Adult Chironomid (Chironomidae: Diptera) Positive Phototactic Behaviour—A Cue for Adult Population Management and Impact on Insect Biodiversity at Lake Trasimeno, Central Italy
Abstract
:1. Introduction
2. Materials and Methods
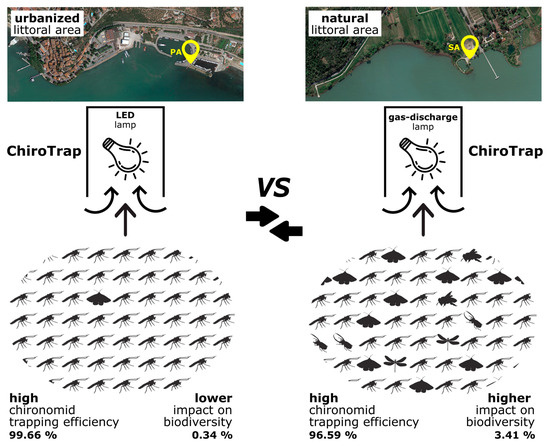
2.1. Study Area
2.2. Sampling Campaign
2.3. Sample Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owens, A.C.S.; Lewis, S.M. The Impact of Artificial Light at Night on Nocturnal Insects: A Review and Synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef] [PubMed]
- Donners, M.; Van Grunsven, R.H.A.; Groenendijk, D.; Van Langevelde, F.; Bikker, J.W.; Longcore, T.; Veenendaal, E. Colors of Attraction: Modeling Insect Flight to Light Behavior. J. Exp. Zool. Pt A 2018, 329, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Chittka, L. The Evolution of Color Vision in Insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Thein, P.P.; Choi, S.-W. Forest Insect Assemblages Attracted to Light Trap on Two High Mountains (Mt. Jirisan and Mt. Hallasan) in South Korea. J. For. Res. 2016, 27, 1203–1210. [Google Scholar] [CrossRef]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; Ffrench-Constant, R.H. Shedding Light on Moths: Shorter Wavelengths Attract Noctuids More than Geometrids. Biol. Lett. 2013, 9, 20130376. [Google Scholar] [CrossRef] [PubMed]
- Van Grunsven, R.H.A.; Donners, M.; Boekee, K.; Tichelaar, I.; Van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E.M. Spectral Composition of Light Sources and Insect Phototaxis, with an Evaluation of Existing Spectral Response Models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Pawson, S.M.; Bader, M.K.-F. LED Lighting Increases the Ecological Impact of Light Pollution Irrespective of Color Temperature. Ecol. Appl. 2014, 24, 1561–1568. [Google Scholar] [CrossRef]
- Zemel, R.S.; Houghton, D. The Ability of Specific-Wavelength LED Lights to Attract Night-Flying Insects. Great Lakes Entomol. 2018, 50, 8. [Google Scholar] [CrossRef]
- De Medeiros, B.A.S.; Barghini, A.; Vanin, S.A. Streetlights Attract a Broad Array of Beetle Species. Rev. Bras. Entomol. 2017, 61, 74–79. [Google Scholar] [CrossRef]
- Haddock, J.K.; Threlfall, C.G.; Law, B.; Hochuli, D.F. Responses of Insectivorous Bats and Nocturnal Insects to Local Changes in Street Light Technology. Austral Ecol. 2019, 44, 1052–1064. [Google Scholar] [CrossRef]
- Larsson, M.; Göthberg, A.; Milberg, P. Night, Light and Flight: Light Attraction in Trichoptera. Insect Conserv. Divers. 2020, 13, 296–302. [Google Scholar] [CrossRef]
- Cambridge, J.E.; Francoeur, L.; Hamilton, G.C. Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Attraction to Various Light Stimuli. Fla. Entomol. 2017, 100, 583–588. [Google Scholar] [CrossRef]
- Endo, N.; Hironaka, M.; Honda, Y.; Iwamoto, T. Combination of UV and Green Light Synergistically Enhances the Attractiveness of Light to Green Stink Bugs Nezara spp. Sci. Rep. 2022, 12, 12279. [Google Scholar] [CrossRef] [PubMed]
