Effect of Bark Biochars on Properties of Steam-Activated Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Activated Carbons
2.3. Scanning Electron Microscopy (SEM)
2.4. Nitrogen Physisorption
2.5. X-ray Fluorescence Analysis (XRF)
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. Thermogravimetric Analysis (TGA)
2.8. Methylene Blue and Methyl Orange Adsorption
2.9. Statistical Analysis
3. Results and Discussion
3.1. Surface Structure
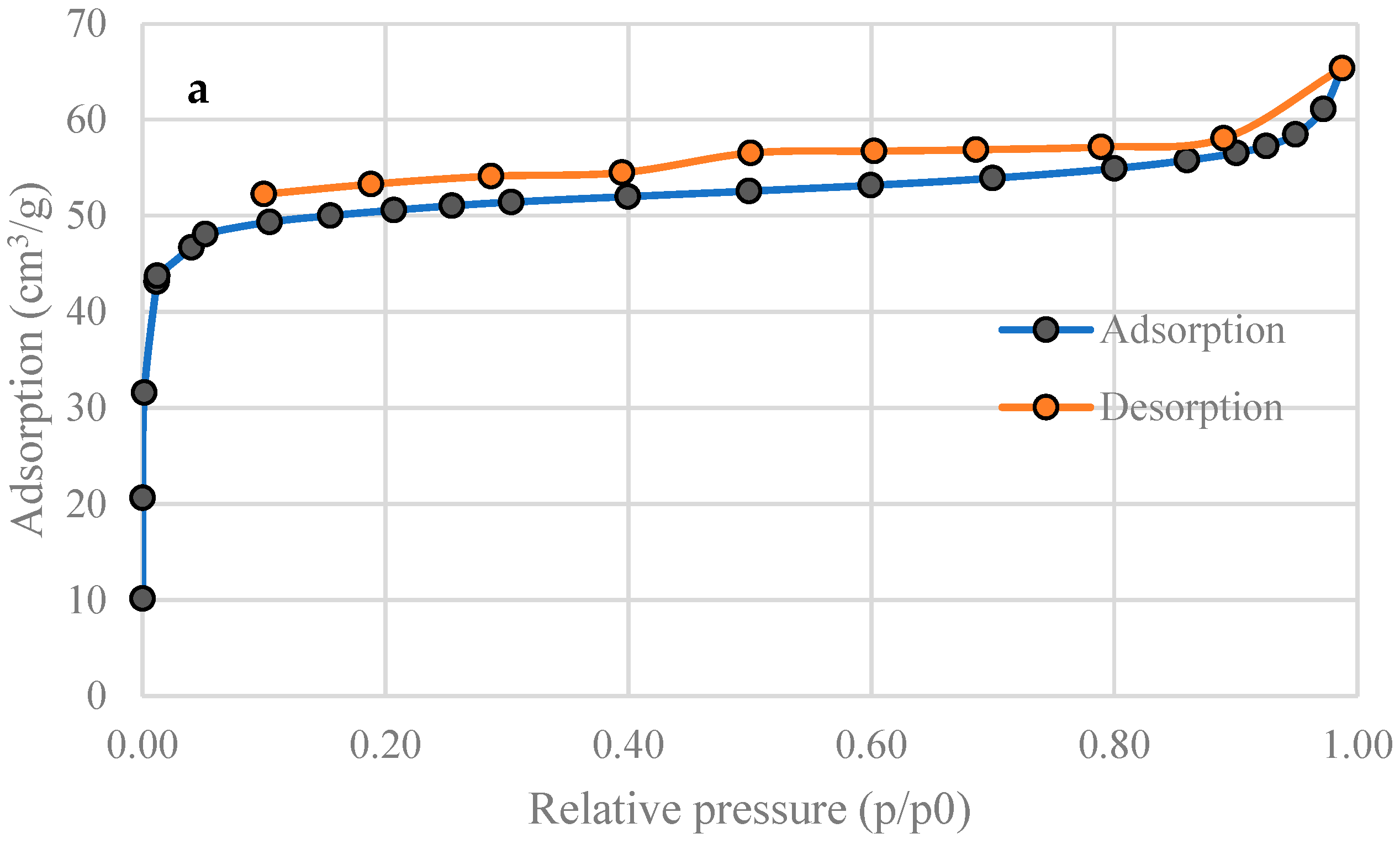
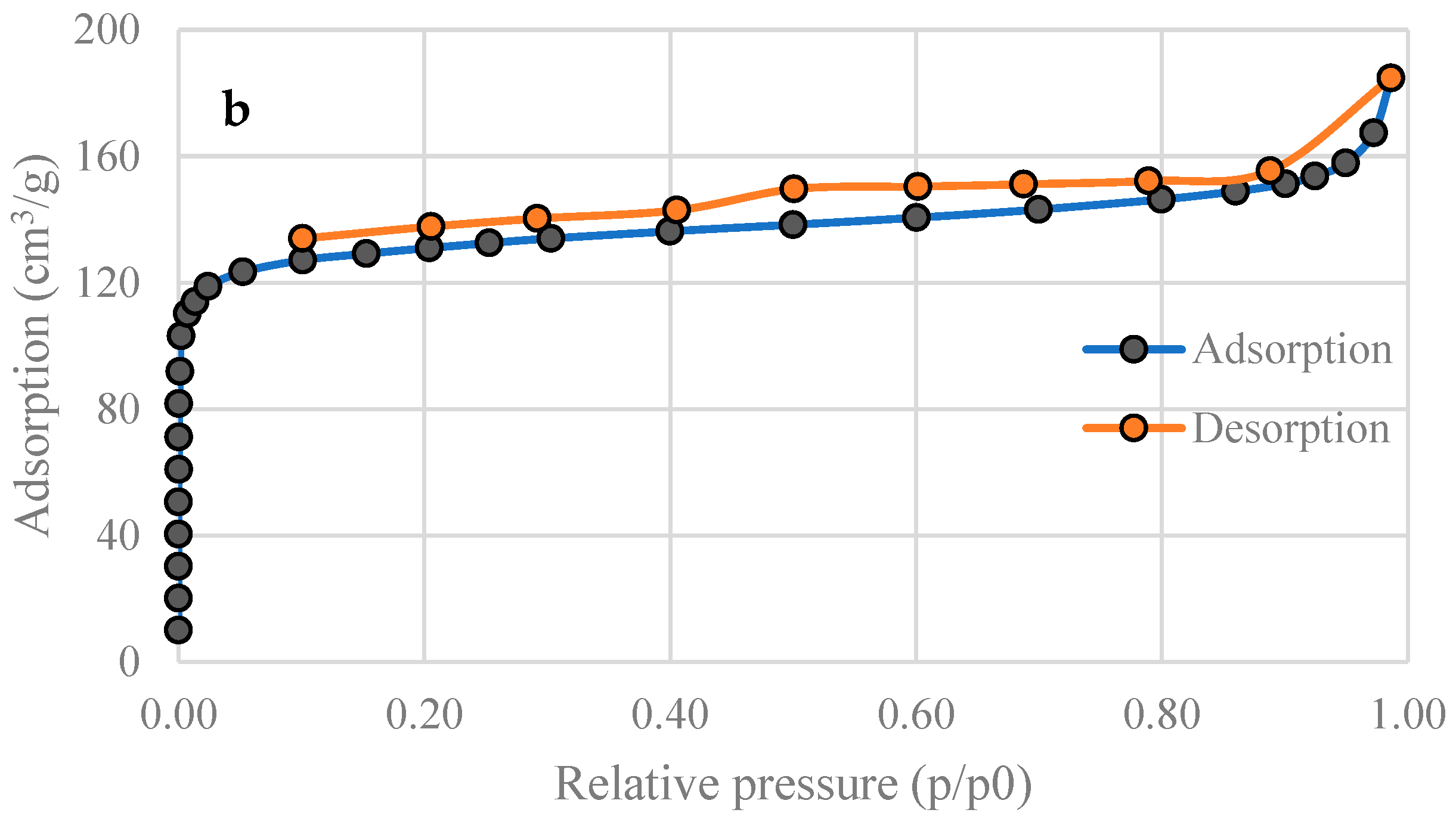
3.2. Specific Surface Area of Activated Carbons
3.3. Inorganic Elemental Composition
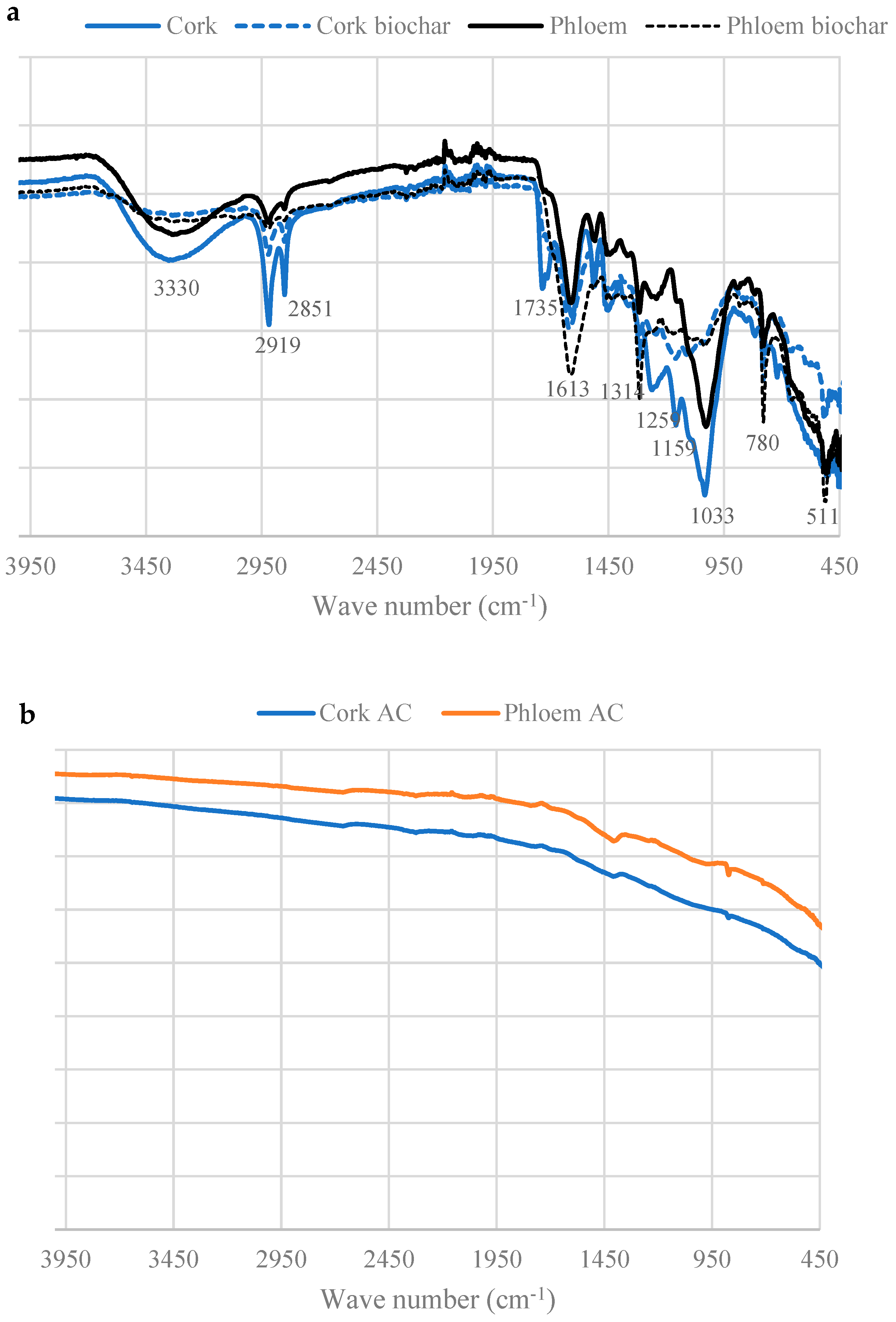
3.4. Surface Functional Groups
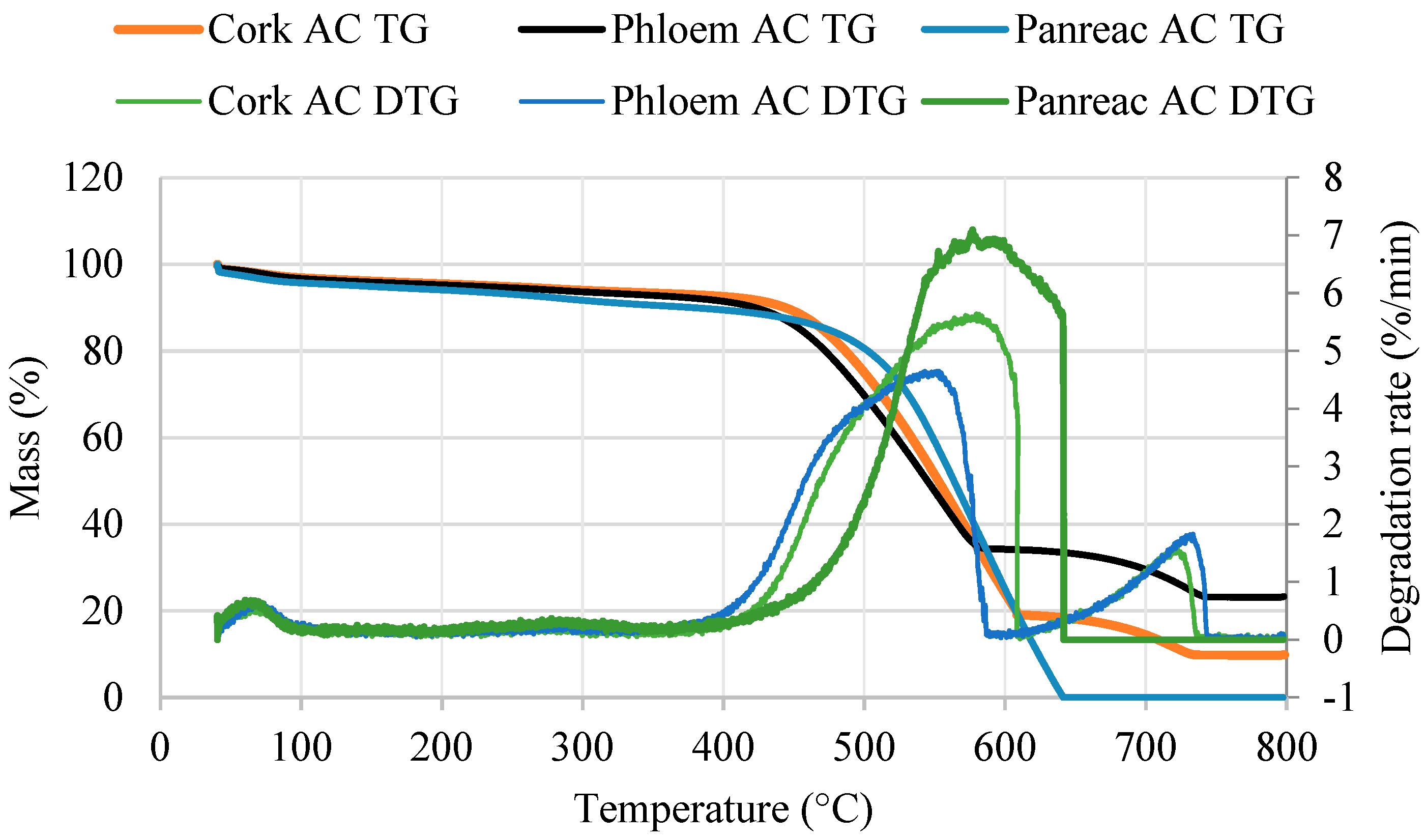
3.5. Thermogravimetric Analysis of Activated Carbons
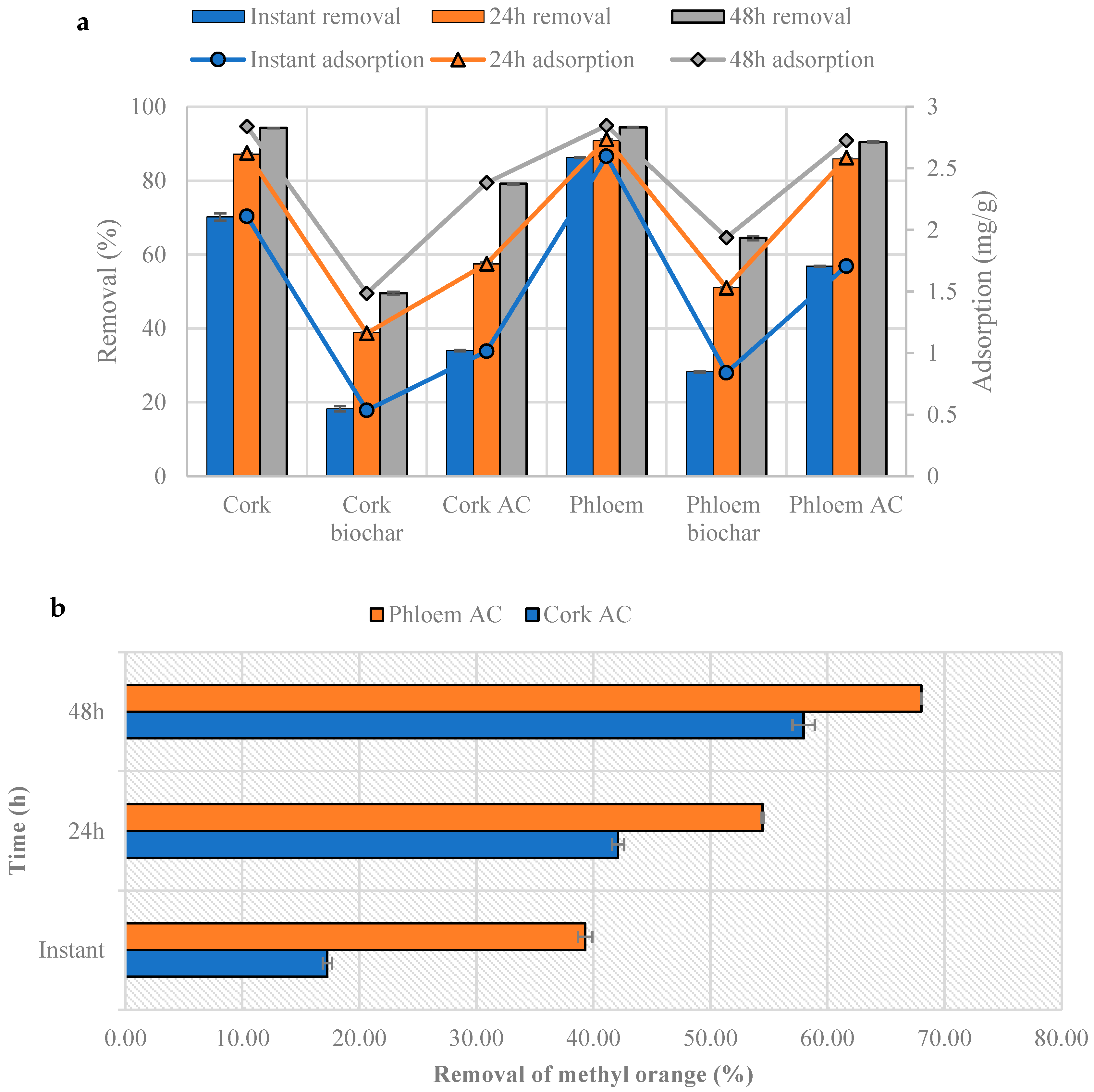
3.6. Methylene Blue and Methyl Orange Adsorption
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, C.H.; Rass-Hansen, J.; Marsden, C.C.; Taarning, E.; Egeblad, K. The renewable chemicals industry. ChemSusChem Chem. Sustain. Energy Mater. 2008, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic biorefineries in Europe: Current state and prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Verma, P. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of hydrochar and process water from the hydrothermal carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.U.; Pereira, H. State-of-the-art char production with a focus on bark feedstocks: Processes, design, and applications. Processes 2021, 9, 87. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1984. [Google Scholar]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.-E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Bläker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of activated carbon adsorbents–state of the art and novel approaches. ChemBioEng Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Le Van, K.; Thi, T.T.L. Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor. Prog. Nat. Sci. Mater. Int. 2014, 24, 191–198. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Schröder, E.; Thomauske, K.; Weber, C.; Hornung, A.; Tumiatti, V. Experiments on the generation of activated carbon from biomass. J. Anal. Appl. Pyrolysis 2007, 79, 106–111. [Google Scholar] [CrossRef]
- Julien, F.; Baudu, M.; Mazet, M. Relationship between chemical and physical surface properties of activated carbon. Water Res. 1998, 32, 3414–3424. [Google Scholar] [CrossRef]
- Nachenius, R.W.; Ronsse, F.; Venderbosch, R.H.; Prins, W. Biomass pyrolysis. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; Volume 42, pp. 75–139. ISBN 0065-2377. [Google Scholar]
- Şen, A.; Leite, C.; Lima, L.; Lopes, P.; Pereira, H. Industrial valorization of Quercus cerris bark: Pilot scale fractionation. Ind. Crops Prod. 2016, 92, 42–49. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical characterization, bioactive and fuel properties of waste cork and phloem fractions from Quercus cerris L. bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Pasztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Şen, A.U.; Nobre, C.; Durão, L.; Miranda, I.; Pereira, H.; Gonçalves, M. Low-temperature biochars from cork-rich and phloem-rich wastes: Fuel, leaching, and methylene blue adsorption properties. Biomass Convers. Biorefin. 2022, 12, 3899–3909. [Google Scholar] [CrossRef]
- Rafatullah, M.; Ahmad, T.; Ghazali, A.; Sulaiman, O.; Danish, M.; Hashim, R. Oil palm biomass as a precursor of activated carbons: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1117–1161. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Zhang, L.; Xiong, Y. Effects of torrefaction on yield and quality of pyrolysis char and its application on preparation of activated carbon. J. Anal. Appl. Pyrolysis 2016, 119, 217–223. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Şen, U.; Longo, A.; Gonçalves, M.; Miranda, I.; Pereira, H. The potential of waste phloem fraction of Quercus cerris bark in biochar production. Environments 2023, 10, 71. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Lorenzen, L.; Van Deventer, J.S.J.; Landi, W.M. Factors affecting the mechanism of the adsorption of arsenic species on activated carbon. Miner. Eng. 1995, 8, 557–569. [Google Scholar] [CrossRef]
- Lu, J.-J.; Chen, W.-H. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Pereira, H.; Rosa, M.E.; Fortes, M.A. The cellular structure of cork from Quercus suber L. IAWA J. 1987, 8, 213–218. [Google Scholar] [CrossRef]
- Pereira, H.; Ferreira, E. Scanning electron microscopy observations of insulation cork agglomerates. Mater. Sci. Eng. A 1989, 111, 217–225. [Google Scholar] [CrossRef]
- Pereira, H.; Baptista, C. Influence of raw-material quality and process parameters in the production of insulation cork agglomerates. Eur. J. Wood Wood Prod. 1993, 51, 301–308. [Google Scholar] [CrossRef]
- Shimada, M.; Iida, T.; Kawarada, K.; Chiba, Y.; Mamoto, T.; Okayama, T. Porous structure of activated carbon prepared from waste newspaper. J. Mater. Cycles Waste Manag. 2000, 2, 100–108. [Google Scholar]
- Yeganeh, M.M.; Kaghazchi, T.; Soleimani, M. Effect of raw materials on properties of activated carbons. Chem. Eng. Technol. Ind. Chem. Equip. Process Eng. 2006, 29, 1247–1251. [Google Scholar] [CrossRef]
- Tamai, H.; Kakii, T.; Hirota, Y.; Kumamoto, T.; Yasuda, H. Synthesis of extremely large mesoporous activated carbon and its unique adsorption for giant molecules. Chem. Mater. 1996, 8, 454–462. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, R.A.; Aroso, I.; Fernandes, E.M.; Pinto, M.L.; Reis, R.L.; Andrade, M.A.; Pires, J.; Silva, S.P.; Carvalho, A.P. Activated carbons prepared from industrial pre-treated cork: Sustainable adsorbents for pharmaceutical compounds removal. Chem. Eng. J. 2014, 253, 408–417. [Google Scholar] [CrossRef]
