Abstract
In modern agricultural production, cattle manure waste recovery is considered as a sustainable approach to agricultural waste management, reducing environmental pollution and chemical fertilizer use. This study aimed to investigate the effects of manure and digestate derived from a pilot-scale livestock waste-recycling system, in combination with a low copper concentration as a fungicide, on the physiological response of lettuce cv Rufus (Lactuca sativa L.) plants and the associated soil microbiome. A five-week microcosm experiment was conducted in a greenhouse under environmental conditions. Lettuce plant performance was assessed in terms of biomass, leaf area index, photosynthetic activity, chlorophyll measurements, lipid peroxidation, total phenolic content, and nutrient uptake. The results suggested that incorporating digestate into the potting soil mix significantly enhanced crop yields compared to the control and manure treatments. The soil microbial activity increased in the presence of fertilizers, improving the soil chemical and biological properties. The addition of copper negatively affected the growth and physiological performance of the lettuce plants under both the control and manure-treated conditions, except for those grown in the presence of digestate, where copper accumulation was reduced. These findings highlight the potential of growing horticultural crops using organic fertilization through livestock waste anaerobic digestate, establishing a waste-to-food recycling system.
1. Introduction
As the human population increases, so does the demand for food, leading to a significant increase in food waste and its environmental impact. Over the last 50 years, worldwide, the use of inorganic fertilizers has significantly increased agricultural production. However, the use of organic fertilizers could be a sustainable way to reuse both organic wastes and manure from livestock farms in order to increase food production. Animal feces and urine contain high percentages of nitrogen (N) (55–95%), phosphorous (P), and potassium (K), which are essential macronutrients for animals, plants, and bacteria [1].
Although livestock waste is a valuable resource and can be used as a biofertilizer, it must be managed correctly to avoid environmental negative impacts, such as water and soil contamination (e.g., excess of organic nutrients and presence of trace micropollutants as antibiotics), air pollution (greenhouse gas emissions), and human health problems (spread of pathogens) [1]. A growing number of intensive livestock farms are turning to anaerobic digestion (AD) technology to solve the problem concerning the disposal of the huge volumes of manure and slurry produced every day. The produced biogas and digestate provide significant energy and fertilizer benefits [2]. Anaerobic digestion is an efficient process in which organic matter breaks down naturally in the absence of oxygen and takes place in a reactor, where the resulting greenhouse gases can be reused rather than released into the atmosphere [1]. The by-product of the AD process is digestate, which can be employed as a biofertilizer returning nutrients to the soil through natural cycles and replacing inorganic fertilizer inputs. Thus, AD can be considered as a process that reduces the use of fossil fuels and inorganic fertilizers, lowers greenhouse gas emissions, and provides a highly efficient method for recycling resources, in line with green and circular economy projects [1,3].
Previous studies have demonstrated that digestate can be a source of macro and micronutrients useful for improving soil quality, promoting biological activity, and improving physiological responses in crop growth [4,5,6]. Nevertheless, digestate, due to its chemical compounds, can also cause slow growth and early senescence in crop plants [7].
Copper (Cu) is an essential micronutrient for plant growth and metabolism, related to many physiological and biochemical processes in plants [8,9]. As a cofactor for many enzymes, Cu plays a key role under stress conditions. Copper is also associated with oxidative phosphorylation, protein trafficking, signal regulation, and lipid and iron metabolism [10]. Indeed, Cu deficiencies can alter several functions of plant metabolism and its excessive concentration can generate phytotoxicity, resulting in the overproduction of reactive oxygen species (ROS) and causing damage to carbohydrates, lipids, proteins, and DNA in respiration, photosynthesis, and enzyme activity that can result in growth and productivity inhibition and imbalances in nutrient uptake [11,12]. Actually, owing to different agricultural practices, such as the use of manure as fertilizer (especially of pig origin) and copper application as a pesticide in vineyards, this metal can accumulate in high concentrations in soils and can become a pollutant [8,9].
In particular, Cu has been applied to vineyards, orchards, and horticultural crops since the late 19th century at doses of several kilograms per hectare to prevent downy mildew [9]. Copper’s antifungal properties were demonstrated in the laboratory in the 1950s, and nowadays, its use is also authorized in organic farming. Copper-based fungicides, such as the Bordeaux mixture, consist of copper (II) sulphate (CuSO4) and quicklime (CaO). Indeed, the average Cu concentration in European agricultural soils has been increasing and it has been estimated to be between 30 and 50 mg kg−1 [8]. The use of copper in agricultural soils has been restricted by the European Commission (EU Reg. 1981/2018) to minimize its potential accumulation in the soil and exposure to non-target organisms, such as plants and animals, so a maximum of 28 kg ha−1 may be applied for plant protection over a 7-year period, with an annual limit of 4 kg ha−1 [9].
Possible copper mobility from soil to plant tissue raises several concerns, including possible adverse effects on herbivorous organisms and humans, which ingest edible plants [9,13].
Lettuce (Lactuca sativa L.), an annual crop belonging to the Asteraceae family, is among the world’s most widely cultivated and consumed vegetables and it is rich in nutrients as well as vitamins, calcium, and fiber. It also has a short growing season, characteristics for which it is considered a model vegetable species [14,15]. Moreover, lettuce is a biological species recommended for soil toxicity testing by the OECD (1984) to evaluate the potential impact of using natural products to limit contaminants and plant growth [16].
The present study aimed to investigate the effects of the application of different combinations of cattle organic soil amendments, manure (M), or digestate (D), and low concentrations of copper on the growth of lettuce plants in terms of biometric (i.e., shoot biomass, shoot height, number of leaves, and surface leaf area), photosynthetic (i.e., chlorophyll content and fluorescence), and biochemical (i.e., lipid peroxidation and total phenol content) parameters, and the uptake of macro- and micro-nutrients highlighting the copper element. Furthermore, the response of the associated soil microbial community (i.e, soil total microbial abundance and soil microbial activity) was analyzed.
2. Materials and Methods
2.1. Plant, Soil Materials, and Experimental Design
Lettuce (Lactuca sativa L.), as one of the most economically important leafy vegetables, was chosen as the model plant species. Twenty-four seedlings of lettuce (cv. “Rufus”), with a height of about 4 cm, were purchased from a local hatchery. The seedlings were transplanted into plastic pots (1 seedling/pot) (1.4 L capacity), previously filled with 900 g of soil. Before transplanting, cattle manure (1% w/w) or digestate (1% w/w) was homogenously added to the soil alone or in combination with 30 mg kg−1 of copper sulphate (CuSO4 5H2O). All residual vegetable matter and stones were manually removed from the soil, and it was air-dried before setting up the microcosm experiment. Distilled water was used to obtain a 29.8% soil humidity, corresponding to 45% of the soil water-holding capacity. Soil was originally collected from an organic local farm, while manure and digestate were obtained from a biogas plant located on a cattle farm in an agricultural area in central Italy. The main physico-chemical characteristics of the soil, manure, and digestate, such as the pH, total organic carbon (Corg) and nitrogen (N) (Table S1), and elements content (Table S2), were measured according to the methods described in [17]. Afterwards, the seedlings were placed in a greenhouse located within the National Research Council (CNR) (42°10′ N, 12°63′ E) in Montelibretti (Rome, Italy), and grown for five weeks (from May to June 2022), where there was a daily average temperature of 26–31 °C during the day and 12–17 °C at night (Figure 1).
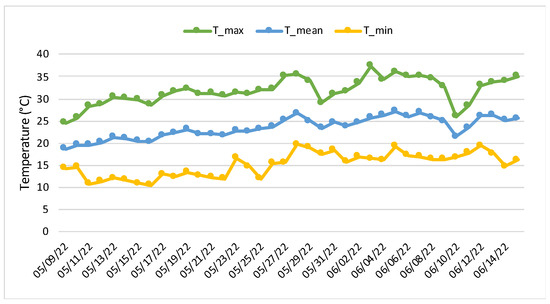
Figure 1.
Temperatures observed during the experimental period in the greenhouse: Maximum (T_max), mean (T_mean) and minimum (T_min).
The soil moisture was maintained at a constant level by daily replenishing lost water with distilled water. A systematic experimental design was established to evaluate the effect of the two amendments used and of the added copper on the plant growth and microbial community response, several experimental conditions were established (Table 1).

Table 1.
Experimental conditions and corresponding acronyms.
A schematic representation of the experimental design is described in Figure 2.
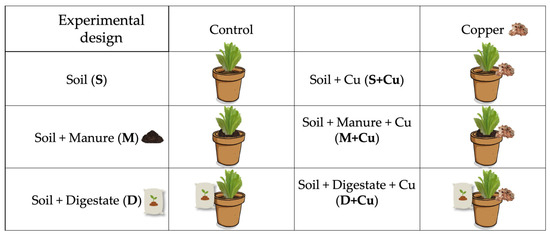
Figure 2.
