Phytoplankton Dynamics in a Large Lagoon: Nutrient Load Reductions, Climate Change, and Cold- and Heatwaves
Abstract
1. Introduction
2. Materials and Methods
2.1. The Oder River and the Oder/Szczecin Lagoon
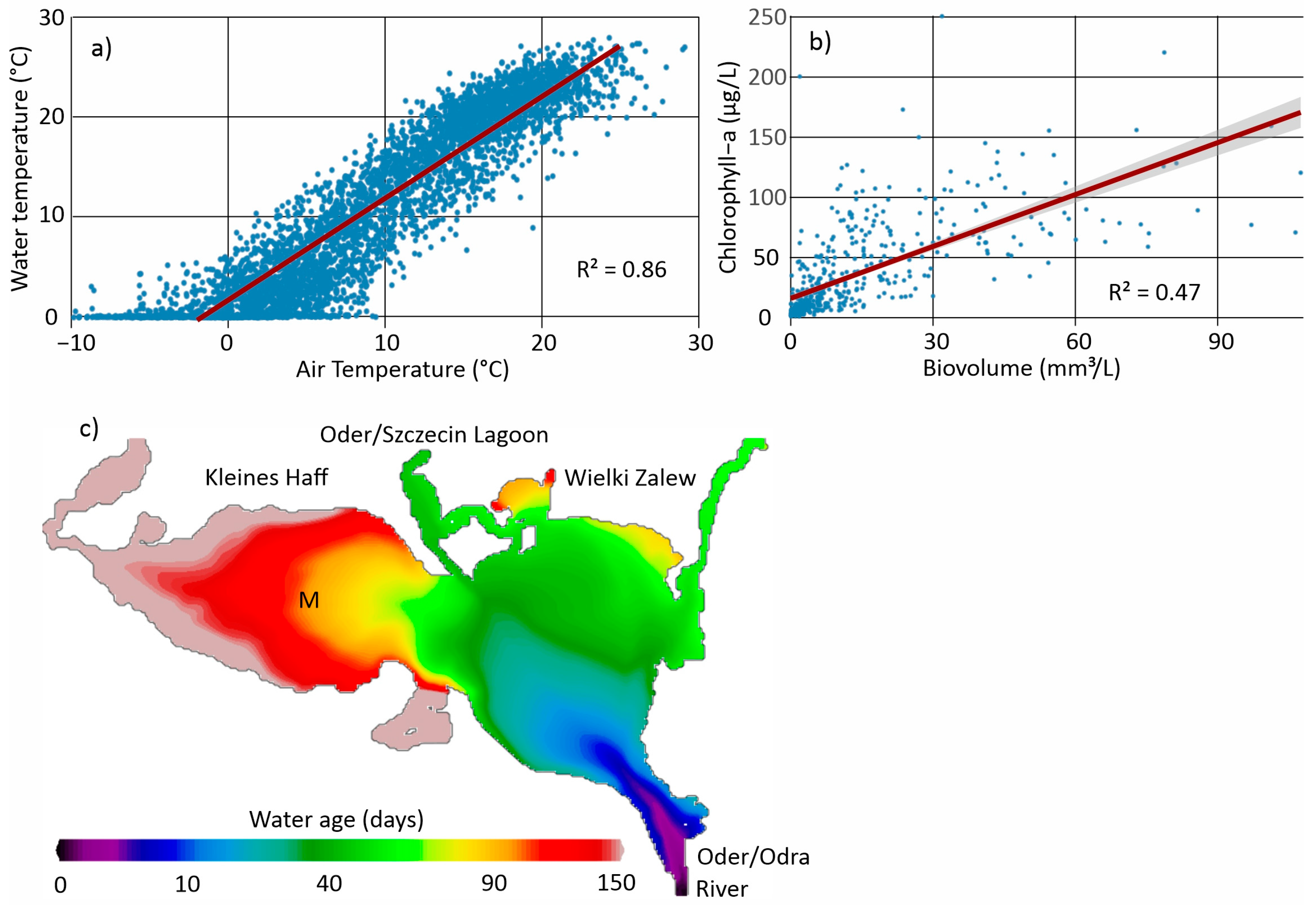
2.2. The Long-Term Monitoring Data and Calculations
2.3. The Ecosystem Model Approach (ERGOM)
3. Results
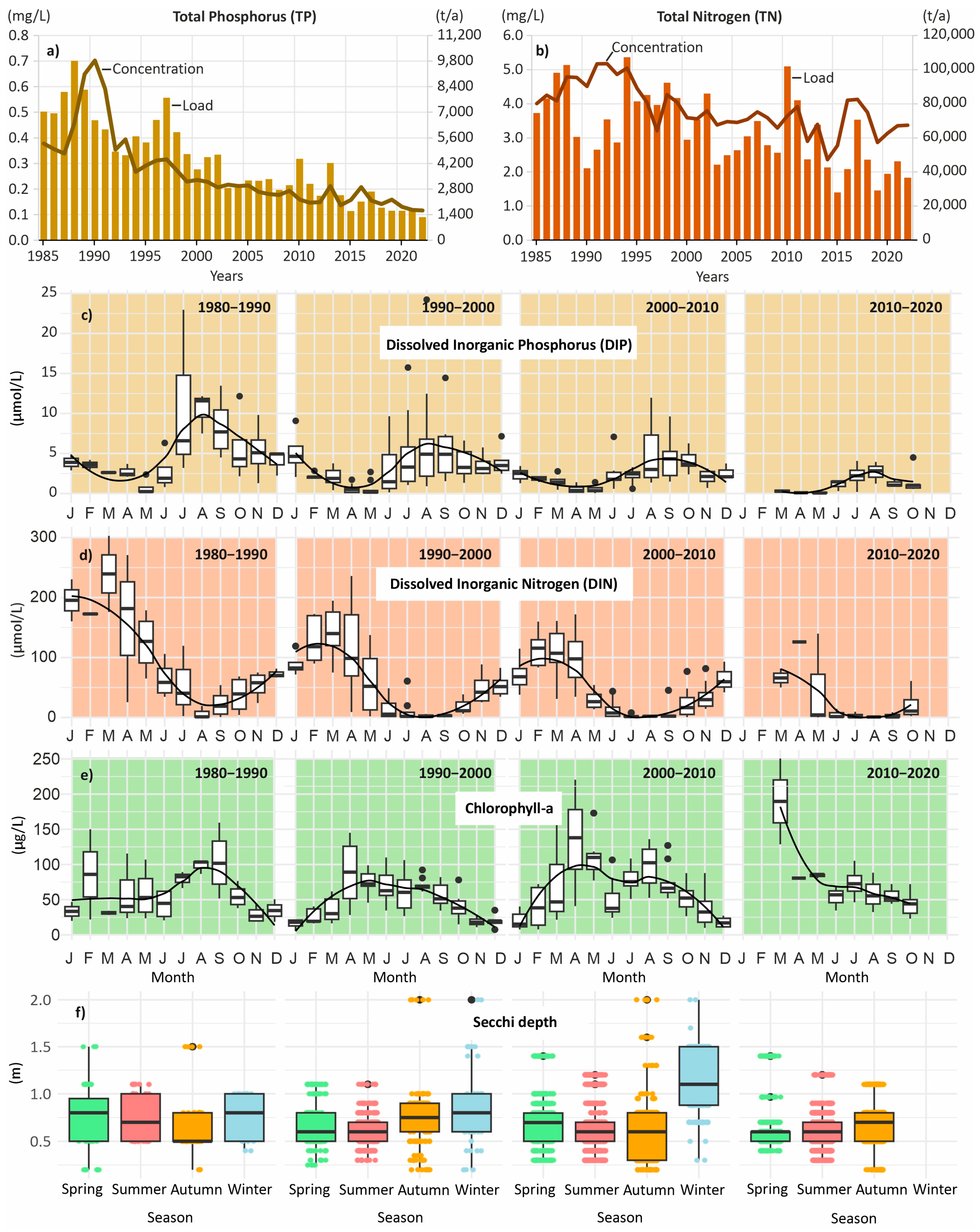
3.1. Long-Term Developments and the Role of Changing External Loads
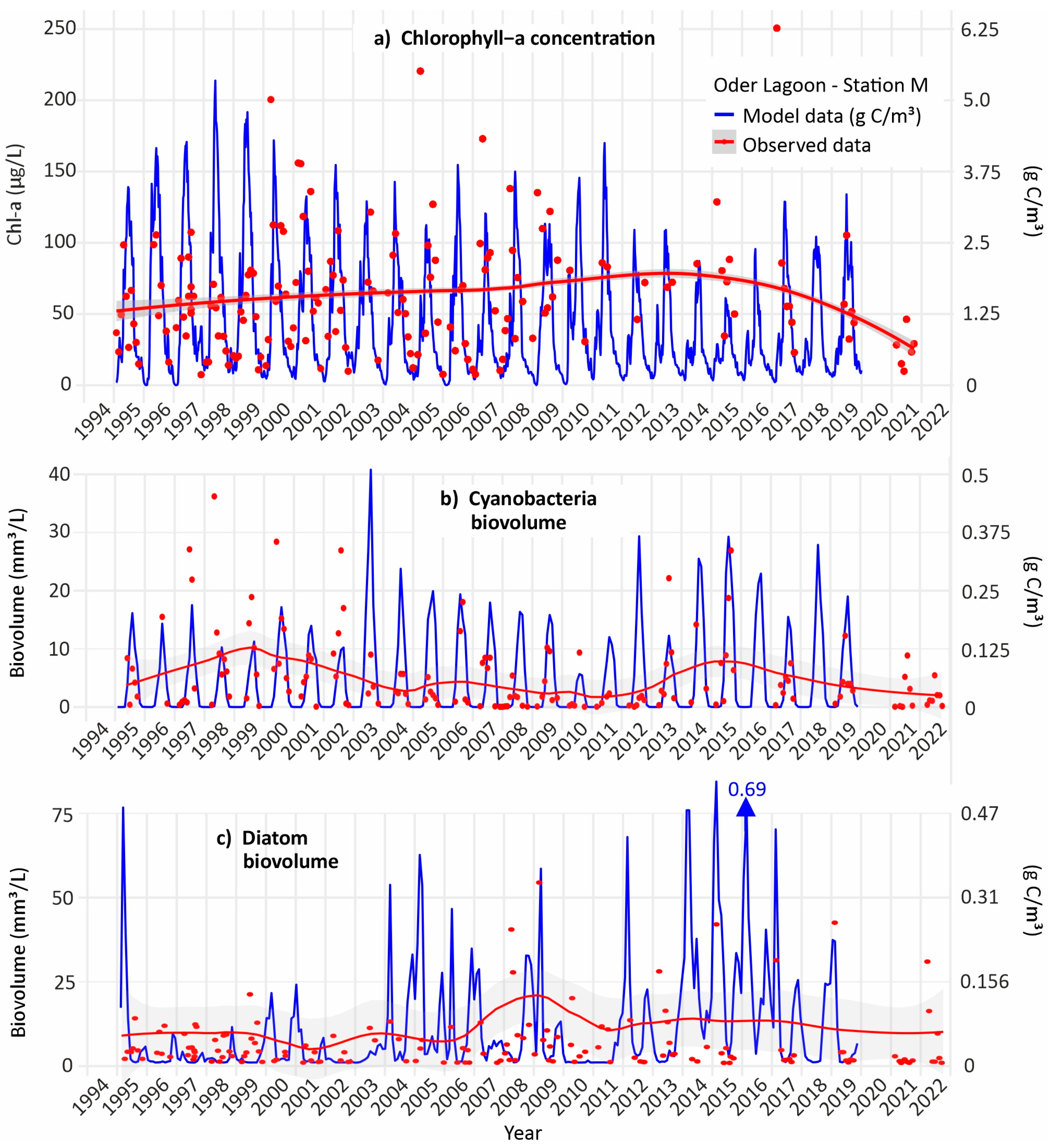
