Honeybees and the One Health Approach
Abstract
1. Introduction
2. Bees’ Contributions
2.1. Ecosystem Services of Bees
2.2. Bees and Food Security
2.3. Bee Products and Human Health
| Type | Application | Effects | Reference |
|---|---|---|---|
| In vitro | Antitumoral agent | 60 kDa protein in Pakistani Sidr honey inhibited angiogenesis in umbilical vein endothelial cells, suggesting its use as a cancer treatment. | [43] |
| Sidr honey has shown antiproliferation activity in cancer cells due to aggregation in G1 phase, increase in apoptosis and necrotic cell death, showing its potential use as an antitumoral agent. | [44] | ||
| Thyme and chestnut honey had little to no effect on the apoptosis of human cancer cells, which was increased through its mixture (10%) with royal jelly or propolis, suggesting its use as a supplement to conventional cancer treatments. | [45] | ||
| In vitro | Antimicrobial agent | Stingless bee honey (Hymenoptera, Apidae, Meliponini) has antimicrobial activity against Gram-positive, Gram-negative bacteria and fungi, with some cases showing stronger activity than the standard antibiotic (ciprofloxacin). | [46] |
| Honey has antimicrobial activity, but it may vary depending on botanical origins and season. | [47] | ||
| Latvian monofloral honey presented antimicrobial activity, higher against Gram-positive than Gram-negative bacteria, with some cases exceeding Manuka honey’s inhibition. | [48] | ||
| Pre-exposure to Sumra and Sidr honey increase antibiotic sensitivity of bacteria and reduced biofilm formation. | [49] | ||
| Manuka honey (rich in methylglyoxal) has a broad-spectrum antimicrobial activity, with both varieties inhibiting bacterial growth but only one having bactericidal and antibiofilm properties. | [50] | ||
| Castanea crenata honey treatment in vitro prevented influenza virus infection in mouse macrophages by inhibiting the expression of viral proteins and increasing the expression in proinflammatory cytokines, while in vivo increase survival, reduced body weight loss, decreased viral replication, reduced inflammatory response, stimulated antiviral response, and prevented infection, presenting protective effects on influenza virus infection in mice. | [51] | ||
| In vitro | Anti-inflammatory agent | Manuka honey natural pteridine derivative 3,6,7-trimethyllumazine (Lepteridine™) shows partial inhibition of a metalloproteinase (MMP) involved in non-healing chronic wounds through a dysregulated proteolytic activity (MMP-9); this activity is not lost during simulated gastrointestinal digestion, which may explain the beneficial anti-inflammatory effects of oral consumption or topical applications. | [52] |
| Honey applied to in vitro cultures of canine, equine, and chicken peripheral blood lymphocytes stimulate proliferation (i.e., moderate stimulant) but also increased cytotoxicity. | [53] | ||
| Stingless bees honey (Melipona, Trigona) inhibits the release of inflammatory mediators from human mast cells, including tumor necrosis factor-a, interleukin-4, and histamine, depending on the botanical origins (for bamboo and rubber tree but not mango and noni honey), which could help treat allergic diseases. | [54] | ||
| In vivo | Isolation and purification of an Alhagi honey polysaccharide (AHPN50-1a), which was shown to reduce colon tissue damage, reduce inflammation, and restore intestinal microbiota in mice, presenting a potential treatment for inflammatory bowel disease. | [55] | |