Representation of the experimental design. Lettuce production during five weeks under different soils: soil alone, cattle manure (1% w/w) and digestate (1% w/w), both homogenously added to the soil alone, and the in combination with 30 mg kg−1 copper sulphate (CuSO4 5H2O). All treatments were tested in quadruplicates, i.e., twenty-four experimental units were used.
Figure 3 shows images of lettuce plants at harvest for each treatment (a) and the layout of the experiment in the greenhouse investigating lettuce growth in different soil conditions (b).
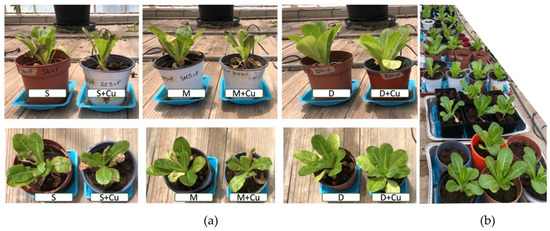
Figure 3.
(a) One representative lettuce plant (side and top view) from each treatment at harvest: S, soil alone; S+Cu, soil and copper; M, manure; M+Cu, manure and copper; D, digestate; D+Cu, digestate and copper and (b) The layout of the experiment in the greenhouse for growing lettuce in different soil conditions.
After five weeks, the lettuce plants were harvested and stored for further analyses. Moreover, aliquots of the rhizosphere soil samples were collected for measurements of the microbiological parameters.
2.2. Plant Growth Analyses
At the end of the experiment, the height (cm) of the lettuce plants and the number of leaves (N° leaves) were measured to assess the plant growth. To quantify the plant shoot biomass, the lettuces were cut from the base with scissors and then weighed. The dry weight (DW) of the lettuce samples was obtained by placing leaves in an oven at 60 °C until a constant mass was reached. The leaf dry mass was then related to its leaf area, calculated using image analysis software (ImageJ, National Institutes of Health, Bethesda, MD, USA), to obtain the specific leaf area (SLA, cm2 g−1 DW). The growth parameters were measured on four biological replicates from each condition (Table 1).
2.3. Determination of Chlorophyll
Photosynthetic pigments of the lettuce plants (0.1 g) were extracted in 96% (v/v) ethanol for 72 h at room temperature in the dark. After centrifugation at 3000× g for 10 min at 4 °C, the absorbance of the supernatant was read spectrophotometrically at 663 and 645 nm (Lambda 35 UV/VIS, Perkin Elmer, Norwalk, CT, USA) [18]. The total chlorophyll content was assessed according to the equations described by Lichtenthaler et al. [19] and the results are expressed in mg of the total chlorophyll per gram of fresh weight plant tissue (mg g−1 FW).
2.4. Chlorophyll Fluorescence Analysis
The chlorophyll a fluorescence transient (OJIP transients) was measured on the fully expanded leaves of the Lactuca sativa plants using a portable fluorometer (FluorPen FP 110-LM/D, Photon System Instruments, Drasov, Czech Republic). The measurements were performed on 60 min dark-adapted leaves with leaf clips, and the fluorescence intensity was measured after the application of a saturating light pulse of 3000 μmol m−2 s−1. These measurements were taken on 10 plants (one leaf per plant) per treatment (S, S+Cu, M, M+Cu, D, and D+Cu), around midday (between 10.00 a.m. and 12.00 p.m.). The obtained data were used in the JIP test [20] to determine various bioenergetic parameters of PSII photochemistry (Table 2).

Table 2.
Abbreviations, formulas, and definitions of the JIP-test parameters.
2.5. Measurement of Lipid Peroxidation Level
Lipid peroxidation was determined by a measurement of the malondialdehyde (MDA) content according to the modified method of Hodges et al. [21]. Frozen samples (0.1 g) were homogenized in a pre-cooled mixer mill (TissueLyser LT, Quiagen, Hielden, German) with 1 mL of 0.1% trichloroacetic acid (TCA) and centrifuged for 10 min at 13,000× g at 4 °C. An assay mixture containing 0.4 mL of supernatant, 1 mL of 0.5% (w/v) thiobarbituric acid (TBA) in 20% (w/v) TCA, and 1 mM ethylenediamine tetraacetic acid (EDTA) was incubated at 80 °C for 30 min and then quickly cooled in an ice bath. Then samples were centrifuged at 13,500× g for 5 min at 4 °C, and the absorbance of the supernatant was measured at 532 nm and 600 nm with a Thermo Multiskan FC Microplate Photometer (Thermo Fisher Scientific; Waltham, MA, USA). Values corresponding to non-specific absorption at 600 nm were subtracted. The MDA concentration was calculated using the extinction coefficient (ε = 155 mM−1 cm−1). The lipid peroxidation levels are expressed as nanomoles of MDA per gram of fresh weight plant tissue (mg g−1 FW).
2.6. Estimation of Total Phenolic Content
Frozen leaves (0.5 g), homogenized in a pre-cooled mortar and pestle, were extracted in 5 mL of 80% (v/v) ethanol, placed on a shaker for 24 h in the dark at 25–30 °C, then centrifuged at 4 °C for 25 min at 4000× g. The total phenolic content was quantified using Folin–Ciocalteu (FC) reagent, with gallic acid as the standard according to the modified protocol of Bernabé-Antonio et al. [22]. Briefly, 20 μL of extract was mixed with 20 μL of 10% FC reagent and 160 μL of 1M Na2CO3. After 20 min of incubation in the dark, the absorbance was measured at 765 nm using a Thermo Multiskan FC Microplate Photometer (Thermo Fisher Scientific; Waltham, MA, USA). The total phenolic content is expressed as mg of gallic acid equivalents (GAE)/g of fresh weight plant tissue (mg GAE g−1 FW) using the gallic acid calibration curve (0.1–0.75 mg mL−1, R2 = 0.97).
2.7. Chemical Analysis
To quantify the chemical element contents in the plants and soils, both oven-dried lettuce leaves and soil samples were weighed and mineralized with a nitric-acid-based microwave digestion method using an EXCEL Microwave digestion system (Preekem Scientific Instruments Co., Ltd., Shanghai, China). Mineralization was performed by treating 250 mg of dried lettuce leaves with 4 mL HNO3 65% (w/w), 2 mL H2O2 30% (v/v), and 3 mL of distilled water. Regarding the metal concentration in the soil, 0.2 g of dried soil samples was digested with 5 mL of HNO3 65% (w/w) and 1 mL of HCl 37% (v/v). Samples were then filtered and analyzed. The determination of elements was performed using inductively coupled plasma optical emission spectrometry at 327.395 nm (ICP-OES, Agilent 5800, USA-LOD = 0.02 mg L−1).
2.8. Bioaccumulation Factor of Copper in Lettuce Plants
The bioaccumulation factor (BAF) was calculated to evaluate the ability of the lettuce plants to extract and accumulate copper from the soil to shoot [23]:
BAF = Cu content in plant shoot (mg kg−1 DW)/Cu content in soil (mg kg−1 DW)
2.9. Tolerance Index of Lettuce Plants toward Copper
The tolerance index (Ti) was measured to assess the growth of the lettuce plants in Cu-contaminated soil (Cu) compared to the control soil (S) [24]:
where FWCu is the fresh weight (g) of shoot lettuce grown in Cu-contaminated soil and FWS is the fresh weight (g) of shoot lettuce grown in non-contaminated soil.
Ti % = (FWCu/FWS) × 100
2.10. Microbiological Analyses of Soil Community
Microbiological analyses of the soil community were performed at the end of the experiment, after 36 days. The total microbial abundance was measured in the soil samples (1 g), previously fixed in 2% formaldehyde (v/v), by the direct epifluorescence count method with a Leica DM LB 30 epifluorescence microscope and using DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride) dye as a fluorescent DNA intercalant [25,26]. The total microbial abundance is expressed as N. cells g−1 dry soil.
Microbial activity was detected by measuring the dehydrogenase activity (DHA) in the soil samples (6 g) using tetrazolium salt, 2,3,5-triphenyl-tetrazolium chloride (TTC) as an artificial terminal hydrogen acceptor in the electron transport chain. TTC was reduced to red-colored triphenylformazan (TPF). TPF was extracted using an organic solvent (ethanol), and the color intensity of the extract was quantified after 24 h at 37 °C in the dark [27] with a Thermo Multiskan FC Microplate Photometer (Thermo Fisher Scientific; Waltham, MA, USA). The intensity of the color was directly proportional to the concentration of the produced TPF. Soil dehydrogenase activity is expressed as μg TPF g−1 dry soil.
