Disentangling the Effects of Multiple Impacts of Natural Flooding on a Riverine Floodplain Lake by Applying the Phytoplankton Functional Approach
Abstract
1. Introduction
2. Materials and Methods
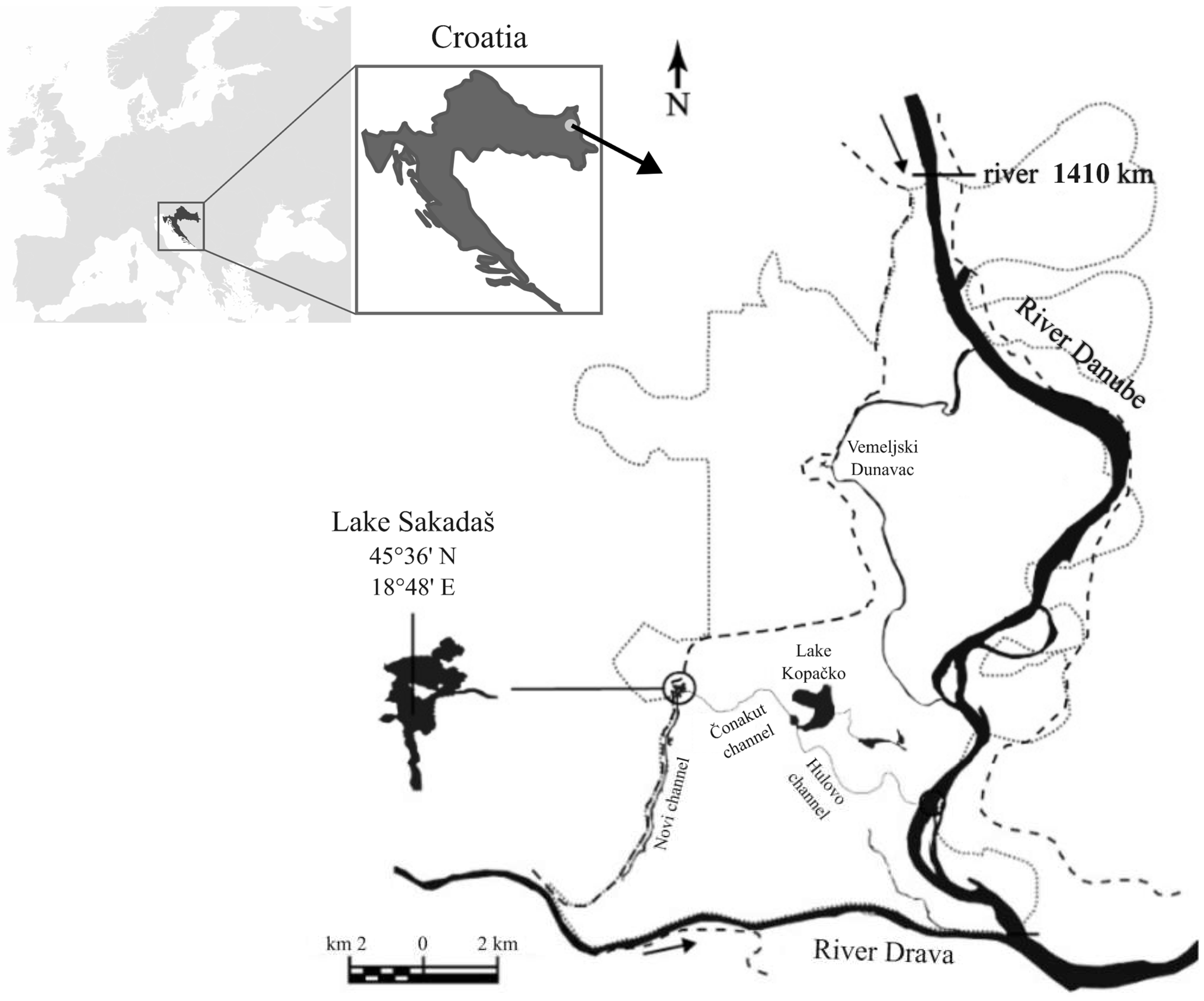
2.1. Study Area
2.2. Sampling and Physicochemical Analyses
2.3. Phytoplankton Analysis
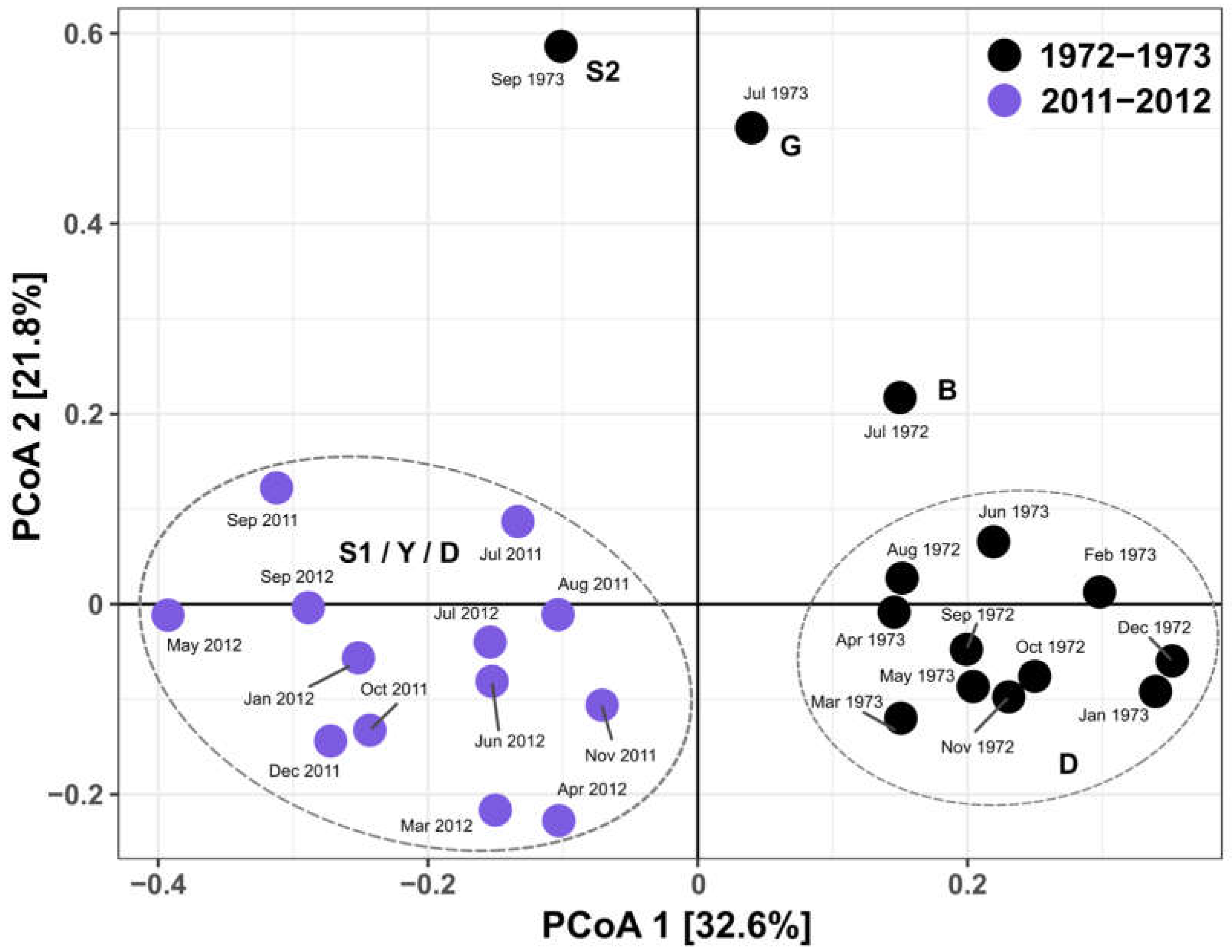
2.4. Statistical Analysis
3. Results
3.1. Flooding Pattern
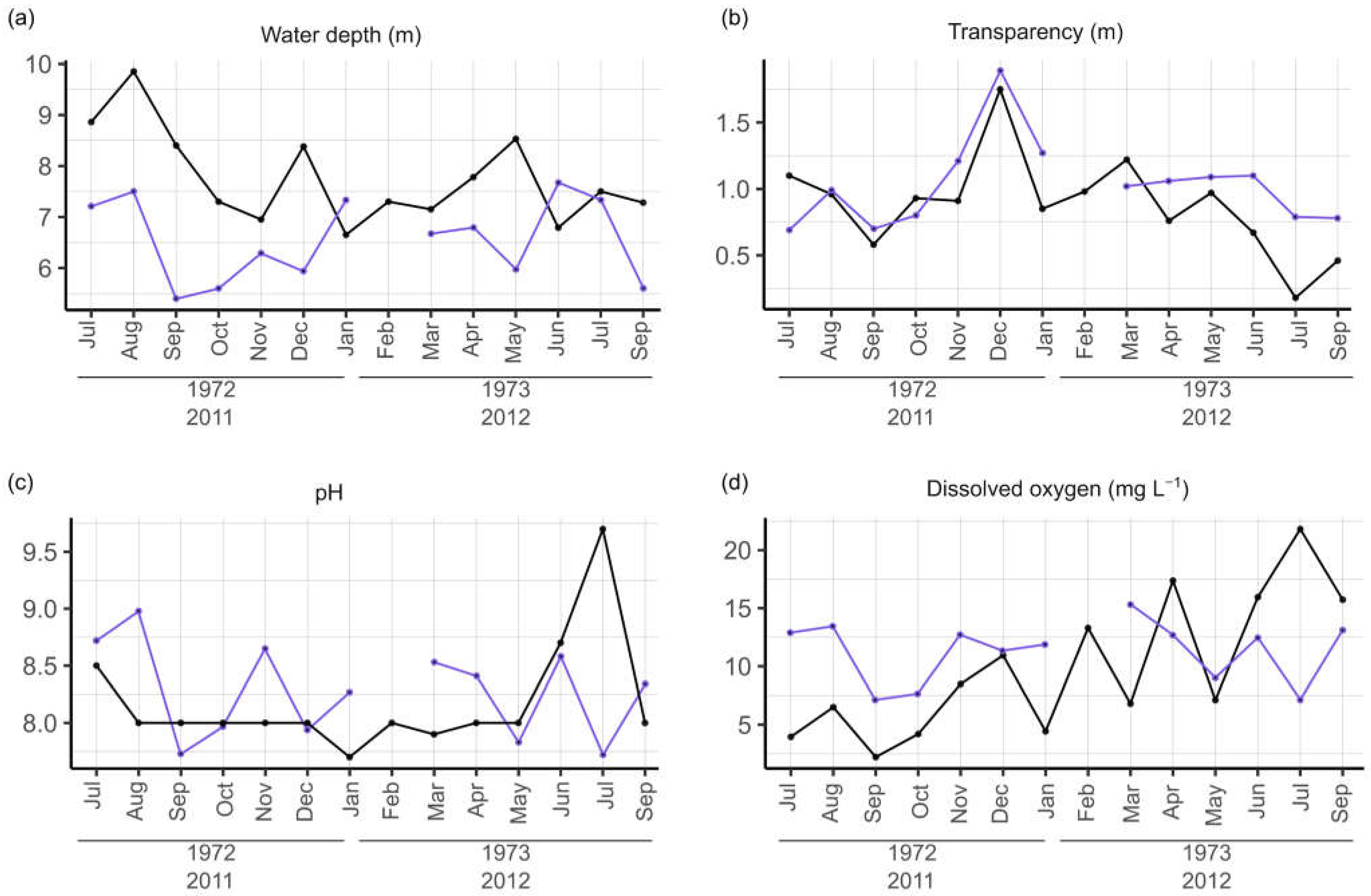
3.2. Physical and Chemical Water Parameters
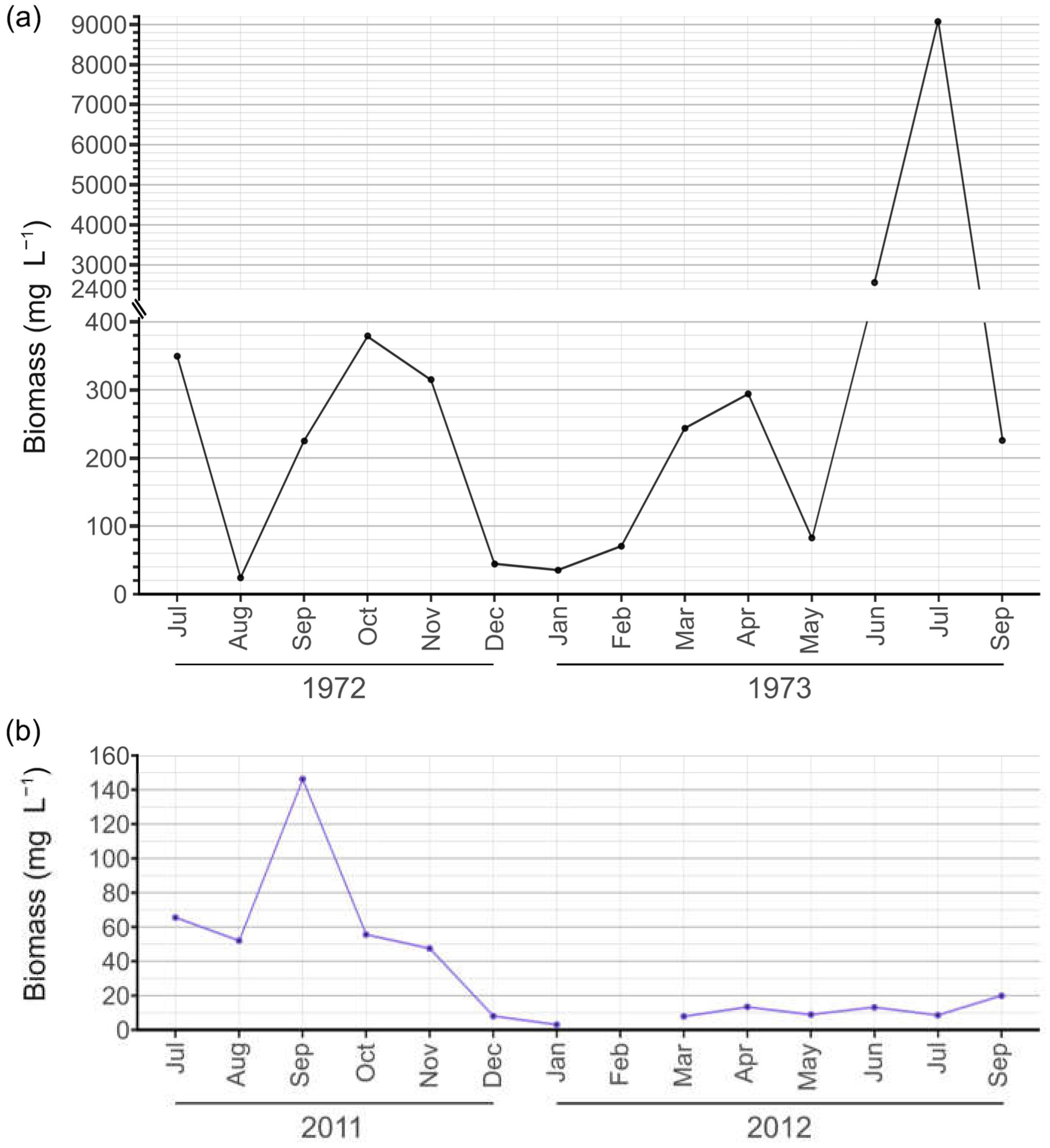
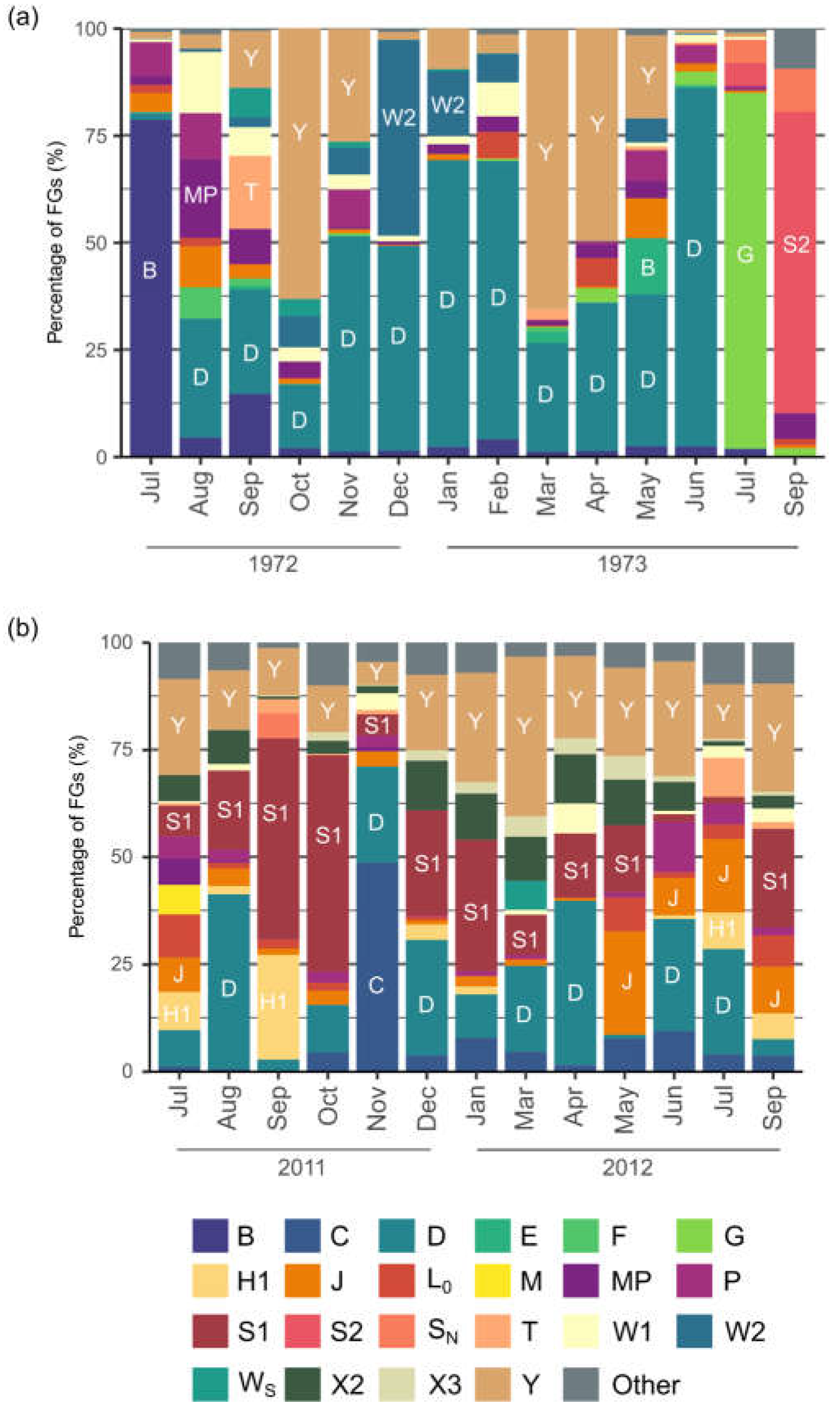
3.3. Phytoplankton Functional Assemblages and Q Index
4. Discussion
4.1. Anthropogenic Influence—Inflow of Agricultural Wastewater
4.2. Influence of Flooding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human Pressures and Ecological Status of European Rivers. Sci. Rep. 2017, 7, 205. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Infante, D.M. Disentangling Effects of Natural Factors and Human Disturbances on Aquatic Systems—Needs and Approaches. Water 2023, 15, 1387. [Google Scholar] [CrossRef]
- European Commission (European Commission, Brussels, Belgium). Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy (Water Framework Directive). Off. J. Eur. Union 2000, L327/1. [Google Scholar]
- Heiskanen, A.; Gromisz, S.; Jaanus, P.; Kauppila, P.; Purina, I.; Sagert, S.; Wasmund, N. Developing Reference Conditions for Phytoplankton in the Baltic Coastal Waters. In Part I: Applicability of Historical and Long-Term Datasets for Reconstruction of Past Phytoplankton Conditions; Technical Report, EUR 21582/EN/1; Joint Research Centre (JRC): Brussels, Belgium, 2005. [Google Scholar]
- Frau, D.; Medrano, J.; Calvi, C.; Giorgi, A. Water Quality Assessment of a Neotropical Pampean Lowland Stream Using a Phytoplankton Functional Trait Approach. Environ. Monit. Assess. 2019, 191, 681. [Google Scholar] [CrossRef]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyos, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Lyche Solheim, A.; Olin, M.; et al. A Hitchhiker’s Guide to European Lake Ecological Assessment and Intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Järvinen, M.; Drakare, S.; Free, G.; Lyche-Solheim, A.; Phillips, G.; Skjelbred, B.; Mischke, U.; Ott, I.; Poikane, S.; Søndergaard, M.; et al. Phytoplankton Indicator Taxa for Reference Conditions in Northern and Central European Lowland Lakes. Hydrobiologia 2013, 704, 97–113. [Google Scholar] [CrossRef]
- Thoms, M.C.; Ogden, R.W.; Reid, M.A. Establishing the Condition of Lowland Floodplain Rivers: A Palaeo-Ecological Approach. Freshw. Biol. 1999, 41, 407–423. [Google Scholar] [CrossRef]
- Globevnik, L.; Januschke, K.; Kail, J.; Snoj, L.; Manfrin, A.; Azlak, M.; Christiansen, T.; Birk, S. Preliminary Assessment of River Floodplain Condition in Europe; ETC/ICM Technical Report 5/2020; European Topic Centre on Inland, Coastal and Marine Waters: Magdeburg, Germany, 2020; pp. 1–121. [Google Scholar]
- Correa, S.B.; van der Sleen, P.; Siddiqui, S.F.; Bogotá-Gregory, J.D.; Arantes, C.C.; Barnett, A.A.; Couto, T.B.A.; Goulding, M.; Anderson, E.P. Biotic Indicators for Ecological State Change in Amazonian Floodplains. Bioscience 2022, 72, 753–768. [Google Scholar] [CrossRef]
- Amoros, C.; Bornette, G. Connectivity and Biocomplexity in Waterbodies of Riverine Floodplains. Freshw. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Maaß, A.-L.; Schüttrumpf, H.; Lehmkuhl, F. Human Impact on Fluvial Systems in Europe with Special Regard to Today’s River Restorations. Environ. Sci. Eur. 2021, 33, 119. [Google Scholar] [CrossRef]
- European Environment Agency. Floodplains: A Natural System to Preserve and Restore; European Environment Agency: Copenhagen, Denmark, 2020; ISBN 9789294802118.