| Isolation and purification of an Alhagi honey polysaccharide (AHPN80), which was shown to improve liver parameters, repair the intestinal barriers, and reduce oxidative stress in mice with alcohol-induced acute liver injury. | [56] | ||
| Case study | Complementary therapy | Postoperative treatment of synovial sepsis in three horses with intraarticular or intrathecal medical-grade honey instillation led to good recoveries (free from lameness in all gaits). | [57] |
| Randomized controlled trial | Gargling with silk-cotton tree or kapok tree honey every 6 h for 10 days after a tonsillectomy reduced pain and the need for analgesics, suggesting its use as a complementary therapy in postoperative patients. | [58] | |
| In vitro | Low toxicity | Treatment of peripheral blood lymphocytes with strawberry tree honey reveals low genotoxic potential, not impairing in vitro proliferation and offering geno- and cytoprotection against a cytotoxic agent damage, showing in vitro safety. | [59] |
| In vivo | Evaluation of repeated dose oral toxicity of Apis cerana honey in Winstar mice testing concentrations of 3–24 g kg−1 body weight/day of honey for 28 days only found decrease in food consumption and body weight in the highest tested concentration and determined the no-observed-adverse-effect level at 12 g kg−1 body weight day. | [60] | |
| In vivo | Negative results | Supplementation of rats undergoing forced swimming tests as a proxy for physical stress with wild bee honey did not result in a significant reduction in antioxidative stress in ovarian follicles. | [61] |
| Manuka honey was applied on clean surgical wounds every day for 15 days on 12 beagle dogs and 12 shorthaired cats, showing no significant different healing than control in cosmetic and histologic evaluations, but showing higher skin thickening and smaller wound area (antimicrobial activity benefits may not be evident in clean surgical wounds). | [62] |
2.4. Bees as Indicators and Sentinels
2.5. Bees in Culture, Science, and Technology
3. Anthropogenic Stressors Affecting Bees
3.1. Detrimental Beekeeping Practices
3.2. Environmental Changes
3.3. Exposure to Pesticides
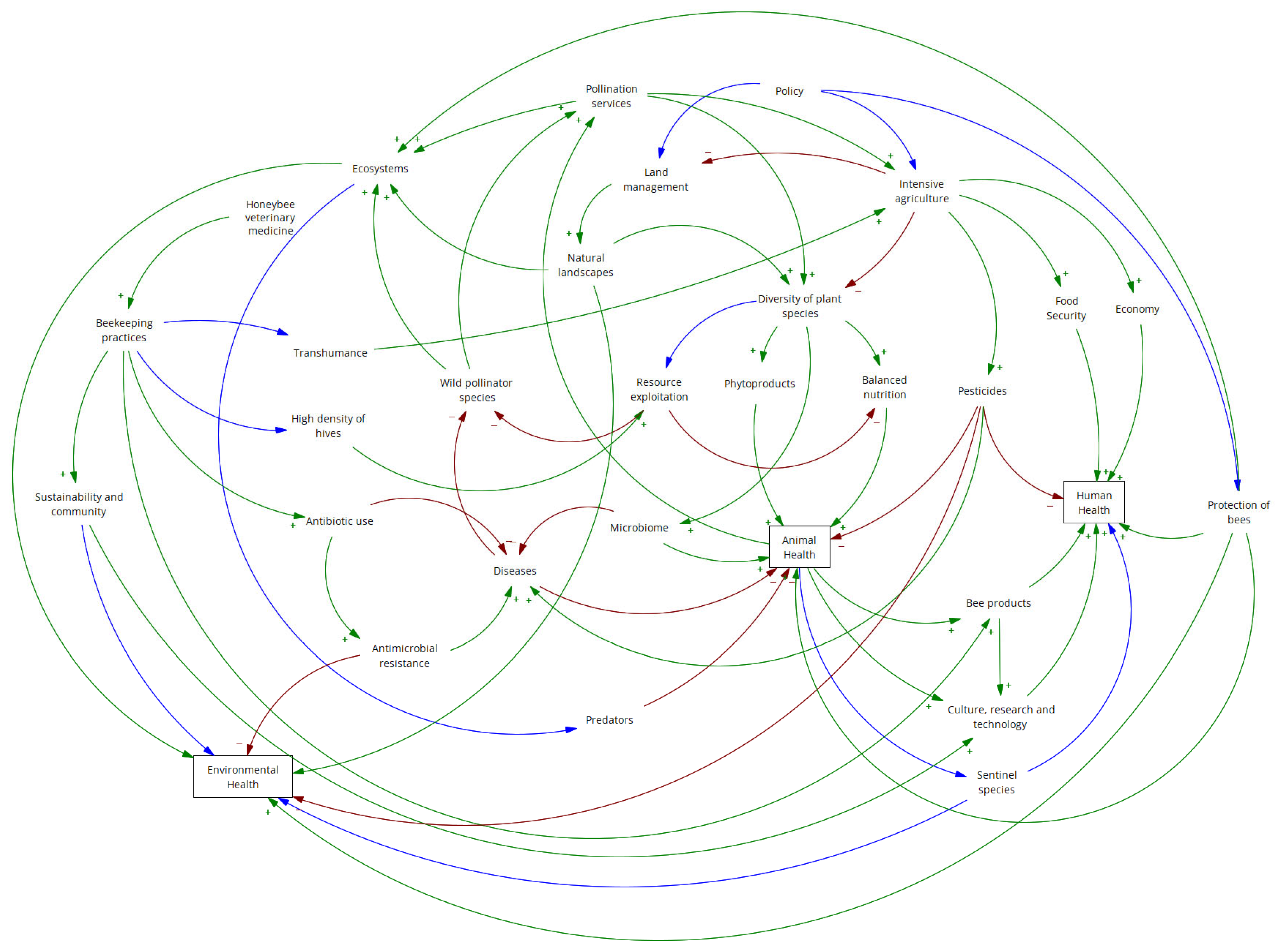
4. Applying a One Health Approach to Bees
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prata, J.C.; Ribeiro, A.I.; Rocha-Santos, T. An Introduction to the Concept of One Health. In One Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–31. [Google Scholar]
- Zinsstag, J.; Crump, L.; Schelling, E.; Hattendorf, J.; Maidane, Y.O.; Ali, K.O.; Muhummed, A.; Umer, A.A.; Aliyi, F.; Nooh, F.; et al. Climate Change and One Health. FEMS Microbiol. Lett. 2018, 365, fny085. [Google Scholar] [CrossRef]
- Heederik, D. The One Health Approach. Environ. Epidemiol. 2019, 3, 157. [Google Scholar] [CrossRef]
- Rabinowitz, P.M.; Gordon, Z.; Holmes, R.; Taylor, B.; Wilcox, M.; Chudnov, D.; Nadkarni, P.; Dein, F.J. Animals as Sentinels of Human Environmental Health Hazards: An Evidence-Based Analysis. Ecohealth 2005, 2, 26–37. [Google Scholar] [CrossRef]
