Abstract
Antarctica is experiencing one of the fastest warming rates globally, profoundly impacting seawater temperature and salinity, with direct consequences for marine life. The present study examined the combined effects of salinity fluctuations at 20, 33 (control salinity), and 41 psu, and temperatures of 2 °C (control temperature) and 8 °C (thermal stress) for 3 days, on the health and physiology of the Antarctic intertidal macroalga Adenocystis utricularis. Photosynthetic activity, photoinhibition, and photoprotective processes were assessed alongside biomarkers of oxidative stress/damage (total ROS, lipid peroxidation, and protein carbonylation) and antioxidant/osmotic response (ascorbate, free amino acids, and proline). The results showed that maximum quantum yield (Fv/Fm) remained stable under both salinity and thermal stress. However, productivity (ETRmax), the photoprotection index (NPQmax), and irradiance saturation (EkETR) were significantly decreased at 8 °C, remaining constant under salinity fluctuations. At 2 °C, oxidative stress and damage were significantly higher under hypo- and hypersalinity conditions. However, at 8 °C, oxidative stress indicators decreased, accompanied by increased ascorbate levels in both hypo- (20 psu) and hypersalinity (41 psu) treatments compared to the control salinity. While warming temperatures negatively altered the oxidative response of A. utricularis at a 33 psu, we report here an interactive effect between salinity and temperature, leading to an altered stress response to salinity fluctuations under thermal stress. This study provides key information to better understand the adaptation of Antarctic intertidal macroalgae to multifactor climate change consequences.
Keywords:
climate change; antarctica; photosynthesis; macroalgae; biomarkers; thermal stress; salinity stress 1. Introduction
In recent decades, global temperatures have risen by approximately 1.25 °C, a trend largely driven by industrialization and the large-scale emissions of greenhouse gases into the atmosphere. According to projections from the Intergovernmental Panel on Climate Change (IPCC) Sixth Assessment Report (AR6), global mean sea surface temperatures are expected to increase by +1.5 to +2 °C by mid-century, with the worst-case scenario predicting a rise of up to +6 °C by the end of the century [1,2]. Such dramatic warming poses significant threats to ecosystems worldwide, and one of the most alarming regions experiencing rapid temperature increases is Antarctica [3].
Global warming is leading to the accelerated melting of glaciers and ice sheets, which in turn, contributes to rising global sea levels, threatening coastal communities and ecosystems worldwide [4]. Glacial melting and seawater evaporation, triggered by temperature increase, lead to significant shifts in salinity within Antarctic intertidal zones, creating highly dynamic environments [5]. These salinity changes, alongside variations in turbidity and water temperature, form complex gradients that can critically impact the distribution, physiology, and survival of marine species [6].
Intertidal ecosystems are among the most vulnerable to climate change impacts [7]. Within these ecosystems, intertidal macroalgae serve as foundational species, contributing to primary productivity, enhancing habitat complexity, and supporting local biodiversity [8,9]. However, their capacity to survive and sustain these critical ecological functions is increasingly threatened by stressors such as salinity fluctuations. For example, climate change-driven salinity declines in the Baltic Sea are expected to shift macroalgae distributions, reduce genetic diversity, and favor green algae over red and brown algae, ultimately impacting ecosystem complexity and higher trophic levels [5]. This underscores the critical need to understand the response of Antarctic intertidal macroalgae to salinity shifts, enabling better predictions of climate change impacts on macroalgae ecosystem services and the biodiversity of this region.
One effective approach to studying the potential effects of abiotic stressors on macroalgae fitness is the use of physiological indicators and stress biomarkers [10]. These tools provide insight into the organism’s cellular response to specific environmental stressors. Abiotic factors, such as fluctuations in temperature and salinity, can trigger the production and accumulation of reactive oxygen species (ROS), leading to oxidative stress and physiological damage in primary producers [11]. ROS interact with a variety of cellular molecules, including membrane lipids (causing lipid peroxidation), proteins (leading to carbonylation), and DNA (resulting in mutations). However, the detrimental effects of ROS can be mitigated by antioxidant defense systems, which encompass both non-enzymatic and enzymatic mechanisms. Non-enzymatic antioxidants, such as glutathione and ascorbate, play a critical role in neutralizing ROS [12], while enzymatic antioxidants, including glutathione reductase (GR), ascorbate peroxidase (APX), and superoxide dismutase (SOD), are essential for scavenging ROS and reducing oxidative stress in many macroalgal species [13]. In addition to oxidative stress, fluctuations in salinity can also result in cell dehydration and a loss of turgor pressure, which further exacerbates stress conditions. To counteract these effects, primary producers, including macroalgae, employ osmoregulatory mechanisms, such as the synthesis of osmoprotectants (e.g., L-proline, L-arginine, and L-asparagine). These compounds help to maintain the osmotic balance and prevent excessive water loss, thus enhancing the organism’s ability to survive under fluctuating salinity conditions [14].
Previous studies have demonstrated the utility of biomarkers in assessing cellular responses of Antarctic macroalgae species, including Monostroma hariotii, Pyropia endiviifolia, and Adenocystis utricularis under heat stress (8 °C vs. 2 °C) over a 5-day period [15]. This study indicated that, even under warmer conditions, intertidal algae exhibited sufficient physiological and metabolic tolerance, which may enhance their survival potential [15]. Similarly, in the Arctic, the macroalga Palmaria palmata displayed relative tolerance to daily irradiance fluctuations and varying salinities (18 and 28 psu), measured by ecophysiological indicators (i.e., photosynthesis performance) [16]. However, its physiological response deteriorated progressively as salinity decreased, likely due to the increased energy demands associated with acclimatization to hyposaline conditions [16]. While a few studies have investigated the effects of elevated temperatures on macroalgae cellular responses [15,17], our understanding of the combined impacts of temperature and salinity stressors on Antarctic macroalgae species remains limited. Thus, a critical knowledge gap remains; while most studies have examined single-factor stressors, the interactive effects of temperature and salinity on Antarctic intertidal macroalgae are still poorly understood.
