Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive
Abstract
1. Introduction
2. Materials and Methods
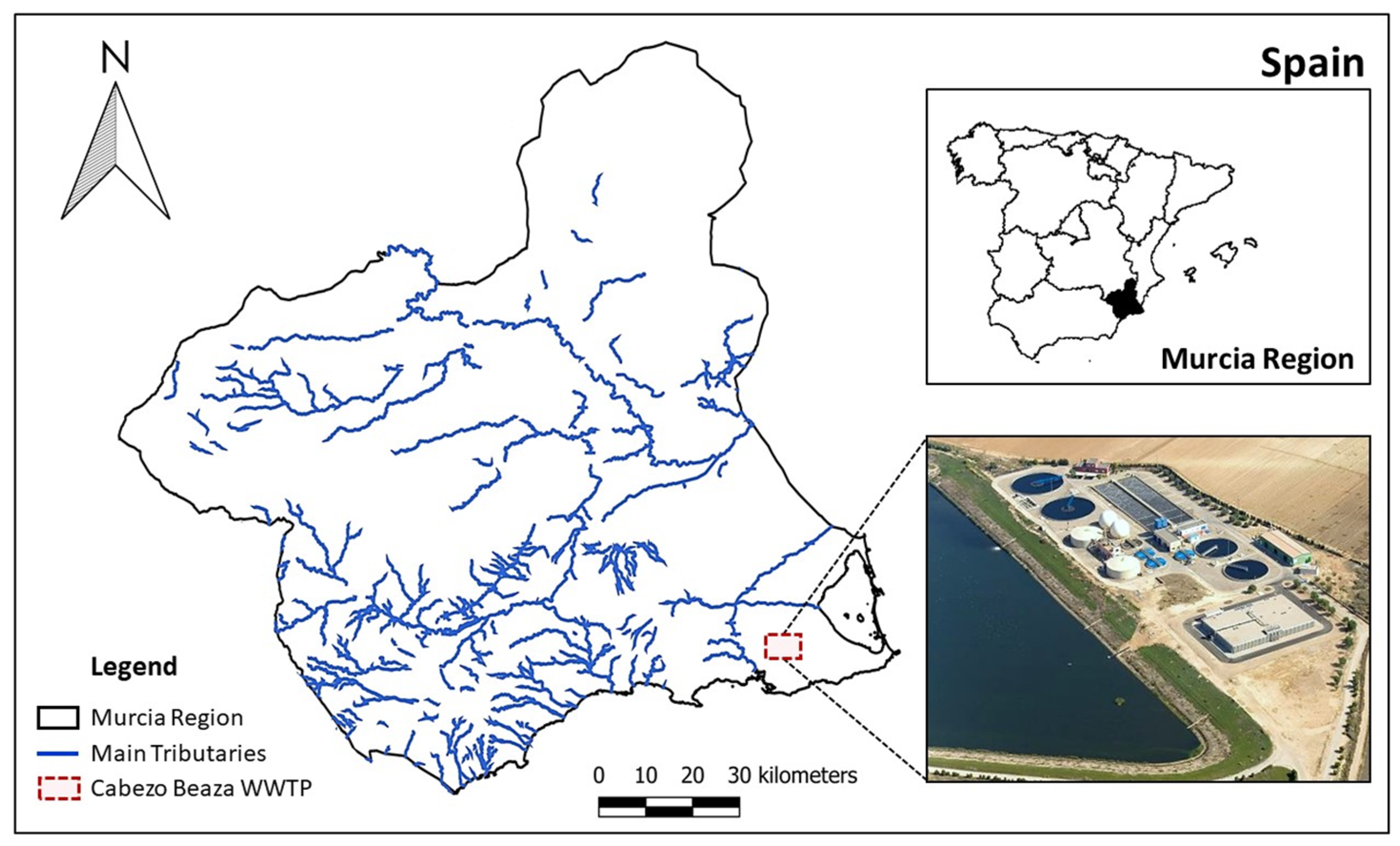
2.1. Description of the WWTP and Lagoons
2.2. Collection of Samples
2.3. Physicochemical Characterization
2.4. Micropollutants Analysis
2.4.1. Micropollutants Characterization
2.4.2. Quantitative Analysis
2.5. Estimation of Removal Efficiency
2.6. Statistical Analysis and Data Treatment
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. Micropollutants Results
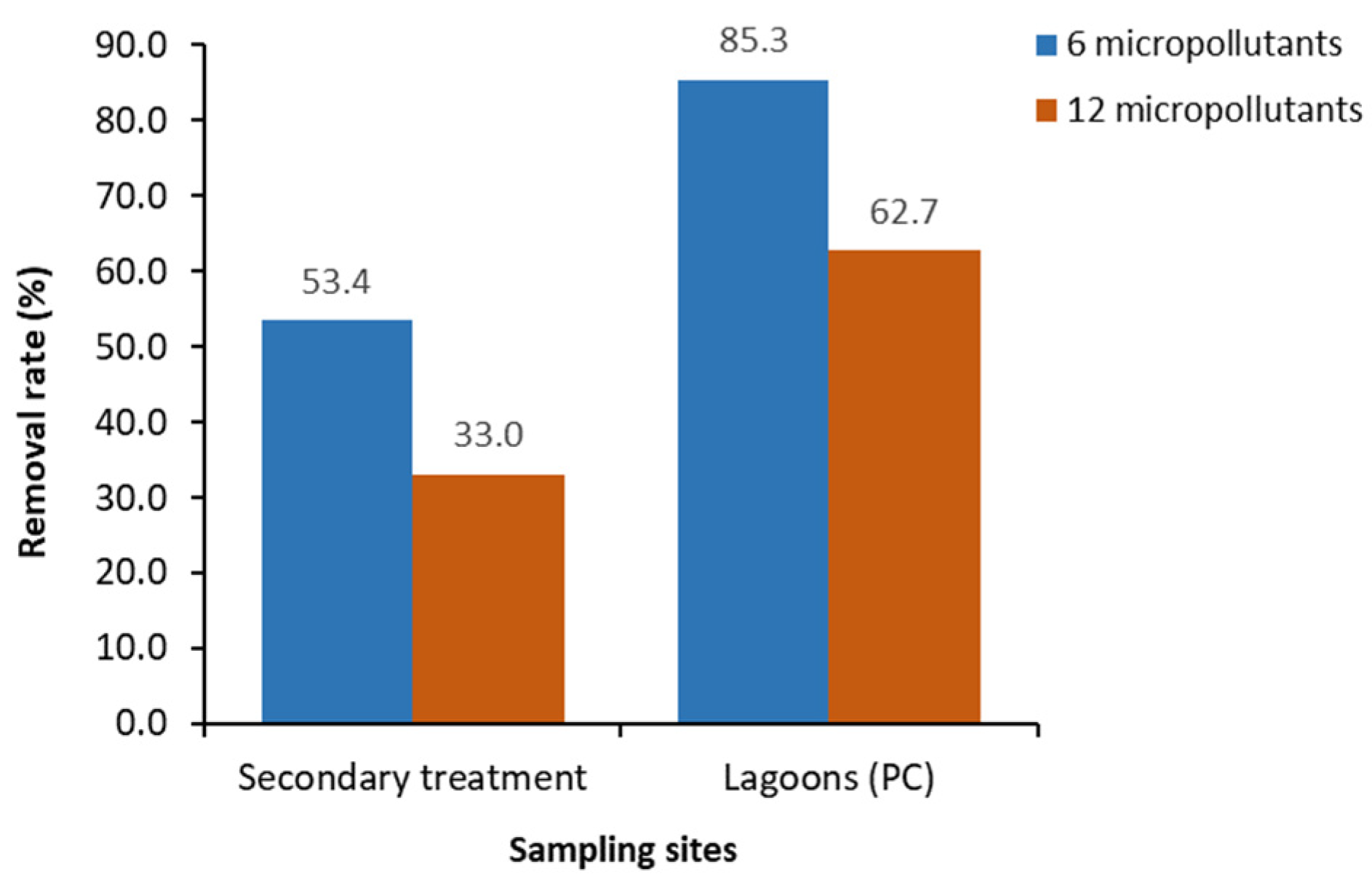
3.3. Lagoons as a Quaternary Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foglia, A.; González-Camejo, J.; Radini, S.; Sgroi, M.; Li, K.; Eusebi, A.L.; Fatone, F. Transforming Wastewater Treatment Plants into Reclaimed Water Facilities in Water-Unbalanced Regions. An Overview of Possibilities and Recommendations Focusing on the Italian Case. J. Clean. Prod. 2023, 410, 137264. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Vithanage, M.; Kapley, A. Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1907/2006—Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)|Safety and Health at Work EU-OSHA. Off. J. Eur. Union 2006. Available online: https://osha.europa.eu/es/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 20 March 2024).