- Murray, E.A.; Bossert, S.; Danforth, B.N. Pollinivory and the Diversification Dynamics of Bees. Biol. Lett. 2018, 14, 20180530. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Chen, Y. Colony Collapse Disorder and Honey Bee Health. In Honey Bee Medicine for the Veterinary Practitioner; Wiley: Hoboken, NJ, USA, 2021; pp. 229–234. [Google Scholar]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; et al. The Honey Bee Apis Mellifera: An Insect at the Interface between Human and Ecosystem Health. Biology 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Salkova, D.; Panayotova-Pencheva, M. Honey Bees and Their Products as Indicators of Environmental Pollution: A Review. Agric. Sci. Technol. 2016, 8, 175–182. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Ortega Polo, R.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Marta Guarna, M. Honey Bees as Biomonitors of Environmental Contaminants, Pathogens, and Climate Change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Muhammad-Bashir, B.; Halimah, B.A. Challenges and Future Perspectives for the Application of One Health. In One Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–343. [Google Scholar]
- Willmer, P.G.; Cunnold, H.; Ballantyne, G. Insights from Measuring Pollen Deposition: Quantifying the Pre-Eminence of Bees as Flower Visitors and Effective Pollinators. Arthropod. Plant Interact. 2017, 11, 411–425. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The Worldwide Importance of Honey Bees as Pollinators in Natural Habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Aslan, C.E.; Liang, C.T.; Galindo, B.; Kimberly, H.; Topete, W. The Role of Honey Bees as Pollinators in Natural Areas. Nat. Areas J. 2016, 36, 478–488. [Google Scholar] [CrossRef]
- Henry, M.; Rodet, G. Controlling the Impact of the Managed Honeybee on Wild Bees in Protected Areas. Sci. Rep. 2018, 8, 9308. [Google Scholar] [CrossRef] [PubMed]
- Piot, N.; Schweiger, O.; Meeus, I.; Yañez, O.; Straub, L.; Villamar-Bouza, L.; De la Rúa, P.; Jara, L.; Ruiz, C.; Malmstrøm, M.; et al. Honey Bees and Climate Explain Viral Prevalence in Wild Bee Communities on a Continental Scale. Sci. Rep. 2022, 12, 1904. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Honey Bees. Available online: https://food.ec.europa.eu/animals/live-animal-movements/honey-bees_en (accessed on 18 June 2024).
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Bareke, T.; Addi, A. Effect of Honeybee Pollination on Seed and Fruit Yield of Agricultural Crops in Ethiopia. MOJ Ecol. Environ. Sci. 2019, 4, 205–209. [Google Scholar] [CrossRef]
- Smith, M.R.; Singh, G.M.; Mozaffarian, D.; Myers, S.S. Effects of Decreases of Animal Pollinators on Human Nutrition and Global Health: A Modelling Analysis. Lancet 2015, 386, 1964–1972. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The Global Stock of Domesticated Honey Bees Is Growing Slower Than Agricultural Demand for Pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop Production in the USA Is Frequently Limited by a Lack of Pollinators. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Tautz, J.; Rostás, M. Honeybee Buzz Attenuates Plant Damage by Caterpillars. Curr. Biol. 2008, 18, R1125–R1126. [Google Scholar] [CrossRef]
- Noiset, P.; Cabirol, N.; Rojas-Oropeza, M.; Warrit, N.; Nkoba, K.; Vereecken, N.J. Honey Compositional Convergence and the Parallel Domestication of Social Bees. Sci. Rep. 2022, 12, 18280. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Ratnieks, F.L.W. Long-range Foraging by the Honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Durak, M.Z. Rapid Detection of Sucrose Adulteration in Honey Using Fourier Transform Infrared Spectroscopy. Spectrosc. Lett. 2019, 52, 267–273. [Google Scholar] [CrossRef]
- European Commission. EU Coordinated Action “From the Hives” (Honey 2021–2022). Available online: https://food.ec.europa.eu/safety/eu-agri-food-fraud-network/eu-coordinated-actions/honey-2021-2022_en (accessed on 18 June 2024).
