Abstract
Caretta caretta (Cheloniidae, Cryptodira) is a species of turtle considered a ‘flagship species’ in the Mediterranean Sea. Unfortunately, the circular marine currents and semi-enclosed configuration of the Mediterranean Sea encourage the accumulation of pollutants (metals, pesticides, etc.) emitted by human activities. Tunisia suffers particularly from coastal urbanisation and industrial development. Metal concentrations (Ag, Al, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn) were measured in distinct tissues (heart, kidney, liver, lung, muscle) of loggerhead turtles stranded in the Gulf of Gabès (Tunisia) to estimate the pollution levels in this emblematic species. High concentrations of arsenic and cadmium were found in marine turtles. Therefore, the differential accumulation of metals was measured in the tissues. For example, the liver appears to be a preferential organ for the accumulation of copper, iron, silver and zinc. In contrast, cobalt and cadmium were more concentrated in the kidneys, while arsenic, chromium and nickel were mainly found in the muscles. Antioxidant enzyme responses (catalase, GPx and SOD) and lipid peroxidation were more expressed in the liver and kidneys than in the muscles.
1. Introduction
Many pollutants (metals, plastics, pesticides, etc.) released by anthropogenic activities (agriculture, industry, wastewater, etc.) are now in the seas and oceans, contaminating and poisoning flora and fauna. Of all the dangerous substances, trace metals are abundant in the marine ecosystem. Because of their widespread use, they continue to increase and persist despite the growing number of laws applied by nations [1,2]. Sea turtles are protected organisms under international agreements and are considered ambassadors of the oceans and “flag species” [3,4]. Therefore, marine turtles have been identified as good bio-accumulators and bio-indicators of environmental health. They have a long lifespan, a diversified diet and migratory behaviour [5,6,7]. Numerous publications report the bioaccumulation of pollutants in marine turtles and their negative effects on reproduction, development/growth and pathologies (cancer, fibropapillomatosis, etc.) [8,9,10,11,12,13,14,15].
Three sea turtle species can easily be observed in the Mediterranean Sea: Caretta caretta (loggerhead turtle), Chelonia mydas (green turtle) and Dermochelys coriacea (leatherback turtle). Dermochelys coriacea appears to be an intrusive species in the Mediterranean Sea, whereas C. caretta and C. mydas are more permanent. Loggerhead turtles are more abundant than green turtles along the Tunisian coasts. The migrations in the Mediterranean basin of C. caretta are well known and mainly established between the coasts of Cyprus, Egypt, Greece, Libya and Tunisia [16]. The neritic feeding and wintering sites of C. caretta are located mainly along the coasts of Tunisia and the Adriatic Sea [16]. The main nesting sites are found along the coasts of Cyprus, France, Italy, Tunisia and Turkey, although nests may be observed on other beaches in the Mediterranean Sea [16,17,18]. Recently, nests have been noted on the French Mediterranean coast. Casale and Mariani (2014) [19] indicated that the distribution pattern of C. caretta in the eastern Mediterranean basin showed two groups of turtles: the first group is dispersed along the Egyptian, Libyan and Turkish coasts, while the second group is found in the Adriatic, Greek, south-eastern (including the Tunisian coasts) and Tyrrhenian Seas.
Several ecotoxicological studies have been carried out on C. caretta and C. mydas, revealing all-time bioaccumulation of metals in their tissues [15,20,21]. For example, for C. caretta, the metal contents have been estimated for populations from Egypt [22], Cyprus [23], Greece [24], Italy [25,26,27,28,29,30,31,32], Spain [5,33,34,35,36] and Turkey [37,38]. Unfortunately, in Tunisia, only one study has been published, and it was based only on Cd, Cu, Hg, and Pb contents in C. caretta collected on the North-East coast [39]. We therefore lack information on the extent of metal accumulation in Tunisian turtles.
To respond to the accumulation and effects of pollutants, turtles can synthesise numerous molecular biomarkers such as antioxidant enzymes. The activities of catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) are modulated by stress conditions. These proteins are involved in the catalysis of reactive oxygen species radicals into water and oxygen. SOD dismutes the free radical superoxide anion into hydroperoxide and oxygen, whereas CAT and GPx catalyse hydroperoxide into oxygen and water. These enzymes have been used as physiological biomarkers in loggerhead turtles [40] and in green turtles [41], as a health monitoring tool.
Our objectives are as follows:
- to fill the knowledge gap on metal pollution (Ag, Al, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn) in loggerhead turtles found in Tunisia;
- to estimate the preferential accumulation of metals in distinct organs (heart, kidney, liver, lung, muscle); and
- to determine the negative effects of environmental pollution in turtles through the lens of antioxidant enzymatic activities (CAT, GPx, SOD) and lipid peroxidation (MDA).
2. Materials and Methods
2.1. Sample Collection
In 2021 and 2022, fourteen stranded loggerhead turtles were recovered along the Sfax (7 individuals) and Gabès (7 individuals) coasts of Tunisia (Figure 1). All the turtles analysed were in non-advanced stages of degradation (stages two and three). Two stranded turtles were still alive when found, but unfortunately, they died at the Sfax Faculty of Sciences rescue centre. In the absence of an established method for determining the age of sea turtles, we relied on recording curved carapace length (CCL) and curved carapace width (CCW) to the nearest centimetre as a means of assessing the size and as a relative indicator of age. The smallest turtle in the study was 21.5 cm long and 19 cm wide, while the largest specimen measured 71 cm and 61 cm, respectively. On average, the turtles were 56.67 ± 12.56 cm long and 52.63 ± 12.02 cm wide. In the bibliography, some authors defined the adult stage as when the CCL > 64 cm [27] or CCL > 70 cm [23,26]. Thus, the turtles in this study were mainly sub-adults, except for one juvenile and one adult (Table 1). Tissue samples were collected from turtles during necropsies, including the heart [number of tissues analysed (n) = 13], kidneys (n = 9), liver (n = 12), lungs (n = 13) and muscle (n = 14). All tissues were packed in sterile polypropylene tubes and frozen at −20 °C until freeze-dried.
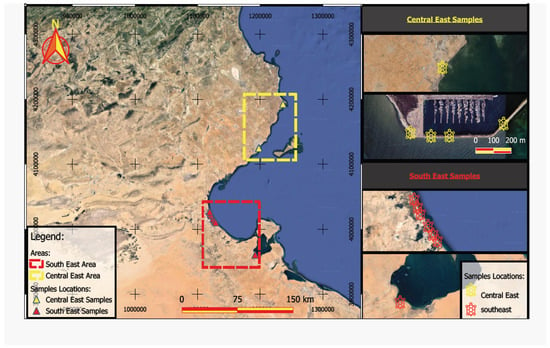
Figure 1.
Localisations of stranded turtles collected along the coasts of the Gulf of Gabès (Tunisia).

Table 1.
Geographical coordinates (longitude/latitude) and biometric parameters (curved carapace length, curved carapace width) of the loggerhead turtles sampled along Tunisia coasts.
2.2. Preparation of Samples for Metal Analyses
Tissues (heart, kidney, lung, liver and muscle) were cut into small pieces and stored at −20 °C in sterile jars. They were then freeze-dried at the Laboratoire de génie enzymatique et de microbiologie de l’Ecole nationale d’ingénieurs de Sfax (Sfax, Tunisia) using a Bioblock Scientific Christ 7 ALPHA 1-2 freeze-dryer (IIIKrich-Cedex, France) for a minimum of 48 h. After freeze-drying, the samples were ground by hand into a fine homogeneous powder using a pestle and mortar and stored safely in sterile, hermetically sealed jars to prevent contamination before being transported to the LIttoral ENvironnement et Sociétés (LIENSs, UMRi 7266 CNRS) laboratory in La Rochelle Université (France).