2.11. Statistical Analysis
All collected data were statistically analyzed by one-way analysis of variance (ANOVA) using R software (version 4.1.2 https://www.r-project.org; accessed on 4 December 2023). Differences among the different treatment means were evaluated by post hoc Tukey’s Honest Significant Difference test (Tukey HSD) with a significance level of p < 0.05. In the figures, the results are presented as box plots with the median value displayed as a line in the middle of the box. Maximum and minimum observations are shown as the endpoint of the upper and lower whiskers, respectively, and outliers are plotted as dots when present. The box plots were made using R ggplot2 package (https://cran.r-project.org/web/packages/ggplot2/citation.html; accessed on 4 December 2023), In the tables, the results are expressed as mean values ± standard deviations (n = 4). The relationships among the plant growth, biochemical parameters, and microbiological data in the soil were analyzed using Principal Component Analysis (PCA).
3. Results and Discussion
3.1. Plant Growth Analyses
The effects of different combinations of cattle organic soil amendments and copper on the lettuce plants growth were analyzed by evaluating the shoot biomass production (Figure 4). In the presence of manure (M) and digestate (D), the plant biomass increased significantly (by 30% and 140%, respectively) compared to the control plants (S). It is worth noting that the lettuces grown on D soil showed the highest shoot biomass. A comparison across separate studies may be quite difficult due to the influence of several variables such as different compositions of biochar, manure or digestate, tested plants and soil, and pot experiment conditions. Nevertheless, our findings were in accordance with Kwoczynski et al. [6], who reported a two-fold increase in the yield of lettuce plants (Lactuca sativa L.) grown in the presence of 5% (w/w) digestate compared to controls [6]. Similarly, the addition of digestate to soil caused an enhancement in biomass yield also in eggplant (Solanum melongena L.) [28], ryegrass (Lolium perenna L.) [29], tomato (Solanum lycopersicum L.) [30], and forage grass (Lolium rigidum L.) [31], resulting in an important source of nutrients. The increase in lettuce shoot biomass might be associated with a slow release of nutrients from the digestate that occurred during the development of the crop, as suggested by Mortola et al. [5]. The addition of a low Cu concentration to the soil (S+Cu) and manure (M+Cu) resulted in a significant reduction in shoot biomass compared to the soil (S) and manure (M) (32.1% and 30.9%, respectively), highlighting a harmful impact of copper sulphate (30 mg kg−1) on biomass production. Instead, no significant difference was shown in the shoot biomass of the lettuce plants grown in the presence of digestate with or without copper. These results are in line with those reported by Gharbi et al. [32] and Shams et al. [33], who observed a decline in the biomass production of lettuce plants exposed to increasing Cu concentrations (100–1000 mg kg−1) for 15 days and 50 and 100 mg kg−1 Cu for 30 days, respectively.
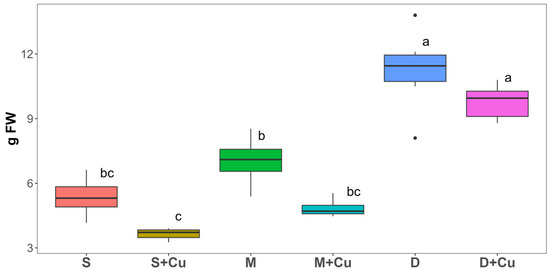
Figure 4.
Shoot biomass (g FW) of lettuce plants grown for five weeks in soil (S), manure (M), and digestate (D) and with the addition of copper (Cu). Box plots labelled with different letters indicate significant differences between different treatments (ANOVA followed by Tukey HSD, p value ≤ 0.05). In the box-plots, the upper and lower whisker endpoints represent the maximum and minimum observations, respectively, outliers are shown as dots. n = 4.
No significant difference in plant height was observed between the soil (S), manure (M), and digestate (D) treatments. A significant downward trend in plant height was detected in S+Cu and M+Cu, at 19.12% and 11.36%, respectively, compared to the S and M conditions. Instead, the addition of copper to digestate significantly increased the height of the lettuce plants (3.8%) (Table 2). The number of leaves significantly increased in the presence of M and D, at 40.9% and 46.5%, respectively, with respect to the lettuce plants grown in the S. Copper addition to S, M, and D did not cause changes in the numbers of leaves compared to the respective uncontaminated soil improvers (Table 3). It is to be noted that the numbers of lettuce leaves in S+Cu were lower than those detected for the plants grown in M+Cu and D+Cu. Considering the specific leaf area (SLA) parameter, no significant difference was observed between the S, M, and D conditions. Accordingly, the addition of copper did not induce any effect on the SLA values (Table 3). The analyzed growth parameters (height, number of leaves, and SLA) were consistent with the previously measured biomass results. Previous studies have indicated that the use of soil organic fertilizer contributes to the better development of crop plants such as lettuce [14], eggplant, Shanghai cabbage [34], and tomato [35]. This might be ascribed to the high nutrient and organic matter contents of amendments, which optimize both the deficit and excess nutrients in the soil [4]. The addition of a low copper amount adversely affected lettuce growth both in the soil and in manure, interfering with plant development and associated metabolic processes [10].

Table 3.
Shoot height (cm), leaf density (N° leaves), and specific leaf area (SLA, cm2 g−1 DW) for lettuces plants collected from different soil conditions: S, M, and D; and in presence of copper (Cu): S+Cu, M+Cu, and D+Cu.
3.2. Chlorophyll Content
A downward trend in the chlorophyll content was observed under the M (9%) treatment compared with S (Figure 5). The addition of copper significantly reduced the chlorophyll content both in S+Cu and M+Cu (18,48% and 12.48%, respectively) compared to their respective uncontaminated soil improvers. A reduction in the photosynthetic pigment content suggested a significant inhibition of photosynthesis, thus affecting the plant growth, as reported by Slamet et al. [36] Indeed, the pattern observed in the plant biomass production (Figure 5) was consistent with that concerning the chlorophyll content (Figure 5). The addition of copper to the soil and manure induced chlorophyll and lettuce biomass reductions, even if the Cu concentration was lower than that reported in the literature (from 200 mg kg−1), which causes toxicity [33]. In agreement with the study by Li et al. [37], low copper concentrations (25–50 mg kg−1) might induce a hormesis effect and a consequent reduction in biomass and pigment contents. The chlorophyll content was significantly increased in lettuce plants grown with D (32%) with respect to S (Figure 5). No significant difference was highlighted in the chlorophyll content measured in the lettuce plants grown with digestate in the absence and presence of copper. The increase in chlorophyll content in the lettuce plants grown with digestate and copper was 30% with respect to S (Figure 5). Chlorophyll is an essential pigment for plant growth and a higher chlorophyll content is usually linked to an increase in plant biomass accumulation. Digestate contains available nitrogen and phosphorus, therefore, its use can stimulate photosynthesis, thus enhancing the chlorophyll content and biomass in a range of vegetable crops, including lettuce (Lactuca sativa L.) [14], tomato (Solanum lycopersicum L.) [38], Spring Wheat (Triticum aestivum L.) [39], and aubergine (Solanum melongena L.) [28].
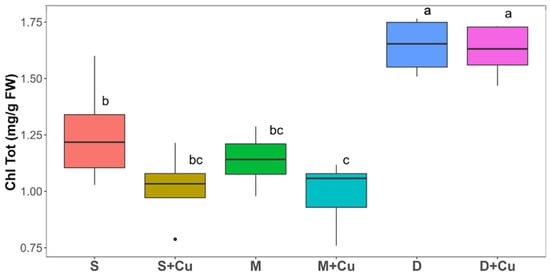
Figure 5.
Total chlorophyll content (mg g−1 FW) of lettuce plants grown for five weeks in soil (S), manure (M), and digestate (D) and with the addition of copper (Cu). Box plots labelled with different letters indicate significant differences between different treatments (ANOVA followed by Tukey HSD, p value ≤ 0.05). In the box-plots, the upper and lower whisker endpoints represent the maximum and minimum observations, respectively, outliers are shown as dots. n = 4.
3.3. Chlorophyll Fluorescence Analysis
In order to study the effect of the soil amendments on the photosynthetic performance of the lettuce plants exposed to a low Cu concentration, the bioenergetic parameters obtained from the JIP test were measured. In particular, some structural and functional parameters are presented in the form of a radar plot (Figure 6), where all values have been normalized to those obtained from the control plants.