- Atanes, E.; Nieto-Márquez, A.; Cambra, A.; Ruiz-Pérez, M.C.; Fernández-Martínez, F. Adsorption of SO2 onto waste cork powder-derived activated carbons. Chem. Eng. J. 2012, 211, 60–67. [Google Scholar] [CrossRef]
- Sessa, F.; Merlin, G.; Canu, P. Pine bark valorization by activated carbons production to be used as VOCs adsorbents. Fuel 2022, 318, 123346. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely fast and efficient methylene blue adsorption using eco-friendly cork and paper waste-based activated carbon adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Lai, Z.; Chen, S.; He, D.; Mu, J. Honeycomb-like cork activated carbon with ultra-high adsorption capacity for anionic, cationic and mixed dye: Preparation, performance and mechanism. Bioresour. Technol. 2022, 357, 127363. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Cardoso, B.; Pires, J.; Brotas de Carvalho, M. Preparation of activated carbons from cork waste by chemical activation with KOH. Carbon 2003, 41, 2873–2876. [Google Scholar] [CrossRef]
- Cardoso, B.; Mestre, A.S.; Carvalho, A.P.; Pires, J. Activated carbon derived from cork powder waste by KOH activation: Preparation, characterization, and VOCs adsorption. Ind. Eng. Chem. Res. 2008, 47, 5841–5846. [Google Scholar] [CrossRef]
- Ochai-Ejeh, F.O.; Bello, A.; Dangbegnon, J.; Khaleed, A.A.; Madito, M.J.; Bazegar, F.; Manyala, N. High electrochemical performance of hierarchical porous activated carbon derived from lightweight cork (Quercus suber). J. Mater. Sci. 2017, 52, 10600–10613. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Gomes, M.; Mestre, A.S.; Pires, J.; de Carvalho, M.B. Activated carbons from cork waste by chemical activation with K2CO3: Application to adsorption of natural gas components. Carbon 2004, 42, 672–674. [Google Scholar] [CrossRef]