- Fischer, C.; Damm, C.; Foeckler, F.; Gelhaus, M.; Gerstner, L.; Harris, R.M.B.; Hoffmann, T.G.; Iwanowski, J.; Kasperidus, H.; Mehl, D.; et al. The “Habitat Provision” Index for Assessing Floodplain Biodiversity and Restoration Potential as an Ecosystem Service—Method and Application. Front. Ecol. Evol. 2019, 7, 483. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Van De Bund, W.; Zampoukas, N.; Hering, D. Three Hundred Ways to Assess Europe’s Surface Waters: An Almost Complete Overview of Biological Methods to Implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Bund, W.V.D.; Solimini, A.G. Ecological Quality Ratios for Ecological Quality Assessment in Inland and Marine Waters; European Commission Joint Research Centre: Ispra, Italy, 2006; pp. 1–22. [Google Scholar]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a Functional Classification of the Freshwater Phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and Misuse in the Application of the Phytoplankton Functional Classification: A Critical Review with Updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Loverde-Oliveira, S.M.; Huszar, V.L.M. Phytoplankton Functional Groups Driven by Alternative States in a Tropical Floodplain Lake (Pantanal, Brazil). Oecologia Aust. 2019, 23, 926–939. [Google Scholar] [CrossRef]
- Machado, K.B.; Teresa, F.B.; Vieira, L.C.G.; Huszar, V.L.d.M.; Nabout, J.C. Comparing the Effects of Landscape and Local Environmental Variables on Taxonomic and Functional Composition of Phytoplankton Communities. J. Plankton Res. 2016, 38, 1334–1346. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.-P.; Miranda, C.A.; de Souza Nogueira, I.; Garnier, J.; Vieira, L.C.G. Interannual Hydrological Variations and Ecological Phytoplankton Patterns in Amazonian Floodplain Lakes. Hydrobiologia 2019, 830, 135–149. [Google Scholar] [CrossRef]
- Stević, F.; Mihaljević, M.; Špoljarić, D. Changes of Phytoplankton Functional Groups in a Floodplain Lake Associated with Hydrological Perturbations. Hydrobiologia 2013, 709, 143–158. [Google Scholar] [CrossRef]
- Yan, G.; Yin, X.; Huang, M.; Wang, X.; Huang, D.; Li, D. Dynamics of Phytoplankton Functional Groups in River-Connected Lakes and the Major Influencing Factors: A Case Study of Dongting Lake, China. Ecol. Indic. 2023, 149, 110177. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Grigorszky, I.; Soróczki-Pintér, É. Use of Phytoplankton Assemblages for Monitoring Ecological Status of Lakes within the Water Framework Directive: The Assemblage Index. Hydrobiologia 2006, 553, 1–14. [Google Scholar] [CrossRef]
- Belkinova, D.; PadisáK, J.; Gecheva, G.; Cheshmedjiev, S. Phytoplankton Based Assessment of Ecological Status of Bulgarian Lakes and Comparison of Metrics within the Water Framework Directive. Appl. Ecol. Environ. Res. 2014, 12, 83–103. [Google Scholar] [CrossRef]
- Poniewozik, M.; Lenard, T. Phytoplankton Composition and Ecological Status of Lakes with Cyanobacteria Dominance. Int. J. Environ. Res. Public Health 2022, 19, 3832. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.N.; Fakioǧlu, Ö.; Dural, B. Phytoplankton Functional Groups Provide a Quality Assessment Method by the Q Assemblage Index in Lake Mogan (Turkey). Turk. J. Bot. 2014, 38, 169–179. [Google Scholar] [CrossRef]
- Ongun Sevindik, T.; Tunca, H.; Gönülol, A.; Yildirim Gürsoy, N.; Küçükkaya, Ş.N.; Durgut Kinali, Z. Phytoplankton Dynamics and Structure, and Ecological Status Estimation by the q Assemblage Index: A Comparative Analysis in Two Shallow Mediterranean Lakes. Turk. J. Bot. 2017, 41, 25–36. [Google Scholar] [CrossRef]
- Çelekli, A.; Öztürk, B. Determination of Ecological Status and Ecological Preferences of Phytoplankton Using Multivariate Approach in a Mediterranean Reservoir. Hydrobiologia 2014, 740, 115–135. [Google Scholar] [CrossRef]
- Vieira, P.C.S.; Cardoso, M.M.L.; da Costa, I.A.S. Vertical and Temporal Dynamics of Phytoplanktonic Associations and the Application of Index Assembly in Tropical Semi-Arid Eutrophic Reservoir, Northeastern Brazil. Acta Limnol. Bras. 2015, 27, 130–144. [Google Scholar] [CrossRef]
- Silva, A.P.C.; da Costa, I.A.S. Biomonitoramento Do Estado Ecológico de Dois Reservatórios Do Semiárido Brasileiro Utilizando Assembleias Fitoplanctônicas (Índice Q). Acta Limnol. Bras. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Becker, V.; Huszar, V.L.M.; Crossetti, L.O. Responses of Phytoplankton Functional Groups to the Mixing Regime in a Deep Subtropical Reservoir. Hydrobiologia 2009, 628, 137–151. [Google Scholar] [CrossRef]
- Hajnal, É.; Padisák, J. Analysis of Long-Term Ecological Status of Lake Balaton Based on the ALMOBAL Phytoplankton Database. Hydrobiologia 2008, 599, 227–237. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Q.; Tan, L.; Kong, L. Phytoplankton Development and Ecological Status during a Cyanobacterial Bloom in a Tributary Bay of the Three Gorges Reservoir, China. Sci. Total Environ. 2011, 409, 3820–3828. [Google Scholar] [CrossRef]