A. utricularis is a brown, pear-shaped seaweed that dominates the mid- and low-intertidal zones along the coast of King George Island, Antarctica, forming extensive meadows [9]. However, the rapid melting of the Collins Glacier [18,19], situated near the coastal zone, could pose a significant threat to the resilience and ecological fitness of their populations in the region. In this study, we investigated the effect of salinity fluctuations—driven by rising temperatures (seawater evaporation) and glacial melting in intertidal pools—under current (2 °C) and projected future (8 °C) sea surface temperature scenarios on the cellular responses of this seaweed. To achieve this, we conducted a laboratory experiment under controlled conditions, utilizing two different temperatures, 2 °C (control) and 8 °C, and three salinity levels, 20 (hyposaline), 33 (control), and 41 (hypersaline) psu, for 3 days. To evaluate the health status of A. utricularis, our study involved multidisciplinary experimental approaches, including photosynthesis indicators along with diagnostic biomarkers, such as parameters for oxidative stress, damage, antioxidant response, and osmolyte production.
2. Materials and Methods
2.1. Samples Collection and Culture Conditions
Samples of A. utricularis were collected in plastic containers from the intertidal zone of Punta Artigas, located in Bahía Fildes, King George Island, Antarctica (62°11′06.2″ S 58°52′42.9″ W) during the Antarctic field campaign ECA-59 (austral summer of 2023) (Figure 1). The experiment was carried out in the facilities of the Professor Julio Escudero Scientific Base belonging to the Chilean Antarctic Institute (INACH), located in Las Estrellas Village on King George Island. Once collected, algae fronds were washed twice with filtered artificial seawater (0.22 µm) and stored in plastic containers for 48 h at 2 °C under dark conditions and constant aeration for acclimation to laboratory conditions. Afterward, approximately ~300 mg of fresh biomass was transferred to 1 L containers with filtered seawater, considering three biological replicates. Salinity treatments consisted of 20 psu (hyposaline condition), 33 psu (control salinity), and 41 psu (hypersaline condition) [16,20]. The hypersaline condition was obtained by adding artificial sea salts (InstantOcean) to filtered seawater until reaching the desired salinity, whereas the hyposaline condition was prepared by diluting filtered seawater with autoclaved ultrapure water. Salinity, temperature, and pH were monitored using a multiparameter probe (Hanna Instruments HI98194; Rhode Island, USA). The experiments were carried out in thermoregulated baths at 2 °C (control temperature) and 8 °C (thermal stress), as described previously [15], with a photoperiod of 20:4 (light: dark), 90 µmol m2 s−1 photosynthetic active radiation (PAR), and constant aeration. After 3 days, the samples were collected and washed twice with filtered distilled water, frozen in liquid nitrogen, and preserved in a dryshipper container until their arrival at the HUB AMBIENTAL UPLA in Valparaíso, Chile, where they were stored at −80 °C until further analyses.

Figure 1.
(A) Collection of A. utricularis samples from an intertidal meadow located at Punta Artigas, Bahía Fildes, King George Island, Antarctica. (B) A. utricularis fronds.
2.2. Photosynthesis and Energy Dissipation as In Vivo Chlorophyll a Fluorescence
The measurement of in vivo chlorophyll a fluorescence from Photosystem II was conducted using a portable pulse-amplitude modulated fluorometer (Junior PAM, Walz GmbH, Effeltrich, Germany). Macroalgae tissues were incubated in 10 mL dark chambers to generate rapid light response curves for each experimental condition. After 15 min of dark adaptation, Fo and Fm values were recorded to calculate the maximum quantum efficiency (Fv/Fm), where Fv is obtained as Fm − Fo. In this context, Fo represents the baseline fluorescence of the thalli after dark adaptation, while Fm corresponds to the peak fluorescence following a high-intensity light pulse exceeding 4000 µmol m−2 s−1 for a few seconds [21]. The electron transport rate (ETR) was evaluated by exposing the samples for 20 s to a series of twelve increasing intensities of actinic white light using the Junior PAM fluorometer (Walz, Germany) [22]. The ETR was then calculated following the formula presented in [21]:
ETR (μmol electron m−2 s−1) = ΔF/F’m × E × A × FII
The effective quantum yield (ΔF/F’m) was defined as ΔF = Fm’ − Ft, where Ft represents the intrinsic fluorescence of the algae under light conditions, and Fm’ is the maximum fluorescence recorded after a saturation pulse in light-incubated algae. E corresponds to the incident PAR irradiance, expressed in µmol photons m−2 s−1, while A denotes the thallus absorptance, representing the fraction of incident light absorbed by the algae [23]. The fraction of chlorophyll associated with Photosystem II (PSII) within the 400–700 nm range, denoted as FII, is considered to be 0.8 in brown macroalgae [24]. The parameters related to electron transport rate (ETR), such as the maximum electron transport rate (ETRmax) and the initial slope of the ETR versus irradiance curve (αETR), which serves as an indicator of photosynthetic efficiency, were derived from the tangential function [25]. Additionally, the saturation irradiance for ETR (EkETR) was determined by identifying the intersection point between ETRmax and αETR. Lastly, non-photochemical quenching (NPQ), used as a measure of photoprotection, was computed following Equation (2) [22]:
NPQ = (Fm − Fm’)/Fm’
The maximum non-photochemical quenching (NPQmax) and the initial rate of increase in NPQ as a function of irradiance (αNPQ) were determined using the tangential function describing NPQ in relation to irradiance [25].
2.3. Total ROS Content
Total ROS levels were measured using the Intracellular ROS fluorometric kit (orange) provided by Sigma-Aldrich. Briefly, 30 mg of previously ground biomass was lysed with 300 µL of 0.5 M HCl, followed by vortexing for 10 min at room temperature. Subsequently, centrifugation was conducted for 5 min at 7500 rpm at 4 °C. Then, 100 µL of the supernatant was combined with 100 µL of 100 mM sodium phosphate buffer (pH 6.8) and 0.5 µL of the kit fluorophore. Additionally, a blank sample was prepared by mixing 100 µL of the supernatant with 100 µL of 100 mM sodium phosphate buffer (pH 6.8), together with an autofluorescence fluorophore blank prepared using 250 µL of 100 mM sodium phosphate buffer (pH 6.8) and 0.5 µL of the fluorophore. Relative fluorescence units (RFUs) were measured at 540 nm (excitation) and 570 nm (emission) in a fluorometric spectrophotometer, Cytation 5 (Agilent Biotek Instruments, Santa Clara, CA, USA). Results were expressed in relative fluorescence units (RFUs) per mg of fresh weight.