2.3. Analyses of Metals
Analyses of metals (Ag, Al, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn) were carried out using a Varian Vista-Pro ICP-OES (La Rochelle, France) and a Thermo Fisher Scientific XSeries 2 ICP-MS (La Rochelle, France). Aliquots weighing 60 and 200 mg were digested using a mixture of 67–70% HNO3 and 34–37% HCl (Fisher, trace metal quality). Acid digestion of the samples was carried out overnight at room temperature, then in a Milestone microwave oven (30 min with a constant increase in temperature up to 120 °C, then 15 min at this temperature). Each sample was prepared with 50 mL of high-quality ultrapure water. For samples weighing < 100 mg, the mixture was 3:1 (v/v) 67–70% HNO3/34–37% HCl, and samples were prepared with up to 25 mL of ultrapure water. Two certified reference materials (CRMs) and a blank, processed and analysed in the same way as the samples, were included in each analysis batch. A standard sample of certified value, TORT-3 (lobster hepatopancreas, NRC, National Research Council of Canada), was used to validate the analytical method. All element concentrations (expressed in μg/g) were based on dry weight (d. w.) measurements. The limit of detection (LOD), the minimum concentration of the analyte that can be detected and the limit of quantification (LOQ), which is the lowest concentration of metal which can be measured with acceptable uncertainty, were determined for each metal.
2.4. Protein Extraction
Ten grams of tissue (heart, kidney, liver, lung or muscle) from each turtle were ground using TRIS-buffered saline (2 mL, pH: 7.4) and placed in a mortar on ice. Centrifugation (10,000× g, 4 °C, 20 min) was performed to separate the cellular components (aqueous phase) from the tissue debris. The supernatant was stored at −20 °C. Proteins were quantified using the Lowry method [42]. Absorbance at 490 nm was measured and protein concentration was determined by comparison with the standard curve (BSA, R2: 0.99).
2.5. Antioxidant Enzymatic Activities and Lipid Peroxidation
Catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities were estimated using colourimetric methods developed by Beauchamp and Fridovich [43], Aebi [44], and Flohe and Gunzler [45], respectively, and absorbance was measured at 240 nm for CAT, 420 nm for GPx, and 560 nm for SOD. SOD activity was expressed in units (U)/mg protein, while CAT and GPx activities were reported in µmol/min/mg protein. Lipid peroxidation was estimated using TBS and TCA as described by Niehaus and Samuelson [46]. Malondialdehyde (MDA) complexed to TCA was measured at 532 nm. The level of MDA was expressed as nmol/mg protein.
2.6. Statistical Analyses
Various statistical analyses were carried out to study the accumulation of metals in Caretta caretta tissues. Descriptive statistical analysis was conducted (e.g., mean, median, standard deviation, Kruskal–Wallis test). When the values of statistical tests were significant, we cited them directly in the figures. PCA was then used to explore correlations (Pearson correlation) between quantitative variables (metal concentrations) and qualitative variables (zones, organs). Statistical analysis was carried out using R software version 4.0.5 [47], with specific packages such as facto extra [48], ellipse [49], ggplot2 [50] and Facto MineR [51].
3. Results
3.1. Metal Contents in C. caretta Tissues
The LOD and LOQ values for all metals analysed are shown in Table 2. The measurement of metal contents revealed that the order of total essential metals in the five tissues analysed was as follows: Fe > Zn > Al > Cu > Mn (Table 3). The order of total non-essential metals in the tissues analysed (heart, kidney, liver, lung and muscle) was As > Cd > Cr > Ni > Co > Pb > Ag (Table 3). Arsenic (As) and cadmium appeared abundantly accumulated. High concentrations of essential metals such as copper, iron and zinc in tissues were also noted.

Table 2.
Limit of detection (LOD) and limit of quantification (LOQ) values of distinct metals (µg/g).

Table 3.
Metal concentrations (mean ± standard deviation) in tissues (heart, kidney, liver, lung and muscle) of Caretta caretta (loggerhead turtle) collected in the Gulf of Gabès (Tunisia). Note: the number of tissues (identical to the number of individuals) analysed is indicated in parenthesis.
Aluminium (Al) primarily accumulates in the lungs and liver and to a lesser extent in the kidneys and muscles (Table 3, Figure 2). Copper, iron and manganese have high levels preferentially in the liver compared with the other organs analysed (Figure 2). Zn was most concentrated in the heart and more or less equally in the liver and muscles.
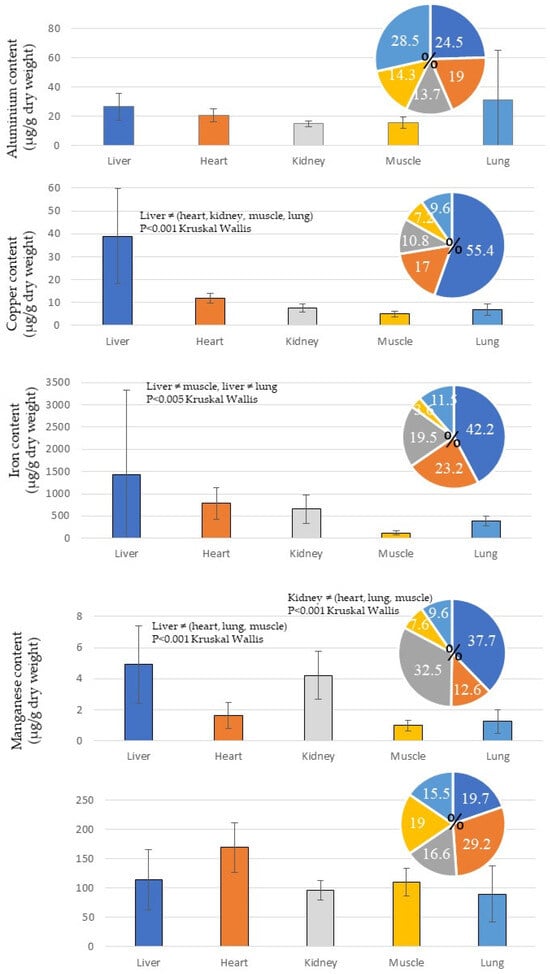
Figure 2.
Concentrations of essential metals (Al, Cu, Fe, Mn, Zn) in tissues of Caretta caretta sampled in Tunisia. Thirteen hearts, 9 kidneys, 12 livers, 13 lungs, and 14 muscles were analysed.
For non-essential metals, arsenic (As) was mainly accumulated in the muscles and to approximately the same extent in the heart, kidneys and lungs (Figure 3). Cadmium (Cd) and cobalt (Co) were concentrated in the kidneys, while silver (Ag) was concentrated in the liver. Chromium (Cr), nickel (Ni) and lead (Pb) were equally distributed in the different tissues (Figure 3). Trends towards correlation of accumulation were noted for the Cd/Co/Mn/Pb and Ag/Al/Cu/Fe/Zn groups (Figure 4). A correlation of accumulation of cadmium and cobalt was particularly noted. Principal component analysis revealed that the accumulation of As/Cr/Ni tended to be opposite to that of the other metals (Figure 4).
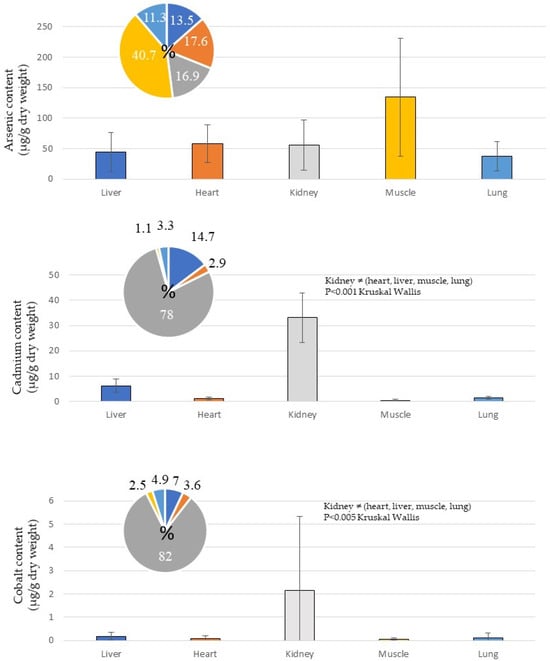
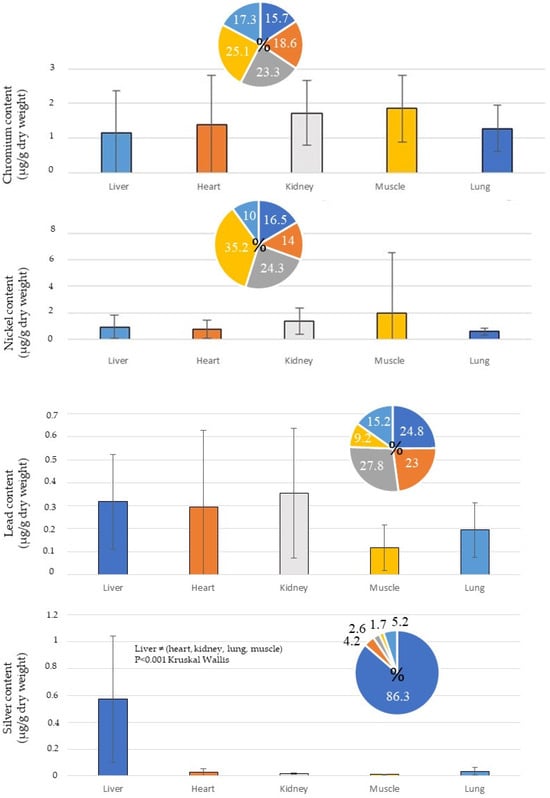
Figure 3.