3.2. Long-Term Phytoplankton Concentration
3.3. Long-Term Phytoplankton Class Composition
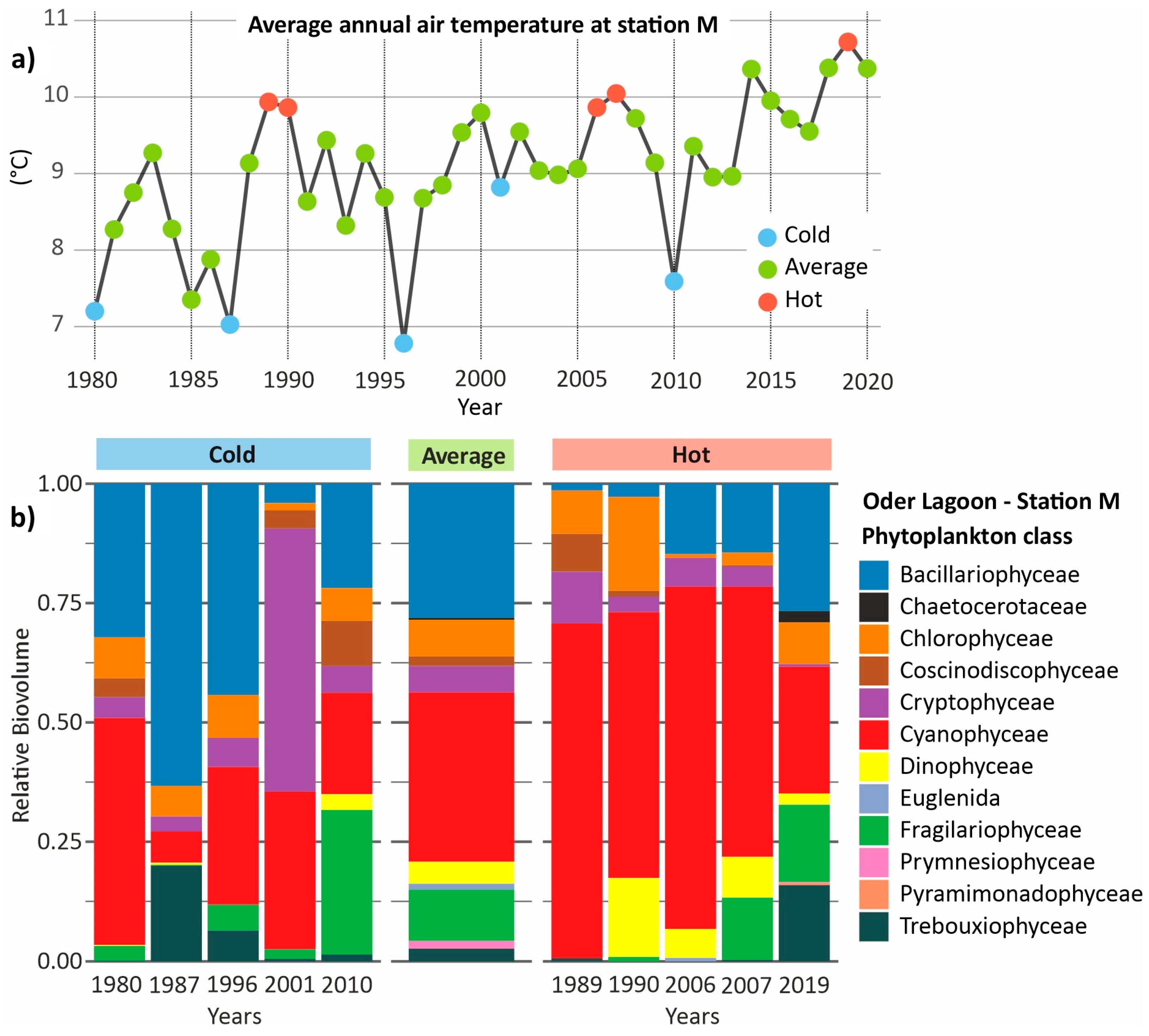
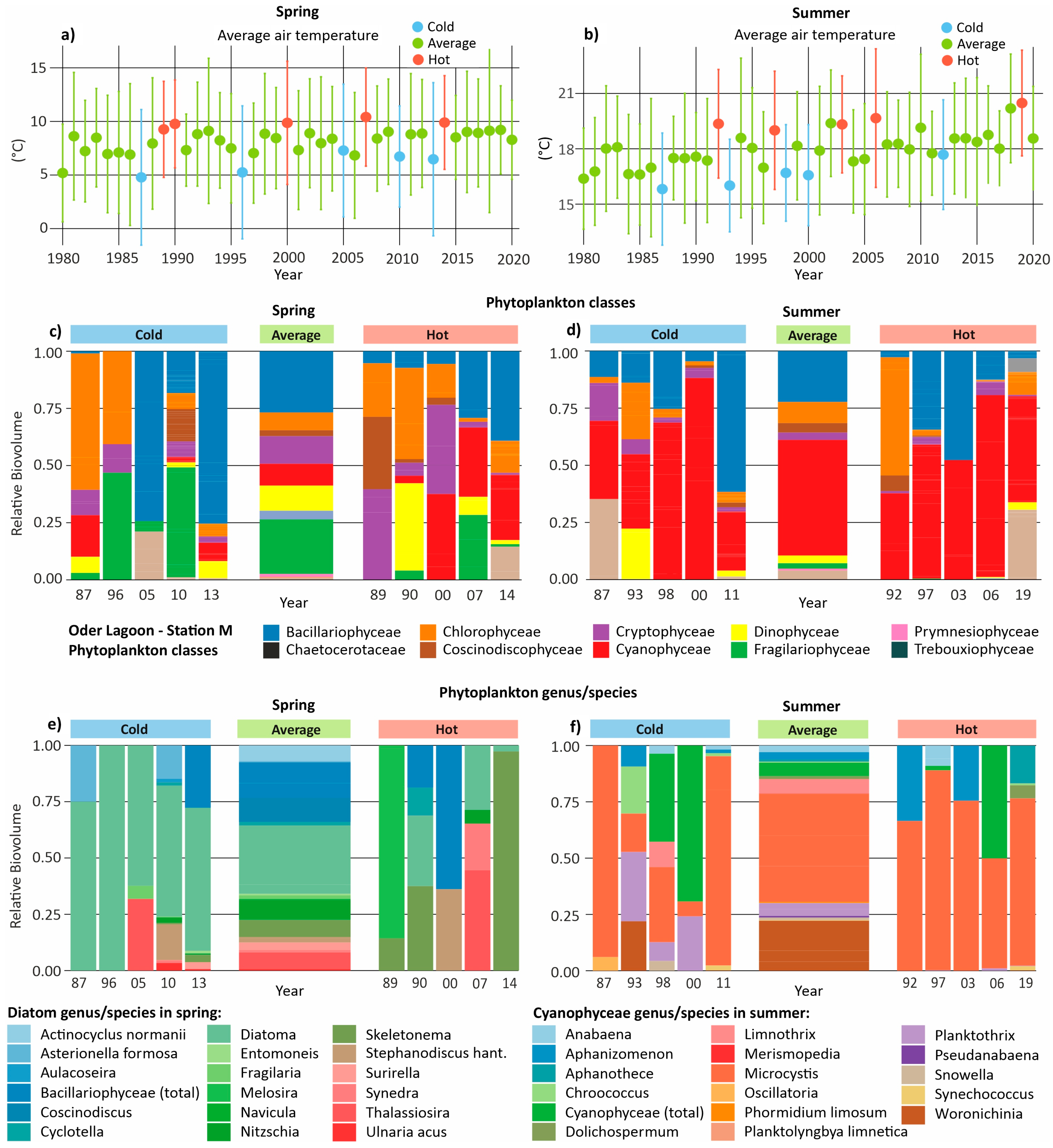
3.4. Phytoplankton in Hot and Cold Years
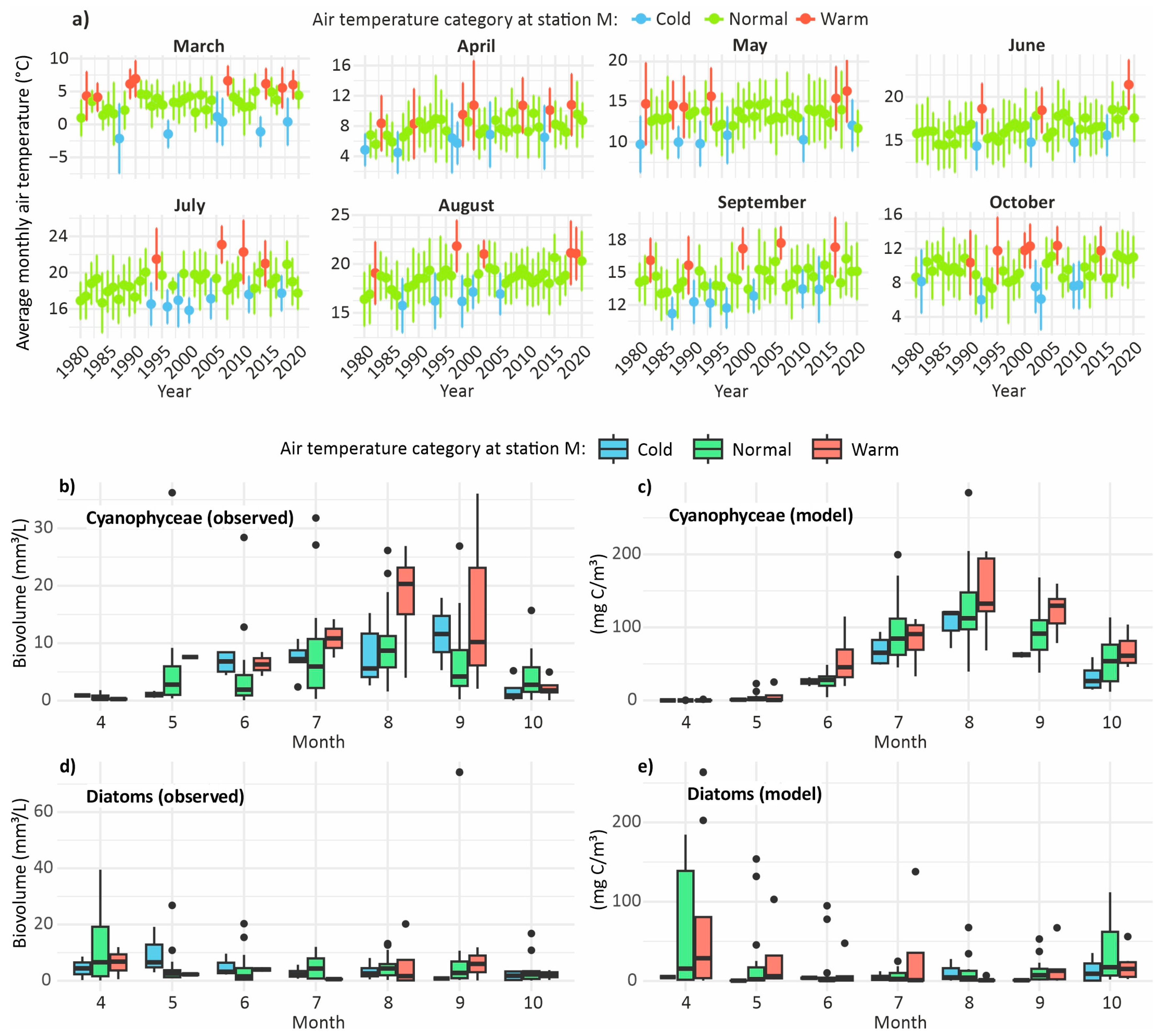
3.5. Phytoplankton in Hot and Cold Seasons
3.6. Cyanobacteria and Diatoms During Hot and Cold Months
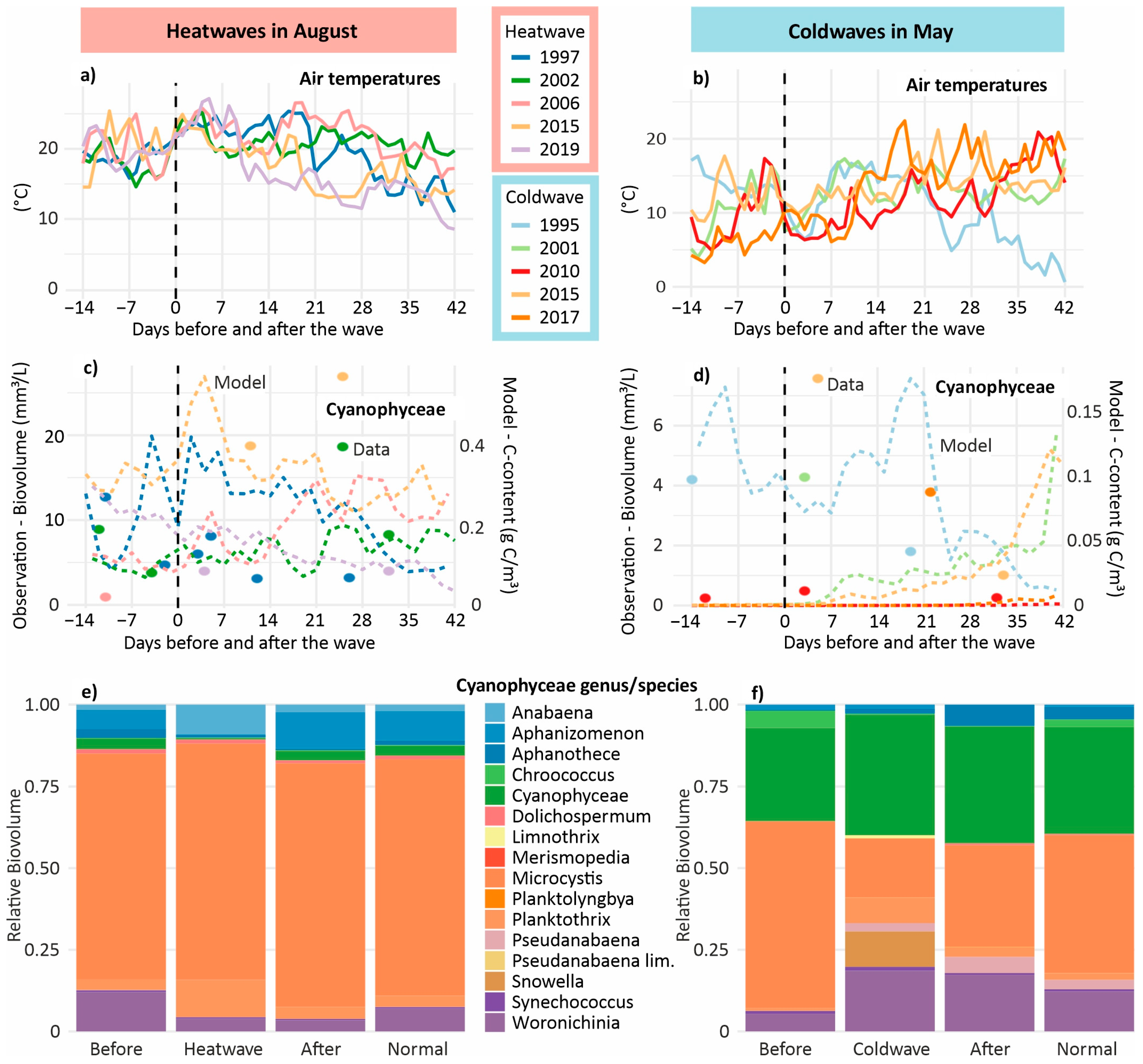
3.7. Phytoplankton During Heat and Cold Waves
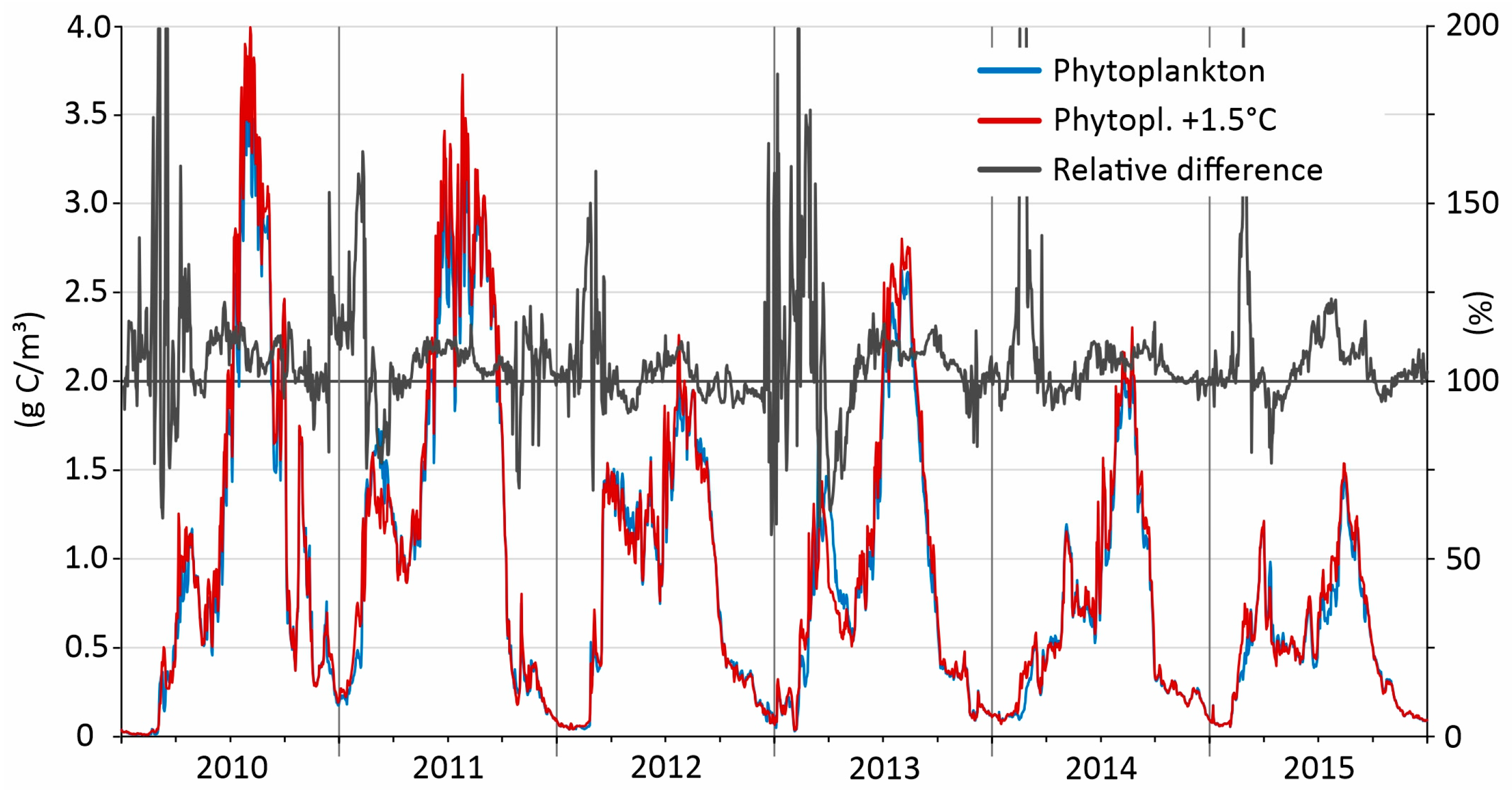
3.8. Phytoplankton Model Simulations—Effects of Increased Temperature