- Frau, D.; Mayora, G.; Devercelli, M. Phytoplankton-Based Water Quality Metrics: Feasibility of Their Use in a Neotropical Shallow Lake. Mar. Freshw. Res. 2018, 69, 1746–1754. [Google Scholar] [CrossRef]
- Abonyi, A.; Leitão, M.; Lançon, A.M.; Padisák, J. Phytoplankton Functional Groups as Indicators of Human Impacts along the River Loire (France). Hydrobiologia 2012, 698, 233–249. [Google Scholar] [CrossRef]
- Gucunski, D. Kvantitativna Istraživanja Fitoplanktona u Upravljanom Prirodnom Rezervatu Kopački Rit. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 1975. [Google Scholar]
- Tadić, L.; Bonacci, O.; Dadić, T. Dynamics of the Kopački Rit (Croatia) Wetland Floodplain Water Regime. Environ. Earth Sci. 2014, 71, 3559–3570. [Google Scholar] [CrossRef]
- Mihaljević, M.; Getz, D.; Tadić, Z.; Živanović, B.; Gucunski, D.; Topić, J.; Kalinović, I.; Mikuska, J. Kopački Rit—Pregled Istraživanja i Bibliografija; Hrvatska Akademija Znanosti i Umjetnosti (HAZU): Zagreb, Croatia, 1999. [Google Scholar]
- Schwarz, U. Landschaftsökologische Charakterisierung des Kopački Rit unter Besonderer Berücksichtigung von Flusslandschaftsformen Sowie Deren Genese und Typologie. Ph.D. Thesis, University of Wien, Wien, Austria, 2005. [Google Scholar]
- Tadić, L.; Tamás, E.A.; Mihaljević, M.; Janjić, J. Potential Climate Impacts of Hydrological Alterations and Discharge Variabilities of the Mura, Drava, and Danube Rivers on the Natural Resources of the MDD UNESCO Biosphere Reserve. Climate 2022, 10, 139. [Google Scholar] [CrossRef]
- OECD. Eutrophication of Waters. Monitoring, Assessment and Control; Organisation for Economic Cooperation and Development: Paris, France, 1982; p. 154. [Google Scholar]
- Mihaljević, M.; Špoljarić, D.; Stević, F.; Cvijanović, V.; Hackenberger Kutuzović, B. The Influence of Extreme Floods from the River Danube in 2006 on Phytoplankton Communities in a Floodplain Lake: Shift to a Clear State. Limnologica 2010, 40, 260–268. [Google Scholar] [CrossRef]
- Horvatić, J.; Mihaljević, M.; Stević, F. Algal Growth Potential of Chlorella Kessleri FOTT et NOV. in Comparison with in Situ Microphytoplankton Dynamics in the Water of Lake Sakadaš Marshes. Period. Biol. 2003, 105, 307–312. [Google Scholar]
- Stević, F.; Mihaljević, M.; Horvatić, J. Interactions between Microphytoplankton of the Danube, Its Sidearms and Wetlands (1426–1388 r. Km, Croatia). Period. Biol. 2005, 107, 299–304. [Google Scholar]
- Mihaljević, M.; Stević, F. Cyanobacterial Blooms in a Temperate River-Floodplain Ecosystem: The Importance of Hydrological Extremes. Aquat. Ecol. 2011, 45, 335–349. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Hustedt, F. Bacillariophyta; Otto Koeltz Science Publishers: Koenigstein, Germany, 1976. [Google Scholar]
- Hindák, F.; Cyrus, Z.; Marvan, P.; Javornicky, P.; Komarek, J.; Etll, H.; Rosa, K.; Sladečkova, A.; Popovsky, J.; Punčocharova, M.; et al. Slatkovodne Riasy. Hindák, F., Ed.; Slovenske Pedagogicke Nakladelstvo: Bratislava, Slovakia, 1978. [Google Scholar]
- Meffert, M.E.; Oberhäuser, R.; Overbeck, J. Morphology and Taxonomy of Oscillatoria redekei (Cyanophyta). Br. Phycol. J. 1981, 16, 107–114. [Google Scholar] [CrossRef]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 1. Introduction. Arch. Für Hydrobiol. Suppl. 1985, 71, 291–302. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Arch. Für Hydrobiol. Suppl. 1988, 80, 327–472. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Modern approach to the classification system of cyanophytes. 4. Nostocales. Algol. Stud. 1989, 56, 247–345. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2024; Available online: http://www.algaebase.org (accessed on 1 June 2024).
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Javornický, P.; Komárková, J. The changes in several parameters of plankton primary productivity in Slapy Reservoir 1960–1967, their mutual correlations and correlations with the main ecological factors. Hydrobiol. Stud. 1973, 2, 155–211. [Google Scholar]
- Sournia, A. Phytoplankton Manual; UNESCO: Paris, Italy, 1978; pp. 1–337. [Google Scholar]
- Gucunski, D.; Popović, Ž. Podaci Za Kvantitativnu Analizu Fitoplanktona Rezervata “Kopački Rit”. Anal. Zavoda Jugoslav. Akad. 1984, 2, 277–298. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 July 2023).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 1 June 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-7; 2020; Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 June 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. Available online: https://dplyr.tidyverse.org/authors.html (accessed on 1 June 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, USA, 2016; Volume 35, ISBN 9780387981406. [Google Scholar]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Bogard, M.J.; Vogt, R.J.; Hayes, N.M.; Leavitt, P.R. Unabated Nitrogen Pollution Favors Growth of Toxic Cyanobacteria over Chlorophytes in Most Hypereutrophic Lakes. Environ. Sci. Technol. 2020, 54, 3219–3227. [Google Scholar] [CrossRef]