Concentrations of non-essential metals (As, Cd, Co, Cr, Ni, Pb) in tissues of Caretta caretta sampled in Tunisia. Thirteen hearts, 9 kidneys, 12 livers, 13 lungs and 14 muscles were analysed.
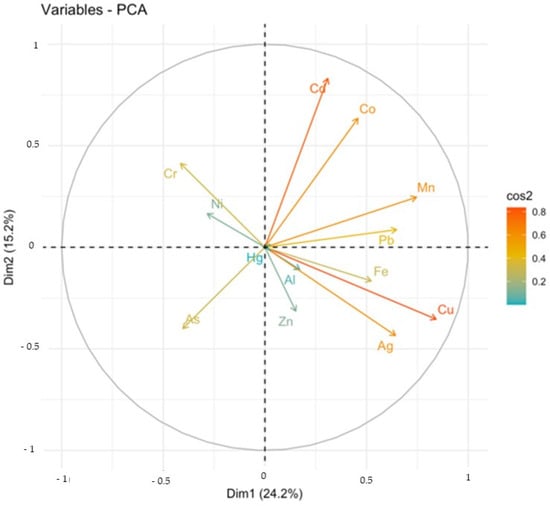
Figure 4.
Principal component analysis for the contents of metals in loggerhead turtles in Tunisia (Gulf of Gabès).
Using metal accumulation in distinct tissues (heart, kidney, liver, lung, muscle) in C. caretta, expressed as percentages, revealed, for example, that cadmium contributes 35.1% in the kidney and 11.7% in the liver, with only 3.5% of non-essential metals accumulating in the lungs (Figure 5). Arsenic (As) accounted for 59% of the non-essential metals in the kidneys and 96.8% of the non-essential metals in the muscles. Zinc (Zn) constituted 43.6% of essential metals in the muscle, 17.4% of essential metals in the lungs, 17% of essential metals in the heart, 12.4% of essential metals in the kidneys, and 7.1% of essential metals in the liver. Iron (Fe) accounted for 47.9% of essential metals in the muscle and 88.5% of essential metals in the liver (Figure 5).
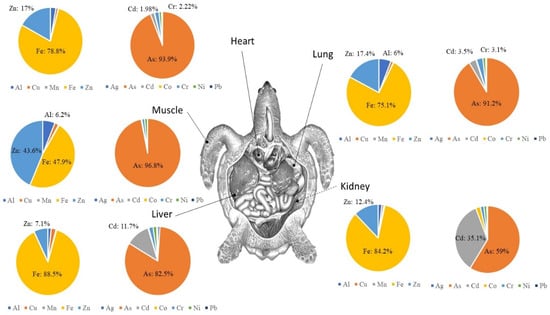
Figure 5.
Metal contribution in distinct tissues (heart, kidney, liver, lung, muscle) of the loggerhead turtle collected along the Tunisian coast (Gulf of Gabès). Note: essential metals (Al, Cu, Fe, Mn, Zn) and non-essential metals (As, Cd, Co, Cr, Ni, Pb) are noted.
3.2. Antioxidant Responses and Lipid Peroxidation in C. caretta
As mentioned in the ‘Materials and Methods’ section, enzyme activities (CAT, GPx and SOD) and lipid peroxidation were measured only if stranded turtles were not degraded (degradation stages two and three). We measured antioxidant response and lipid denaturation in the kidney, liver (organs involved in detoxification) and muscle (Table 4). Antioxidant responses were expressed in all three tissues. SOD activity was significantly higher in the liver than in the muscle (Figure 6). No significant difference in SOD activity was obtained between the kidney and the muscle because there was a high standard deviation. Therefore, CAT activity was not significantly differentiable between tissues. Lipid peroxidation was recorded in three tissues, in the order of kidney (398 ± 258 nmoles/mg protein) > liver (108 ± 72.2 nmoles/mg protein) > muscle (31.8 ± 16.2 nmoles/mg protein) (Figure 7), showing a differential lipid denaturation related to the antioxidant response level in distinct tissues. It is important to remember that these antioxidant activities and lipid denaturation levels reflect responses to the overall environmental pollution encountered by turtles.

Table 4.
Antioxidative enzymatic responses (catalase, GPx, and SOD) and lipid peroxidation (MDA) in tissues (kidney, liver, muscle) of loggerhead turtles collected in the Gulf of Gabès (Tunisia).
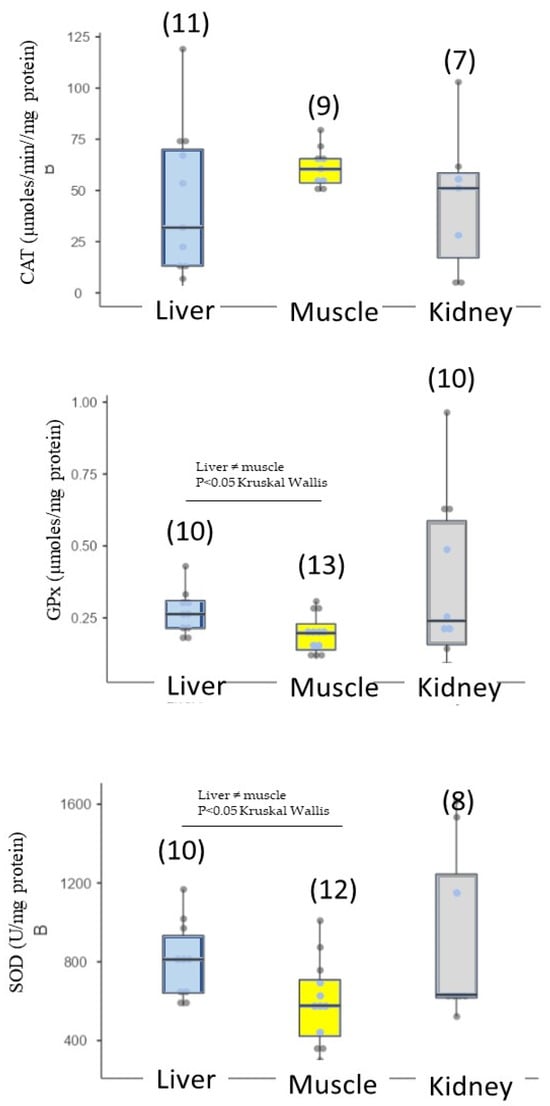
Figure 6.
Antioxidative enzymatic responses (CAT, GPx, SOD) in loggerhead turtles sampled along the Gulf of Gabès (Tunisia).
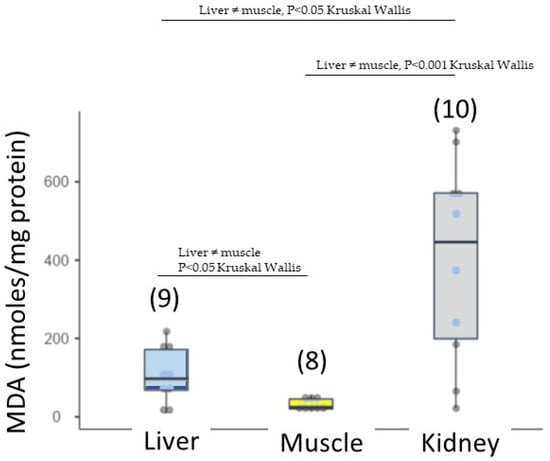
Figure 7.
Lipid peroxidation in loggerhead turtles sampled along the Gulf of Gabès (Tunisia).