4. Discussion
4.1. Long-Term Development of the Western Oder Lagoon
4.2. Phytoplankton in the Oder Lagoon—A Comparison
4.3. Limitations of the Phytoplankton Data
4.4. The Model Approach—Opportunities and Weaknesses
4.5. Towards an Improved Monitoring of Phytoplankton
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meier, H.E.M.; Kniebusch, M.; Dieterich, C.; Gröger, M.; Zorita, E.; Elmgren, R.; Myrberg, K.; Ahola, M.P.; Bartosova, A.; Bonsdorff, E.; et al. Climate change in the Baltic Sea region: A summary. Earth Syst. Dyn. 2022, 13, 457–593. [Google Scholar] [CrossRef]
- Rutgersson, A.; Jaagus, J.; Schenk, F.; Stendel, M. Observed changes and variability of atmospheric parameters in the Baltic Sea region during the last 200 years. Clim. Res. 2014, 61, 177–190. [Google Scholar] [CrossRef]
- Kniebusch, M.; Meier, H.E.M.; Neumann, T.; Börgel, F. Temperature variability of the Baltic Sea since 1850 and attribution to atmospheric forcing variables. J. Geophys. Res. Oceans 2019, 124, 4168–4187. [Google Scholar] [CrossRef]
- Dutheil, C.; Meier, H.E.M.; Gröger, M.; Börgel, F. Understanding past and future sea surface temperature trends in the Baltic Sea. Clim. Dyn. 2021, 58, 3021–3039. [Google Scholar] [CrossRef]
- Siegel, H.; Gerth, M. Sea Surface Temperature in the Baltic Sea 2018. HELCOM Baltic Sea Environment Fact Sheets 2019; 7p. Available online: https://helcom.fi/wp-content/uploads/2020/07/BSEFS-Sea-Surface-Temperature-in-the-Baltic-Sea-2018.pdf (accessed on 17 February 2022).