- Kumar, N.; Ranjan, R.; Kumar, Y.; Patel, S.S.; Sai Krishna, V.; Appaiah, A.; Gupta, K.; Panchariya, P. Discrimination of Various Pure Honey Samples and Its Adulterants Using FTIR Spectroscopy Coupled with Chemometrics. In Proceedings of the 2021 7th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 19–20 March 2021; IEEE: New York, NY, USA, 2021; pp. 808–811. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Ropciuc, S.; Oroian, M. Advanced Characterization of Monofloral Honeys from Romania. Agriculture 2022, 12, 526. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey Antibacterial Activity: A Neglected Aspect of Honey Quality Assurance as Functional Food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Mustar, S.; Ibrahim, N. A Sweeter Pill to Swallow: A Review of Honey Bees and Honey as a Source of Probiotic and Prebiotic Products. Foods 2022, 11, 2102. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.P.; Mitchell, P.; Wells, C.; Russell, M. Honey Supplementation and Exercise: A Systematic Review. Nutrients 2019, 11, 1586. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Chézeries, J.-F. A Saúde Pelo Mel e Produtos Da Colmeia; Litexa Editora: Lisbon, Portugal, 1984; ISBN 9789725781029. [Google Scholar]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The Antiviral and Immunomodulatory Activities of Propolis: An Update and Future Perspectives for Respiratory Diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.A.; Ripari, N.; Conte, F.L.; Honorio, M.d.S.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An Overview about Apitherapy and Its Clinical Applications. Phytomed. Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Bilò, B.M.; Bonifazi, F. Epidemiology of Insect-Venom Anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Aw Yong, P.Y.; Islam, F.; Harith, H.H.; Israf, D.A.; Tan, J.W.; Tham, C.L. The Potential Use of Honey as a Remedy for Allergic Diseases: A Mini Review. Front. Pharmacol. 2021, 11, 599080. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Wahid, M.; Nazeer, M.; Qadir, A.; Azmi, M.B. Investigating the Protein-Based Therapeutic Relationship between Honey Protein SHP-60 and Bevacizumab on Angiogenesis: Exploring the Synergistic Effect through In Vitro and in Silico Analysis. ACS Omega 2024, 9, 17143–17153. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Patel, M.; Althomali, O.W.; Sheeha, B. Bin In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer. Nutrients 2023, 15, 3448. [Google Scholar] [CrossRef]
- Sánchez-Martín, V.; Morales, P.; Iriondo-DeHond, A.; Hospital, X.F.; Fernández, M.; Hierro, E.; Haza, A.I. Differential Apoptotic Effects of Bee Product Mixtures on Normal and Cancer Hepatic Cells. Antioxidants 2023, 12, 615. [Google Scholar] [CrossRef]
- Mduda, C.A.; Muruke, M.H.; Hussein, J.M. Antimicrobial Properties of Honeys Produced by Stingless Bees (Hymenoptera, Apidae, Meliponini) from Different Vegetation Zones of Tanzania. Int. J. Trop. Insect Sci. 2023, 43, 1563–1581. [Google Scholar] [CrossRef]
- Rikohe, I.F.; Mlozi, S.H.; Ngondya, I.B. Seasons and Bee Foraging Plant Species Strongly Influence Honey Antimicrobial Activity. J. Agric. Food Res. 2023, 12, 100622. [Google Scholar] [CrossRef]
- Skadiņš, I.; Labsvārds, K.D.; Grava, A.; Amirian, J.; Tomsone, L.E.; Ruško, J.; Viksna, A.; Bandere, D.; Brangule, A. Antimicrobial and Antibiofilm Properties of Latvian Honey against Causative Agents of Wound Infections. Antibiotics 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Aldarhami, A.; Bazaid, A.S.; Qanash, H.; Ahmad, I.; Alshammari, F.H.; Alshammari, A.M.; Alshammari, A.H.; Aljanfawe, F.M.; Aldamiri, B.; Aldawood, E.; et al. Effects of Repeated In-Vitro Exposure to Saudi Honey on Bacterial Resistance to Antibiotics and Biofilm Formation. Infect. Drug Resist. 2023, 16, 4273–4283. [Google Scholar] [CrossRef]