- Lima-Neto, A.R.; Costa-Neta, B.M.; Da Silva, A.A.; Brito, J.M.; Aguiar, J.V.C.; Ponte, I.S.; Silva, F.S. The Effect of Luminous Intensity on the Attraction of Phlebotomine Sand Flies to Light Traps. J. Med. Entomol. 2018, 55, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Nuisance Chironomids and Their Control: A Review. Bull. Entomol. Soc. Am. 1980, 26, 3–16. [Google Scholar] [CrossRef]
- Ali, A.; Stanley, B.H.; Chaudhuri, P.K. Attraction of Some Adult Midges (Diptera: Chironomidae) of Florida to Artificial Light in the Field. Fla. Entomol. 1986, 69, 644. [Google Scholar] [CrossRef]
- Kokkinn, M.; Williams, W. An Experimental Study of Phototactic Responses of Tanytarsus barbitarsis Freeman (Diptera: Chironomidae. Mar. Freshw. Res. 1989, 40, 693. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Nagai, Y.; Mushya, T.; Higashino, M.; Taniguchi, Y. Phototaxis of Propsilocerus akamusi (Diptera: Chironomidae) From a Shallow Eutrophic Lake in Response to Led Lamps. J. Am. Mosq. Control Assoc. 2017, 33, 128–133. [Google Scholar] [CrossRef]
- Armitage, P.D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae: Biology and Ecology of Non-Biting Midges; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Pfitzner, W.P.; Beck, M.; Weitzel, T.; Becker, N. The Role of Mosquitoes in the Diet of Adult Dragon and Damselflies (Odonata). J. Am. Mosq. Control Assoc. 2015, 31, 187–189. [Google Scholar] [CrossRef]
- Lencioni, V.; Cranston, P.S.; Makarchenko, E. Recent Advances in the Study of Chironomidae: An Overview. J. Limnol. 2018. [Google Scholar] [CrossRef]
- Theissinger, K.; Kästel, A.; Elbrecht, V.; Makkonen, J.; Michiels, S.; Schmidt, S.; Allgeier, S.; Leese, F.; Brühl, C. Using DNA Metabarcoding for Assessing Chironomid Diversity and Community Change in Mosquito Controlled Temporary Wetlands. MBMG 2018, 2, e21060. [Google Scholar] [CrossRef]
- Adler, P.; Courtney, G. Ecological and Societal Services of Aquatic Diptera. Insects 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Goretti, E.; Coletti, A.; Di Veroli, A.; Di Giulio, A.M.; Gaino, E. Artificial Light Device for Attracting Pestiferous Chironomids (Diptera): A Case Study at Lake Trasimeno (Central Italy). Ital. J. Zool. 2011, 78, 336–342. [Google Scholar] [CrossRef]
- Robertson, B.A.; Horváth, G. Color Polarization Vision Mediates the Strength of an Evolutionary Trap. Evol. Appl. 2019, 12, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. A Concise Review of Chironomid Midges (Diptera: Chironomidae) as Pests and Their Management. J. Vector Ecol. 1996, 21, 105–121. [Google Scholar]
- Failla, A.; Vasquez, A.; Fujimoto, M.; Ram, J. The Ecological, Economic and Public Health Impacts of Nuisance Chironomids and Their Potential as Aquatic Invaders. Aquat. Invasions 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Goretti, E.; Pallottini, M.; Pagliarini, S.; Catasti, M.; Porta, G.L.; Selvaggi, R.; Gaino, E.; Di Giulio, A.M.; Ali, A. Use of Larval Morphological Deformities in Chironomus plumosus (Chironomidae: Diptera) as an Indicator of Freshwater Environmental Contamination (Lake Trasimeno, Italy). Water 2020, 12, 1. [Google Scholar] [CrossRef]