4. Discussion
The Mediterranean is a semi-enclosed sea, with circular marine currents encouraging the permanent accumulation of pollutants emitted by human activities (agriculture, industry, tourism, urban populations) from 22 countries [52,53]. Tunisia suffers particularly from coastal urbanisation (64% of the population lives along the coast) and industrial development [54]. The release of metals into the environment has been observed for several decades, with industrial development (petrochemical production, mineral extraction) and the discharge of wastewater [55]. Tanneries, factories and phosphate industries (such as Tunisian Chemical Group, SIAPE and Granuphos) are now considered sources of pollution in the Gulf of Gabès. Thus, metal accumulation in ecosystems has been reported [56,57,58,59,60,61]. Our investigation on stranded C. caretta (mainly sub-adults) collected (Figure 1, Table 1) along the shores of the Gulf of Gabès revealed metal (Ag, Al, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Zn) contents in distinct tissues (heart, kidney, liver, lung, muscle) (Table 3). Although comparisons with previous studies should be made with caution, as levels of metals in the environment vary over time and according to location, we compared our results with others established for C. caretta in the Mediterranean Sea from 2004 to 2023 (Egypt [22], Italy [25,26,27,29,30], Spain [28,33,34], Tunisia [39], and Turkey [37,38]). In addition, comparing metal accumulation is tricky because the amount of metal accumulated can depend on the age of the turtles (estimated by the curve carapace length). The size of the turtles mentioned in the publications varies highly. In this case, comparing our results with other publications was difficult. For example, the turtles analysed by Abdallah [23] or El Hili et al. [39] were mainly adults (the curved carapace length ranged from 70.6 to 85.8 cm and 80. 9 ± 16.12 cm, respectively). In contrast, many publications mentioned analyses based on sub-adults [24,25,28,29,36,37]. Sometimes, the CCL measures showed that the turtle samples included sub-adults and adults, such as Canzanella et al. [26], Esposito et al. [27], and Febrer et al. [33].
Aluminium (Al): More than 50% of aluminium was accumulated in the lungs (28.5%) and the liver (24.5%) (Figure 2, Table 3). The lungs appeared to be a significant concentrator of Al (in comparison, lung: 28.5%; liver: 24.5%; heart: 19%; muscle: 14.3%; and kidney: 13.7%) (Figure 2).
Copper (Cu) was mainly accumulated in the liver (55.4%), and in decreasing order as follows: heart (17%) > kidney (10.8%) > lung (9.6%) > muscle (7.2%) (Figure 2). Thus, the liver seemed to be a preferential organ for the accumulation of copper. This observation was in accord with Andreani et al. [20] and D’ilio et al. [62]. The Cu concentration in the kidney (7.58 ± 1.71 µg/g d. w.), the liver (39 ± 20.7 µg/g d. w.) and muscle (5.05 ± 1.3 µg/g d. w.) (Table 2) was also higher than the copper concentrations previously recorded in Tunisian turtles (North-East region) in 2019 (kidney: 6.07 ± 5.07 µg/g d. w.; liver: 9.56 ± 8.1 µg/g d. w.) [39]; in Turkey in 2021 (liver: 27.72 ± 15.83 µg/g d. w.) [38]; in Spain in 2008 (kidney: 3.77 ± 3.50 µg/g d. w.; liver: 21.60 ± 8.03 µg/g d. w) [34]; in Italy in 2005 (kidney: 2.6 ± 0.7 µg/g d. w.; liver: 37.3 ± 8.7 µg/g d. w.; muscle: 2.7 ± 1.4 µg/g d. w.) [29]; and in Turkey in 2004 (kidney: 2.08 ± 0.60 µg/g d. w.; liver: 2.98 ± 0.90 µg/g d. w.; muscle: 1.5 ± 0.82 µg/g d. w.) [37]. On the contrary, the Italian turtles sampled in 2022 exhibited high Cu concentrations in their tissues (kidney: 12.74 ± 13.16 µg/g d. w.; liver: 64.57 ± 89.19 µg/g d. w.) [30].
Iron (Fe) was abundant in the tissues of C. caretta (Figure 5) because, like other essential metals, it is involved in many molecular structures and is primordial to physiological mechanisms. It was mainly concentrated in the liver (42.2%) and in the heart (23.2%) (Figure 2). The Fe contents were as follows: liver (1426.65 ± 1906.59 µg/g d. w.) > heart (782.65 ± 357.43 µg/g d. w.) > kidney (657 ± 319.99 µg/g d. w.) > lung (388.44 ± 111.56 µg/g d. w.) > muscle (121.02 ± 51.98 µg/g d. w.) (Table 3). These values were higher than those obtained in C. caretta collected in Italy in 2022 (heart: 399.24 ± 203.35 µg/g d. w.; kidney: 205.86± 30.08 µg/g d. w.), except for the liver: 4096.55 ± 3510.14 µg/g d. w. [30]. Therefore, the turtles found in Turkey had lower Fe concentrations in 2004 (kidney: 15.31 ± 3.87 µg/g d. w.; liver: 15.74 ± 4.79 µg/g d. w.; lung: 15.61 ± 3.65 µg/g d. w.; muscle: 20.62 ± 2.55 µg/g d. w.) [37].
Manganese (Mn) is rarely measured in tissues of C. caretta in the Mediterranean Sea. In this study, we show that a proportion superior to 70% of Mn was accumulated in the liver (37.7%) and the kidney (32.5%) (Figure 2). The Mn concentrations were 1.63 ± 0.8 µg/g d. w. in the heart, 4.23 ± 1.5 µg/g d. w. in the kidney, 4.91 ± 2.47 µg/g d. w. in the liver, 1.26 ± 0.75 µg/g d. w. in the lung, and 0.99 ± 0.39 µg/g d. w. in muscle (Table 3). The Mn contents observed in C. caretta from Italy in 2022 were slightly superior (heart: 2.01 ± 0.28 µg/g d. w.; kidney: 5.61 ± 7.22 µg/g d. w.; liver: 8.63 ± 5.30 µg/g d. w.) [30], and from Turkey in 2021 (liver: 5.08 ± 4.06 µg/g d. w.; muscle: 1.53± 0.81 µg/g d. w.) [38].
Zinc (Zn) was mainly accumulated in the liver (Figure 2), which confirmed the observations of D’ilio et al. [62] and Gardner et al. [63]. The Zn concentrations measured in C. caretta in this study were as follows: heart (169.20 ± 42.11 µg/g d. w.), kidney (96.53 ± 16.34 µg/g d. w.), liver (114.23 ± 51.36 µg/g d. w.), lung (89.90 ± 47.47 µg/g d. w.), and muscle (110.14 ± 23.55 µg/g d. w.) (Table 3). These Zn contents were lower than those obtained in Italy in 2022 (heart: 209.24 ± 31.74 µg/g d. w.; kidney: 100.97 ± 20.75 µg/g d. w.; liver: 127.11 ± 67.72 µg/g d. w.) [30], but superior to the Zn levels found in C. caretta from Turkey in 2021 (liver: 83.21 ± 52.17 µg/g d. w.; muscle: 91.09 ± 33.01 µg/g d. w.) [38]. Therefore, the Zn concentrations found in the loggerhead turtles collected in Spain were lower in 2008 (kidney: 27.88 ± 26.77 µg/g d. w.; liver: 107.3 ± 82.51 µg/g d. w.; muscle: 65.39 ± 28.3 µg/g d. w.) [35] and in 2010 (kidney: 32.64 ± 12.25 µg/g d. w.; liver: 30.23 ± 12.24 µg/g d. w.; muscle: 113.29 ± 267.33 µg/g d. w.) [28], like in Italy in 2005 (kidney: 97.0 ±31.7 µg/g d. w.; liver: 66.0 ± 42.7 µg/g d. w.; muscle: 107.0 ± 26.1 µg/g d. w.) [29].
We also determined the concentrations of non-essential metals, as these elements cannot be involved in cellular mechanisms and their accumulation in tissues reflects a potential physiological disturbance. The non-essential metals most frequently cited in publications on C. caretta in the Mediterranean are arsenic (As), cadmium (Cd), lead (Pb) and mercury (Hg). In our study, we estimated levels of Ag (silver), As, Cd, Co (cobalt), Cr (chromium), Ni (nickel) and Pb (lead). Unfortunately, we had a technical problem and could not obtain the Hg content of our turtle tissues.