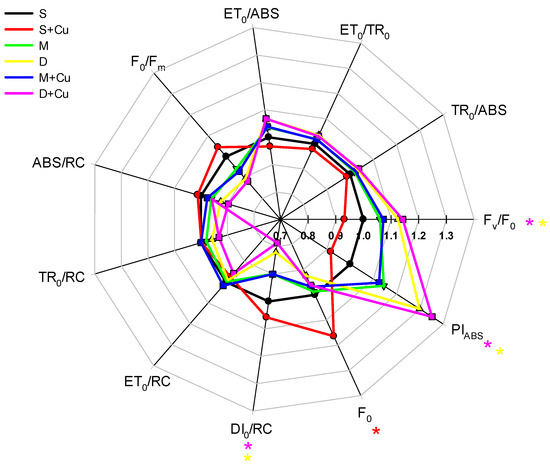
Figure 6.
Changes in the shape of the radar plot JIP-test parameters induced by different amendment treatments with or without the addition of a low copper concentrations applied to Lactuca sativa plants and measured at the end of the treatment. The data are the average of ten replicates and report the values with respect to plants grown in the control condition (S = control = 1). Red, pink, and yellow asterisks indicate significant differences (LSD test, p < 0.05) between control plants (S) and those exposed to S+Cu, D, or D+Cu, respectively.
A chlorophyll a fluorescence-transient analysis is a useful method for examining the physiological aspects of structure and activity, particularly in the PSII [20]. It has been extensively employed to assess damage to the plant photosynthetic system caused by various types of stress [40]. The exposure of lettuce plants to a low copper concentration, as in S+Cu, caused a slight change in most of the parameters analyzed, except for F0, which exhibited a significant increase compared to the control (S) plants (Figure 6). As reported in the literature, the absence of an F0 variation indicates a good ability of the treated plants to maintain the efficiency of energy transfer between the pigments of the antenna and the PSII reaction center without structural damage at the photosystem level [41]. However, an increase in F0 can be interpreted as indicating irreversible damage to PSII caused by uncontrolled dissipation of heat that produces an excess of excitation energy [42,43], while a decrease in F0 is, on the contrary, an indication of a high-energy dissipation in the minor antenna [44]. The data relating to the specific energy fluxes, such as the absorption flux per reaction center (ABS/RC), trapped energy flux per reaction center (TR0/RC), electron transport flux per reaction center (ET0/RC), and dissipated energy flux per reaction center (DI0/RC), measured to determine the photosynthetic performance of the active PSII reaction centers of the lettuce plants subjected to Cu treatment (S+Cu), did not show significant variations with respect to the control plants (Figure 6). Nevertheless, although copper treatment was found to not significantly impact the other analyzed parameters in the S+Cu plants, such as the maximum quantum yield of primary photochemical reactions (TR0/ABS), the maximum quantum yield for electron transport (ET0/ABS), the probability that an electron moves further than QA (ET0/TR0), and the light energy used for thermal energy dissipation (F0/Fm), both the (Fv/F0) and the performance index PIABS showed a slight decrease compared to the control, indicating a reduction in the efficiency of water splitting by the PSII and the overall vital state of the plant [45].
The exposure of lettuce plants to manure amendments in the soil, without or with the addition of a low copper concentration (M or M+Cu, respectively), resulted in a partial improvement in the plant physiological status. Compared to the plants grown in the S+Cu treatment, some of the analyzed parameters in the plants grown under the M and M+Cu treatments showed a positive impact on the plant viability but did not significantly differ from the control plants. These results are consistent with the biomass and chlorophyll content data, which indicate similar grown patterns across the treatments (S, S+Cu, M, and M+Cu).
On the contrary, the exposure of the lettuce plants to digestate amendments in soil, without or with the addition of a low copper concentration (D or D+Cu, respectively), significantly enhanced the plant physiological performance.
Several chlorophyll fluorescence parameters measured in the plants grown under the D or D+Cu treatments were found to be significantly different from those of the control plants (S), indicating a beneficial effect on the plant viability due to the digestate amendment.
Indeed, in our study, the functionality of both photosystems I and II was noticeably higher in the D- and D+Cu-treated lettuce plants, as indicated by the increase in the performance index PIABS and in the Fv/F0 values compared to the control [14]. Additionally, the decrease in the dissipated energy flux per reaction center (DI0/RC) indicated a greater ability to use the energy by the active RCs [46]. The data on the biomass and chlorophyll content confirm these findings, showing an increase in biomass production and pigment content in the plants treated with D or D+Cu compared to the controls.
The results obtained in this experiment are consistent with previous studies that demonstrate the positive impact of digestate addition on crop growth [47,48,49]. Notably, significant increases in biomass have been reported for wheat (Triticum aestivum L.) [50], giant Napier (Pennisetum purpureum Schumach) [51], tomato (Solanum lycopersicum L.), and cucumber (Cucumis sativus L.) [52].
3.4. Measurement of Lipid Peroxidation Levels
The malondialdehyde (MDA) content increased significantly in the manure (M) and digestate (D) conditions (by 32% and 86%, respectively) compared to the control condition (S), (Table 3). Malondialdehyde is a natural by-product of cell membrane lipid peroxidation, usually analyzed to assess the damage caused by oxidative stress in plant tissues [14]. The addition of low concentrations of copper significantly reduced the MDA content in the lettuces grown in S+Cu (22%) compared to soil (S). In the M+Cu condition, the measured MDA values were significantly reduced (66%) when compared with M, and no significant difference was detected compared to the control (S), highlighting a partial improvement of the plant physiological performance, strictly consistent with previous results concerning the chlorophyll fluorescence analysis. The MDA content in the lettuces planted in D+Cu did not differ significantly from the digestate (D) conditions, maintaining high MDA levels. If MDA levels persist to be high, irreversible modifications to proteins and nucleic acids occur. However, if the scavenging system composed of enzymatic and non-enzymatic systems synergistically works to maintain intra- and extracellular redox homoeostasis, MDA increases, and its maintenance may represent an acclimation process rather than damage, as suggested by Morales et al. [53] and Liang et al. [54]. Consequently, MDA might act as a protection factor exerting a positive role by activating the regulatory genes involved in plant defense and development according to Tagnon et al. [55]. Additional parameters, such as growth measurements and chlorophyll fluorescence analyses, in plants grown under the D and D+Cu treatments showed a beneficial effect on plant vitality due to the presence of digestate when compared to the S and M conditions with and without copper.
3.5. Estimation of Total Phenolic Content
Lettuces of different varieties have different total phenol contents due to genetic characteristics and different agronomic practices, such as fertilizer application, which could influence the nutritional status of the crop, modulating the antioxidant and phenol contents [56]. Phenols are secondary metabolites used by plants, along with antioxidants, to defend against oxidative or abiotic stresses [28]. Lettuces grown with digestate (D) and digestate plus copper (D+Cu) showed the highest content of total phenolic content, (20% and 23% respectively) compared to the control condition (S). The phenolic content in the lettuce plants did not change significantly between the different soil conditions, with or without copper addition (Table 4). These findings agreed with the results obtained for cucumber [57] and lettuce plants [58], where organic fertilizers enhanced the total phenol content, since they are rich in carbon and phenols, and stimulated plant antioxidant activity. Phenols can be found in organic fertilizer from the biological degradation of several xenobiotic substances, like pesticides, or naturally occurring aromatic amino acids and polymers in plant materials such as lignin, tannins, and humic acids [28].

Table 4.
Measurement of lipid peroxidation levels (MDA, nmol MDA g−1 FW) and estimation of total phenolic content (TPC, mg GAE g−1 FW) for lettuces plants collected from different soil conditions: S, M, and D; and in presence of copper (Cu): S+Cu, M+Cu, and D+Cu.
3.6. Copper Determination, Bioaccumulation Factor, and Tolerance Index in Lettuce
Copper is an essential element for plant nutrition, needed as a cofactor for many enzymatic activities catalyzing redox reactions and for photosynthetic functions. Excess copper induces high levels of reactive oxygen species (ROS) and negatively affects the photosystem during photosynthesis [10]. About Cu concentration in soil any differences were detected in S, M and D (Table S2). Although copper addition increased its concentration in different soils, no significant difference was observed between them (Table S2). The lettuce plants grown under the control condition (S) showed the lowest Cu accumulation (1.1 ± 0.2 mg kg−1) (Table 5). Compared to the S condition, in lettuce plants grown with M and D, a marked increase in Cu concentration was observed, following the order of D > M > S. The addition of a low Cu concentration to different soils (S+Cu, M+Cu, and D+Cu) induced a significant enhancement in copper accumulation in the lettuce plants, following the order of M+Cu > D+Cu > S+Cu > S.

Table 5.