- Pallottini, M.; Pagliarini, S.; Catasti, M.; La Porta, G.; Selvaggi, R.; Gaino, E.; Spacone, L.; Di Giulio, A.M.; Ali, A.; Goretti, E. Population Dynamics and Seasonal Patterns of Chironomus plumosus (Diptera, Chironomidae) in the Shallow Lake Trasimeno, Central Italy. Sustainability 2023, 15, 851. [Google Scholar] [CrossRef]
- Pallottini, M.; Pagliarini, S.; Catasti, M.; La Porta, G.; Selvaggi, R.; Gaino, E.; Spacone, L.; Di Giulio, A.M.; Ali, A.; Goretti, E. Role of Chironomus plumosus (Diptera, Chironomidae) Population in the Central Zone of the Shallow Lake Trasimeno (Italy). Sustainability 2023, 15, 5540. [Google Scholar] [CrossRef]
- Longcore, T.; Aldern, H.L.; Eggers, J.F.; Flores, S.; Franco, L.; Hirshfield-Yamanishi, E.; Petrinec, L.N.; Yan, W.A.; Barroso, A.M. Tuning the White Light Spectrum of Light Emitting Diode Lamps to Reduce Attraction of Nocturnal Arthropods. Phil. Trans. R. Soc. B 2015, 370, 20140125. [Google Scholar] [CrossRef]
- Justice, M.J.; Justice, T.C. Attraction of Insects to Incandescent, Compact Fluorescent, Halogen, and Led Lamps in a Light Trap: Implications for Light Pollution and Urban Ecologies. Entomol. News 2016, 125, 315–326. [Google Scholar] [CrossRef]
- Pohe, S.R.; Winterbourn, M.J.; Harding, J.S. Comparison of Fluorescent Lights with Differing Spectral Properties on Catches of Adult Aquatic and Terrestrial Insects. N. Z. Entomol. 2018, 41, 1–11. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Becker, J.; Peter, S.; Heller, S.; Hölker, F. Long-Term Comparison of Attraction of Flying Insects to Streetlights after the Transition from Traditional Light Sources to Light-Emitting Diodes in Urban and Peri-Urban Settings. Sustainability 2019, 11, 6198. [Google Scholar] [CrossRef]
- Brehm, G.; Niermann, J.; Jaimes Nino, L.M.; Enseling, D.; Jüstel, T.; Axmacher, J.C.; Warrant, E.; Fiedler, K. Moths Are Strongly Attracted to Ultraviolet and Blue Radiation. Insect Conserv Divers. 2021, 14, 188–198. [Google Scholar] [CrossRef]
- Carannante, D.; Blumenstein, C.S.; Hale, J.D.; Arlettaz, R. LED lighting threatens adult aquatic insects: Impact magnitude and distance thresholds. Ecol. Solut. Evid. 2021, 2, e12053. [Google Scholar] [CrossRef]
- Manu, M.; Lotrean, N.; Onete, M.; Nicoara, R.; Badescu, F. Monitoring of the Callimorpha (Euplagia) quadripunctaria (Poda, 1761) (Insecta: Lepidoptera) in the Macin Mountains National Park (Romania). In The Novelt Results of the Institute of Biology Bucharest into Fields of Ecology, Microbiology and Citobiology; Ars Docendi: Bucharest, Romania, 2018. [Google Scholar]
- Trizzino, M. Gli Artropodi Italiani in Direttiva Habitat: Biologia, Ecologia, Riconoscimento e Monitoraggio; Cierre Grafica Editore: Verona, Italy, 2013; ISBN 978-88-95351-94-0. [Google Scholar]
- Harvey, D.J.; Gange, A.C. Size Variation and Mating Success in the Stag Beetle, Lucanus cervus. Physiol. Entomol. 2006, 31, 218–226. [Google Scholar] [CrossRef]
- Harvey, D.J.; Gange, A.C.; Hawes, C.J.; Rink, M. Bionomics and Distribution of the Stag Beetle, Lucanus cervus (L.) across Europe*: European Distribution of L. cervus. Insect Conserv. Divers. 2011, 4, 23–38. [Google Scholar] [CrossRef]