- Jagadeeswaran, I.; Sriram, H. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC). In Medical Device Guidelines and Regulations Handbook; Springer International Publishing: Berlin, Germany, 2022; pp. 261–295. [Google Scholar] [CrossRef]
- López-Ruiz, S.; González-Gómez, F. Regenerate and Reuse Water in Spain: Facts and Politics. In Water Management and Circular Economy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 177–196. [Google Scholar] [CrossRef]
- Pistocchi, A.; Alygizakis, N.A.; Brack, W.; Boxall, A.; Cousins, I.T.; Drewes, J.E.; Finckh, S.; Gallé, T.; Launay, M.A.; McLachlan, M.S.; et al. European Scale Assessment of the Potential of Ozonation and Activated Carbon Treatment to Reduce Micropollutant Emissions with Wastewater. Sci. Total Environ. 2022, 848, 157124. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2021; Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX%3A52021DC0400 (accessed on 20 March 2024).
- Council of the European Union. Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast). Letter to the Chair of the European Parliament Committee on the Environment, Public Health and Food Safety (ENVI); Council of the European Union: Brussels, Belgium, 2024; pp. 1–148. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=consil%3AST_7108_2024_INIT (accessed on 18 March 2024).
- Wang, K.; Li, K.-Z.; Zhou, Y.-Y.; Liu, Z.-H.; Xue, G.; Gao, P. Adsorption characteristics of typical PPCPs onto river sediments and its influencing factors. Huan Jing Ke Xue 2015, 36, 847–854. [Google Scholar]
- Kavitha, V. Global Prevalence and Visible Light Mediated Photodegradation of Pharmaceuticals and Personal Care Products (PPCPs)—A Review. Results Eng. 2022, 14, 100469. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of Environmental Wastes: The Role of Microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; Lee, J.; Barathi, S. Microbial Degradation and Transformation of PPCPs in Aquatic Environment: A Review. Heliyon 2023, 9, e18426. [Google Scholar] [CrossRef]
- ESAMUR. Estación Depuradora de Aguas Residuales CABEZO BEAZA (CARTAGENA). Available online: https://www.esamur.com/esamur/edar/16A (accessed on 10 February 2024).
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization/International Electrotechnical Committee: Geneva, Switzerland, 2006.
- Götz, C.; Otto, J.; Singer, H. Überprüfung Des Reinigungseffekts. Auswahl Geeigneter Organischer Spurenstoffe. Aqua Gas 2015, 2, 34–40. [Google Scholar]
- Jezko, P.; Zufkova, V.; Remko, M. Modelling of Absorption, Distribution and Physicochemical Properties of AT1 Receptor Antagonists. Acta Fac. Pharm. Univ. Comenianae 2015, 62, 20–31. [Google Scholar] [CrossRef]
- DIN 38407-47; Bestimmung Ausgewählter Arzneimittelwirkstoffe Und Weitere Organischer Stoffe—Verfahren Mittels HPLC-MS/MS Oder—HRMS Nach Direktinjektion. Beuth Verlag: Berlin, Germany, 2017.