- Stramska, M.; Białogrodzka, J. Spatial and temporal variability of sea surface temperature in the Baltic Sea based on 32 years (1982–2013) of satellite data. Oceanologia 2015, 57, 223–235. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid warming of large marine ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Meier, H.E.M.; Dieterich, C.; Gröger, M.; Dutheil, C.; Börgel, F.; Safonova, K.; Christensen, O.B.; Kjellström, E. Oceanographic regional climate projections for the Baltic Sea until 2100. Earth Syst. Dyn. 2022, 13, 159–199. [Google Scholar] [CrossRef]
- Gröger, M.; Arneborg, L.; Dieterich, C.; Höglund, A.; Meier, H.E.M. Summer hydrographic changes in the Baltic Sea, Kattegat and Skagerrak projected in an ensemble of climate scenarios downscaled with a coupled regional ocean–sea ice–atmosphere model. Clim. Dyn. 2019, 53, 5945–5966. [Google Scholar] [CrossRef]
- Gröger, M.; Dieterich, C.; Meier, H.E.M. Is interactive air–sea coupling relevant for simulating the future climate of Europe? Clim. Dyn. 2021, 56, 491–514. [Google Scholar] [CrossRef]
- Viitasalo, M.; Bonsdorff, E. Global climate change and the Baltic Sea ecosystem: Direct and indirect effects on species, communities and ecosystem function. Earth Syst. Dyn. 2022, 13, 711. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef]
- Hochfeld, I.; Hinners, J. Phytoplankton adaptation to steady or changing environments affects marine ecosystem functioning. Biogeosciences 2024, 21, 5591–5611. [Google Scholar] [CrossRef]
- Hayes, N.M.; Haig, H.A.; Simpson, G.L.; Leavitt, P.R. Effects of lake warming on the seasonal risk of toxic cyanobacteria exposure. Limnol. Oceanogr. Lett. 2020, 5, 10164. [Google Scholar] [CrossRef]
- Richardson, R.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Change Biol. 2020, 26, e14701. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; More, M. Cyanobacterial harmful algal bloom toxin microcystin and increased Vibrio occurrence as climate-change-induced biological co-stressors: Exposure and disease outcomes via their interaction with gut–liver–brain axis. Toxins 2023, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.C.; Newton, A.; Tett, P.; Fernandes, T.F. How will shallow coastal lagoons respond to climate change? A modelling investigation. Estuar. Coast. Shelf Sci. 2011, 95, 98–104. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. Categorizing and naming marine heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Galli, G.; Solidoro, C.; Lovato, T. Marine heat waves hazard 3D maps and the risk for low motility organisms in a warming Mediterranean Sea. Front. Mar. Sci. 2017, 4, 136. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jennings, E.; Shatwell, T.; Golub, M.; Pierson, D.C.; Maberly, S.C. Lake heatwaves under climate change. Nature 2021, 589, 402–407. [Google Scholar] [CrossRef]
- Benthuysen, J.A.; Oliver, E.C.J.; Chen, K.; Wernberg, T. Editorial: Advances in Understanding Marine Heatwaves. Front. Mar. Sci. 2020, 7, 147. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.G.; Sen Gupta, A.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.J.; Moore, P.J.; et al. Marine heatwaves. Annu. Rev. Mar. Sci. 2021, 13, 313–342. [Google Scholar] [CrossRef] [PubMed]
- Safonova, K.; Meier, H.E.M.; Gröger, M. Summer heatwaves on the Baltic Sea seabed contribute to oxygen deficiency in shallow areas. Commun. Earth Environ. 2024, 5, 106. [Google Scholar] [CrossRef]
- Humborg, C.; Geibel, M.C.; Sun, X.; McCrackin, M.; Mörth, C.-M.; Stranne, C.; Jakobsson, M.; Gustafsson, B.; Sokolov, A.; Norkko, A.; et al. High emissions of carbon dioxide and methane from the coastal Baltic Sea at the end of a summer heat wave. Front. Mar. Sci. 2019, 6, 493. [Google Scholar] [CrossRef]
- Bergkemper, V.; Weisse, T. Phytoplankton response to the summer 2015 heat wave—A case study from pre-alpine Lake Mondsee, Austria. Inland Waters 2017, 7, 88–99. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Zhang, Y.; Shi, K.; Qian, H.; Yang, H.; Niu, Y.; Qin, B.; Zhu, G.; Woolway, R.I.; et al. The unprecedented 2022 extreme summer heatwaves increased harmful cyanobacteria blooms. Sci. Total Environ. 2023, 896, 165312. [Google Scholar] [CrossRef]
- Jeppesen, E.; Pierson, D.; Jennings, E. Effect of extreme climate events on lake ecosystems. Water 2021, 13, 282. [Google Scholar] [CrossRef]
- Soulié, T.; Vidussi, F.; Mas, S.; Mostajir, B. Functional and structural responses of plankton communities toward consecutive experimental heatwaves in Mediterranean coastal waters. Sci. Rep. 2023, 13, 8050. [Google Scholar] [CrossRef]
- Chen, W.; Nielsen, A.; Andersen, T.K.; Hu, F.; Chou, Q.; Søndergaard, M.; Jeppesen, E.; Trolle, D. Modeling the ecological response of a temporarily summer-stratified lake to extreme heatwaves. Water 2020, 12, 94. [Google Scholar] [CrossRef]
- Rutgersson, A.; Kjellström, E.; Haapala, J.; Stendel, M.; Danilovich, I.; Drews, M.; Jylhä, K.; Kujala, P.; Larsén, X.G.; Halsnæs, K.; et al. Natural hazards and extreme events in the Baltic Sea region. Earth Syst. Dyn. 2022, 13, 251. [Google Scholar] [CrossRef]
- Aoki, L.R.; Brisbin, M.M.; Hounshell, A.G.; Kincaid, D.W.; Larson, E.I.; Sansom, B.J.; Shogren, A.J.; Smith, R.S.; Sullivan-Stack, J. Preparing aquatic research for an extreme future: Call for improved definitions and responsive, multidisciplinary approaches. BioScience 2022, 72, 508–520. [Google Scholar] [CrossRef]
- Diamond, D.; Relyea, R.A.; McCaul, M. Understanding and mitigating global change with aquatic autonomous sensors: A fifty-year perspective. Front. Sens. 2023, 4, 1284043. [Google Scholar] [CrossRef]
- LUNG. Monitoringprogramm zur Überwachung der Oberflächengewässer und des Grundwassers in Mecklenburg-Vorpommern im Zeitraum 2022–2027; Landesamt für Umwelt, Naturschutz und Geologie Mecklenburg-Vorpommern: Güstrow, Germany, 2024; Available online: https://www.lung.mv-regierung.de/static/LUNG/dateien/fachinformationen/wasser/Gewaesserueberwachung/monitoringprogramm_2022-2027.pdf (accessed on 15 April 2025).
- Filiz, N.; Işkın, U.; Beklioğlu, M.; Öğlü, B.; Cao, Y.; Davidson, T.A.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Phytoplankton community response to nutrients, temperatures, and a heat wave in shallow lakes: An experimental approach. Water 2020, 12, 3394. [Google Scholar] [CrossRef]
- Radziejewska, T.; Schernewski, G. The Szczecin (Oder-) Lagoon. In Ecology of Baltic Coastal Waters; Schiewer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 115–129. [Google Scholar] [CrossRef]
- Neumann, T.; Schernewski, G.; Friedland, R. Transformation Processes in the Oder Lagoon as Seen from a Model Perspective. EGUsphere 2025. [Google Scholar] [CrossRef]
- HELCOM. State of the Baltic Sea. Third HELCOM Holistic Assessment 2016–2021; Baltic Sea Environment Proceedings No. 194; HELCOM: Helsinki, Finland, 2023; Available online: https://helcom.fi/wp-content/uploads/2023/10/State-of-the-Baltic-Sea-2023.pdf (accessed on 15 April 2025).