- Patnukao, P.; Pavasant, P. Activated carbon from Eucalyptus camaldulensis Dehn bark using phosphoric acid activation. Bioresour. Technol. 2008, 99, 8540–8543. [Google Scholar] [CrossRef] [PubMed]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S., Jr. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 2019, 7, 103396. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-52967-1. [Google Scholar]
- Gergova, K.; Petrov, N.; Eser, S. Adsorption properties and microstructure of activated carbons produced from agricultural by-products by steam pyrolysis. Carbon 1994, 32, 693–702. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar]
- Yun, C.H.; Park, Y.H.; Oh, G.H.; Park, C.R. Contribution of inorganic components in precursors to porosity evolution in biomass-based porous carbons. Carbon 2003, 41, 2009–2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Zhang, P.; Gao, H.; Ou, C.; Kong, X. Production of activated carbons from four wastes via one-step activation and their applications in Pb2+ adsorption: Insight of ash content. Chemosphere 2020, 245, 125587. [Google Scholar] [CrossRef]
- Asyana, V.; Haryanto, F.; Fitri, L.A.; Ridwan, T.; Anwary, F.; Soekersi, H. Analysis of urinary stone based on a spectrum absorption FTIR-ATR. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Yogyakarta, Indonesia, 2016; Volume 694, pp. 1–5. [Google Scholar]
- Siipola, V.; Tamminen, T.; Källi, A.; Lahti, R.; Romar, H.; Rasa, K.; Keskinen, R.; Hyväluoma, J.; Hannula, M.; Wikberg, H. Effects of biomass type, carbonization process, and activation method on the properties of bio-based activated carbons. BioResources 2018, 13, 5976–6002. [Google Scholar] [CrossRef]