Copper content in leaves (Cu, mg kg−1 DW), bioaccumulation factor (BAF) of Cu (BAFCu), and tolerance index (Ti) for lettuces plants collected from different soil conditions: S, M, and D; and in presence of copper (Cu): S+Cu, M+Cu, and D+Cu.
Based on the Cu contents in the plant shoot and soil, the bioaccumulation factor (BAF) and tolerance index (Ti) were calculated to evaluate the metal uptake and plant tolerance to Cu stress. The highest BAF was detected in the lettuce plants grown with M soil (0.23 ± 0.09 mg kg−1) compared to the different uncontaminated soils (Table 5). The addition of a low Cu concentration caused a significant enhancement in the BAF value in the lettuces grown with S+Cu compared to the S condition, not statistically different from those occurring in the M+Cu and D+Cu soils. It is well known that the BAF value depends on the tested metal, plant species, soil pollution level, and soil acidity, as reported by Sipter et al. [58,59]. Nevertheless, in this study, the BAF value in the plant leaves was <1, revealing that the lettuces were poor accumulators [60,61] (Table 5). The plant capability to limit Cu’s damaging effects was very different among soil conditions, as evidenced by the tolerance index (Ti) (Table 5). The lettuce plants grown in D+Cu soil showed the highest Ti values with respect to the S+Cu and M+Cu conditions, even though the respective BAF values were similar. These results are consistent with data concerning plant growth and physiological parameters, suggesting that the presence of digestate in the soil reduced Cu’s phytotoxic effect, improving the plant physiological status. It is worth noting that the lowest Ti was observed in the lettuces planted in S+Cu, which corresponded to a 3.5-fold increase in the BAF value compared to the S conditions and a decrease in the growth parameters and chlorophyll content. Although the Cu concentration at which toxic effects become evident is plant-species- and soil-type-dependent [62], our findings agree with previous studies where plants with Ti values of <100 experienced stress due to metal contamination along with a net reduction in plant biomass. Conversely, the plant species with a Ti of >100 developed tolerance along with a net biomass increase and improved physiological performance [63,64].
3.7. Macro- and Micro-Nutrient Content in Lettuce Plants
It is well established that plant growth and nutritional quality are related to mineral accumulation. Macronutrients such as Ca, K, Mg, P, and S carry out important functions in several metabolic pathways, including photosynthesis, growth, and stress tolerance, and are required in large quantities by plants [65]. Compared to macronutrients, micronutrients (Cu, Fe, Mn, Na, Zn, Al, Cd, Cr, Ni, and Si) are required in relatively lesser amounts and, likewise, they play key roles in plant growth and metabolism [66]. The levels of chemical elements recorded in the lettuce plants used in this study were all below the WHO safe limit of the selected heavy metals in vegetables for human consumption [67]. Macro- and micronutrient uptake in the lettuce leaves was reported as a radar plot (Figure 7), where all values were normalized to those obtained from the control plants (S) (Table S4). In general, the uptake of macro- and micronutrients varied significantly in the lettuce plants treated with different soil amendments in the absence or presence of a low Cu concentration, resulting to be higher in plants grown in S+Cu. Compared to the control plants (S), the presence of the amendments mainly affected the uptake of micronutrients, such as Cr, Ni, Cu, and Cd. In particular, under the M treatment, the leaves of lettuce plants showed reductions to 36.7% and 41.7% in Ni and Cd concentrations, respectively, whereas an enhancement was observed in Cr and Cu uptake (up to 45.7% and 91%, respectively) with respect to the control (S). Instead, in the lettuce plants exposed to D, marked reductions were detected in Ni and Cd uptake (up to 50% and 66%, respectively) whereas Cr and Cu concentrations increased to 25% and more than twice, respectively, compared to the control (S). It is worth noting that, under the D and D+Cu treatments, the plants showed significant decreases also in Al, Zn, Na, Mn, and Fe uptake (13–16%, 14–20%, 10–12%, 14–17%, and 17–19%, respectively). As described in the literature, the uptake of inorganic nutrients and metals is dependent on both the metal and soil, and strongly influenced by the amount of water transpired by the plant. As a consequence of the rise in temperature and the subsequent increase in transpiration, there is the potential for an increase in the transfer of copper from the soil to the plant [16]. The reduction in micronutrients in the lettuce leaves could likely be ascribed to their lower solubility due to the higher digestate pH compared to the soil one (Table S1), as reported by Olszyk et al. [68], who observed a decrease in Fe, Mn, and Zn concentrations, mainly, in lettuce plants grown in the presence of several biochars as amendments. Similarly, a reduction in Fe uptake was detected in baby leaf lettuce under different digestate treatments [69,70]. The addition of a low Cu concentration (M+Cu and D+Cu) caused in the plant leaves a two-fold increase in Cu uptake with respect to the control (S). Moreover, as reported previously, our findings highlighted that the Cu concentration was higher in the plants grown in M+Cu compared to those exposed to S+Cu and D+Cu.
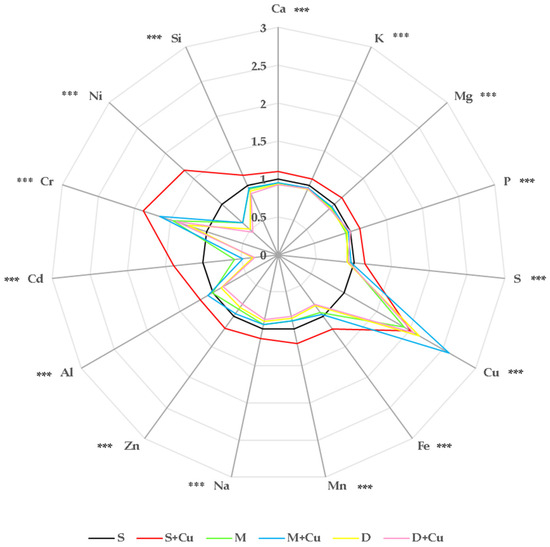
Figure 7.
Changes in the shape of the radar plot relative to plant uptake of macro- and micronutrients induced by different amendment treatments with or without the addition of a low copper concentrations applied to lettuce plants and measured at the end of the treatment. The data are the average of four replicates and report the values with respect to plants grown in the control condition (S = control = 1). Asterisks indicate significant differences (Tukey HSD, p < 0.05) between control plants (S) and those exposed to M, M+Cu, D, and D+Cu.
3.8. Microbiological Analyses of Soil Community
At the end of the experiment, after five weeks, the total microbial abundance was not significantly different between the tested soils (S, M, and D) (Figure 8a). The addition of a low Cu concentration significantly reduced the microbial abundance in the S+Cu soil (36%) whereas a significant enhancement was observed in the M+Cu soil (35%) compared to S and M, respectively. The higher microbial abundance in M+Cu with respect to S+Cu could be ascribed to the presence of copper-resistant bacteria in the manure [71]. Moreover, organic soil amendments could immobilize metals, reducing their bioavailability, due to the presence of humic acids able to chelate a wide variety of metals, including copper [72]. No significant difference in microbial abundance was highlighted between the D and D+Cu soils.
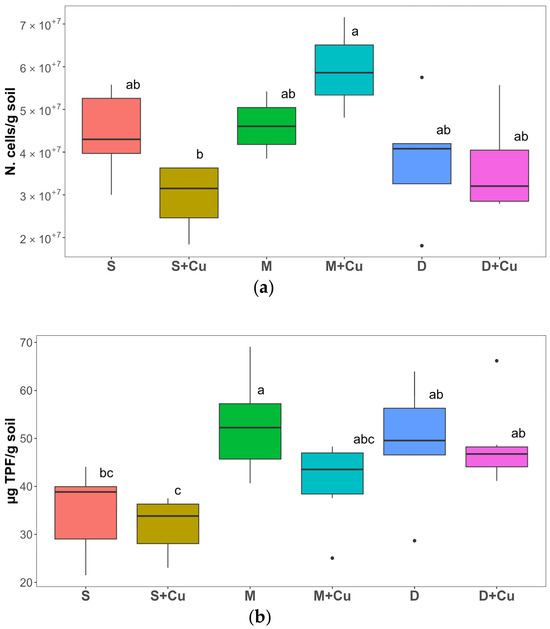
Figure 8.
(a) Soil total microbial abundance (N. cells g−1 dry soil) and (b) soil microbial activity (ug TPF g−1 dry soil) measured in soil (S), manure (M), and digestate (D) and with the addition of copper (Cu) after five weeks of experimentation. Box plots labelled with different letters indicate significant differences between different treatments (ANOVA followed by Tukey HSD, p value ≤ 0.05). In the box-plots, the upper and lower whisker endpoints represent the maximum and minimum observations, respectively, outliers are shown as dots. n = 4.