2.4. Quantification of Lipid Oxidative Damage
The quantification of thiobarbituric acid reactive substances (TBARSs) as an indicator of lipid peroxidation was conducted following the method described in [15]. Initially, 150 mg of biomass previously ground in liquid nitrogen was lysed with 200 µL of 10% TCA solution by adding four 3 mm glass beads and vortexed for 15 min at room temperature. After centrifugation at 14,000× g for 25 min at 4 °C, 100 µL of the supernatant was mixed with 100 µL of 0.5% TBA solution. The samples were incubated for 45 min at 95 °C in a thermoregulated bath, followed by cooling on ice for 5 min. The absorbance of the final mixture was measured at 532 nm using a SPECTROstarNano Microplate Reader (BMG-LABTECH). This measurement was conducted in a 96-well microplate, with 200 μL of the mixture per plate. The standard curve was constructed using a commercial malondialdehyde standard (MDA) (Sigma-Aldrich, Darmstadt, Germany).
2.5. Quantification of Protein Oxidative Damage
The quantification of carbonyl groups in proteins is a parameter to evaluate their oxidative damage by using the 2,4-dinitrophenylhydrazine (DNPH) method [26]. Briefly, 30 mg of fresh biomass was homogenized in liquid nitrogen and then mixed with 300 µL of FAPRB lysis buffer (Plant Total RNA Purification Mini Kit 50 preps FAVORGEN) and 300 µL of 0.5 M HCl, vortexed for 10 min at room temperature, and centrifuged at 10,000 g for 15 min at 4 °C. For total protein content, 5 µL of supernatant was mixed with 250 µL of Bradford reagent, incubated at 37 °C for 2 min for subsequent reading in the BMG-LABTECH SPECTROstar Nano spectrophotometer at 596 nm. For carbonylated proteins, 300 µL was rescued in a new tube, and 60 µL of 10 mM DNPH was added. After a 10 s vortex, the mixture was incubated in the dark for 30 min at room temperature. Then, 360 µL of 4 °C cooled 20% TCA was added and incubated for 15 min on ice. After vortexing for 10 s, the tube was centrifuged for 5 min at 10,000× g at 4 °C. The supernatant was carefully removed, and the pellet was washed with 1 mL of ethanol/ethyl acetate (1:1). After another round of vortexing for 10 s, the tube was centrifuged for 1 h at 10,000× g at 4 °C. Following removal of the supernatant, the pellet was inverted and air-dried for 20 min. Then, the pellet was resuspended in 500 µL of 6 M guanidine hydrochloride and incubated at 37 °C for 15 min. A 10 s vortex was performed halfway through the incubation period and again at the end. Finally, the sample was centrifuged at 10,000× g for 5 min at 4 °C. In a UV plate, 150 µL of the supernatant and 150 µL of 6 M guanidine (quantification of carbonylated proteins) were added. Measurements were performed in the SPECTROstarNano spectrophotometer (BMG Labtech, Ortenberg, Germany) at 366 nm. The values were replaced in the Lambert–Beer equation (cuvette length 0.8 cm and DNPH–carbonyl complex molar extinction coefficient 22,000 M−1 cm−1). The results are expressed as nmoles of carbonylated proteins in mg of total proteins.
2.6. Quantification of Free Amino Acids and Proline
The quantification of ninhydrin-positive substances (NPSs) was used as an approximation for the quantification of free amino acids and proline related to the osmotic stress response (osmolytes compatible with water) [26]. Briefly, 100 mg of previously ground biomass was homogenized with liquid nitrogen and lysed with 400 µL of 0.1 M HCl by adding four 3 mm glass beads and vortexing for 10 min at room temperature. Then, samples were centrifuged at 7000 rpm for 15 min at 4 °C. A volume of 350 µL of the supernatant was mixed with 350 µL 100 mM sodium citrate buffer, pH 4.8, and 290 µL of the previous mixture, and this was mixed with 10 µL of 2% ninhydrin. Then, samples were incubated at 95 °C for 20 min and allowed to cool to room temperature for 10 min. Finally, 150 µL of incubated sample was diluted with 150 µL of 100 mM sodium citrate buffer, pH 4.8, and measured in a spectrophotometer, SPECTROstarNano, at 560 nm for free amino acid content and 403 nm for proline content. The concentrations were established by comparison with a standard curve constructed with a commercial solution of free amino acids obtained from Sigma-Aldrich, and free amino acid levels are expressed in µmoles of free amino acids per mg of fresh weight.
2.7. Quantification of Ascorbate and Dehydroascorbate
The quantification of ascorbate in its reduced (ASC) and oxidized (DHA) forms was evaluated through the reduction in the Fe (III)-2,4,6-tripyridyl-S-triazine (TPTZ) complex [15]. Briefly, 100 mg of previously ground biomass was homogenized with liquid nitrogen and lysed with 300 µL of 0.1 M HCl, 300 µL of FAPRB lysis buffer (Plant Total RNA Purification Mini Kit 50 preps FAVORGEN), and four 3 mm glass beads, with vortexing for 10 min at room temperature. The samples were centrifuged at 17,800× g for 15 min at 4 °C. To measure reduced ascorbate (ASC), 10 µL of supernatant was mixed with 290 µL of the FRAP solution (250 mM sodium acetate buffer, pH 3.6, 0.83 mM TPTZ, and 1.7 mM FeCl3) and measured immediately at 593 nm. For total ascorbate (ASC + DHA), 250 µL of the lysed supernatant was mixed with 2.5 mL of 100 mM dithiothreitol (DTT) and incubated for one hour at room temperature. The reaction was stopped with 2.5 µL of 5% N-ethylmaleimide. Then, 10 µL of the reaction was mixed with 290 µL of the FRAP solution and measured at 593 nm. DHA was quantified by a simple subtraction of total ascorbate and reduced ascorbate. Concentrations were established by comparison with a calibration curve generated with commercial L-ascorbic acid obtained from Sigma-Aldrich as a standard. Results were expressed in µmoles of ascorbate per mg of fresh weight.
2.8. Statistical Analysis
Statistical analyses were performed in R version 4.3.0. Interactive effects between physiological variables (Fv/Fm, αETR, ETRmax, EkETR, and NPQmax) were analyzed using two-way ANOVA [27]. This test was performed for A. utricularis, including temperature (two levels) and salinity (three levels) as fixed factors for photosynthetic variables (n = 3), with a level of probability applied in the statistical analyses at p < 0.05. Homogeneity of variance was tested using Cochran tests and by visual inspection of the residuals. Student–Newman–Keuls tests (SNK) were performed after significant ANOVA interactions. For stress biomarkers, normality and homogeneity tests were evaluated using the Shapiro–Wilk and the Bartlett statistical tests, respectively. Two-way ANOVA with a confidence interval of 95%, together with a Tukey test, was carried out to establish specific statistical differences between treatments.