- Donald, D.B.; Bogard, M.J.; Finlay, K.; Bunting, L.; Leavitt, P.R. Phytoplankton-Specific Response to Enrichment of Phosphorus-Rich Surface Waters with Ammonium, Nitrate, and Urea. PLoS ONE 2013, 8, e53277. [Google Scholar] [CrossRef]
- Munawar, M.; Zafar, A.R. A Preliminary Study of Vertical Movement of Eudorina Elegans and Trinema Lineare during a Bloom Caused by Them. Hydrobiologia 1967, 29, 140–148. [Google Scholar] [CrossRef]
- Millie, D.F.; Fahnenstiel, G.L.; Bressie, J.D.; Pigg, R.J.; Rediske, R.R.; Klarer, D.M.; Tester, P.A.; Litaker, W.R. Late-Summer Phytoplankton in Western Lake Erie (Laurentian Great Lakes): Bloom Distributions, Toxicity, and Environmental Influences. Aquat. Ecol. 2009, 43, 915–934. [Google Scholar] [CrossRef]
- Kiss, I. Investigation of the Water Blooms of Eudorina Elegans in the Dead-Arm of the River Tisza at the Community Mártély. Tiscia 1977, 12, 37–47. [Google Scholar]
- Padisák, J.; Naselli-Flores, L. Phytoplankton in Extreme Environments: Importance and Consequences of Habitat Permanency. Hydrobiologia 2021, 848, 157–176. [Google Scholar] [CrossRef]
- El-Bestawy, E.; Bellinger, E.G.; Sigee, D.C. Elemental Composition of Phytoplankton in a Subtropical Lake: X-ray Microanalytical Studies on the Dominant Algae Spirulina Platensis (Cyanophyta) and Cyclotella Meneghiniana (Bacillariophyceae). Eur. J. Phycol. 1996, 31, 157–166. [Google Scholar] [CrossRef]
- Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 9789400738, pp. 677–705. [Google Scholar]
- Fužinato, S.; Fodora, A.; Subakov-Simić, G. Arthrospira Fusiformis (Voronichin) Komarek et Lund (Cyanoprokaryota) A New Species for Europe. Arch. Hydrobiol. Suppl. Algol. Stud. 2010, 134, 17–24. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An Extension of the Flood Pulse Concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F.; Horvatić, J.; Hackenberger Kutuzović, B. Dual Impact of the Flood Pulses on the Phytoplankton Assemblages in a Danubian Floodplain Lake (Kopački Rit Nature Park, Croatia). Hydrobiologia 2009, 618, 77–88. [Google Scholar] [CrossRef]
- Bouska, K.L.; Houser, J.N.; De Jager, N.R.; Drake, D.C.; Collins, S.F.; Gibson-Reinemer, D.K.; Thomsen, M.A. Conceptualizing Alternate Regimes in a Large Floodplain-River Ecosystem: Water Clarity, Invasive Fish, and Floodplain Vegetation. J. Environ. Manag. 2020, 264, 110516. [Google Scholar] [CrossRef]
- Yang, Y.; Stenger-Kovács, C.; Padisák, J.; Pettersson, K. Effects of Winter Severity on Spring Phytoplankton Development in a Temperate Lake (Lake Erken, Sweden). Hydrobiologia 2016, 780, 47–57. [Google Scholar] [CrossRef]
- Vieira, A.A.H.; Ortolano, P.I.C.; Giroldo, D.; Oliveira, M.J.D.; Bittar, T.B.; Lombardi, A.T.; Sartori, A.L.; Paulsen, B.S. Role of Hydrophobic Extracellular Polysaccharide of Aulacoseira Granulata (Bacillariophyceae) on Aggregate Formation in a Turbulent and Hypereutrophic Reservoir. Limnol. Oceanogr. 2008, 53, 1887–1899. [Google Scholar] [CrossRef]
- Weilhoefer, C.L.; Pan, Y.; Eppard, S. The Effects of River Floodwaters on Floodplain Wetland Water Quality and Diatom Assemblages. Wetlands 2008, 28, 473–486. [Google Scholar] [CrossRef]
- Tolotti, M.; Boscaini, A.; Salmaso, N. Comparative Analysis of Phytoplankton Patterns in Two Modified Lakes with Contrasting Hydrological Features. Aquat. Sci. 2010, 72, 213–226. [Google Scholar] [CrossRef]
- Ma, C.; Chula Mwagona, P.; Yu, H.; Sun, X.; Liang, L.; Al-Ghanim, K.A.; Mahboob, S. Spatial and Temporal Variation of Phytoplankton Functional Groups in Extremely Alkaline Dali Nur Lake, North China. J. Freshw. Ecol. 2019, 34, 91–105. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Lengyel, E.; Buczkó, K.; Tóth, F.M.; Crossetti, L.O.; Pellinger, A.; Doma, Z.Z.; Padisák, J. Vanishing World: Alkaline, Saline Lakes in Central Europe and Their Diatom Assemblages. Inland Waters 2014, 4, 383–396. [Google Scholar] [CrossRef]
- Mihaljević, M.; Stević, F.; Špoljarić-Maronić, D.; Žuna Pfeiffer, T. Application of Morpho-Functional Classifications in the Evaluation of Phytoplankton Changes in the Danube River. Acta Zool. Bulg. Suppl. 2014, 7, 153–158. [Google Scholar]
- Padisák, J.; Borics, G.; Fehér, G.; Grigorszky, I.; Oldal, I.; Schmidt, A.; Zámbóné-Doma, Z. Dominant Species, Functional Assemblages and Frequency of Equilibrium Phases in Late Summer Phytoplankton Assemblages in Hungarian Small Shallow Lakes. Hydrobiologia 2003, 502, 157–168. [Google Scholar] [CrossRef]