Both cattle organic soil amendments, manure (M) and digestate (D), showed higher soil microbial activity, 51.24% and 41.31%, respectively, when compared to the non-amended soil (S). Following Cu addition (S+Cu, M+Cu, and D+Cu), a downward trend in dehydrogenase activity was observed compared to the respective soils without amendments (S, M, and D, respectively) (Figure 8b). Dehydrogenase activity (DHA) is a biomarker related to soil fertility and measures the metabolic state of soil microbial communities. In addition, this enzyme reflects the total range of oxidative activity of soil microorganisms [73]. Soil microflora contributes to the improvement of plant physiological performance status. Any application of a toxicant that might affect the growth of soil microorganisms can induce alterations in the general activity of enzymes, such as dehydrogenase [74]. As reported in previous works, DHA activity is increased by the addition of organic amendments in the soil [73], such as the use of manure to fertilize winter rye, potato, oats, barley, and red clover in crop rotations. Heavy metals, including copper, might negatively affect soil bacterial communities by reducing their populations, changing their structure and diversity, decreasing soil enzyme activity, and interfering in plant–soil–metal associations [70].
3.9. Principal Component Analysis
The first two components of the PCA analysis explained 68.3% of the overall variance in the lettuce dataset (Dim 1: 43.1%; Dim 2: 25.2%) (Figure 9). The contribution (contrib) values of the plant performance parameters showed that shoot biomass, MDA, total chlorophyll, number of leaves, DHA, and DAPI were well represented in Dim1 and Dim2, in contrast with SLA, TPC, and the copper content in leaves (Figure 9a). A correlation analysis of the plant performance parameters (Figure S1) demonstrated that shoot biomass, MDA, total chlorophyll, number of leaves, and DHA were significantly positively correlated with Dim1, while SLA, TPC, DAPI, and leaves’ copper content were significantly negatively correlated with Dim1. A correlation analysis of Dim2 revealed that TPC, DAPI, DHA, and leaves’ number correlated positively, whereas SLA, copper content in leaves, shoot biomass, MDA, and Chl Tot were negatively correlated. The outcomes of the PCA clearly discriminated the samples according to soil composition, showing six clusters, which reflected the different soil conditions plus the control (S) (Figure 9b). The profile of the lettuce plants grown in D and D+Cu clustered in a Dim1-positive direction, while those grown in S, S+Cu, and M+Cu clustered in a Dim1-negative direction. Plants grown in D and D+Cu were separated from the control (S) and manure (M) plants grown with or without copper towards number of leaves, shoot biomass, Chl tot, MDA, and DHA. Similarly, separation of the control (S) and manure (M) plants grown with or without copper was observed towards TPC, copper content in leaves, SLA, and DAPI. Treatment with D and D+Cu resulted in a better physiological performance in the lettuce plants with respect to the manure treatments and control conditions. The second axis (Dim2) was most closely related to DAPI and TPC, and the samples segregation on Dim2 showed that manure had the highest microbial abundance.
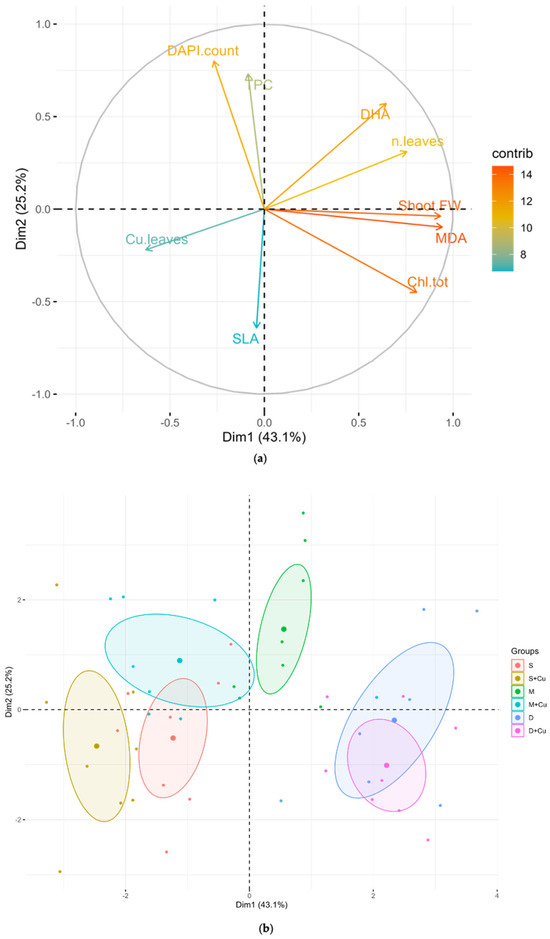
Figure 9.
(a) Variable correlation plot, constructed using the following variables: shoot fresh biomass (Shoot FW), total chlorophyll content (Chl Tot), specific leaf area (SLA), malondialdehyde content (MDA), copper content in leaves (Cu leaves), leaves number (n.leaves), total phenolic content (TPC), soil total microbial abundance (DAPI count), and soil microbial dehydrogenase activity (DHA). The length of each vector represents the contribution of the corresponding variable on the principal component (Dim1 and Dim2), while the direction of each vector indicates the correlation between the variable and the principal components. Vector colors represent the contribution of the corresponding variable to the overall variability. (b) Principal component analysis (PCA) showing samples’ segregation by soil composition (S, S+Cu, M, M+Cu, D, and D+Cu). Data points are grouped into colored ellipses representing a 95% confidence interval. Enlarged points within ellipses represent the centroids of the sample distributions grouped by soil composition. The legend provides a color code for each group.
4. Conclusions
This study investigated the effects of growing lettuce plants with different combinations of cattle organic soil amendments, cattle manure (M) or digestate (D), and low concentrations of copper, like those found in agricultural soils used for the cultivation of horticultural species treated with, i.e., copper-based fungicides. The results showed that both manure and digestate affected the lettuce plants very differently. While manure showed negative effects overall, the application of digestate resulted in an increased yield and improved the photosynthetic performance. Digestate application might have buffered the decrease in soil pH caused by fertilization, potentially mitigating soil acidification compared to manure amendment. In the M and M+Cu conditions, a decreased lettuce growth, a higher Cu bioaccumulation, and a reduced tolerance index were associated with increasing the Cu concentration in the soil, suggesting an increased level of Cu phytotoxicity in the plants and a detrimental impact compared to the plants grown in digestate and in the digestate-copper amended soil.
Our greenhouse pot study aligns with existing research on digestate’s potential, but field trials are necessary for real-world applicability.
In low- and middle-income countries, digestate offers a more advantageous alternative to manure for replacing chemical fertilizers. As a renewable and inexpensive source of nutrients and energy derived from organic materials, it promotes a circular economy. Large-scale validation is needed to confirm the benefits of anaerobic digestion for a circular economy, as shown by research on energy and fertilizer production from various organic materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments11070134/s1; Table S1: Main physico-chemical characteristics of soil, manure and digestate; Table S2: Elements content (mg kg−1) in different soils; Table S3: Elements content in leaves of lettuce plants grown in different soil conditions; Figure S1: Correlation between the variable and the principal components in PCA analyses.
Author Contributions
Conceptualization, P.G., A.B.C. and M.A.I.; methodology, P.G., A.B.C. and M.A.I.; validation, C.D.C. and M.A.I.; formal analysis, C.D.C., A.N., B.C., F.P., D.G. and V.I.; investigation C.D.C., A.N. and F.P.; resources, P.G., A.B.C., F.P. and M.A.I.; writing—original draft preparation, C.D.C., F.P., V.I. and M.A.I.; writing—review and editing, C.D.C., A.N., B.C., F.P., V.I., P.G., A.B.C. and M.A.I.; visualization, C.D.C., F.P., V.I. and M.A.I.; supervision, M.A.I.; funding acquisition, A.B.C. and M.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was carried out within the following research initiatives: (i) the Italian Ministry of University and Research, PON “Ricerca e Innovazione” 2014-2020 DM 1061/2021 Azione IV.5 “Dottorati su tematiche Green” (PhD scholarship awarded by Sapienza University of Rome to CDC); (ii) “Gruppi di Ricerca 2020” SMART-BREED Project A0375E0166 (POR FESR LAZIO 2014-2020); (iii) Agritech National Research Center, European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022)—SPOKE 1, Task 1.2.3.
Data Availability Statement
All the data supporting the reported results can be found in this article.