- Clare, J.; Lindley, M.R.; Ratcliffe, E. The Antimicrobial and Antibiofilm Abilities of Fish Oil Derived Polyunsaturated Fatty Acids and Manuka Honey. Microorganisms 2024, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-B.; Kim, S.-G.; Kim, Y.S.; Kim, B.; Han, S.M.; Lee, H.J.; Choi, H.M.; Choi, J.-G. Castanea Crenata Honey Reduces Influenza Infection by Activating the Innate Immune Response. Front. Immunol. 2023, 14, 1157506. [Google Scholar] [CrossRef]
- Lin, B.; Nair, S.; Fellner, D.M.J.; Nasef, N.A.; Singh, H.; Negron, L.; Goldstone, D.C.; Brimble, M.A.; Gerrard, J.A.; Domigan, L.; et al. The Leptospermum Scoparium (Mānuka)-Specific Nectar and Honey Compound 3,6,7-Trimethyllumazine (LepteridineTM) That Inhibits Matrix Metalloproteinase 9 (MMP-9) Activity. Foods 2023, 12, 4072. [Google Scholar] [CrossRef] [PubMed]
- Turn, J.T.; Mayer, J.; Nagata, K.; Banovic, F.; Meichner, K.; Hurley, D.J.; Koslowski, E.; Gogal, R.M., Jr. Impact of Apitherapy on Canine, Equine, and Chicken Lymphocytes, In Vitro. Vet. Immunol. Immunopathol. 2024, 268, 110700. [Google Scholar] [CrossRef]
- Yong, P.Y.A.; Yip, A.J.W.; Islam, F.; Hong, H.J.; Teh, Y.E.; Tham, C.L.; Tan, J.W. The Anti-Allergic Potential of Stingless Bee Honey from Different Botanical Sources via Modulation of Mast Cell Degranulation. BMC Complement Med. Ther. 2023, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Y.; Lv, Z.; Taoerdahong, H.; Li, G.; Li, J.; Zhao, X.; Jin, X.; Chang, J. Structural Characterization of a Polysaccharide from Alhagi Honey and Its Protective Effect against Inflammatory Bowel Disease by Modulating Gut Microbiota Dysbiosis. Int. J. Biol. Macromol. 2024, 259, 128937. [Google Scholar] [CrossRef]
- Song, J.; Zhao, X.; Bo, J.; Lv, Z.; Li, G.; Chen, Y.; Liang, J.; Zhang, C.; Jin, X.; Liu, C.; et al. A Polysaccharide from Alhagi Honey Protects the Intestinal Barrier and Regulates the Nrf2/HO-1-TLR4/MAPK Signaling Pathway to Treat Alcoholic Liver Disease in Mice. J. Ethnopharmacol. 2024, 321, 117552. [Google Scholar] [CrossRef]
- Terschuur, J.A.; Coomer, R.P.C.; McKane, S.A. Administration Safety of Medical-Grade Honey (MGH) in Septic Synovial Structures in Horses: 3 Cases. Can. J. Vet. Res. 2023, 87, 153–156. [Google Scholar]
- Lubis, A.S.; Herwanto, H.R.Y.; Rambe, A.Y.M.; Munir, D.; Asroel, H.A.; Ashar, T.; Lelo, A. The Effect of Honey on Post-Tonsillectomy Pain Relief: A Randomized Clinical Trial. Braz. J. Otorhinolaryngol. 2023, 89, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Jurič, A.; Brčić Karačonji, I.; Gašić, U.; Milojković Opsenica, D.; Prđun, S.; Bubalo, D.; Lušić, D.; Vahčić, N.; Kopjar, N. Protective Effects of Arbutus unedo L. Honey in the Alleviation of Irinotecan-Induced Cytogenetic Damage in Human Lymphocytes—An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 1903. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, P.; Zheng, S.; Nie, Y.; Zhang, W.; Feng, Y.; Ning, J.; Li, G.; Gao, S. A 28-Day Subacute Oral Toxicity Study of Apis cerana (Fabricius) Honey in Wistar Rats. Int. Food Res. J. 2023, 30, 1481–1494. [Google Scholar] [CrossRef]
- Plumeriastuti, H.; Widjiati; Proboningrat, A.; Sajida, M.V.P. SOD2 and HIF-1α Expression in Rat Ovaries (Rattus norvegicus) Administered with Forest Bee Honey (Apis dorsata) Following Physical Stress. Bali Med. J. 2023, 12, 1835–1839. [Google Scholar]
- Gouletsou, P.G.; Zacharopoulou, T.; Skampardonis, V.; Georgiou, S.G.; Doukas, D.; Galatos, A.D.; Flouraki, E.; Dermisiadou, E.; Margeti, C.; Barbagianni, M.; et al. First-Intention Incisional Wound Healing in Dogs and Cats: A Controlled Trial of Dermapliq and Manuka Honey. Vet. Sci. 2024, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Di Noi, A.; Casini, S.; Campani, T.; Cai, G.; Caliani, I. Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 1863. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481. [Google Scholar] [CrossRef]