3. Results
3.1. Physiological Responses of A. utricularis to Salinity Fluctuations and Elevated Temperatures
Physiological responses in A. utricularis to temperature and salinity were assessed using photosynthetic parameters (Figure 2). The Fv/Fm ratio, as a photoinhibition index, showed no significant variation in response to changes in temperature or salinity (Figure 2A). The highest irradiance at the saturation point of the curve (EkETR) was significantly affected by temperature and salinity fluctuations compared to the control, whereas the other treatments showed no statistically significant differences among them (Figure 2B). Photosynthetic efficiency (αETR) increased significantly when exposed to 8 °C and under 20 and 33 psu (Figure 2C). The maximal electron transport rate (ETRmax) was significantly higher under control temperature conditions, regardless of salinity levels (Figure 2D). Finally, maximal non-photochemical quenching (NPQmax), as the photoprotection index, was significantly higher under control temperature conditions, irrespective of salinity (Figure 3).
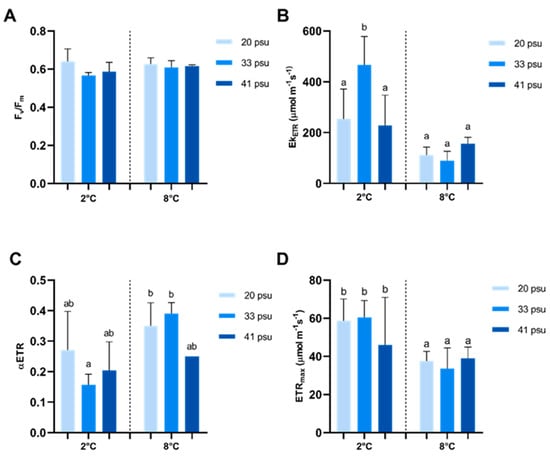
Figure 2.
Effect of temperature and salinity on photosynthetic performance in A. utricularis. (A) Maximum quantum yield, Fv/Fm; (B) irradiance of saturation, EkETR; (C) photosynthetic efficiency, αETR; and (D) maximal electron transport rate, ETRmax. The determination of photosynthetic parameters was conducted at 2 °C (control) and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters indicate significant differences between treatments (p < 0.00032).
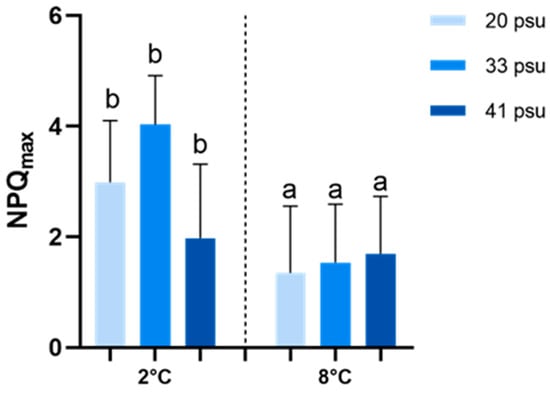
Figure 3.
Effect of temperature and salinity on maximal non-photochemical quenching (NPQmax) in A. utricularis. This parameter was determined at 2 °C and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters indicate significant differences between treatments (p < 0.00392).
3.2. Oxidative Stress and Damage in A. utricularis Under Salinity Fluctuations and Elevated Temperatures
The total ROS content in A. utricularis fronds varied significantly across different salinity conditions and between temperature scenarios (Figure 4). According to ANOVA results, salinity and temperature exhibited a highly significant interaction. At 2 °C, ROS content was significantly higher under hyposaline conditions compared to both the control and hypersaline conditions. In contrast, at 8 °C, ROS accumulation was significantly lower under hyposaline conditions than in the control and hypersaline conditions. Additionally, at 20 psu, ROS levels decreased at 8 °C compared to 2 °C, whereas at 33 psu and 41 psu, ROS levels increased. However, all salinity treatments at 8 °C showed higher ROS accumulation compared to the control (2 °C, 33 psu) (Figure 4).
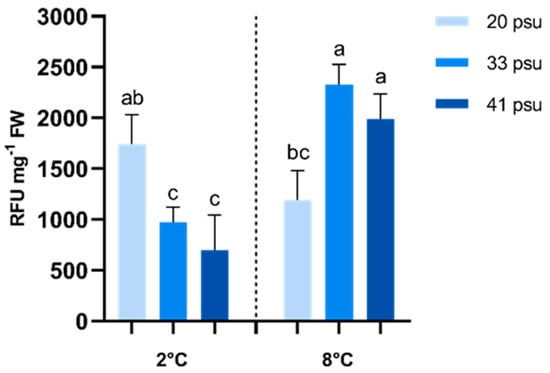
Figure 4.
Effect of temperature and salinity on total ROS content in A. utricularis. The determination of ROS was conducted at 2 °C (control) and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters represent significant differences between treatments (p < 0.0456).
Oxidative stress damage was assessed by measuring lipid peroxidation in cell membranes and protein oxidation levels; these two parameters responded differently to salinity and temperature (Figure 5). In the case of lipid peroxidation, salinity and temperature exhibited a highly significant interaction according to the two-way ANOVA analyses. At 2 °C, lipid peroxidation significantly increased under both hypo- and hypersaline conditions compared to the control salinity. Conversely, at 8 °C, hypo- and hypersaline conditions resulted in lower levels of lipid peroxidation compared to the control. Additionally, at 20 psu and 41 psu, TBARS levels decreased at 8 °C compared to 2 °C, whereas at 33 psu, these increased. However, all salinity treatments at 8 °C showed elevated TBARS levels compared to the control (2 °C, 33 psu), although this difference was not statistically significant in the 41 psu treatment (Figure 5A).
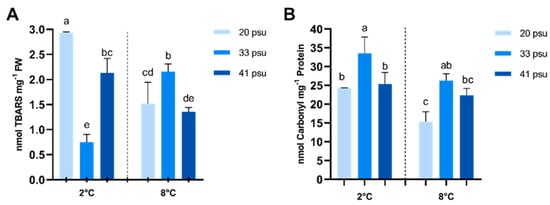
Figure 5.
Effect of temperature and salinity on oxidative damage to macromolecules in A. utricularis. (A) Quantification of peroxidized lipids (TBARS). (B) Quantification of protein carbonylation. The determination of oxidative damage parameters was conducted at 2 °C (control) and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters represent significant differences between treatments (p < 0.00046 and p < 0.00089).