- Zhou, S.; Brown, R.C.; Bai, X. The use of calcium hydroxide pretreatment to overcome agglomeration of technical lignin during fast pyrolysis. Green Chem. 2015, 17, 4748–4759. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, K. Development of high surface area mesoporous activated carbons from herb residues. Chem. Eng. J. 2011, 167, 148–154. [Google Scholar] [CrossRef]
- Moreno-Piraján, J.C.; Garcia-Cuello, V.S.; Giraldo, L. The removal and kinetic study of Mn, Fe, Ni and Cu ions from wastewater onto activated carbon from coconut shells. Adsorption 2011, 17, 505–514. [Google Scholar] [CrossRef]
- Jawad, A.H.; Ismail, K.; Ishak, M.A.M.; Wilson, L.D. Conversion of Malaysian low-rank coal to mesoporous activated carbon: Structure characterization and adsorption properties. Chin. J. Chem. Eng. 2019, 27, 1716–1727. [Google Scholar] [CrossRef]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-carbon-supported gold–cesium (I) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. Chempluschem 2015, 80, 196–201. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Suteu, D.; Malutan, T. Industrial Cellolignin Wastes as Adsorbent for Removal of Methylene Blue Dye from Aqueous Solutions. BioResources 2013, 8, 427–446. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Kokhdan, S.; Ghaedi, A.; Moosavian, S. Comparison of multiwalled carbon nanotubes and activated carbon for efficient removal of methyl orange: Kinetic and thermodynamic investigation. Fresenius Environ. Bull. 2011, 20, 219–234. [Google Scholar]
- Huang, C.P.; Fu, P.L.K. Treatment of arsenic (V)-containing water by the activated carbon process. J. Water Pollut. Control Fed. 1984, 56, 233–242. [Google Scholar]




| Cork AC | Phloem AC | |
|---|---|---|
| SBET (m2 g−1) | 201 | 515 |
| Total pore volume (Adsorption, cm3 g−1) | 0.101 | 0.285 |
| Total pore volume (Desorption, cm3 g−1) | 0.101 | 0.285 |
| Average pore diameter (nm) | 2.00 | 2.22 |