- Quintero, M.; Sánchez, C.; Soria, I. Eliminación de las pérdidas de agua en embalses con el uso combinado de impermeabi-lización y cubiertas flotantes. In Proceedings of the XXXVIII Congreso Nacional de Riegos, Cartagena, Spain, 3–5 November 2021. [Google Scholar] [CrossRef]
- Wijaya, I.M.W.; Soedjono, E.S. Physicochemical Characteristic of Municipal Wastewater in Tropical Area: Case Study of Surabaya City, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 135, 012018. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; González-Mazo, E.; Petrovic, M.; Barceló, D.; Brownawell, B.J. Occurrence, Distribution and Partitioning of Nonionic Surfactants and Pharmaceuticals in the Urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef]
- Xu, G.; Lu, K.; Li, Z.; Li, P.; Liu, H.; Cheng, S.; Ren, Z. Temporal Stability and Periodicity of Groundwater Electrical Conductivity in Luohuiqu Irrigation District, China. Clean—Soil Air Water 2015, 43, 995–1001. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Meulepas, R.J.W.; van Veelen, H.P.J.; Rijnaarts, H.H.M.; Sutton, N.B. Influence of Redox Condition and Inoculum on Micropollutant Biodegradation by Soil and Activated Sludge Communities. Sci. Total Environ. 2023, 897, 165233. [Google Scholar] [CrossRef]
- Burke, V.; Greskowiak, J.; Grünenbaum, N.; Massmann, G. Redox and Temperature Dependent Attenuation of Twenty Organic Micropollutants—A Systematic Column Study. Water Environ. Res. 2017, 89, 155–167. [Google Scholar] [CrossRef]
- Ghattas, A.K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic Biodegradation of (Emerging) Organic Contaminants in the Aquatic Environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, H.; Zhang, R.; Zhu, N. Removal of Ammonia Nitrogen from Wastewater: A Review. Trans. ASABE 2019, 62, 1767–1778. [Google Scholar] [CrossRef]
- Wang, F.; Bai, Y.; Yang, F.; Zhu, Q.; Zhao, Q.; Zhang, X.; Wei, Y.; Liao, H. Degradation of Nitrogen, Phosphorus, and Organic Matter in Urban River Sediments by Adding Microorganisms. Sustainability 2021, 13, 2580. [Google Scholar] [CrossRef]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the Degradation, Sorption, and Negative Mass Balances of Pharmaceuticals and Personal Care Products during Wastewater Treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Angeles, L.F.; Mullen, R.A.; Huang, I.J.; Wilson, C.; Khunjar, W.; Sirotkin, H.I.; McElroy, A.E.; Aga, D.S. Assessing Pharmaceutical Removal and Reduction in Toxicity Provided by Advanced Wastewater Treatment Systems. Environ. Sci. Water Res. Technol. 2019, 6, 62–77. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Callejón, M.; Alonso, E. Occurrence of Pharmaceutically Active Compounds during 1-Year Period in Wastewaters from Four Wastewater Treatment Plants in Seville (Spain). J. Hazard. Mater. 2009, 164, 1509–1516. [Google Scholar] [CrossRef]
- Sari, S.; Ozdemir, G.; Yangin-Gomec, C.; Zengin, G.E.; Topuz, E.; Aydin, E.; Pehlivanoglu-Mantas, E.; Okutman Tas, D. Seasonal Variation of Diclofenac Concentration and Its Relation with Wastewater Characteristics at Two Municipal Wastewater Treatment Plants in Turkey. J. Hazard. Mater. 2014, 272, 155–164. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Ejhed, H.; Fång, J.; Hansen, K.; Graae, L.; Rahmberg, M.; Magnér, J.; Dorgeloh, E.; Plaza, G. The Effect of Hydraulic Retention Time in Onsite Wastewater Treatment and Removal of Pharmaceuticals, Hormones and Phenolic Utility Substances. Sci. Total Environ. 2018, 618, 250–261. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union L 2015, 78, 40–42. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission. Off. J. Eur. Union L 2018, 141, 9–12. [Google Scholar]
- European Commission. Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2022, 197, 117–120. [Google Scholar]