Protein oxidation, measured as carbonylated proteins, followed a different pattern. Although both temperature and salinity showed a significant effect according to two-way ANOVA analyses, no significant interaction between salinity and temperature was observed. At 2 °C, lower levels of protein carbonylation were observed under hypo- and hypersaline conditions compared to the control. This trend persisted at 8 °C, where protein carbonylation remained elevated under control salinity conditions, although the difference was not significant with the 41 psu treatment. Additionally, all salinity treatments at 8 °C showed a tendency toward lower carbonylated protein levels compared to the control (2 °C, 33 psu), although not statistically significant in the 33 psu treatment (Figure 5B).
3.3. Osmotic and Antioxidant Responses in A. utricularis Under Salinity Fluctuations and High Temperatures
Osmolytes parameters, free amino acids (NPSs), and proline showed different responses to salinity and temperature fluctuations. According to the two-way ANOVA, salinity and temperature had no significant effect on NPS levels (Figure 6A). In the case of proline, however, salinity and temperature exhibited a highly significant interaction. At 2 °C, the hyposaline condition exhibited a tendency toward higher proline concentrations compared to both the control and hypersaline conditions, although not statistically significant. In contrast, at 8 °C, the hyposaline condition showed the lowest proline levels, but these differences were also not significant. Additionally, at 20 psu, proline levels significantly decreased at 8 °C compared to 2 °C, while at 33 psu and 41 psu, no significant differences in proline levels were observed across temperatures (Figure 6B).
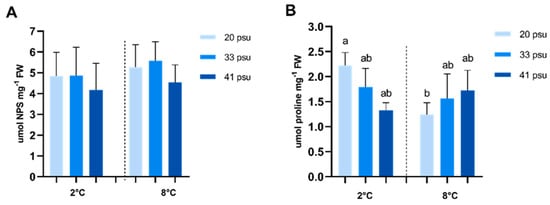
Figure 6.
Effect of temperature and salinity on free amino acids and proline content in A. utricularis. (A) Quantification of ninhydrin-positive substances (NPSs) to determine osmolytes compatible with water. (B) Proline quantification to determine response to salinity stress. Both parameters were determined at 2 °C (control) and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters represent significant differences between treatments (p < 0.000879 and p < 0.000776).
Antioxidant response was assessed by measuring ascorbate levels in both its reduced (ASC) and oxidized (DHA) forms (Figure 7). Total ascorbate levels (ASC + DHA) were significantly influenced by temperature, as indicated by two-way ANOVA, while no significant effects of salinity or interactions between salinity and temperature were observed. Total ascorbate levels were consistently lower at 8 °C compared to 2 °C within the same salinity treatments. Although these differences were not statistically significant, the only significant differences were observed between 20 psu at 2 °C and 33 psu at 8 °C. At both 2 °C and 8 °C, the highest total ascorbate levels were recorded under hyposaline conditions. DHA levels were also influenced by temperature, being consistently lower at 8 °C compared to 2 °C within the same salinity treatments. Although not statistically significant, a different trend was observed at 2 °C vs. 8 °C. At 2 °C, total ascorbate levels were higher under low salinity conditions (20 psu), whereas at 8 °C, higher values were obtained at 20 and 41 psu. Finally, the ASC/DHA ratio was higher at 8 °C, indicating an efficient reduction in ascorbate at this temperature.
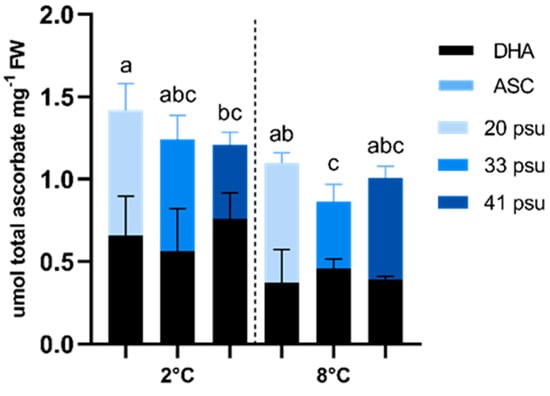
Figure 7.
Effect of temperature and salinity on ascorbate (ASC in blue) and dehydroascorbate (DHA in black) in A. utricularis. Both parameters were determined at 2 °C (control) and 8 °C, at three salinity levels: 20 (hyposaline), 33 (control), and 41 psu (hypersaline). The data are presented as mean ± SD, with n = 3. Lowercase letters represent significant differences between treatments (p < 0.00073).
4. Discussion
Despite the ecological significance of Antarctic intertidal macroalgae, cellular and molecular studies investigating their responses to salinity fluctuations and rising temperatures remain scarce. This knowledge gap limits our understanding of the mechanisms underlying their physiological and biochemical adaptations to the rapidly changing environmental conditions driven by climate change. In this study, we conducted a controlled short-term experiment to understand the cellular responses of an ecologically relevant macroalgae species of the intertidal zone to salinity and thermal stresses.
4.1. Photosynthetic Performance in A. utricularis Under Salinity Fluctuations and Elevated Temperatures
Photosynthetic efficiency in A. utricularis remained relatively high across all treatments, with Fv/Fm values around 0.6 [28], showing no significant differences. This indicates an ability to tolerate short-term temperature and salinity fluctuations. The lack of temperature effects aligns with findings of the authors of [15], who reported similar results for this species, exposed to 8 °C over the same period.
Regarding other photosynthetic parameters, the ecophysiological responses appear to be modulated only by temperature. Photosynthetic parameters did not show significant differences across the three salinity treatments. However, ETRmax, a proxy for productivity, and NPQmax, an indicator of photoprotection, decreased under elevated temperature, in contrast to what was reported in [15]. This decline may be driven by various biological mechanisms, such as pigment content, and should be addressed in further research. The NPQmax results suggest that heat dissipation could be an important mechanism in this species, alongside the use of the xanthophyll cycle as a photoprotection strategy [17]. In general, based on photosynthetic performance and ecophysiological parameters, the intertidal macroalgae A. utricularis exhibits biological viability and reduced vulnerability under increased temperature conditions, highlighting its potential adaptation to thermal and salinity stress.
4.2. Oxidative Stress and Damage in A. utricularis Under Salinity Fluctuations and Elevated Temperatures
Both temperature and salinity are well-known triggers of oxidative stress in marine photoautotrophs. Temperature increases beyond critical thresholds induce oxidative stress in these species, significantly affecting marine organisms in polar ecosystems [29]. Similarly, salinity fluctuations can cause oxidative stress in macroalgae [30,31]. Although the specific effects of temperature and salinity interactions on oxidative stress responses in macroalgae remain unexplored, evidence from Arctic macroalgae has shown that such interactions can influence their physiological and biochemical status [32], suggesting that oxidative stress responses could also be impacted.