- IKSO. Zweite Aktualisierung des Bewirtschaftungsplans für die Internationale Flussgebietseinheit; IKSO: Wrocław, Poland, 2022; Available online: http://www.mkoo.pl/download.php?fid=6996&lang=DE (accessed on 15 April 2025).
- Bundesregierung. Verordnung zum Schutz der Oberflächengewässer (Oberflächengewässerverordnung—OGewV); Federal Ministry of Justice and Consumer Protection: Berlin, Germany, 2016. Available online: https://www.gesetze-im-internet.de/ogewv_2016/OGewV.pdf (accessed on 15 April 2025).
- Grenzgewässerkommission. Bericht über die Beschaffenheit der Deutsch–Polnischen Grenzgewässer 2022; Wasser Blick: Loddin, Germany, 2024; Available online: https://www.wasserblick.net/servlet/is/1/ (accessed on 15 April 2025).
- Neumann, T.; Radtke, H.; Cahill, B.; Schmidt, M.; Rehder, G. Non-Redfieldian Carbon Model for the Baltic Sea (ERGOM Version 1.2)—Implementation and Budget Estimates. Geosci. Model Dev. 2022, 15, 8473–8540. [Google Scholar] [CrossRef]
- European Commission. Marine Biodiversity Modelling Study; Publications Office of the European Union: Luxembourg, 2022; Available online: https://data.europa.eu/doi/10.2777/213731 (accessed on 15 April 2025).
- Neumann, T. Model Code and Boundary Data for “Non-Redfield Carbon Model for the Baltic Sea (ERGOM Version 1.2)—Implementation and Budget Estimates” [Code]; Zenodo: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Geyer, B.; Rockel, B. coastDat-2 COSMO-CLM Atmospheric Reconstruction [Data Set]; World Data Center for Climate (WDCC): Hamburg, Germany, 2013. [Google Scholar] [CrossRef]
- Friedland, R.; Schernewski, G.; Gräwe, U.; Greipsland, I.; Palazzo, D.; Pastuszak, M. Managing Eutrophication in the Szczecin (Oder) Lagoon—Development, Present State and Future Perspectives. Front. Mar. Sci. 2019, 5, 521. [Google Scholar] [CrossRef]
- Bartoli, M.; Žilius, M.; Ruginis, T.; Vybernaite-Lubiene, I.; Petkuvienė, J.; Daunys, D. Seasonal Nitrogen Cycling Fuels Cyanobacteria Blooms in a Hypertrophic Lagoon. Front. Environ. Sci. 2024, 12, 1384114. [Google Scholar] [CrossRef]
- Pilkaitytė, R.; Razinkovas, A. Seasonal Changes in Phytoplankton Composition and Nutrient Limitation in a Shallow Baltic Lagoon. Boreal Environ. Res. 2007, 12, 551–559. [Google Scholar]
- Kownacka, J.; Krzymińska, J.; Jasser, I.; Grabowska, M.; Hutorowicz, A. Phytoplankton Structure in the Vistula Lagoon (Southern Baltic Sea) and the Turning Point in Its Development. Oceanologia 2020, 62, 313–324. [Google Scholar] [CrossRef]
- Saraiva, S.; Markus Meier, H.E.; Andersson, H.; Höglund, A.; Dieterich, C.; Gröger, M.; Hordoir, R.; Eilola, K. Baltic Sea ecosystem response to various nutrient load scenarios in present and future climates. Clim. Dyn. 2019, 52, 3369–3387. [Google Scholar] [CrossRef]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton Primary Production in the World’s Estuarine–Coastal Ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Schumann, R.; Schiewer, U.; Schubert, H. Phytoplankton Composition and Production during the Winter–Spring Transition in the Darss–Zingst Bodden Chain, Southern Baltic Sea. Estuar. Coast. Shelf Sci. 2005, 62, 169–181. [Google Scholar] [CrossRef]
- Schiewer, U. Darß-Zingst Boddens, Northern Rügener Boddens and Schlei. In Ecology of Baltic Coastal Waters; Schiewer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 35–86. [Google Scholar] [CrossRef]
- Poppeschi, C.; Charria, G.; Daniel, A.; Verney, R.; Rimmelin-Maury, P.; Retho, M.; Goberville, E.; Grossteffan, E.; Plus, M. Interannual variability of the initiation of the phytoplankton growing period in two French coastal ecosystems. Biogeosciences 2022, 19, 5667–5687. [Google Scholar] [CrossRef]
- Gasiūnaitė, Z.R.; Daunys, D.; Olenin, S.; Razinkovas, A. The Curonian Lagoon. In Ecology of Baltic Coastal Waters; Schiewer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 197–216. [Google Scholar] [CrossRef]
- Wasmund, N.; Tuimala, J.; Suikkanen, S.; Vandepitte, L.; Kraberg, A. Long-term trends in phytoplankton composition in the western and central Baltic Sea. J. Mar. Syst. 2011, 87, 145–159. [Google Scholar] [CrossRef]
- Overlingė, D.; Toruńska-Sitarz, A.; Cegłowska, M.; Błaszczyk, A.; Szubert, K.; Pilkaitytė, R.; Mazur-Marzec, H. Phytoplankton of the Curonian Lagoon as a New Interesting Source for Bioactive Natural Products—Special Impact on Cyanobacterial Metabolites. Biomolecules 2021, 11, 1139. [Google Scholar] [CrossRef]
- Vahtera, E.; Conley, D.J.; Gustafsson, B.G.; Kuosa, H.; Pitkänen, H.; Savchuk, O.P.; Tamminen, T.; Viitasalo, M.; Voss, M.; Wasmund, N.; et al. Internal Ecosystem Feedbacks Enhance Nitrogen-Fixing Cyanobacteria Blooms and Complicate Management in the Baltic Sea. AMBIO 2007, 36, 186–194. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Svedén, J.B.; Garbaras, A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen Fixation by Cyanobacteria Stimulates Production in Baltic Food Webs. AMBIO 2015, 44, 413–426. [Google Scholar] [CrossRef]
- Olofsson, M.; Klawonn, I.; Karlson, B. Nitrogen Fixation Estimates for the Baltic Sea Indicate High Rates for the Previously Overlooked Bothnian Sea. AMBIO 2021, 50, 203–214. [Google Scholar] [CrossRef]
- Žilius, M.; Vybernaite-Lubiene, I.; Vaiciute, D.; Overlingė, D.; Grinienė, E.; Zaiko, A.; Bonaglia, S.; Liskow, I.; Voss, M.; Andersson, A.; et al. Spatiotemporal Patterns of N2 Fixation in Coastal Waters Derived from Rate Measurements and Remote Sensing. Biogeosciences 2021, 18, 1857–1871. [Google Scholar] [CrossRef]
- Pimentel, J.S.M.; Giani, A. Microcystin Production and Regulation under Nutrient Stress in Cyanobacteria. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef]