| Whole Bark/Bark Fraction | Activation Method | BET Surface Area of Activated Carbon (m2 g−1) | Reference | ||
|---|---|---|---|---|---|
| Material | Species | Activation Type | Activation Agent | ||
| Cork | Quercus cerris | Physical | H2O | 201 | This work |
| Phloem | Quercus cerris | Physical | H2O | 515 | This work |
| Cork | Quercus suber | Physical | H2O | 750 | [36] |
| Cork | Quercus suber | Physical | CO2 | 76 | [37] |
| Bark | Pine | Physical | CO2 | 868 | [38] |
| Cork | Quercus suber | Chemical | NaOH (alkaline wastewater) | 1670 | [39] |
| Cork | Quercus suber | Chemical | KOH | 3403 | [40] |
| Cork | Quercus suber | Chemical | KOH | 251–1336 | [41] |
| Cork | Quercus suber | Chemical | KOH | 584 | [37] |
| Cork | Quercus suber | Chemical | KOH | 948 | [36] |
| Cork | Quercus suber | Chemical | KOH | 1336 | [42] |
| Cork | Quercus suber | Chemical | KOH | 1081 | [43] |
| Cork | Quercus suber | Chemical | K2CO3 | 907 | [36] |
| Cork | Quercus suber | Chemical | K2CO3 | 1279 | [44] |
| Bark | Pine | Chemical | K2CO3 | 1499 | [38] |
| Bark | Eucalyptus camaldulensis | Chemical | H3PO4 | 1239 | [45] |
| Bark | Pine | Chemical | H3PO4 | 3342 | [38] |
| Bark | Acacia mearnsii | Chemical | ZnCl2 | 414 | [46] |
| Elements | Cork | Cork Biochar | Cork AC | Phloem | Phloem Biochar | Phloem AC |
|---|---|---|---|---|---|---|
| Mo | 8.7 | 8.4 | 8.3 | 8.2 | 7.9 | 9.6 |
| Zr | 8.4 | 4.8 | 0 | 8.2 | 10.9 | 15.4 |
| Sr | 68.4 | 115.8 | 213.9 | 95.9 | 162.5 | 268.6 |
| U | 6.2 | 6.3 | 4.2 | 4.7 | 5.8 | 1.4 |
| Rb | 3.8 | 4.3 | 4.4 | 6.3 | 8.5 | 9.7 |
| Zn | 0 | 0 | 28.7 | 66.1 | 32.9 | 52.4 |
| W | 38.8 | 39.2 | 10.5 | 35.3 | 0 | 18.6 |
| Cu | 45.9 | 39.9 | 41.3 | 21.9 | 24.4 | 35.1 |
| Ni | 0 | 0 | 0 | 0 | 0 | 33.5 |
| Cr | 0 | 0 | 0 | 31.7 | 0 | 162.9 |
| V | 12.0 | 8.4 | 0 | 77.0 | 72.5 | 9.9 |
| Ti | 2799.1 | 255.3 | 295.9 | 409.9 | 359.1 | 766.7 |