| Danube Water Level | 1972–1973 | 2011–2012 | |
|---|---|---|---|
| Maximum | 5.84 | 4.91 | |
| Minimum | 0.55 | −0.30 | |
| Mean | 2.48 | 2.14 | |
| Flooded area (%) * | Flooding duration (days/period) | ||
| 3.0–3.5 | 20 | 46 | 42 |
| 3.5–4.0 | 40 | 43 | 34 |
| 4.0–5.0 | 75 | 38 | 17 |
| >5.0 | >90 | 27 | 0 |
| Total >3 m | 155 | 100 | |
| >3 in continuum | 80 | 28 | |
| Parameter (µg L−1) | Min | Max | Mean |
|---|---|---|---|
| Ammonium (NH4+) | <5 | 454 | 127 |
| Nitrates (NO3−) | 20 | 3950 | 710 |
| Nitrites (NO2−) | 5 | 83.6 | 25 |
| Organic nitrogen (orgN) | 323 | 5300 | 1701 |
| Total nitrogen (TN) | 588 | 5682 | 2524 |
| Total phosphorus (TP) | 61 | 422 | 209 |
| Functional Groups | Species |
|---|---|
| A | Acanthoceras zachariasii (Brun) Sim. (*), Cyclotella sp. (#) |
| B | Aulacoseira italica (Ehrenb.) Sim. (*), Lindavia comta (Kütz.) Nakov, Gullory, Julius, Theriot & Alverson (#) |
| C | Asterionella formosa Hass. (*), Stephanocyclus meneghinianus (Kütz.) Kulikovskiy, Genkal & Kociolek (#) |
| D | Cyclostephanos dubius (Hust.) Round (*), Stephanodiscus hantzschii Grun., Ulnaria acus (Kütz.) Aboal (#), Ulnaria ulna (Nitz.) Comp. |
| E | Dinobryon divergens var. angulatum (Seligo) Brunnth. (*), Dinobryon divergens Imh. (#) |
| F | Oocystis marssonii Lemm. (*), Micractinium bornhemiense (W.Conrad) Korshikov (#) |
| G | Eudorina elegans Ehrenb. (*), Pleodorina illinoisensis Kofoid (*), Pandorina morum (O.F.Müller) Bory (*) |
| H1 | Dolichospermum planctonicum (Brunnth.) Wacklin, L.Hoff. & Kom. (*), Cuspidothrix issatschenkoi (Usachev) P.Rajaniemi, Kom., R.Willame, P.Hrouzek, K.Kastovská, L.Hoffm. & K.Sivonen (#), Dolichospermum sigmoideum (Nygaard) Wacklin, L.Hoffm. & Kom. (#), Aphanizomenon flos-aquae Ralfs ex Born. & Flah. (#), Dolichospermum solitarium (Kleb.) Wacklin, L.Hoffm. & Kom. (#) |
| H2 | Gloeotrichia sp. (*) |
| J | Pediastrum boryanum var. boryanum (Turp.) Menegh. (*), Tetradesmus lagerheimii M.J.Wynne & Guiry (#), Coelastrum microporum Nägeli (#) |
| K | Aphanothece elabens (Bréb. ex Menegh.) Elenkin |
| L0 | Peridinium cinctum (O.F.Müller) Ehrenb., Apocalathium aciculiferum (Lemm.) Craveiro, Daugbjerg, Moestrup & Calado (#) |
| M | Microcystis aeruginosa (Kütz.) Kütz., Microcystis wesenbergii (Kom.) Kom. ex Kom. (*) |
| MP | Brachysira exilis (Kütz.) Round & D.G.Mann (*), Gyrosigma macrum (W.Smith) J.W.Griffith & Henfrey (*), Oscillatoria tenuis C.Agardh ex Gomont (*), Amphora ovalis (Kütz.) Kütz. (#) |
| N | Cosmarium sp. (*), Cosmarium phaseolus Bréb. ex Ralfs (#) |
| P | Aulacoseira granulata (Ehrenb.) Sim., Staurastrum sp. (*), Closterium macilentum Bréb. (*) |
| S1 | Limnothrix redekei (Goor) Meffert, Planktothrix agardhii (Gom.) Anag. & Kom. (#), Pseudanabaena limnetica (Lemm.) Kom. (#) |
| S2 | Limnospira sp. (*) |
| SN | Raphidiopsis mediterranea Skuja (*), Raphidiopsis raciborskii (Wołoszyńska) Aguilera & al. (#) |
| T | Mougeotia spp. (*), Binuclearia lauterbornii (Schmidle) Proschkina-Lavrenko (#) |
| TB | Navicula rhynchocephala Kütz. (*), Navicula sp. (#) |
| W1 | Lepocinclis ovum (Ehrenb.) Lemm., Euglena texta (Duj.) Hübner (#) |
| W2 | Strombomonas annulata (Daday) Deflandre (*), Trachelomonas volvocina (Ehrenb.) Ehrenb., Trachelomonas crenulatocollis Maskell (*) |
| WS | Synura uvella Ehrenb. |
| X1 | Pseudodidymocystis planctonica (Korshikov) E.Hegewald & Deason |
| X2 | Chlamydomonas globosa J.W.Snow (*), Chlamydomonas sp. (#), Carteria sp. (#), Rhodomonas sp. (#), Rhodomonas lacustris Pasch. & Rutt. (#) |
| X3 | Chrysococcus rufescens Klebs (#) |
| Y | Cryptomonas erosa Ehrenb., Cryptomonas ovate Ehrenb. (#), Cryptomonas sp. (#), Naiadinium polonicum (Wolosz.) S.Carty (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaljević, M.; Kajan, K. Disentangling the Effects of Multiple Impacts of Natural Flooding on a Riverine Floodplain Lake by Applying the Phytoplankton Functional Approach. Environments 2024, 11, 216. https://doi.org/10.3390/environments11100216
Mihaljević M, Kajan K. Disentangling the Effects of Multiple Impacts of Natural Flooding on a Riverine Floodplain Lake by Applying the Phytoplankton Functional Approach. Environments. 2024; 11(10):216. https://doi.org/10.3390/environments11100216
Chicago/Turabian StyleMihaljević, Melita, and Katarina Kajan. 2024. "Disentangling the Effects of Multiple Impacts of Natural Flooding on a Riverine Floodplain Lake by Applying the Phytoplankton Functional Approach" Environments 11, no. 10: 216. https://doi.org/10.3390/environments11100216
APA StyleMihaljević, M., & Kajan, K. (2024). Disentangling the Effects of Multiple Impacts of Natural Flooding on a Riverine Floodplain Lake by Applying the Phytoplankton Functional Approach. Environments, 11(10), 216. https://doi.org/10.3390/environments11100216