- Tonk, L.; Visser, P.M.; Christiansen, G.; Dittmann, E.; Snelder, E.O.; Wiedner, C.; Mur, L.R.; Huisman, J. The Microcystin Composition of Planktothrix agardhii Changes with Light Intensity. Appl. Environ. Microbiol. 2005, 71, 553–560. [Google Scholar] [CrossRef]
- Silveira, S.B.; Odebrecht, C. Effects of Salinity and Temperature on Growth and Nodularin Production in Nodularia spumigena. Front. Mar. Sci. 2019, 6, 339. [Google Scholar] [CrossRef]
- Trombetta, T.; Vidussi, F.; Mas, S.; Parin, D.; Simier, M.; Mostajir, B. Water Temperature Drives Phytoplankton Blooms in Coastal Waters. PLoS ONE 2019, 14, e0214933. [Google Scholar] [CrossRef]
- Spilling, K.; Olli, K.; Lehtoranta, J.; Kremp, A.; Tamelander, T.; HELCOM Phytoplankton Group. Shifting Diatom–Dinoflagellate Dominance During Spring Bloom in the Baltic Sea. Front. Mar. Sci. 2018, 5, 327. [Google Scholar] [CrossRef]
- Almén, A.K.; Tiselius, P.; Rydberg, L. Temperature-Related Timing of the Spring Bloom: Contrasts between Cold and Warm Years. J. Sea Res. 2020, 160, 101887. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Ecological Studies 535; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-Model of Seasonal Succession of Planktonic Events in Fresh Waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Hašler, P.; Poulíčková, A. Diurnal Changes in Vertical Distribution and Morphology of a Natural Population of Planktothrix agardhii (Gom.) Anagnostidis et Komárek (Cyanobacteria). Hydrobiologia 2003, 506, 195–201. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Mur, L.R.; Walsby, A.E. Diurnal Changes in Buoyancy and Vertical Distribution in Populations of Microcystis in Two Shallow Lakes. J. Plankton Res. 1991, 13, 419–436. [Google Scholar] [CrossRef]
- Hall, N.S.; Whipple, A.C.; Paerl, H.W. Vertical Spatio-Temporal Patterns of Phytoplankton Due to Migration Behaviors in Two Shallow, Microtidal Estuaries: Influence on Phytoplankton Function and Structure. Estuar. Coast. Shelf Sci. 2015, 162, 7–21. [Google Scholar] [CrossRef]
- Cloern, J.E. Phytoplankton Bloom Dynamics in Coastal Ecosystems: A Review with Some General Lessons from Sustained Investigation of San Francisco Bay. Rev. Geophys. 1996, 34, 127–168. [Google Scholar] [CrossRef]
- Borics, G.; Abonyi, A.; Krasznai, E.; Várbíró, G.; Grigorszky, I.; Szabó, S.; Deák, C.; Tóthmérész, B. Small-Scale Patchiness of the Phytoplankton in a Lentic Oxbow. J. Plankton Res. 2011, 33, 973–981. [Google Scholar] [CrossRef]
- Jacobs, P.; Serre-Fredj, L.; Koeman, R.; van den Oever, A.; Peck, M.A.; Philippart, C.J.M. Impacts of Counting Protocols for Light Microscopy on Estimates of Biodiversity and Algal Density of Phytoplankton. Limnol. Oceanogr. Methods 2024, 22, 930–949. [Google Scholar] [CrossRef]
- Cozzoli, F.; Stanca, E.; Selmeczy, G.B.; Francé, J.; Varkitzi, I.; Basset, A. Sensitivity of Phytoplankton Metrics to Sample-Size: A Case Study on a Large Transitional Water Dataset (WISER). Ecol. Indic. 2017, 82, 558–573. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-Based Community Ecology of Phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Weithoff, G.; Beisner, B.E.; von Elert, E.; Reynolds, C.S.; Spaak, P. Measures and Approaches in Trait-Based Phytoplankton Community Ecology—From Freshwater to Marine Ecosystems. Front. Mar. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Kruk, C.; Huszar, V.L.M.; Peeters, E.T.H.M.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A Morphological Classification Capturing Functional Variation in Phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Felip, M.; Catalan, J. The Relationship Between Phytoplankton Biovolume and Chlorophyll in a Deep Oligotrophic Lake: Decoupling in Their Spatial and Temporal Maxima. J. Plankton Res. 2000, 22, 91–105. [Google Scholar] [CrossRef]
- Gilabert, J.; Socorro, A.; Ferriol, C.; Sanpera, C.; Abad, M. Short-Term Variability of the Planktonic Size Structure in a Mediterranean Hypersaline Coastal Lagoon (Mar Menor). J. Plankton Res. 2001, 23, 219–226. [Google Scholar] [CrossRef]
- Gui, J. Phytoplankton Carbon to Chlorophyll a Model Development: A Review and Perspective. Front. Mar. Sci. 2024, 11, 1466072. [Google Scholar] [CrossRef]
- Baylón, M.; Ramirez, J.L. A Comparison of eDNA Metabarcoding and Microscopy Techniques to Analyze Algal Diversity in Lake Titicaca, Peru. Diversity 2025, 17, 560. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Z.; Lin, K.; Lv, S.; Liu, Y.; Lu, W.; Liu, L. Comparative Analysis of Light Microscopy and High-Throughput Sequencing for Phytoplankton Detection in Rivers Flowing into the Sea. Water 2025, 17, 1559. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schernewski, G.; Schneider, M.; Neumann, T.; von Weber, M. Phytoplankton Dynamics in a Large Lagoon: Nutrient Load Reductions, Climate Change, and Cold- and Heatwaves. Environments 2025, 12, 370. https://doi.org/10.3390/environments12100370
Schernewski G, Schneider M, Neumann T, von Weber M. Phytoplankton Dynamics in a Large Lagoon: Nutrient Load Reductions, Climate Change, and Cold- and Heatwaves. Environments. 2025; 12(10):370. https://doi.org/10.3390/environments12100370
Chicago/Turabian StyleSchernewski, Gerald, Maria Schneider, Thomas Neumann, and Mario von Weber. 2025. "Phytoplankton Dynamics in a Large Lagoon: Nutrient Load Reductions, Climate Change, and Cold- and Heatwaves" Environments 12, no. 10: 370. https://doi.org/10.3390/environments12100370
APA StyleSchernewski, G., Schneider, M., Neumann, T., & von Weber, M. (2025). Phytoplankton Dynamics in a Large Lagoon: Nutrient Load Reductions, Climate Change, and Cold- and Heatwaves. Environments, 12(10), 370. https://doi.org/10.3390/environments12100370