- Wermelinger, B.; Wyniger, D.; Forster, B. First Records of an Invasive Bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a New Pest on Woody Ornamentals and Fruit Trees? Mitteilungen-Schweiz. Entomol. Ges. 2008, 81, 1. [Google Scholar] [CrossRef]
- Maistrello, L.; Vaccari, G.; Costi, E. Primi Rinvenimenti in Italia Della Cimice Esotica Halyomorpha halys, Una Nuova Minaccia per La Frutticoltura. ATTI Giornate Fitopatol. 2014, 1, 283–288. [Google Scholar]
- Hoebeke, R.; Carter, M. Halymoropha halys (Heteroptera: Pentatomidae): A Polyphagous Plant Pest from Asia Newly Detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Egri, Á.; Mészáros, Á.; Kriska, G.; Fail, J. Dichromacy in the Brown Marmorated Stink Bug? Spectral Sensitivity of the Compound Eyes and Phototaxis of Halyomorpha halys. J. Pest Sci. 2023, 1–10. [Google Scholar] [CrossRef]
- Bencivenga, G.; Zerunian, Z.; Tito, S.; Sorcini, S.; Luna, M.; Pallottini, M.; Goretti, E. Initial Survey of the Nocturnal Macromoths of the Umbria (Central Italy). In 1° Congresso Nazionale Congiunto SITE-UZI-SIB-Biodiversity: Concepts New Tools Future Challenges; UZI: Milano, Italy, 2016. [Google Scholar]


| Passignano sul Trasimeno (PA) | Period | Collection | Total Biomass | Total Biomass | Chironomid Biomass | Chironomid Biomass | Other Biomass | Other Biomass | Other Biomass (Identified) | Other Biomass (Identified) |
| 2019 | (days) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | |
| PA1 | 8 March–18 March | 11 | 12.97 | 1.18 | 9.47 | 0.86 | 3.50 | 0.32 | 3.097 | 0.282 |
| PA2 | 19 March–29 April | 42 | 10.94 | 0.26 | 10.63 | 0.25 | 0.31 | 0.01 | 0.036 | 0.001 |
| PA3 | 30 April–17 May | 18 | 47.78 | 2.65 | 47.74 | 2.65 | 0.038 | 0.00 | 0.038 | 0.002 |
| PA4 | 18 May–5 June | 19 | 166.11 | 8.74 | 165.74 | 8.72 | 0.37 | 0.02 | 0.178 | 0.009 |
| PA5 | 6 June–18 June | 13 | 606.19 | 46.63 | 604.93 | 46.53 | 1.26 | 0.10 | 0.039 | 0.003 |
| 19 June–2 July | ||||||||||
| 3 July–9 July | ||||||||||
| PA6 | 12 July–01 August | 42 | 697.65 | 16.61 | 696.77 | 16.59 | 0.88 | 0.02 | 0.646 | 0.015 |
| PA7 | 2 August–9 August | 8 | 311.97 | 39.00 | 311.79 | 38.97 | 0.18 | 0.02 | 0.009 | 0.001 |
| PA8 | 10 August–22 August | 13 | 314.99 | 24.23 | 314.42 | 24.19 | 0.57 | 0.04 | 0.031 | 0.002 |
| PA9 | 23 August–6 September | 15 | 145.15 | 9.68 | 144.90 | 9.66 | 0.25 | 0.02 | 0.027 | 0.002 |
| PA10 | 7 September–10 September | 4 | 875.01 | 218.75 | 874.27 | 218.57 | 0.74 | 0.19 | 0.137 | 0.034 |
| PA11 | 11 September–10 October | 30 | 538.07 | 17.94 | 537.51 | 17.92 | 0.56 | 0.02 | 0.091 | 0.003 |
| Total | 215 | 3726.83 | --- | 3718.17 | --- | 8.66 | --- | 4.33 | --- | |
| Mean | 19.55 | 338.80 | 35.06 | 338.02 | 34.99 | 0.79 | 0.07 | 0.39 | 0.032 | |
| Minimum | 4 | 10.94 | 0.26 | 9.47 | 0.25 | 0.04 | 0.00 | 0.01 | 0.001 | |
| Maximum | 42 | 875.01 | 218.75 | 874.27 | 218.57 | 3.50 | 0.32 | 3.10 | 0.282 | |
| Sant’Arcangelo (SA) | Period | Collection | Total biomass | Total biomass | Chironomid biomass | Chironomid biomass | Other biomass | Other biomass | Other biomass (identified) | Other biomass (identified) |
| 2019 | (days) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | |
| SA1 | 8 March–18 March | 11 | 34.06 | 3.10 | 14.28 | 1.30 | 19.78 | 1.80 | 17.98 | 1.63 |
| SA2 | 19 March–30 April | 43 | 44.47 | 1.03 | 38.68 | 0.90 | 5.79 | 0.13 | 4.88 | 0.11 |
| SA3 | 1 May–17 May | 17 | 118.08 | 6.95 | 115.24 | 6.78 | 2.84 | 0.17 | 2.84 | 0.17 |
| SA4 | 18 May–5 June | 19 | 473.86 | 24.94 | 466.71 | 24.56 | 7.15 | 0.38 | 6.84 | 0.36 |
| SA5 | 6 June–18 June | 13 | 447.93 | 34.46 | 439.95 | 33.84 | 7.98 | 0.61 | 7.03 | 0.54 |
| 19 June–29 June | ||||||||||
| SA6 | 12 July–1 August | 32 | 744.19 | 16.61 | 724.71 | 22.65 | 19.48 | 0.61 | 17.98 | 0.56 |
| SA7 | 2 August–9 August | 8 | 175.43 | 21.93 | 171.11 | 21.39 | 4.32 | 0.54 | 3.93 | 0.49 |
| SA8 | 10 August–22 August | 13 | 408.62 | 31.43 | 396.07 | 30.47 | 12.55 | 0.97 | 8.31 | 0.64 |
| SA9 | 23 August–6 September | 15 | 182.35 | 12.16 | 176.57 | 11.77 | 5.78 | 0.39 | 5.07 | 0.34 |
| SA10 | 7 September–10 September | 4 | 394.29 | 98.57 | 386.31 | 96.58 | 7.98 | 2.00 | 6.99 | 1.75 |
| SA11 | 11 September–11 October | 31 | 347.35 | 11.20 | 326.53 | 10.53 | 20.82 | 0.67 | 17.92 | 0.58 |
| Total | 206 | 3370.63 | --- | 3256.16 | --- | 114.47 | --- | 99.77 | --- | |
| Mean | 18.73 | 306.42 | 23.85 | 296.01 | 23.71 | 10.41 | 0.75 | 9.07 | 0.65 | |
| Minimum | 4 | 34.06 | 1.03 | 14.28 | 0.90 | 2.84 | 0.13 | 2.84 | 0.11 | |
| Maximum | 43 | 744.19 | 98.57 | 724.71 | 96.58 | 20.82 | 2.00 | 17.98 | 1.75 | |
| Passignano sul Trasimeno (PA) | Period | Collection | Total biomass | Total biomass | Chironomid biomass | Chironomid biomass | Other biomass | Other biomass | Other biomass (identified) | Other biomass (identified) |
| 2020 | (days) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | |
| PA1 | 22 April–4 May | 13 | 362.47 | 27.88 | 361.89 | 27.84 | 0.58 | 0.04 | 0.390 | 0.030 |
| PA2 | 5 May–27 May | 23 | 399.11 | 17.35 | 398.80 | 17.34 | 0.31 | 0.01 | 0.027 | 0.001 |
| PA3 | 28 May–11 June | 15 | 47.54 | 3.17 | 47.54 | 3.17 | 0.00 | 0.00 | 0.000 | 0.000 |
| PA4 | 12 June–23 June | 12 | 57.38 | 4.78 | 56.69 | 4.72 | 0.69 | 0.06 | 0.296 | 0.025 |
| PA5 | 24 June–10 July | 17 | 604.28 | 35.55 | 597.80 | 35.16 | 6.48 | 0.38 | 0.639 | 0.038 |
| PA6 | 11 July–28 July | 18 | 608.48 | 33.80 | 606.79 | 33.71 | 1.69 | 0.09 | 0.415 | 0.023 |
| PA7 | 29 July–7 August | 10 | 233.17 | 23.32 | 232.25 | 23.23 | 0.92 | 0.09 | 0.165 | 0.017 |
| PA8 | 8 August–2 September | 26 | 260.53 | 10.02 | 259.50 | 9.98 | 1.03 | 0.04 | 0.321 | 0.012 |
| PA9 | 3 September–16 September | 14 | 133.63 | 9.55 | 133.42 | 9.53 | 0.21 | 0.02 | 0.063 | 0.004 |
| PA10 | 17 September–9 October | 23 | 65.36 | 2.84 | 64.09 | 2.79 | 1.27 | 0.06 | 0.180 | 0.008 |
| Total | 171 | 2771.95 | --- | 2758.77 | --- | 13.18 | --- | 2.50 | --- | |
| Mean | 17.10 | 277.20 | 16.83 | 275.88 | 16.75 | 1.32 | 0.08 | 0.25 | 0.02 | |
| Minimum | 10 | 47.54 | 2.84 | 47.54 | 2.79 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 26 | 608.48 | 35.55 | 606.79 | 35.16 | 6.48 | 0.38 | 0.64 | 0.04 | |
| Sant’Arcangelo (SA) | Period | Collection | Total biomass | Total biomass | Chironomid biomass | Chironomid biomass | Other biomass | Other biomass | Other biomass (identified) | Other biomass (identified) |