Arsenic (As) was abundant in the tissues of C. caretta from the Gulf of Gabès. Arsenic was the non-essential metal most accumulated in the turtle’s tissues, accounting for 59% (kidney) to 96.8% (muscle) (Figure 5). Arsenic contents were 134.24 ± 97.11 µg/g d. w. had concentrations in the muscle, 58.10 ± 31.27 µg/g d. w. in the heart, 55.75 ± 41.08 µg/g d. w. in the kidney, 44.37 ± 31.61 µg/g d. w. in the liver and 37.27 ± 24.04 µg/g d. w. in the lung (Table 3). The mean arsenic content estimated in this study was much higher than in the other Mediterranean countries. The higher level of arsenic found in muscles confirmed the observations of Abdallah [22], Canzanella et al. [26], and Esposito et al. [27]. The As concentrations found in C. caretta sampled in Spain were lower (kidney: 34.72 ± 31.79 µg/g d. w.; liver: 12.73 ± 13.03 µg/g d. w.; muscle: 40.95 ± 37.32 µg/g d. w.) [28]. Arsenic released by anthropogenic activities (for example, the mining industry) is absorbed by organisms and transferred via the marine food web. Thus, arsenic contamination is probably directly linked to diet, as loggerhead turtles are carnivores. It has been shown that many prey items (crustaceans, molluscs, jellyfish, fish, etc.) are potential sources of arsenic [32,64,65]. Thus, marine animals, like sea turtles, absorb the organic forms of arsenic, although their ability to accumulate the inorganic arsenic from seawater appears limited [66]. The As values found in C. caretta from Tunisia were higher than those established in Egypt in 2023 (kidney: 0.272 ± 0.014 µg/g d. w.; liver: 0.94 ± 0.01 µg/g d. w.; muscle: 0.267 ± 0.04 µg/g d. w.) [22]; in Italy (2021) (heart: 70.58 ± 30.03 µg/g d. w.; kidney: 166.17 ± 74.74 µg/g d. w.; liver: 77.27 ± 45.21 µg/g d. w.) [30]; and Turkey (2004: kidney: 18.99 ± 10.24 µg/g d. w.; liver: 14.23 ± 7.60 µg/g d. w.; lung: 8.66 ± 7.72 µg/g d. w.; muscle: 20.80 ± 9.97 µg/g d. w.; 2017: kidney: 1.99 ± 1.66 µg/g d. w.; liver: 1.87 ± 1.45 µg/g d. w.; and 2021: liver: 37.60 ± 37.61 µg/g d. w.; muscle: 72.85 ± 39.32 µg/g d. w.) [37,38]. It could be interesting to assess the concentrations of arsenic in multiple preys of C. caretta along the Tunisian coast to determine whether the marine ecosystem is highly contaminated with this metal.
Cadmium (Cd) was most concentrated in the kidney (78% of non-essential metal accumulated in tissues), reflecting chronic exposure and a slow elimination by filtration [67]. High Cd content probably induces high metallothionein synthesis (small non-enzymatic proteins involved in the metal regulation in detoxification) [17,20,28,68,69,70]. The sources of cadmium can also be prey [71], like arsenic. The distinct Cd concentrations in the loggerhead turtles collected in the Gulf of Gabès (Tunisia) were 1.23 ± 0.59 µg/g d. w. in the heart, 33.17± 9.78 µg/g d. w. in the kidney, 6.27± 2.62 µg/g d. w. in the liver, 1.43 ± 0.51 µg/g d. w. in the lung, and 0.45 ± 0.48 µg/g d. w. in the muscle (Table 3, Figure 3). These Cd contents appeared lower than those determined in C. caretta in Egypt in 2023 (kidney: 42.49 ± 11.61 µg/g d. w.; liver: 6.45 ± 3.95 µg/g d. w.; muscle: 0.97± 0.54 µg/g d. w.) [22], in Italy (2022, heart: 1.90 ± 0.45 µg/g d. w.; kidney: 39.96 ± 41.86 µg/g d. w.; liver: 10.21 ± 11.04 µg/g d. w. [30]; 2005, kidney: 57.2 ± 34.6 µg/g d. w.; liver: 19.3 ± 34.2 µg/g d. w. [29]); and in Tunisia in 2019 (kidney: 78.13± 59.6 µg/g d. w.; liver: 8.31± 3.22 µg/g d. w.) [39]. On the contrary, the highest Cd concentrations were established in Italy in 2021 (kidney: 8.78 ± 7.28 µg/g d. w.; liver: 1.49 ± 1.37 µg/g d. w.; muscle: 0.11 ± 0.16 µg/g d. w.) [26], in Spain in 2010 (kidney: 6.88 ± 6.26 µg/g d. w.; liver: 0.81 ± 0.48 µg/g d. w.; muscle: 0.08 ± 0.06 µg/g d. w.) [28]; in Turkey (2004, liver: 10.84 ± 3.89 µg/g d. w.; lung: 5.71 ± 3.56 µg/g d. w.; muscle: 3.57 ± 5.86 µg/g d. w. [37]; 2021, liver: 4.59 ± 2.98 µg/g d. w.; muscle: 0.48 ± 0.91 µg/g d. w.) [38]). Sometimes, the Cd concentrations estimated in our study were superior or inferior to others related to the organ, for example to those determined in turtles collected in Spain in 2008 (kidney: 31.47 ± 70.75 µg/g d. w.; liver: 23.38 ± 53.66 µg/g d. w.; muscle: 0.20 ± 0.14 µg/g d. w.) [34] and in 2021 (kidney: 8.98 ± 6.08 µg/g d. w.; liver: 9.75 ± 6.81 µg/g d. w.; muscle: 27.17 ± 24.05 µg/g d. w.) [36].
The concentrations of silver (Ag) and cobalt (Co) in sea turtles are not commonly cited in publications. Cobalt and silver are mainly concentrated in the kidney (Co: 82% of the non-essential metals) and the liver (Ag: 86.2% of the non-essential metals), respectively (Table 3, Figure 3), although the other metals seemed approximately equally bioaccumulated between the tissues (Figure 3). A correlation of accumulation was found between Ag/Cu and Cd/Co (Figure 4). For silver (Ag contents in this study: heart: 0.03 ± 0.03 µg/g d. w.; kidney: 0.02 ± 0.01 µg/g d. w.; liver: 0.57 ± 0.47 µg/g d. w.; lung: 0.03 ± 0.03 µg/g d. w.; muscle: 0.01 ± 0 µg/g d. w.), the same observation was made with the Ag values published on C. caretta in Italy in 2022 (heart: 0.05 ± 0.04 µg/g d. w.; kidney: 0.12 ± 0.18 µg/g d. w.; liver: 0.45 ± 0.29 µg/g d. w.) [30]. The quantities of these metals in the tissues of turtles seemed negligible every time. However, little is known about the toxicity of silver and cobalt in sea turtles. Thus, low quantities could induce putative physiological disturbances. Studying the toxicity of these metals in reptiles seems worthwhile.
Chromium (Cr) (heart: 1.37 ± 1.43 µg/g d. w.; kidney: 1.72 ± 0.94 µg/g d. w.; liver: 1.16 ± 1.20 µg/g d. w.; lung: 1.28 ± 0.67 µg/g d. w.; muscle: 1.85 ± 0.95 µg/g d. w.) and nickel (Ni) (heart: 0.77 ± 0.69 µg/g d. w.; kidney: 1.35 ± 0.99 µg/g d. w.; liver: 0.92 ± 0.89 µg/g d. w.; lung: 0.55 ± 0.26 µg/g d. w.; muscle: 1.95 ± 4.64 µg/g d. w.) concentrations (Table 2) were compared to those measured in Italy [30] and Turkey [37], which are unique measures from the Mediterranean Sea. Our Cr contents in C. caretta from Tunisia were higher than those published on loggerhead turtles found in Italy in 2022 (liver: 0.12 ± 0.35 µg/g d. w.) [30], but lower if we compared them to those measured in sea turtles in Turkey in 2004 (kidney: 2.06 ± 0.59 µg/g d. w.; liver: 2.77 ± 0.93 µg/g d. w.; lung: 2.54 ± 0.83 µg/g d. w.; muscle: 1.51 ± 0.78 µg/g d. w.) [37]. Nickel appeared higher in the turtles from Turkey collected in 2004 (kidney: 9.63 ± 2.43 µg/g d. w.; liver: 11.56 ± 3.08 µg/g d. w.; lung: 10.08 ± 3.97 µg/g d. w.; muscle: 10.02 ± 3.73 µg/g d. w.) [37]. It was mentioned that Cr and Ni could induce negative effects in the turtles [72,73,74,75]. Therefore, it seems worthwhile to develop future studies on the systematic measurement of these metals in marine animals, such as sea turtles, to enhance our understanding of their bioaccumulation and negative effects on ecosystems [76].
