic apparatus in response to environmental stresses [
8,
20]. During the dry season, we observed that clones V19, F8, MIU and OEM presented the lowest Fv/Fm values, with averages of 0.587, 0.627, 0.683 and 0.705, respectively. This indicates a lower tolerance of these genotypes to the adverse conditions imposed during this period and compromised photochemical activity. These reductions in Fv/Fm and PSII effective quantum yield efficiency (ΔF/Fm′) were accompanied by an increase in non-photochemical quenching (NPQ) values. This increase represents an adaptive response of plants to stress, through the dissipation of excess light energy in the form of heat, as a protective mechanism against photoinhibition [
20].
The variations in chlorophyll fluorescence parameters observed in forage cactus clones during the dry period reflect the reduction in the photosynthetic rate and stomatal conductance, which compromised the functioning of the Calvin–Benson cycle. As a consequence, there was less consumption of ATP and NADPH, in addition to a reduction in the regeneration of electron acceptors, such as NADP
+ [
21]. Given this imbalance between the capture of light energy and its use in biochemical processes, an increase in non-photochemical quenching values was observed, indicating greater dissipation of excess energy in the form of heat. This mechanism contributed to a reduction in the ETR, functioning as a photoprotective strategy. The modulation of NPQ and ETR acts as a defense system to prevent the excessive formation of reactive oxygen species (ROS) and thus avoid oxidative damage to the photosynthetic apparatus. As a result of this regulation, forage cactus clones were able to maintain, even during the dry season, values of the photochemical extinction coefficient (qP) similar to those observed in the rainy season. The qP, measured under light, expresses the proportion of PSII reaction centers in the open state and the electron transfer used for the production of ATP and NADPH [
8,
22]. Jardim et al. [
8], when evaluating the genotypic differences in the photochemical activity of forage cactus clones, observed that two clones of the genus
Opuntia (V19 e F8) and a clone of the genre
Nopalea (MIU) were the most sensitive to environmental stresses characteristic of semiarid regions. This sensitivity was evidenced by reductions in the values of ETR, NPQ, qP and ΔF/Fm′, indicating impairment of photochemical activities in these genotypes.
During the rainy–dry transition period, we clearly observed the lowest values of ETR, NPQ, qP and ΔF/Fm′, indicating greater light and thermal stress recorded in this interval. Despite this, Fv/Fm values remained above 0.8, suggesting that the maximum efficiency of photosystem II was not significantly compromised. According to Heyduk [
10], plants with CAM, in addition to using photoprotective strategies common to C3 and C4 plants, such as increased NPQ, have developed additional mechanisms that favor tolerance to high light. Among these mechanisms is the ability to maintain basal metabolic activity, which allows photosynthetic functionality to be prolonged even under extreme environmental conditions, avoiding prolonged photoinhibition. In addition to photochemical protection mechanisms, cacti also have structural adaptations that contribute to their survival in adverse environments, such as the presence of mucilaginous cells for water storage, fast-growing roots, and highly controlled transpiration [
8].
Our findings show that, during the dry season, clones IPA, OEM, F8 and V19 presented greater impairment of the photochemical apparatus, characterized by an increase in the initial fluorescence and non-photochemical dissipation values, associated with a reduction in the Fv/Fm and Fm parameters (
Figure 5). These results suggest limited photosystem II efficiency and possible structural damage to light-harvesting complexes in response to environmental stress [
8,
17,
18,
19]. On the other hand, we observed that all the clones evaluated presented superior physiological performance during the rainy season, reflected in higher rates of net photosynthesis, transpiration, stomatal conductance and effective quantum yield of PSII. This positive response, seen with the help of PCA, was associated with more favorable environmental conditions during this period, such as higher relative humidity and rainfall, as well as lower average and maximum temperatures, VPD and lower incidence of global solar radiation, factors that contributed to preserving the integrity and functioning of the photosynthetic apparatus [
7,
14].
Thus, the results of this study reinforce that the photosynthetic and photochemical performance of forage cactus clones is closely linked to the interaction between water availability, temperature, radiation and intrinsic characteristics of each genotype. In semiarid environments, the maintenance of photosynthetic efficiency depends not only on the capacity for nocturnal fixation of CO2 and water accumulation in cladodes, but also the activation of photoprotective mechanisms and plasticity to adjust gas exchange and energy dissipation according to environmental conditions. The MIU, OEM and IPA clones showed a smaller drop in performance during the dry season, demonstrating greater physiological resilience and agronomic potential, being promising candidates for use in resilient production systems.