| Sc | 218.3 | 398.1 | 568.3 | 299.2 | 438.6 | 510.4 |
| Ba | 0 | 0 | 0 | 0 | 0 | 15.0 |
| Sn | 16.9 | 4.0 | 0 | 3.5 | 15.3 | 0 |
| Cd | 20.0 | 20.9 | 16.4 | 20.8 | 21.2 | 20.4 |
| Pd | 7.7 | 8.1 | 6.4 | 8.1 | 7.8 | 6.7 |
| Ag | 13.2 | 5.4 | 6.0 | 6.1 | 3.9 | 7.2 |
| Nb | 12.1 | 11.3 | 9.9 | 10.3 | 10.0 | 11.2 |
| Cork AC | Phloem AC | Commercial AC | |
|---|---|---|---|
| Ash content (%) | 9.9 | 23.3 | 0 |
| Burnout temperature (°C) | 725.8 | 735.8 | 639.7 |
| Mass loss (%) | |||
| 100–400 °C | 4.3 | 5.3 | 6.3 |
| 400–650 °C | 74.4 | 58.3 | 89.4 |
| 650–750 °C | 8.3 | 9.9 | 0 |
| Cork AC | Phloem AC | |
|---|---|---|
| Mean removal (%) | 47.99 | 65.82 |
| Variance | 466.70 | 385.41 |
| Observations | 6 | 6 |
| Pearson correlation | 0.94 | |
| Hypothesized mean difference | 0 | |
| df | 5 | |
| t Stat | −5.76 | |
| P(T ≤ t) one-tail | 0.0011 | |
| t Critical one-tail | 2.0150 | |
| P(T ≤ t) two-tail | 0.0022 | |
| t Critical two-tail | 2.5706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, U.; Nobre, C.; Martins, M.; Gonçalves, M.; Pereira, H. Effect of Bark Biochars on Properties of Steam-Activated Carbons. Environments 2024, 11, 2. https://doi.org/10.3390/environments11010002
Şen U, Nobre C, Martins M, Gonçalves M, Pereira H. Effect of Bark Biochars on Properties of Steam-Activated Carbons. Environments. 2024; 11(1):2. https://doi.org/10.3390/environments11010002
Chicago/Turabian StyleŞen, Umut, Catarina Nobre, Marta Martins, Margarida Gonçalves, and Helena Pereira. 2024. "Effect of Bark Biochars on Properties of Steam-Activated Carbons" Environments 11, no. 1: 2. https://doi.org/10.3390/environments11010002
APA StyleŞen, U., Nobre, C., Martins, M., Gonçalves, M., & Pereira, H. (2024). Effect of Bark Biochars on Properties of Steam-Activated Carbons. Environments, 11(1), 2. https://doi.org/10.3390/environments11010002