- Taylor, M.P.; Gillings, M.M.; Fry, K.L.; Barlow, C.F.; Gunkel-Grillion, P.; Gueyte, R.; Camoin, M. Tracing Nickel Smelter Emissions Using European Honey Bees. Environ. Pollut. 2023, 335, 122257. [Google Scholar] [CrossRef]
- Kritsky, G. Beekeeping from Antiquity through the Middle Ages. Annu. Rev. Entomol. 2017, 62, 249–264. [Google Scholar] [CrossRef]
- Turner, J.H.; Maryanski, A. The Deep Origins of Society: An Assessment of E.O. Wilson’s Genesis. Int. Sociol. 2019, 34, 536–551. [Google Scholar] [CrossRef]
- Wilson, E.O.; Hölldobler, B. Eusociality: Origin and Consequences. Proc. Natl. Acad. Sci. USA 2005, 102, 13367–13371. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Cook, C.N.; Zayed, A. Effects of Group Size on Learning and Memory in the Honey Bee Apis mellifera. J. Exp. Biol. 2019, 222, jeb193888. [Google Scholar] [CrossRef] [PubMed]
- Sponsler, D.B.; Bratman, E.Z. Beekeeping in, of or for the City? A Socioecological Perspective on Urban Apiculture. People Nat. 2021, 3, 550–559. [Google Scholar] [CrossRef]
- Menzel, R. The Honeybee as a Model for Understanding the Basis of Cognition. Nat. Rev. Neurosci. 2012, 13, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Avarguès-Weber, A.; Garcia, J.E.; Greentree, A.D.; Dyer, A.G. Numerical Ordering of Zero in Honey Bees. Science 2018, 360, 1124–1126. [Google Scholar] [CrossRef]
- Laomettachit, T.; Liangruksa, M.; Termsaithong, T.; Tangthanawatsakul, A.; Duangphakdee, O. A Model of Infection in Honeybee Colonies with Social Immunity. PLoS ONE 2021, 16, e0247294. [Google Scholar] [CrossRef] [PubMed]
- Cridge, A.G.; Lovegrove, M.R.; Skelly, J.G.; Taylor, S.E.; Petersen, G.E.L.; Cameron, R.C.; Dearden, P.K. The Honeybee as a Model Insect for Developmental Genetics. Genesis 2017, 55, e23019. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the Effects of Sublethal Pesticide Exposure on Honey Bees: A Role for Probiotics as Mediators of Environmental Stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Engel, P.; Kwong, W.K.; McFrederick, Q.; Anderson, K.E.; Barribeau, S.M.; Chandler, J.A.; Cornman, R.S.; Dainat, J.; de Miranda, J.R.; Doublet, V.; et al. The Bee Microbiome: Impact on Bee Health and Model for Evolution and Ecology of Host-Microbe Interactions. mBio 2016, 7, e02164. [Google Scholar] [CrossRef]
- Zheng, H.; Steele, M.I.; Leonard, S.P.; Motta, E.V.S.; Moran, N.A. Honey Bees as Models for Gut Microbiota Research. Lab. Anim. 2018, 47, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, H.; Sasaki, M. Perspectives of Multi-modal Contribution of Honeybee Resources to Our Life. Entomol. Res. 2008, 38, S15–S21. [Google Scholar] [CrossRef]
- Srinivasan, M.V. Honeybees as a Model for the Study of Visually Guided Flight, Navigation, and Biologically Inspired Robotics. Physiol. Rev. 2011, 91, 413–460. [Google Scholar] [CrossRef] [PubMed]
- Sabo, C.; Yavuz, E.; Cope, A.; Gurney, K.; Vasilaki, E.; Nowotny, T.; Marshall, J.A.R. An Inexpensive Flying Robot Design for Embodied Robotics Research. In Proceedings of the 2017 International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May 2017; IEEE: New York, NY, USA, 2017; pp. 4171–4178. [Google Scholar]
- Thakoor, S.; Moro, J.M.; Chahl, J.; Hine, B.; Zornetzer, S. BEES: Exploring Mars with Bioinspired Technologies. Computer 2004, 37, 38–47. [Google Scholar] [CrossRef]
- Hoogendoorn, M.; Schut, M.C.; Treur, J. Modeling Decentralized Organizational Change in Honeybee Societies. In Advances in Artificial Life; Springer: Berlin/Heidelberg, Germany, 2007; pp. 615–624. [Google Scholar]
- Hewlett Packard Enterprise. Swarm Intelligence. Available online: https://www.hpe.com/pt/en/what-is/swarm-intelligence.html (accessed on 18 June 2024).