Metal bioaccumulation facilitates oxidative stress [77,78]. Oxidative stress is the result of oxidoreduction imbalance and allows the production of reactive oxygen species (ROS), such as superoxide radical anions (O2−), hydrogen peroxide (H2O2), and peroxyl radicals (ROO−) [79]. These elements degrade cellular compounds, such as proteins and lipids, and induce DNA damage [80]. Even if it is preferable to use blood to estimate the antioxidant responses, Cortes-Gomez et al. [81] demonstrated on Lepidochhelys olivacea that we can measure enzymatic activities in dead turtles. In our study, we observed high lipid peroxidation in the kidneys and liver (Table 4, Figure 7) of C. caretta, two organs involved in detoxification and filtration processes. The antioxidant cellular mechanisms include enzymatic proteins (catalase, glutathione peroxidase, superoxide dismutase) and other elements such as vitamins and pigments (for example, carotenoids). The enzymes require metals (Cu, Fe, Mn, Ni, Zn) to activate their catalytic sites [82]. Thus, unsurprisingly, we found an increase in antioxidant enzyme activity in the tissues of turtles accumulating high levels of metals, such as the liver (Table 4, Figure 6). The induction of antioxidant enzymes after metal exposure was also noted in the turtle Mauremys reevesii after exposure to cadmium [83]. Labrada-Martagon et al. [84] suggested that lipid peroxidation (LPO) and antioxidant enzyme activities in sea turtles could be used as biomarkers of environmental pollution. Future investigations could use blood samples of Caretta caretta to estimate cell damage (LPO, micronuclei, etc.) and enzyme activity, to take a non-destructive approach. These analytical methods were used successfully on wild populations of Lepidochelys olivacea [84] and Eretmochelys imbricata [85].
In perspective, the study of pollution and its effects on the Caretta caretta populations in the Mediterranean Sea could include the measurement of multiple pollutant (metals, HAP, pesticides) concentrations in distinct organs (integrating the brain, fat, gonads and thyroid) and the evaluation of the negative effects on the nervous, reproductive, digestive, endocrine and respiratory systems.
5. Conclusions
Loggerhead turtles (Caretta caretta), collected along the coast of the Gulf of Gabès (Tunisia), revealed a high concentration of metals (such as arsenic and cadmium). In particular, the liver and kidneys are preferential organs for the accumulation of metals. Antioxidant responses (CAT, GPx, SOD) and lipid peroxidation were measured in the kidney, liver and lower muscle, suggesting oxidative stress in these tissues.
Author Contributions
Conceptualisation, M.H. and V.L.; methodology, M.H., C.B. and V.L.; validation, I.J.; formal analysis, I.J. and V.L.; investigation, M.H.; resources, M.H. and V.L.; data curation, M.H.; writing—original draft preparation, M.H. and V.L.; writing—review and editing, I.J. and V.L.; visualisation, I.J.; supervision, V.L.; project administration, I.J. and V.L.; funding acquisition, I.J. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Campus France, Partenariat Hubert Curien UTIQUE program 2021–2023 (grant number: 46155XA). Thus, the work was financially supported by the MEAE (Ministère de l’Europe et des Affaires Etrangères, France), the MESR (Ministère de l’Enseignement Supérieur et de la Recherche, France), and the MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique, Tunisia). Therefore, Society for conservation biology (Africa section graduate student research award) offered grant to this study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the members of the Association Sauvegarde des Zones Humides du Sud Tunisien for their precious help during work with stranding sea turtles. We would like to thank the “plateforme analyse élémentaire” from the LIENSs laboratory UMR7266 (La Rochelle, France).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haynes, D.; Johnson, J.E. Organochlorine, Heavy Metal and Polyaromatic Hydrocarbon Pollutant Concentrations in the Great Barrier Reef (Australia) Environment: A Review. Mar. Pollut. Bull. 2000, 41, 267–278. [Google Scholar] [CrossRef]
- Simantiris, N.; Andreanidou, K.; Sampson, G. Over 30 years of monitoring and implementing the Bern Convention’s recommendations for the protection of Mediterranean Sea turtles. Mar. Policy 2024, 168, 106319. [Google Scholar] [CrossRef]
- Maret, W. The Metals in the Biological Periodic System of the Elements: Concepts and Conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Novillo, O.; Pertusa, J.F.; Tomás, J. Exploring the Presence of Pollutants at Sea: Monitoring Heavy Metals and Pesticides in Loggerhead Turtles (Caretta caretta) from the Western Mediterranean. Sci. Total Environ. 2017, 598, 1130–1139. [Google Scholar] [CrossRef]
- Duncan, E.M.; Akbora, H.D.; Baldi, P.; Beton, D.; Broderick, A.C.; Cicek, B.A.; Crowe-Harland, C.; Davey, S.; DeSerisy, T.; Fuller, W.J.; et al. Marine turtles as bio-indicators of plastic pollution in the eastern Mediterranean. Mar. Pollut. Bull. 2024, 201, 116141. [Google Scholar] [CrossRef] [PubMed]
- Caracappa, S.; Persichetti, M.F.; Piazza, A.; Caracappa, G.; Gentile, A.; Marineo, S.; Crucitti, D.; Arculeo, M. Incidental Catch of Loggerhead Sea Turtles (Caretta caretta) along the Sicilian Coasts by Longline Fishery. PeerJ 2018, 6, e5392. [Google Scholar] [CrossRef] [PubMed]
- Kuzukiran, O.; Simsek, I.; Kara, E.; Yurdakok-Dikmen, B.; Boztepe, U.G.; Toprak, M.; Filazi, A. Investigation of some persistent organic pollutants in loggerhead Sea turtles (Caretta caretta). Mar. Pollut. Bull. 2024, 205, 116670. [Google Scholar] [CrossRef] [PubMed]
- Garcè, A.; Pires, I. Fibropapillomatosis on Sea turtles, a sentinel of ecosystem health? Environ. Sci. Proc. 2022, 24, 1. [Google Scholar] [CrossRef]
- Dujon, A.M.; Schofield, G.; Venegas, R.M.; Thomas, F.; Ujvari, B. Sea turtles in the cancer risk landscape: A global meta-analysis of fibropapillomatosis prevalence and associated risk factors. Pathogens 2021, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, A. Progress on the impact of persistent pollutants on marine turtles: A review. J. Mar. Sci. Eng. 2023, 11, 266. [Google Scholar] [CrossRef]
- Barraza, A.D.; Finlayson, K.A.; Leusch, F.D.L.; van de Merwe, J.P. Systematic review of reptile reproductive toxicology to inform future research directions on endangered or threatened species, such as Sea turtles. Environ. Pollut. 2021, 286, 117470. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.M.; Broderick, A.C.; Critchell, K.; Galloway, T.S.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Santillo, D.; Tucker, A.D.; Whiting, S.; et al. Plastic pollution and small juvenile marine turtles: A potential evolutionary trap. Front. Mar. Sci. 2021, 8, 699521. [Google Scholar] [CrossRef]
- Tanabe, L.K.; Scott, K.; Dasari, V.; Berumen, M. An assessment of heavy metals in green Sea turtle (Chelonia mydas) hatchlings from Saudi Arabia’s largest rookery, Ras Baridi. PeerJ 2022, 10, e13928. [Google Scholar] [CrossRef]
- Morao, I.F.C.; Simoes, T.; Casado, R.B.; Vieira, S.; Ferreira-Airaud, B.; Caliani, I.; Di Noi, A.; Casini, S.; Fossi, M.C.; Lemos, M.F.L.; et al. Metal accumulation in female green sea turtles (Chelonia mydas) from Eastern Atlantic affects their egg quality with potential implications for embryonic development. Sci. Total Environ. 2024, 931, 172710. [Google Scholar] [CrossRef] [PubMed]