Acknowledgments
We thank: the Orto di Fabiana company (Rome, Italy) for providing L. sativa seedlings and soil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ruiz, D.; San Miguel, G.; Corona, B.; Gaitero, A.; Domínguez, A. Environmental and Economic Analysis of Power Generation in a Thermophilic Biogas Plant. Sci. Total Environ. 2018, 633, 1418–1428. [Google Scholar] [CrossRef]
- Mazzurco Miritana, V.; Patrolecco, L.; Barra Caracciolo, A.; Visca, A.; Piccinini, F.; Signorini, A.; Rosa, S.; Grenni, P.; Garbini, G.L.; Spataro, F.; et al. Effects of Ciprofloxacin Alone or in Mixture with Sulfamethoxazole on the Efficiency of Anaerobic Digestion and Its Microbial Community. Antibiotics 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Podjanapon, P.; Jamaree, T.; Sakhonwasee, S. Optimization of Liquid Digestate Fertigation and Light Intensity for Lettuce Cultivation in Closed Plant Production System. Environ. Control Biol. 2023, 61, 9–16. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Mallamaci, C.; Attinà, E.; Muscolo, A. Using Digestate as Fertilizer for a Sustainable Tomato Cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Mortola, N.; Romaniuk, R.; Cosentino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.; Bres, P.; Riera, N.; Roba, M.; Butti, M.; et al. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Kwoczynski, Z.; Burdová, H.; Al Souki, K.S.; Čmelík, J. Extracted Rapeseed Meal Biochar Combined with Digestate as a Soil Amendment: Effect on Lettuce (Lactuca sativa L.) Biomass Yield and Concentration of Bioavailable Element Fraction in the Soil. Sci. Hortic. 2024, 329, 113041. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential Sources of Anthropogenic Copper Inputs to European Agricultural Soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper Accumulation in Agricultural Soils: Risks for the Food Chain and Soil Microbial Populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and Biochemical Responses of Suaeda Fruticosa to Cadmium and Copper Stresses: Growth, Nutrient Uptake, Antioxidant Enzymes, Phytochelatin, and Glutathione Levels. Environ. Sci. Pollut. R. 2015, 22, 13058–13069. [Google Scholar] [CrossRef]
- Zvezdanovic, J.; Markovic, D.; Nikolic, G. Different Possibilities for the Formation of Complexes of Copper and Zinc with Chlorophyll inside Photosynthetic Organelles: Chloroplasts and Thylakoids. J. Serb. Chem. Soc. 2007, 72, 1053–1062. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, Toxicity and Tolerance in Plants and Management of Cu-Contaminated Soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Fedeli, R.; Celletti, S.; Loppi, S.; Vannini, A. Comparison of the Effect of Solid and Liquid Digestate on the Growth of Lettuce (Lactuca sativa L.) Plants. Agronomy 2023, 13, 782. [Google Scholar] [CrossRef]
- Faran, M.; Nadeem, M.; Manful, C.F.; Galagedara, L.; Thomas, R.H.; Cheema, M. Agronomic Performance and Phytochemical Profile of Lettuce Grown in Anaerobic Dairy Digestate. Agronomy 2023, 13, 182. [Google Scholar] [CrossRef]
- Peijnenburg, W.; Baerselman, R.; de Groot, A.; Jager, T.; Leenders, D.; Posthuma, L.; Van Veen, R. Quantification of Metal Bioavailability for Lettuce (Lactuca sativa L.) in Field Soils. Arch. Environ. Contam. Toxicol. 2000, 39, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the Belowground Microbial Community in a Poplar-Phytoremediation Strategy of a Multi-Contaminated Soil. Front. Microbiol. 2020, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guo, Z.; Xu, Z. Determined Methods of Chlorophyll from Lemma Paucicostata. Exp. Technol. Manag. 2007, 24, e31. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar]
- Bernabé-Antonio, A.; Sánchez-Sánchez, A.; Romero-Estrada, A.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Fuentes-Talavera, F.J.; Hurtado-Díaz, I.; Alvarez, L.; Cruz-Sosa, F. Establishment of a Cell Suspension Culture of Eysenhardtia platycarpa: Phytochemical Screening of Extracts and Evaluation of Antifungal Activity. Plants 2021, 10, 414. [Google Scholar] [CrossRef]
- Huang, W.-L.; Chang, W.-H.; Cheng, S.-F.; Li, H.-Y.; Chen, H.-L. Potential Risk of Consuming Vegetables Planted in Soil with Copper and Cadmium and the Influence on Vegetable Antioxidant Activity. Appl. Sci. 2021, 11, 3761. [Google Scholar] [CrossRef]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Giuliano, G.; Grenni, P.; Cremisini, C.; Ciccoli, R.; Ubaldi, C. Effect of Urea on Degradation of Terbuthylazine in Soil. Environ. Toxicol. Chem. 2005, 24, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in Microbial Community Structure and Functioning of a Semiarid Soil Due to the Use of Anaerobic Digestate Derived Composts and Rosemary Plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Grenni, P.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Barra Caracciolo, A. Effects of Wood Amendments on the Degradation of Terbuthylazine and on Soil Microbial Community Activity in a Clay Loam Soil. Water Air Soil. Pollut. 2012, 223, 5401–5412. [Google Scholar] [CrossRef]
- Vaish, B.; Srivastava, V.; Singh, U.K.; Gupta, S.K.; Chauhan, P.S.; Kothari, R.; Singh, R.P. Explicating the Fertilizer Potential of Anaerobic Digestate: Effect on Soil Nutrient Profile and Growth of Solanum melongena L. Environ. Technol. Innov. 2022, 27, 102471. [Google Scholar] [CrossRef]
- Coelho, J.J.; Hennessy, A.; Casey, I.; Woodcock, T.; Kennedy, N. Responses of Ryegrass, White Clover, Soil Plant Primary Macronutrients and Microbial Abundance to Application of Anaerobic Digestates, Cattle Slurry and Inorganic N-Fertiliser. Appl. Soil. Ecol. 2019, 144, 112–122. [Google Scholar] [CrossRef]
- Cristina, G.; Camelin, E.; Tommasi, T.; Fino, D.; Pugliese, M. Anaerobic Digestates from Sewage Sludge Used as Fertilizer on a Poor Alkaline Sandy Soil and on a Peat Substrate: Effects on Tomato Plants Growth and on Soil Properties. J. Environ. Manag. 2020, 269, 110767. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Mancilla-Leytón, J.M.; Jiménez-Rodríguez, A.; Borja, R.; Rincón, B. Reuse of the Digestate Obtained from the Biomethanization of Olive Mill Solid Waste (OMSW) as Soil Amendment or Fertilizer for the Cultivation of Forage Grass (Lolium rigidum Var. Wimmera). Sci. Total Environ. 2021, 792, 148465. [Google Scholar] [CrossRef]
- Gharbi, F.; Rejeb, S.; Ghorbal, M.H.; Morel, J.L. Plant Response to Copper Toxicity as Affected by Plant Species and Soil Type. J. Plant Nutr. 2005, 28, 379–392. [Google Scholar] [CrossRef]
- Shams, M.; Ekinci, M.; Turan, M.; Dursun, A.; Kul, R.; Yildirim, E. Growth, Nutrient Uptake and Enzyme Activity Response of Lettuce (Lactuca sativa L.) to Excess Copper. Environ. Sustain. 2019, 2, 67–73. [Google Scholar] [CrossRef]
- Jin, K.; Ran, Y.; Alengebawy, A.; Yang, G.; Jia, S.; Ai, P. Agro-Environmental Sustainability of Using Digestate Fertilizer for Solanaceous and Leafy Vegetables Cultivation: Insights on Fertilizer Efficiency and Risk Assessment. J. Environ. Manag. 2022, 320, 115895. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Z.; Zhu, X.; Li, G.; Zhang, W.; Chen, Y.; Ren, L.; Luo, S.; Lin, H.; Zhou, H.; et al. Improved Tomato Yield and Quality by Altering Soil Physicochemical Properties and Nitrification Processes in the Combined Use of Organic-Inorganic Fertilizers. Eur. J. Soil. Biol. 2022, 109, 103384. [Google Scholar] [CrossRef]
- Slamet, W.; Purbajanti, E.D.; Darmawati, A.; Fuskhah, E. Leaf Area Index, Chlorophyll, Photosynthesis Rate of Lettuce (Lactuca sativa L.) under N-Organic Fertilizer. Indian. J. Agric. Res. 2017, 51, 365–369. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Qiu, Y.; Zhao, Q.; Chen, D.; Wu, Z.; Peng, A.; Niazi, N.K.; Trakal, L.; Sakrabani, R.; Gao, B.; et al. Lead and Copper-Induced Hormetic Effect and Toxicity Mechanisms in Lettuce (Lactuca sativa L.) Grown in a Contaminated Soil. Sci. Total Environ. 2020, 741, 140440. [Google Scholar] [CrossRef] [PubMed]