- Landgraf, T.; Rojas, R.; Nguyen, H.; Kriegel, F.; Stettin, K. Analysis of the Waggle Dance Motion of Honeybees for the Design of a Biomimetic Honeybee Robot. PLoS ONE 2011, 6, e21354. [Google Scholar] [CrossRef] [PubMed]
- Crane, E. Recent Research on the World History of Beekeeping. Bee World 1999, 80, 174–186. [Google Scholar] [CrossRef]
- Paris, E.H.; Castrejon, V.B.; Walker, D.S.; Lope, C.P. The Origins of Maya Stingless Beekeeping. J. Ethnobiol. 2020, 40, 386–405. [Google Scholar] [CrossRef]
- Weiss, K. Experiences with Plastic Combs and Foundation. Bee World 1983, 64, 56–62. [Google Scholar] [CrossRef]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; Brosi, B.J.; de Roode, J.C. Reduced Density and Visually Complex Apiaries Reduce Parasite Load and Promote Honey Production and Overwintering Survival in Honey Bees. PLoS ONE 2019, 14, e0216286. [Google Scholar] [CrossRef]
- Raymann, K.; Shaffer, Z.; Moran, N.A. Antibiotic Exposure Perturbs the Gut Microbiota and Elevates Mortality in Honeybees. PLoS Biol. 2017, 15, e2001861. [Google Scholar] [CrossRef]
- Rodet, G. The Man and the Bees: A Coviability Issue—Beekeeping Can It Be Intensively Farmed? In Coviability of Social and Ecological Systems: Reconnecting Mankind to the Biosphere in an Era of Global Change; Springer International Publishing: Cham, Switzerland, 2019; pp. 305–327. [Google Scholar]
- Jacques, A.; Laurent, M.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A Pan-European Epidemiological Study Reveals Honey Bee Colony Survival Depends on Beekeeper Education and Disease Control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [PubMed]
- Sonmez Oskay, G.; Uygur, G.S.; Oskay, D.; Arda, N. Impact of Stress Factors Internal and External to the Hive on Honey Bees and Their Reflection on Honey Bee Products: A Review. J. Apic. Res. 2023, 1–16. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef]
- Chauzat, M.-P.; Laurent, M.; Riviere, M.-P.; Saugeon, C.; Hendrikx, P.; Ribiere-Chabert, M. A Pan-European Epidemiological Study on Honeybee Colony Losses 2012–2013. Available online: https://food.ec.europa.eu/system/files/2017-04/la_bees_epilobee-report_2012-2013.pdf (accessed on 18 June 2024).