Our study identified significant interactions between salinity and temperature across the responses of various biomarkers in A. utricularis, including ROS and TBARS, where temperature strongly influenced the species’ response to salinity. At the control temperature (2 °C), the species exhibited higher stress under hyposaline conditions compared to the control salinity (33 psu), as evidenced by higher ROS and TBARS levels, whereas at 8 °C, the opposite trend was observed. This suggests that the studied species may have a greater capacity to withstand short-term hyposaline stress under future scenarios of elevated sea temperatures, while also highlighting its vulnerability to hyposaline conditions under current temperature regimes. Our findings also suggest that salinity could influence the species’ ability to cope with thermal stress. At 20 psu, ROS and TBARS levels were lower under future climate scenario temperatures compared to current conditions, while at control salinity (33 psu), these levels significantly increased. Therefore, under hyposaline conditions, the species may exhibit a higher capacity to adapt to higher temperatures. Effects of salinity on modifying resilience to other abiotic stresses have been observed in other species like Palmaria palmata, which demonstrated significant tolerance to daily irradiance fluctuations, though this resilience diminished under hyposaline conditions [16]. Taken together, our findings and previous studies highlight the critical importance of investigating the combined effects of multiple stressors on marine primary producers.
Despite the interaction effects mentioned above, it is important to highlight that, after the short-term exposure period studied (3 days), the species consistently exhibited higher ROS and TBARS levels, along with lower total ascorbate and DHA levels, at elevated temperatures (20 psu, 33 psu, and 41 psu, at 8 °C) when compared to the control conditions (33 psu at 2 °C). This could suggest a potentially reduced fitness of A. utricularis under future climate change scenarios, regardless of salinity conditions. The observed short-term effects of temperature are consistent with previous studies on this species, which reported a slight increase in oxidative stress indicators, such as hydrogen peroxide (H2O2) and TBARS, during short-term exposure to elevated temperatures (3 days at 8 °C). However, these oxidative stress parameters were found to decrease or return to control levels during mid-term exposure (5 days), indicating the presence of adaptive responses to thermal stress [15].
4.3. Osmotic and Antioxidant Responses in A. utricularis Under Salinity Fluctuations and High Temperatures
Osmolytes, such as proline, despite their role related to the response to hypersaline stress, are also key indicators of antioxidant capacity in response to other abiotic stressors, enhancing the co-accumulation of antioxidant molecules, bolstering resistance to oxidative stress [33,34]. In A. utricularis, our results show an interactive effect of temperature and salinity on proline levels. These peaked under hyposaline conditions at 2 °C and also reached higher values when exposed to hypersaline conditions at 8 °C. These results suggest that, under current low-temperature conditions, proline may function primarily as an antioxidant molecule. However, in future warmer scenarios, it appears to shift its role to its canonical function as an osmolyte, helping to prevent dehydration at hypersaline conditions. Under low temperatures, the increase in proline coincided with elevated ROS and TBARS levels under hyposaline conditions, supporting its potential antioxidant role in redox buffering and membrane stabilization. Under warming, the strongest proline accumulation observed at hypersalinity is consistent with its canonical osmoprotective function in mitigating dehydration stress [33,34,35]. This context-dependent shift aligns with previous evidence indicating that proline can act both as a stress signal and a protective metabolite when organisms are exposed to combined abiotic stressors [35].
The ascorbate–glutathione cycle also plays a pivotal role in antioxidant defense mechanisms in macroalgae, mirroring processes observed in terrestrial plants [36]. Our results indicate that temperature and salinity have an interactive effect on ascorbate levels. ASC accumulated under both hypo- and hypersalinity conditions at 8 °C. In contrast, at 2 °C, ascorbate showed either no significant differences or was lower under hypo- and hypersalinity conditions compared to the control, respectively. Additionally, the ascorbate-to-dehydroascorbate ratio (ASC/DHA) showed a similar trend, suggesting efficient maintenance of reducing power within the cycle to withstand high temperatures. This indicates that the antioxidant response of A. antioxidant response to hypo- and hypersalinity would be better at higher temperatures. Therefore, A. utricularis may efficiently cope with salinity changes under a climate change scenario, highlighting the capacity of macroalgae to optimize antioxidant responses under multiple stressors. Data from [15] suggest that these mechanisms allow Antarctic macroalgae to maintain adequate metabolite levels to counteract ROS induced by temperature variations. Similarly, the Arctic marine macroalga Polysiphonia arctica demonstrated an enhanced enzymatic activity, particularly APX and catalase (CAT), and production of novel antioxidant compounds, which contributed to their resilience against environmental stressors [37]. Additionally, the tropical red alga Hypnea musciformis also exhibited elevated glutathione (GSH) and ASC synthesis at higher annual temperatures. This robust antioxidant system enabled the macroalgae to tolerate oxidative stress fluctuations characteristic of intertidal habitats during seasonal changes [38].
Despite the better adaptability to salinity shown at higher temperatures in A. utricularis, it is important to highlight that, after the short-term exposure period studied (3 days), the species presented lower total ascorbate levels, at elevated temperatures, when compared to the control conditions (33 psu at 2 °C). This could suggest a potentially reduced fitness of A. utricularis under future climate change scenarios, regardless of salinity conditions. However, under salinity fluctuations at 8 °C, total ascorbate levels were slightly higher compared to control salinity, suggesting that the combined stress conditions triggered ascorbate accumulation. Indeed, it has been evidenced that a combination of abiotic stresses, such as salinity, heavy metals, and desiccation, among others, could enhance the biosynthesis of metabolites and promote macroalgae adaptability against stressful conditions [11].
Although these results are valuable for understanding A. utricularis’s short-term response to thermal and salinity stresses, it is important to mention that ecosystem resilience cannot be predicted solely by examining individual species’ responses, as interspecies interactions may enhance the overall resilience of an ecological system. For example, Arctic mixed kelp communities have shown remarkable short-term tolerance to multiple climate stressors, including warming, freshening, and reduced light availability [7]. Although light availability remains the primary factor affecting net community productivity, the combined effects of these stressors did not significantly impact productivity or photosynthetic efficiency in short-term experiments [7]. Interestingly, these results contrast with previous studies on individual species, which reported significant sensitivity to environmental stressors [39,40,41]. Therefore, the antioxidant responses of macroalgae are diverse and dynamic, tailored to specific ecological niches and stressors, resulting in a complex spectrum of tolerance and adaptation among species.