- Pistocchi, A.; Andersen, H.R.; Bertanza, G.; Brander, A.; Choubert, J.M.; Cimbritz, M.; Drewes, J.E.; Koehler, C.; Krampe, J.; Launay, M.; et al. Treatment of Micropollutants in Wastewater: Balancing Effectiveness, Costs and Implications. Sci. Total Environ. 2022, 850, 157593. [Google Scholar] [CrossRef]
- Kienle, C.; Werner, I.; Fischer, S.; Lüthi, C.; Schifferli, A.; Besselink, H.; Langer, M.; McArdell, C.S.; Vermeirssen, E.L.M. Evaluation of a Full-Scale Wastewater Treatment Plant with Ozonation and Different Post-Treatments Using a Broad Range of in Vitro and in Vivo Bioassays. Water Res. 2022, 212, 118084. [Google Scholar] [CrossRef]
- Postigo, C.; Gil-Solsona, R.; Herrera-Batista, M.F.; Gago-Ferrero, P.; Alygizakis, N.; Ahrens, L.; Wiberg, K. A step forward in the detection of byproducts of anthropogenic organic micropollutants in chlorinated water. Trends Environ. Anal. Chem. 2021, 32, e00148. [Google Scholar] [CrossRef]
- Shao, B.; Shen, L.; Liu, Z.; Tang, L.; Tan, X.; Wang, D.; Weiming, Z.; Wu, T.; Pan, Y.; Zhang, X.; et al. Disinfection byproducts formation from emerging organic micropollutants during chlorine-based disinfection processes. Chem. Eng. J. 2023, 455, 140476. [Google Scholar] [CrossRef]
- Mahmoud, G.A.-E. Microbial Scavenging of Heavy Metals Using Bioremediation Strategies. In Rhizobiont in Bioremediation of Hazardous Waste; Springer: Singapore, 2021; pp. 265–289. [Google Scholar] [CrossRef]
- Men, Y.; Achermann, S.; Helbling, D.E.; Johnson, D.R.; Fenner, K. Relative Contribution of Ammonia Oxidizing Bacteria and Other Members of Nitrifying Activated Sludge Communities to Micropollutant Biotransformation. Water Res. 2017, 109, 217–226. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Stravs, M.A.; Pomati, F.; Hollender, J. Exploring Micropollutant Biotransformation in Three Freshwater Phytoplankton Species. Environ. Sci. Process. Impacts 2017, 19, 822–832. [Google Scholar] [CrossRef]
- Southwell, R.V.; Hilton, S.L.; Pearson, J.M.; Hand, L.H.; Bending, G.D. Water Flow Plays a Key Role in Determining Chemical Biodegradation in Water-Sediment Systems. Sci. Total Environ. 2023, 880, 163282. [Google Scholar] [CrossRef] [PubMed]
- Gattullo, C.E.; Bährs, H.; Steinberg, C.E.W.; Loffredo, E. Removal of Bisphenol A by the Freshwater Green Alga Monoraphidium Braunii and the Role of Natural Organic Matter. Sci. Total Environ. 2012, 416, 501–506. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Biodegradation of Levofloxacin by an Acclimated Freshwater Microalga, Chlorella Vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Singh, S.; Acharyya, T.; Gopinath, A. Phytoplankton Ecology in Indian Coastal Lagoons: A Review. Coast Res Libr. 2022, 38, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Alisawi, H.A.O. Performance of Wastewater Treatment during Variable Temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef]
- Díaz-López, C.; Jódar, J.; Verichev, K.; Rodríguez, M.L.; Carpio, M.; Zamorano, M. Dynamics of Changes in Climate Zones and Building Energy Demand. A Case Study in Spain. Appl. Sci. 2021, 11, 4261. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A Review of Factors Affecting the Release and Bioavailability of Contaminants during Sediment Disturbance Events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef]
- Dhir, B. Effective Control of Waterborne Pathogens by Aquatic Plants. In Waterborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 339–361. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Removal of Nitrogen from Wastewater Using Microalgae and Microalgae–Bacteria Consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of Ozonation for Pharmaceuticals and Personal Care Products Removal from Water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar]
- ANSE. Conservación de las Lagunas de Beaza—ANSE—Asociación de Naturalistas del Sureste. Available online: https://www.asociacionanse.org/proyectos/beaza/ (accessed on 20 February 2024).