- Casale, P.; Broderick, A.C.; Caminas, J.A.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.J.; Godley, B.J.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea turtles: Current knowledge and priorities for conservation and research. Endang. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Mancino, C.; Canestrelli, D.; Maiorano, L. Going west: Range expansion for loggerhead Sea turtles in the Mediterranean Sea under climate change. Glob. Ecol. Conserv. 2022, 38, e02264. [Google Scholar] [CrossRef]
- Mancino, C.; Hochscheid, S.; Maiorano, L. Increase of nesting habitat suitability for green turtles in the warming Mediterranean Sea. Sci. Rep. 2023, 13, 19906. [Google Scholar] [CrossRef]
- Casale, P.; Mariani, P. The first Lost year’ of Mediterranean Sea turtles: Dispersal patterns indicate sub-regional management units for conservation. Mar. Ecol. Prog. Ser. 2014, 498, 263–274. [Google Scholar] [CrossRef]
- Andreani, G.; Santoro, M.; Cottignoli, S.; Fabbri, M.; Carpenè, E.; Isani, G. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) Sea turtles. Sci. Total Environ. 2008, 390, 287–294. [Google Scholar] [CrossRef]
- Godley, B.J.; Thompson, D.R.; Furness, R.W. Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar. Pollut. Bull. 1999, 38, 497–502. [Google Scholar] [CrossRef]
- Abdallah, M.A.M. Bioaccumulation and biomagnifications of toxic metals in tissues of loggerhead turtles (Carettta caretta) from the Mediterranean Sea coast, Egypt. Sci. Rep. 2023, 13, 7995. [Google Scholar] [CrossRef]
- Celik, S.; Beton, D.; Cicek, B.A.; Snape, R.T.E.; Baskale, E. Heavy metal accumulation of pre-adult loggerhead turtle and green turtle in Northern Cyprus. SSRN Electron. J. 2022, 316, 120482. [Google Scholar] [CrossRef]
- Antoniou, Z.; Dassenakis, M.; Panagopoulos, D.; Sofouli, E. Copper and manganese in loggerhead turtles (Caretta caretta) tissues in the Mediterranean. Mediterr. Mar. Sci. 2004, 5, 109–116. [Google Scholar]
- Cammilleri, G.; Galluzzo, F.G.; Pulvirenti, A.; Pantano, L.; Calabrese, V.; Gentile, A.; Cumbo, V.; Macaluso, A.; Vella, A.; Ferrantelli, V. Toxic metals in loggerhead Sea turtle (Caretta caretta) stranded freshly dead along Sicilian coasts. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Canzanella, S.; Danese, A.; Mandato, M.; Lucifora, G.; Riverso, C.; Federico, G.; Gallo, P.; Esposito, M. Concentrations of trace elements in tissues of loggerhead turtles (Caretta caretta) from the Tyrrhenian and the Ionian coastlines (Calabria, Italy). Environ. Sci. Pollut. Res. 2021, 28, 26545–26557. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; De Roma, A.; Sansone, D.; Capozzo, D.; Iaccarino, D.; di Nocera, F.; Gallo, P. Non-essential toxic element (Cd, As, Hg and Pb) levels in muscle, liver and kidney of loggerhead Sea turtles (Caretta caretta) stranded along the southwestern coasts of the Tyrrhenian Sea. Comp. Biochem. Physiol. C. 2020, 231, 108725. [Google Scholar] [CrossRef] [PubMed]
- Jerez, S.; Motas, M.; Canovas, R.A.; Talavera, J.; Almela, R.M.; Bayon del Rio, A. Distribution of heavy metals and essentials elements in loggerhead turtles (Caretta caretta) from Spanish Mediterranean coastline. Chemosphere 2010, 78, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, F.; Caurant, F.; Bustamante, P.; Bentivegna, F. Trace elements (Cd, Cu, Hg, Se, Zn) accumulation and tissue distribution in loggerhead turtles (Caretta caretta) from Western Mediterranean Sea (Southern Italy). Chemosphere 2005, 58, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Arculeo, M.; Arizza, V.; Pace, A.; Melfi, R.; Caracappa, S.; Caracappa, G.; Vullo, C.; Cambera, I.; Visconti, G.; et al. Impact of heavy metals in eggs and tissues of C. caretta along the Sicilian coast (Mediterranean Sea). Environments 2022, 9, 88. [Google Scholar] [CrossRef]
- Storelli, M.M.; Ceci, E.; Marcotrigiano, G.O. Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimens beached along the Adriatic Sea (Italy). Bull. Environ. Contam. Toxicol. 1998, 60, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Marcotrigiano, G.O. Total organic and inorganic arsenic from marine turtles (Caretta caretta) beached along the Italian coast (South Adriatic Sea). Bull. Environ. Contam. Toxicol. 2000, 65, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Febrer-Serra, M.; Renga, E.; Fernandez, G.; Lassnig, N.; Tejada, S.; Capo, X.; Pinya, S.; Sureda, A. First report of heavy metal presence in the muscular tissue of loggerhead turtles Caretta caretta (Linnaeus, 1758) from the Balearic Sea (Balearic Islands, Spain). Environ. Sci. Pollut. Res. 2020, 27, 39651–39656. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, A.J.; Gomez-Ramirez, P.; Martinez-Lopez, E.; Hernandez-Garcia, A. Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the Southwestern Mediterranean (Spain). Ecotoxicol. Environ. Saf. 2008, 72, 557–563. [Google Scholar] [CrossRef]
- Gomez-Ramirez, P.; Espin, S.; Navas, I.; Martinez-Lopez, E.; Jimenez, P.; Maria-Mojica, P.; Penalver, J.; Garcia-Fernandez, A.J. Mercury and organochlorine pesticides in tissues of loggerhead Sea turtles (Caretta caretta) stranded along the Southwestern Mediterranean coastline (Andalusia, Spain). Bull. Environ. Contam. Toxicol. 2020, 104, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, E.; Herrero, D.; Lopez-Berenguer, G.; Penalver, J. Total arsenic concentrations in Sea turtle tissues from the Mediterranean coast of Spain. Bull. Environ. Contam. Toxicol. 2021, 107, 820–826. [Google Scholar] [CrossRef]
- Kaska, Y.; Celik, A.; Bag, H.; Aureggi, M.; Ozel, K.; Elci, A.; Kaska, A. Heavy metal monitoring in stranded Sea turtles along the Mediterranean coast of Turkey. Fresenius Environ. Bull. 2004, 13, 769–776. [Google Scholar]
- Aymak, C.; Ucar, A.H.; Ergene, S. Distribution of heavy metals in tissues of stranded loggerhead turtles (Caretta caretta) on Kazanh Beach, turkey, North-Western. J. Zool. 2021, 17, 82–91. [Google Scholar]
- El Hili, H.A.E.; Mzoughi, N.; Karaa, S.; Chouba, L. Distribution of trace metals (Cd, Hg, Pb, Cu) and polycyclic aromatic hydrocarbons (PAH) in loggerhead turtles (Reptilia: Testudines: Cheloniidae: Caretta caretta (Linnaeus, 1758)) tissues stranded along the North Tunisian coasts. Int. J. Mar. Biol. Res. 2019, 3, 1–6. [Google Scholar]
- Pinya, S.; Renga, E.; Fernandez, G.; Mateu-Vicens, G.; Tejada, S.; Capo, X.; Sureda, A. Physiological biomarkers in loggerhead turtles (Caretta caretta) as a tool for monitoring sanitary evolution in marine recovery centres. Sci. Total Environ. 2024, 755, 143930. [Google Scholar] [CrossRef]
- Morao, I.F.C.; Lemos, M.F.L.; Felix, R.; Vieira, S.; Barata, C.; Novais, S.C. Stress response markers in the blood of São Tomé green Sea turtles (Chelonia mydas) and their relation with accumulated metal levels. Environ. Pollut. 2022, 293, 118490. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Niehaus, W.G.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef]
- Turkozan, O.; Ozdilek, S.Y.; Ergene, S.; Ucar, A.H.; Sonmez, B.; Yilmaz, C.; Kacar, Y.; Aymak, C. Strandings of Loggerhead (Caretta caretta) and Green (Chelonia mydas) Sea Turtles along the Eastern Mediterranean Coast of Turkey. Herpetol. J. 2013, 23, 11–15. [Google Scholar]
- Breitwieser, M.; Viricel, A.; Churlaud, C.; Guillot, B.; Martin, E.; Stenger, P.-L.; Huet, V.; Fontanaud, A.; Thomas-Guyon, H. First Data on Three Bivalve Species Exposed to an Intra-Harbour Polymetallic Contamination (La Rochelle, France). Comp. Biochem. Physiol. C 2017, 199, 28–37. [Google Scholar] [CrossRef]
- Core Team R. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online: http://www.R-project.org (accessed on 1 March 2022).