Increasing the rate of exploration and study of Antarctic macroalgae is crucial, as these species play key ecological roles and may hold unique biochemical adaptations. Expanding our knowledge in this region will enhance understanding of ecosystem functioning under changing environmental conditions and provide valuable insights into biodiversity and resilience in polar marine systems.
5. Conclusions
The ability of macroalgae such as A. utricularis to activate stress response mechanisms under multiple environmental stressors highlights their potential for adaptation to fluctuating conditions. Under current temperature conditions, this species exhibits heightened oxidative stress and damage in hyposaline environments. However, this pattern reverses under higher temperature scenarios, where the species demonstrates higher tolerance to hyposaline conditions. Notably, this species experiences the least oxidative stress under control conditions (2 °C at 33 psu) compared to any other scenario studied. This observation suggests that A. utricularis may be less suited to the elevated sea temperatures expected with climate change, regardless of salinity levels. Nevertheless, within a warmer temperature scenario, this species appears better adapted to salinity fluctuations (i.e., hyposaline environment) than to control (33 psu), potentially driving shifts in its distribution and relative abundance toward lower-salinity regions. These findings indicate possible ecological implications, but this hypothesis requires further validation through long-term experimental studies. Understanding how these organisms adapt to interactive stressors, including warming, freshening, salinity changes, and UVR, will be essential for predicting the resilience of polar ecosystems. Additionally, integrating molecular, biochemical, and ecological approaches can provide a comprehensive understanding of the mechanisms driving adaptation and resilience in these vital ecosystems.
Author Contributions
F.M.: Methodology, formal analysis, data curation, visualization, writing—original draft preparation. P.T.M.: Methodology, investigation, formal analysis, writing—review and editing. A.U.: Methodology, visualization, formal analysis, writing—review and editing, funding acquisition. P.S.M.C.-P.: formal analysis, methodology, visualization, writing—review and editing. C.R.: formal analysis, validation, writing—review and editing. P.A.-M.: formal analysis, methodology, writing—review and editing. V.M.: Conceptualization, methodology, visualization, writing—review and editing. N.P.N.: Conceptualization, methodology, formal analysis, writing—review and editing. C.A.S.: writing—review and editing, resources. C.L.: conceptualization, validation, writing—review and editing. C.T.: Methodology, formal analysis. B.C.: Methodology, formal analysis, visualization, writing—review and editing. K.G.-P.: Methodology, formal analysis, visualization, writing—review and editing. G.B.P.-H.: Formal analysis, supervision, conceptualization. F.R.-R.: Conceptualization, resources, methodology, visualization, supervision, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the INACH RT_30-21 grant from the Instituto Antártico Chileno, Chile. VM was funded by Fondecyt Regular N°1211977 and COPAS COASTAL ANID FB210021. CL was funded by the projects ANID InES I + D 2021, grant number INID210013, and the Marie Curie Postdoctoral Fellowship HORIZON-MSCA-2022-PF-01 project, grant number 101106387, and CL acknowledges the Severo Ochoa Centre of Excellence accreditation (CEX2019-000928-S), funded by AEI 10.13039/501100011033. BC acknowledges Fondecyt Regular N°1221264 and ANID-Milenio-NCN2023_054. KG was financed by the UPLA inner doctoral scholarship UPA22991. Data obtained for total ROS quantification were granted by Cytation 5 equipment from the Fondequip EQM160131 project of the University of Playa Ancha, Chile.
Data Availability Statement
The data that support the results of this study can be obtained from the corresponding author upon request.
Acknowledgments
During the preparation of this manuscript/study, the author(s) used ChatGPT4 to improve the readability and language of the text. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Convey, P.; Peck, L.S. Antarctic environmental change and biological responses. Sci. Adv. 2019, 5, eaaz0888. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Siegert, M.; Atkinson, A.; Banwell, A.; Brandon, M.; Convey, P.; Davies, B.; Downie, R.; Edwards, T.; Hubbard, B.; Marshall, G.; et al. The Antarctic Peninsula under a 1.5 °C global warming scenario. Front. Environ. Sci. 2019, 7, 102. [Google Scholar] [CrossRef]
- Hill, E.A.; Gudmundsson, G.H.; Chandler, D.M. Ocean warming as a trigger for irreversible retreat of the Antarctic ice sheet. Nat. Clim. Chang 2024, 14, 1165–1171. [Google Scholar] [CrossRef]
- Röthig, T.; Trevathan-Tackett, S.M.; Voolstra, C.R.; Ross, C.; Chaffron, S.; Durack, P.J.; Warmuth, L.M.; Sweet, M. Human-induced salinity changes impact marine organisms and ecosystems. Glob. Change Biol. 2023, 29, 4731–4749. [Google Scholar] [CrossRef]
- Umbert, M.; De Andrés, E.; Sánchez, M.; Gabarró, C.; Hoareau, N.; González-Gambau, V.; García-Espriu, A.; Olmedo, E.; Raj, R.P.; Xie, J.; et al. Contribution of satellite sea surface salinity to the estimation of liquid freshwater content in the Beaufort Sea. Ocean Sci. 2024, 20, 279–291. [Google Scholar] [CrossRef]
- Miller, C.A.; Gazeau, F.; Lebrun, A.; Gattuso, J.P.; Alliouane, S.; Urrutti, P.; Schlegel, R.W.; Comeau, S. Productivity of mixed kelp communities in an Arctic fjord exhibit tolerance to a future climate. Sci. Total Environ. 2024, 930, 172571. [Google Scholar] [CrossRef] [PubMed]
- Watt, C.A.; Scrosati, R.A. Seaweeds as ecosystem engineers. Bull. Ecol. Soc. Am. 2014, 95, 250–251. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Antarctic Seaweeds: Diversity, Adaptation and Ecosystem Services, Adaptation and Ecosystem Services; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Kaviraj, A.; Unlu, E.; Gupta, A.; El Nemr, A. Biomarkers of environmental pollutants. BioMed Res. Int. 2014, 2014, 806598. [Google Scholar] [CrossRef]