- Laurino, D.; Lioy, S.; Carisio, L.; Manino, A.; Porporato, M. Vespa Velutina: An Alien Driver of Honey Bee Colony Losses. Diversity 2019, 12, 5. [Google Scholar] [CrossRef]
- Peck, D.T. The Parasitic Mite Varroa destructor. In Honey Bee Medicine for the Veterinary Practitioner; Wiley: Hoboken, NJ, USA, 2021; pp. 235–251. [Google Scholar]
- de Jongh, E.J.; Harper, S.L.; Yamamoto, S.S.; Wright, C.J.; Wilkinson, C.W.; Ghosh, S.; Otto, S.J.G. One Health, One Hive: A Scoping Review of Honey Bees, Climate Change, Pollutants, and Antimicrobial Resistance. PLoS ONE 2022, 17, e0242393. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, L.; Brown, M.J.F.; Doublet, V. One Health Implications of Infectious Diseases of Wild and Managed Bees. J. Invertebr. Pathol. 2021, 186, 107506. [Google Scholar] [CrossRef]
- Negri, P.; Villalobos, E.; Szawarski, N.; Damiani, N.; Gende, L.; Garrido, M.; Maggi, M.; Quintana, S.; Lamattina, L.; Eguaras, M. Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects 2019, 10, 401. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and Nosema Ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Bretagnolle, V.; Gaba, S. Weeds for Bees? A Review. Agron. Sustain. Dev. 2015, 35, 891–909. [Google Scholar] [CrossRef]
- Alberoni, D.; Favaro, R.; Baffoni, L.; Angeli, S.; Di Gioia, D. Neonicotinoids in the Agroecosystem: In-Field Long-Term Assessment on Honeybee Colony Strength and Microbiome. Sci. Total Environ. 2021, 762, 144116. [Google Scholar] [CrossRef]
- Flores, J.M.; Gil-Lebrero, S.; Gámiz, V.; Rodríguez, M.I.; Ortiz, M.A.; Quiles, F.J. Effect of the Climate Change on Honey Bee Colonies in a Temperate Mediterranean Zone Assessed through Remote Hive Weight Monitoring System in Conjunction with Exhaustive Colonies Assessment. Sci. Total Environ. 2019, 653, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, B.; Greco, V.; Piras, C.; Ceniti, C.; Paonessa, M.; Musella, V.; Bava, R.; Palma, E.; Morittu, V.M.; Spina, A.A.; et al. The Bee Gut Microbiota: Bridging Infective Agents Potential in the One Health Context. Int. J. Mol. Sci. 2024, 25, 3739. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Radcliffe, A.; Stout, J.C.; Wright, G.A. Bees Prefer Foods Containing Neonicotinoid Pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süßenbach, D.; Del Aguila, M.; Ercolano, V.; et al. Revised Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis mellifera, Bombus spp. and Solitary bees). EFSA J. 2023, 21, 3295. [Google Scholar] [CrossRef]
- Zaluski, R.; Justulin, L.A.; Orsi, R.d.O. Field-Relevant Doses of the Systemic Insecticide Fipronil and Fungicide Pyraclostrobin Impair Mandibular and Hypopharyngeal Glands in Nurse Honeybees (Apis mellifera). Sci. Rep. 2017, 7, 15217. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate Perturbs the Gut Microbiota of Honey Bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Zafeiraki, E.; Manea-Karga, E.; Anastasiadou, P.; Machera, K. Pesticide Residues and Metabolites in Greek Honey and Pollen: Bees and Human Health Risk Assessment. Foods 2023, 12, 706. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A. Application of Analytical Methods in Authentication and Adulteration of Honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. SDG Indicators Data Portal. Available online: https://www.fao.org/sustainable-development-goals-data-portal/data/indicators/2.1.1-prevalence-of-undernourishment/en#:~:text=Global%20hunger%20remained%20relatively%20unchanged,world%20faced%20hunger%20in%202022 (accessed on 18 June 2024).
- Sun, H.; Li, H.; Zhang, X.; Liu, Y.; Chen, H.; Zheng, L.; Zhai, Y.; Zheng, H. The Honeybee Gut Resistome and Its Role in Antibiotic Resistance Dissemination. Integr. Zool. 2023, 18, 1014–1026. [Google Scholar] [CrossRef]
- Rodrigues, H.; Leite, M.; Oliveira, B.; Freitas, A. Antibiotics in Honey: A Comprehensive Review on Occurrence and Analytical Methodologies. Open Res. Eur. 2024, 4, 125. [Google Scholar] [CrossRef]
- Croppi, S.; Yu, L.; Robinette, C.S.; Hassler, E.E.; Newmark, A.J.; Scott, A.; Cazier, J.; Song, J.; Formato, G. Impact of Legislation on Antibiotic Use and Awareness of Beekeepers. J. Apic. Sci. 2021, 65, 265–277. [Google Scholar] [CrossRef]
- Donkersley, P.; Elsner-Adams, E.; Maderson, S. A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Vet. Sci. 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Mañes, A.M.; Formato, G.; Mortarino, M.; Toporcak, J. Veterinarians and Beekeeping: What Roles, Expectations and Future Perspectives?—A Review Paper. Vet. Arh. 2021, 91, 437–443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prata, J.C.; Martins da Costa, P. Honeybees and the One Health Approach. Environments 2024, 11, 161. https://doi.org/10.3390/environments11080161
Prata JC, Martins da Costa P. Honeybees and the One Health Approach. Environments. 2024; 11(8):161. https://doi.org/10.3390/environments11080161
Chicago/Turabian StylePrata, Joana C., and Paulo Martins da Costa. 2024. "Honeybees and the One Health Approach" Environments 11, no. 8: 161. https://doi.org/10.3390/environments11080161
APA StylePrata, J. C., & Martins da Costa, P. (2024). Honeybees and the One Health Approach. Environments, 11(8), 161. https://doi.org/10.3390/environments11080161