- Tiong, Y.W.; Sharma, P.; Xu, S.; Bu, J.; An, S.; Foo, J.B.L.; Wee, B.K.; Wang, Y.; Lee, J.T.E.; Zhang, J.; et al. Enhancing Sustainable Crop Cultivation: The Impact of Renewable Soil Amendments and Digestate Fertilizer on Crop Growth and Nutrient Composition. Environ. Pollut. 2024, 342, 123132. [Google Scholar] [CrossRef] [PubMed]
- Pranckietienė, I.; Navickas, K.; Venslauskas, K.; Jodaugienė, D.; Buivydas, E.; Žalys, B.; Vagusevičienė, I. The Effect of Digestate from Liquid Cow Manure on Spring Wheat Chlorophyll Content, Soil Properties, and Risk of Leaching. Agronomy 2023, 13, 626. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Pietrini, F.; Carnevale, M.; Beni, C.; Zacchini, M.; Gallucci, F.; Santangelo, E. Effect of Different Copper Levels on Growth and Morpho-Physiological Parameters in Giant Reed (Arundo donax L.) in Semi-Hydroponic Mesocosm Experiment. Water 2019, 11, 1837. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Cascio, C.; Pollastrini, M.; Gravano, E.; Gerosa, G.; Marzuoli, R.; Nali, C.; Lorenzini, G.; Salvatori, E.; et al. Ozone Stress in Woody Plants Assessed with Chlorophyll a Fluorescence. A Critical Reassessment of Existing Data. Environ. Exp. Bot. 2011, 73, 19–30. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Hazlett, T.L.; Debrunner, P.G. Govindjee Comparative Time-Resolved Photosystem II Chlorophyll a Fluorescence Analyses Reveal Distinctive Differences between Photoinhibitory Reaction Center Damage and Xanthophyll Cycle-Dependent Energy Dissipation. Photochem. Photobiol. 1996, 64, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, D.; Soni, V. Performance of Chlorophyll a Fluorescence Parameters in Lemna Minor under Heavy Metal Stress Induced by Various Concentration of Copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Suorsa, M.; Jajoo, A.; Tikkanen, M.; Aro, E.-M. Light-Harvesting II Antenna Trimers Connect Energetically the Entire Photosynthetic Machinery—Including Both Photosystems II and I. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tian, Y.; Sun, Y.; Dong, L. Effects of Biogas Slurry Irrigation on Growth, Photosynthesis, and Nutrient Status of Perilla frutescens Seedlings. Commun. Soil. Sci. Plant Anal. 2013, 44, 3381–3390. [Google Scholar] [CrossRef]
- Malav, L.C.; Khan, S.A.; Gupta, N. Impacts of Biogas Slurry Application on Soil Environment, Yield and Nutritional Quality of Baby Corn. Soc. Plant Res. 2015, 74, 194. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, J.; Cui, J.; Wang, Y.; Zhou, J.; Ye, M.; Sun, M. Effects of Biogas Slurry Application on Peanut Yield, Soil Nutrients, Carbon Storage, and Microbial Activity in an Ultisol Soil in Southern China. J. Soils Sediments 2016, 16, 449–460. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.; Ibrahim, M.H.; Hashim, R. Land Application of Sewage Sludge: Physicochemical and Microbial Response. Rev. Environ. Contam. Toxicol. 2011, 214, 41–61. [Google Scholar]
- Tan, F.; Zhu, Q.; Guo, X.; He, L. Effects of Digestate on Biomass of a Selected Energy Crop and Soil Properties. J. Sci. Food Agric. 2021, 101, 927–936. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, S.; Wang, Y.; Wang, R. Advantages of the Integrated Pig-Biogas-Vegetable Greenhouse System in North China. Ecol. Eng. 2005, 24, 175–183. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Qian, R.; Wang, D.; Liu, L.; Sun, C.; Lin, X. Lipid-Derived Aldehydes: New Key Mediators of Plant Growth and Stress Responses. Biology 2022, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- Tagnon, M.D.; Simeon, K.O. Aldehyde Dehydrogenases May Modulate Signaling by Lipid Peroxidation-Derived Bioactive Aldehydes. Plant Signal Behav. 2017, 12, e1387707. [Google Scholar] [CrossRef] [PubMed]
- Nicoletto, C.; Santagata, S.; Zanin, G.; Sambo, P. Effect of the Anaerobic Digestion Residues Use on Lettuce Yield and Quality. Sci. Hortic. 2014, 180, 207–213. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè, A.; Muscolo, A. Use of Digestate as an Alternative to Mineral Fertilizer: Effects on Growth and Crop Quality. Arch. Agron. Soil. Sci. 2019, 65, 700–711. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Gong, P.; Imazumi, Y.; Na, R.; Shimizu, N. Comparative Effects of Mineral Fertilizer and Digestate on Growth, Antioxidant System, and Physiology of Lettuce under Salt Stress. Hortic. Environ. Biotechnol. 2023, 64, 379–391. [Google Scholar] [CrossRef]
- Sipter, E.; Auerbach, R.; Gruiz, K.; Mathe-Gaspar, G. Change of Bioaccumulation of Toxic Metals in Vegetables. Commun. Soil. Sci. Plant Anal. 2009, 40, 285–293. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Metal(Loid) Induced Toxicity and Defense Mechanisms in Spinacia oleracea L.: Ecological Hazard and Prospects for Phytoremediation. Ecotoxicol. Environ. Saf. 2019, 183, 109570. [Google Scholar] [CrossRef]
- Kikis, C.; Thalassinos, G.; Antoniadis, V. Soil Phytomining: Recent Developments—A Review. Soil. Syst. 2024, 8, 8. [Google Scholar] [CrossRef]
- Christiansen, K.S.; Borggaard, O.K.; Holm, P.E.; Vijver, M.G.; Hauschild, M.Z.; Peijnenburg, W.J.G.M. Experimental Determinations of Soil Copper Toxicity to Lettuce (Lactuca sativa) Growth in Highly Different Copper Spiked and Aged Soils. Environ. Sci. Pollut. R 2015, 22, 5283–5292. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Heavy Metal Phytoremediation from a Meta-Analytical Perspective. Environ. Pollut. 2007, 147, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Baretta, D.; Becegato, V.A.; Almeida, V.d.C.; Paulino, A.T. Copper/Zinc Bioaccumulation and the Effect of Phytotoxicity on the Growth of Lettuce (Lactuca sativa L.) in Non-Contaminated, Metal-Contaminated and Swine Manure-Enriched Soils. Water Air Soil. Pollut. 2017, 228, 152. [Google Scholar] [CrossRef]
- Niazi, P.; Monib, A. Function of Macronutrients in Plant Growth and Human. IJSDR Res. J. 2023, 8, 1265. [Google Scholar]
- Gomes, D.G.; Pieretti, J.C.; Rolim, W.R.; Seabra, A.B.; Oliveira, H.C. Advances in Nano-Based Delivery Systems of Micronutrients for a Greener Agriculture. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 111–143. [Google Scholar]
- Codex Stan 193-1995; General Standard for Contaminants and Toxins in Food and Feed. Codex Allimentarius: Rome, Italy, 2014. Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 28 April 2024).
- Olszyk, D.M.; Shiroyama, T.; Novak, J.M.; Cantrell, K.B.; Sigua, G.; Watts, D.W.; Johnson, M.G. Biochar Affects Essential Nutrients of Carrot Taproots and Lettuce Leaves. HortScience 2020, 55, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ntinas, G.K.; Bantis, F.; Koukounaras, A.; Kougias, P.G. Exploitation of Liquid Digestate as the Sole Nutrient Source for Floating Hydroponic Cultivation of Baby Lettuce (Lactuca sativa) in Greenhouses. Energies 2021, 14, 7199. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Q.; Chen, Z.; Fu, Q.; Bao, H. Response of Antibiotic Resistance to the Co-Exposure of Sulfamethoxazole and Copper during Swine Manure Composting. Sci. Total Environ. 2022, 805, 150086. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of Soil Amendments to Contaminated Soils for Heavy Metal Immobilization and Improved Soil Quality—A Critical Review. Soil. Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Järvan, M.; Edesi, L.; Adamson, A.; Võsa, T. Soil Microbial Communities and Dehydrogenase Activity Depending on Farming Systems. Plant Soil. Environ. 2014, 60, 459–463. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Moore, F.; González, M.-E.; Khan, N.; Curaqueo, G.; Sanchez-Monedero, M.; Rilling, J.; Morales, E.; Panichini, M.; Mutis, A.; Jorquera, M.; et al. Copper Immobilization by Biochar and Microbial Community Abundance in Metal-Contaminated Soils. Sci. Total Environ. 2018, 616–617, 960–969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).