- Kassambara, A.; Mundt, F. Facto Extra: Extract and Visualize the Results of Multivariate Data Analyses (Version 1.0. 5). 2017, 5. Available online: https://www.rdocumentation.org/packages/factoextra/versions/1.0 (accessed on 15 June 2024).
- Ellipse, D.M. Functions for Drawing Ellipses and Ellipse-like Confidence Regions. Statistical Package. 2007. Available online: https://cran.r-project.org/web/packages/ellipse/ellipse.pdf (accessed on 15 June 2024).
- El-Geziry, T.M.; Bryden, I.G. The circulation pattern in the Mediterranean Sea: Issues for modeller consideration. J. Oper. Oceanogr. 2010, 3, 39–46. [Google Scholar] [CrossRef]
- Harrabi, M.; Varela Della Giustina, S.; Aloulou, F.; Rodriguez-Mozaz, S.; Barcelo, D.; Elleuch, B. Analysis of multiclass antibiotic residues in urban wastewater in Tunisia. Environ. Nanotechnol. Monit. Manag. 2018, 10, 163–170. [Google Scholar] [CrossRef]
- WWF. Le Littoral Tunisien. 2020. Available online: https://wwf.panda.org/frwwf_action_zones/tunisie/littoral_tunisien/ (accessed on 14 July 2021).
- Nriagu, J.O. A history of global metal pollution. Science 1996, 272, 223. [Google Scholar] [CrossRef]
- Kaste, O.; Skarbovik, E.; Greipsland, I.; Gundersen, C.B.; Austnes, K.; Skancke, L.B.; Guerrero, J.-L.; Sample, J.E. The Norwegian River Monitoring Programme—Water Quality Status and Trends; Norsk Institutt for Vannforskning: Oslo, Norway, 2017. [Google Scholar]
- La Colla, N.S.; Botte, S.E.; Simonetti, P.; Negrin, V.L.; Serra, A.V.; Marcovecchio, J.E. Water, sediments and fishes: First multicompartment assessment of metal pollution in a coastal environment from the SW Atlantic. Chemosphere 2021, 282, 131131. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Athmouni, K.; Metais, I.; Ayadi, H.; Leignel, V. The Mediterranean limpet Patella caerulea (Gastropoda, Mollusca) to assess marine ecotoxicological risk: A case study of Tunisian coasts contaminated by metals. Environ. Sci. Pollut. Res. 2022, 29, 28339–28358. [Google Scholar] [CrossRef]
- Annabi, A.; Bardelli, R.; Vizzini, S.; Mancinelli, G. Baseline assessment of heavy metals content and trophic position of the invasive blue swimming crab Portunus segnis (Forskal, 1775) in the Gulf of Gabès (Tunisia). Mar. Pollut. Bull. 2018, 136, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Suarez, I.; Lozano-Bilbao, E.; Hardisson, A.; Paz, S.; Gutièrrez, A.J. Metal and trace element concentrations in cetaceans worldwide: A review. Mar. Pollut. Bull. 2023, 192, 115010. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Conti, G.O.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals in fish from the Mediterranean Sea: Potential impact on diet. Med. Diet 2015, 547–562. [Google Scholar] [CrossRef]
- D’ilio, S.; Mattei, D.; Blasi, M.F.; Alimonti, A.; Bogialli, S. The occurrence of chemical elements and POPs in loggerhead turtles (Caretta caretta): An overview. Mar. Poll. Bull. 2011, 62, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.C.; Fitzgerald, S.L.; Vargas, B.A.; Rodríguez, L.M. Heavy metal accumulation in four species of Sea turtles from the Baja California peninsula, Mexico. Biometals 2006, 19, 91–99. [Google Scholar] [CrossRef]
- Bustamante, P.; Caurant, F.; Fowler, S.W.; Miramand, P. Cephalopods as a vector for the transfer of cadmium to top marine predators in the north-east Atlantic Ocean. Sci. Total Environ. 1998, 220, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristali, A.; Copat, C. Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts: A health risk assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef]
- Kalia, H.-M.; Khambholja, D.B. Arsenic contents and its biotransformation in the marine environment. In Handbook of Arsenic Toxicology; Academic Press: Cambridge, MA, USA, 2015; pp. 675–700. [Google Scholar]
- Rie, M.T.; Lendas, K.A.; Callard, I.P. Cadmium: Tissue distribution and binding protein induction in the painted turtle Chrysemys picta. Comp. Biochem. Physiol. C 2001, 130, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.C.; Varela, A.S.; Barcarolli, I.F.; Bianchini, A. Concentrations and distributions of metals in tissues of stranded green Sea turtles (Chelonia mydas) from the southern Atlantic coast of Brazil. Sci. Total Environ. 2014, 466–467, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Guirlet, E.; Das, K. Cadmium toxicokinetics and bioaccumulation in turtles: Trophic exposure of Trachemys scripta elegans. Ecotoxicology 2012, 21, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jakimska, A.; Konieczka, P.; Skóra, K.; Namieśnik, J. Bioaccumulation of metals in tissues of marine animals, part II: Metal concentrations in animal tissues. Pol. J. Environ. Stud. 2011, 20, 1127–1146. [Google Scholar]
- Caurant, F.; Bustamante, P.; Bordes, M.; Miramand, P. Bioaccumulation of metals in tissues of marine animals, part II: Metal concentrations in animal tissues. Pol. J. Environ. Stud. 1999, 38, 1085–1091. [Google Scholar]
- Wise, S.S.; Xie, H.; Fukuda, T.; Thompson, W.D.; Wise, J.P. Hexavalent chromium is cytotoxic and genotoxic to hawksbill Sea turtle cells. Toxicol. Appl. Pharmacol. 2014, 279, 113–118. [Google Scholar] [CrossRef]
- Lam, J.C.; Tanabe, S.; Chan, S.K.; Lam, M.H.; Martin, M.; Lam, P.K. Levels of trace elements in green turtle eggs collected from Hong Kong: Evidence of risks due to selenium and nickel. Environ. Pollut. 2006, 144, 790–801. [Google Scholar] [CrossRef]
- Chakraborty, R.; Renu, K.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Mirza, A.K.; Vellingiri, B.; Iyer, M.; Dey, A.; Gopalakrishnan, A.V. Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed. Pharmacother. 2022, 151, 113119. [Google Scholar] [CrossRef] [PubMed]
- DesMarais, T.L.; Costa, M. Mechanisms of Chromium-Induced Toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gissi, F.; Stauber, J.L.; Binet, M.T.; Golding, L.A.; Adams, M.S.; Schlekat, C.E.; Garman, E.R.; Jolley, D.F. A review of nickel toxicity to marine and estuarine tropical biota with particular reference to the Southeast Asian and Melanesian regions. Environ. Pollut. 2016, 218, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Byeon, E.; Kang, H.-M.; Yoon, C.; Lee, J.-S. Toxicity mechanisms of arsenic compounds in aquatic organisms. Aquat. Toxicol. 2021, 237, 105901. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Schiedber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Gomez, A.; Morcillo, P.; Guardiola, F.A.; Espinosa, C.; Esteban, M.A.; Cuesta, A.; Girondot, M.; Romero, D. Molecular oxidative stress markers in olive ridley turtles (Lepidochelys olivacea) and their relation to metal concentrations in wild populations. Environ. Pollut. 2018, 233, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD(SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Dong, A.; Huo, J.; Yan, J.; Dong, A.; Liu, B. Lipid peroxidation of kidney of the turtle Mauremys reevesii caused by cadmium. Environ. Sci. Pollut. Res. 2021, 28, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Labrada-Martagon, V.; Rodriguez, P.A.T.; Mendez-Rodriguez, L.C.; Zenteno-Savin, T. Oxidative stress indicators and chemical contaminants in East Pacific green turtles (Chelonia mydas) inhabiting two foraging coastal lagoons in the Baja California peninsula. Comp. Biochem. Physiol. C 2011, 154, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, P.I.; Vieira, L.R.; Ku-Peralta, W.; Morgado, F.; Rendon-von Osten, J. Oxidative stress biomarkers and organochlorine pesticides in nesting female hawksbill turtles Eretmochelys imbricata from Mexican coast (Punta Xen, Mexico). Environ. Sci. Pollut. Res. 2018, 25, 23809–23816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).