- Kaur, M.; Saini, K.C.; Ojah, H.; Sahoo, R.; Gupta, K.; Kumar, A.; Bast, F. Abiotic stress in algae: Response, signaling and transgenic approaches. J. Appl. Phycol. 2022, 34, 1843–1869. [Google Scholar] [CrossRef]
- Moenne, A.; González, A.; Sáez, C.A. Mechanisms of metal tolerance in marine macroalgae, with emphasis on copper tolerance in Chlorophyta and Rhodophyta. Aquat. Toxicol. 2016, 176, 30–37. [Google Scholar] [CrossRef]
- Ren, C.G.; Liu, Z.Y.; Wang, X.L.; Qin, S. The seaweed holobiont: From microecology to biotechnological applications. Microb. Biotechnol. 2022, 15, 738–754. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Moenne, F.; Rodríguez-Rojas, F.; Pardo, D.; Lavergne, C.; Moenne, A.; Brown, M.T.; Huovinen, P.; Gómez, I.; Navarro, N.; et al. Antarctic intertidal macroalgae under predicted increased temperatures mediated by global climate change: Would they cope? Sci. Total Environ. 2020, 740, 140379. [Google Scholar] [CrossRef] [PubMed]
- Marambio, J.; Rosenfeld, S.; Bischof, K. Hyposalinity affects diurnal photoacclimation patterns in the rhodophyte Palmaria palmata under mimicked Arctic summer conditions. J. Photochem. Photobiol. 2022, 11, 100124. [Google Scholar] [CrossRef]
- Sáez, C.A.; Troncoso, M.; Navarrete, C.; Rodríguez-Rojas, F.; Navarro, N.; Trabal, A.; Lavergne, C.; Pardo, D.; Brown, M.T.; Gómez, I.; et al. Photoprotective responses of three intertidal Antarctic macroalgae to short-term temperature stress. Front. Mar. Sci. 2023, 10, 1223853. [Google Scholar] [CrossRef]
- Piccini, C.; Bertoglio, F.; Sommaruga, R.; Martínez de La Escalera, G.; Pérez, L.; Bugoni, L.; Bergamino, L.; Evangelista, H.; García-Rodríguez, F. Prokaryotic richness and diversity increased during Holocene glacier retreat and onset of an Antarctic Lake. Commun. Earth Environ. 2024, 5, 94. [Google Scholar] [CrossRef]
- Parodi, C.; Cerpa, L.; Zhu, Z.; Zhang, J.; Muniz, P.; Venturini, N. Tracking suspended particulate organic matter biochemistry from glacial meltwater runoff to coastal waters of an Antarctic fjord. Mar. Chem. 2024, 267, 104455. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Hwang, J.Y.; Kim, Y.K.; Kim, T.; Kim, I.N.; Kang, S.; Kim, J.H.; Rhee, J.S. Physiological and molecular responses of Antarctic harpacticoid copepod Tigriopus kingsejogensis to salinity fluctuations—A multiregional study. Environ. Res. 2022, 204, 112075. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence As a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, J.M.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Conde-Alvarez, R.; Gómez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light. intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Pérez-Hernández, G.; Morales, D.; Pereira-Rojas, J.; Díaz, M.J.; Blanco-Murillo, F.; Sola, I.; Rámila, C.; González, C.; González, K.; Sánchez-Lizaso, J.L.; et al. The halotolerant white sea anemone Anthothoe chilensis, highly abundant in brine discharges zones, as a promising biomonitoring species for evaluating the impacts of desalination plants. Desalination 2024, 581, 117612. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar] [CrossRef]
- Rothäusler, E.; Rugiu, L.; Jormalainen, V. Forecast climate change conditions sustain growth and physiology but hamper reproduction in range-margin populations of a foundation rockweed species. Mar. Environ. Res. 2018, 141, 205–213. [Google Scholar] [CrossRef]
- Gordillo, F.J.; Carmona, R.; Jiménez, C. A Warmer Arctic Compromises Winter Survival of Habitat-Forming Seaweeds. Front. Mar. Sci. 2022, 8, 750209. [Google Scholar] [CrossRef]
- Luo, M.B.; Liu, F. Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. J. Exp. Mar. Biol. Ecol. 2011, 409, 223–228. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, F.; López-Marras, A.; Celis-Plá, P.S.; Muñoz, P.; García-Bartolomei, E.; Valenzuela, F.; Sáez, C.A. Ecophysiological and cellular stress responses in the cosmopolitan brown macroalga Ectocarpus spp. as biomonitoring tools for assessing desalination brine impacts. Desalination 2020, 489, 114527. [Google Scholar] [CrossRef]
- Diehl, N.; Karsten, U.; Bischof, K. Impacts of combined temperature and salinity stress on the endemic Arctic brown seaweed Laminaria solidungula J. Agardh. Polar Biol. 2020, 43, 647–656. [Google Scholar] [CrossRef]
- Barera, S.; Forlani, G. The role of proline in the adaptation of eukaryotic microalgae to environmental stress: An underestimated tool for the optimization of algal growth. J. Appl. Phycol. 2023, 35, 1635–1648. [Google Scholar] [CrossRef]
- Xing, H.; Li, Q.; Zhao, Y.; Gao, H.; Li, L.; Zhang, Y.; Yu, X. Exogenous proline boosts the co-accumulation of astaxanthin and biomass in stress-induced Haematococcus pluvialis. Bioresour. Technol. 2023, 369, 128488. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Dummermuth, A. Antioxidative properties of marine macroalgae from the Arctic Antioxidative Eigenschaften mariner Makroalgen der Arktis. Ber. Polarforsch. Meeresforsch. Rep. Polar Mar. Res. 2003, 458, 9–11. [Google Scholar]
- Maharana, D.; Das, P.B.; Verlecar, X.N.; Pise, N.M.; Gauns, M. Oxidative stress tolerance in intertidal red seaweed Hypnea musciformis (Wulfen) in relation to environmental components. Environ. Sci. Pollut. Res. 2015, 22, 18741–18749. [Google Scholar] [CrossRef] [PubMed]
- Filbee-Dexter, K.; Wernberg, T.; Grace, S.P.; Thormar, J.; Fredriksen, S.; Narvaez, C.N.; Feehan, C.J.; Norderhaug, K.M. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Sci. Rep. 2020, 10, 13388. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, A.; Comeau, S.; Gazeau, F.; Gattuso, J.P. Impact of climate change on Arctic macroalgal communities. Glob. Planet. Change 2022, 219, 103980. [Google Scholar] [CrossRef]
- Niedzwiedz, S.; Bischof, K. Glacial retreat and rising temperatures are limiting the expansion of temperate kelp species in the future Arctic. Limnol. Oceanogr. 2023, 68, 816–830. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).