| Category | Micropollutants | CAS N° | LogKow (25 °C) | pKa |
|---|---|---|---|---|
| Category 1 (substances that can be very easily treated) | Amisulpride | 71675-85-9 | – | 9.37 a |
| Carbamazepine | 298-46-4 | 2.45 b | <1–13.9 a | |
| Citalopram | 59729-33-8 | 1.39 a | 9.38–9.78 a | |
| Clarithromycin | 81103-11-9 | 3.16 a | 8.99 a | |
| Diclofenac | 15307-86-5 | 4.51 a | 4.15–4.3 a | |
| Hydrochlorothiazide | 58-93-5 | -0.07 a | 7.9–9.2 a | |
| Metoprolol | 37350-58-6 | 1.88 b | 9.5–10.09 a | |
| Venlafaxine | 93413-69-5 | 3.20 a | 9.56–9.7 a | |
| Category 2 (substances that can be easily disposed of) | Benzotriazole | 95-14-7 | 1.44 a | 8.37 a |
| Candesartan | 139481-59-7 | 4.79 a | 3.82–3.92 c | |
| Irbesartan | 138402-11-6 | 5.31 a | 4.08–4.29 a | |
| Methylbenzotriazole | ||||
| (4-Methylbenzotriazole | 29878-31-7 | – | 8.74 b | |
| 5-Methylbenzotriazole) | 136-85-6 | – | 8.74 b |
| WWTP | Lagoons | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Influent | Effluent | Lagoon 1 (L1) | Lagoon 2 (L2) | Post Chlorination (PC) | Removal Rate WWTP (%) | Removal Rate PC (%) |
| pH | 7.45 | 7.17 | 7.84 | 8.10 | 8.09 | NA | NA |
| Electrical Conductivity (EC) | 2467.23 | 2132.52 | 2141.33 | 2209.14 | 2148.98 | NA | NA |
| Oxidation–Reduction Potential (ORP) | −236.18 | 106.18 | 89.08 | 50.80 | 149.14 | NA | NA |
| Suspended Solids (SS) | 388.10 | 9.22 | 10.00 | 10.00 | 9.28 | 97.63 | 97.61 |
| Chemical Oxygen Demand (COD) | 725.28 | 35.06 | 33.00 | 31.00 | 25.00 | 95.17 | 96.55 |
| Biochemical Oxygen Demand (BOD5) | 364.09 | 6.83 | 5.00 | 5.00 | 7.96 | 98.13 | 97.81 |
| Ammoniacal nitrogen (NH4-N) | 65.17 | 19.23 | 20.80 | 17.80 | 9.67 | 70.49 | 85.16 |
| Nitrate nitrogen (NO3-N) | 2.50 | 31.69 | 3.50 | 2.53 | 12.75 | NA | NA |
| Total Nitrogen | 76.82 | 27.99 | 26.00 | 22.00 | 10.35 | 63.56 | 86.53 |
| Total Phosphorous | 10.67 | 1.18 | 1.35 | 2.10 | 1.15 | 88.94 | 89.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Gamboa, L.; Martínez-López, S.; Ayuso-García, L.M.; Lahora, A.; Martínez-Alcalá, I. Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive. Environments 2024, 11, 105. https://doi.org/10.3390/environments11060105
Díaz-Gamboa L, Martínez-López S, Ayuso-García LM, Lahora A, Martínez-Alcalá I. Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive. Environments. 2024; 11(6):105. https://doi.org/10.3390/environments11060105
Chicago/Turabian StyleDíaz-Gamboa, Lissette, Sofía Martínez-López, Luis Miguel Ayuso-García, Agustín Lahora, and Isabel Martínez-Alcalá. 2024. "Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive" Environments 11, no. 6: 105. https://doi.org/10.3390/environments11060105
APA StyleDíaz-Gamboa, L., Martínez-López, S., Ayuso-García, L. M., Lahora, A., & Martínez-Alcalá, I. (2024). Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive. Environments, 11(6), 105. https://doi.org/10.3390/environments11060105