| 2020 | (days) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | (g) | (g/day) | |
| SA1 | 23 April–4 May | 12 | 101.35 | 8.45 | 93.56 | 7.80 | 7.79 | 0.65 | 6.22 | 0.52 |
| SA2 | 5 May–27 May | 23 | 482.19 | 20.96 | 465.14 | 20.22 | 17.05 | 0.74 | 14.15 | 0.62 |
| SA3 | 28 May–11 June | 15 | 448.40 | 29.89 | 434.03 | 28.94 | 14.37 | 0.96 | 7.78 | 0.52 |
| SA4 | 12 June–23 June | 12 | 606.82 | 50.57 | 599.35 | 49.95 | 7.47 | 0.62 | 5.56 | 0.46 |
| SA5 | 24 June–10 July | 17 | 1276.53 | 75.09 | 1253.40 | 73.73 | 23.13 | 1.36 | 18.04 | 1.06 |
| SA6 | 11 July–28 July | 18 | 1270.88 | 70.60 | 1244.20 | 69.12 | 26.68 | 1.48 | 14.27 | 0.79 |
| SA7 | 29 July–7 August | 10 | 220.46 | 22.05 | 206.59 | 20.66 | 13.87 | 1.39 | 11.02 | 1.10 |
| SA8 | 8 August–2 September | 26 | 207.35 | 7.98 | 185.83 | 7.15 | 21.52 | 0.83 | 17.54 | 0.67 |
| SA9 | 3 September–16 September | 14 | 450.71 | 32.19 | 423.43 | 30.25 | 27.28 | 1.95 | 24.93 | 1.78 |
| SA10 | 17 September–9 October | 23 | 161.73 | 7.03 | 141.81 | 6.17 | 19.92 | 0.87 | 16.92 | 0.74 |
| Total | 170 | 5226.42 | --- | 5047.34 | --- | 179.08 | --- | 136.43 | --- | |
| Mean | 17.00 | 522.64 | 32.48 | 504.73 | 31.40 | 17.91 | 1.08 | 13.64 | 0.83 | |
| Minimum | 10 | 101.35 | 7.03 | 93.56 | 6.17 | 7.47 | 0.62 | 5.56 | 0.46 | |
| Maximum | 26 | 1276.53 | 75.09 | 1253.40 | 73.73 | 27.28 | 1.95 | 24.93 | 1.78 |
| Passignano sul Trasimeno (PA) | 2019: 215 Days | 2020: 171 Days | |||||||
| Other Biomass (Identified) | Total | Total | % | % | Total | Total | % | % | |
| (Taxa) | (N) | (g) | (N) | (g) | (N) | (g) | (N) | (g) | |
| Insecta | Blattodea | --- | --- | --- | --- | --- | --- | --- | --- |
| Insecta | Coleoptera | 1 | 0.27 | 0.98 | 6.26 | 18 | 0.305 | 15.00 | 12.22 |
| Insecta | Dermaptera | 1 | 0.02 | 0.98 | 0.37 | --- | --- | --- | --- |
| Insecta | Diptera | 19 | 0.17 | 18.63 | 3.88 | 19 | 0.090 | 15.83 | 3.61 |
| Insecta | Ephemeroptera | 9 | 0.01 | 8.82 | 0.12 | 1 | 0.004 | 0.83 | 0.14 |
| Insecta | Hemiptera | 3 | 0.07 | 2.94 | 1.58 | 12 | 0.070 | 10.00 | 2.79 |
| Insecta | Hymenoptera | 6 | 0.13 | 5.88 | 2.95 | 13 | 0.174 | 10.83 | 6.99 |
| Insecta | Lepidoptera | 42 | 0.70 | 41.18 | 16.12 | 45 | 0.482 | 37.50 | 19.31 |
| Insecta | Mantodea | --- | --- | --- | --- | --- | --- | --- | --- |
| Insecta | Neuroptera | 1 | 0.00 | 0.98 | 0.07 | 1 | 0.002 | 0.83 | 0.09 |
| Insecta | Odonata | 2 | 0.01 | 1.96 | 0.31 | 2 | 0.009 | 1.67 | 0.34 |
| Insecta | Orthoptera | --- | --- | --- | --- | --- | --- | --- | --- |
| Insecta | Trichoptera | 3 | 0.00 | 2.94 | 0.10 | --- | --- | --- | --- |
| Arachnida | Araneae | 8 | 0.24 | 7.84 | 5.56 | 3 | 0.158 | 2.50 | 6.35 |
| Gastropoda | Pulmonata | 7 | 2.71 | 6.86 | 62.68 | 6 | 1.202 | 5.00 | 48.16 |
| Total | 102 | 4.33 | 100 | 100 | 120 | 2.495 | 100 | 100 | |
| Sant’Arcangelo (SA) | 2019: 206 days | 2020: 170 days | |||||||
| Other biomass (identified) | Total | Total | % | % | Total | Total | % | % | |
| (Taxa) | (N) | (g) | (N) | (g) | (N) | (g) | (N) | (g) | |
| Insecta | Blattodea | --- | --- | --- | --- | 1 | 0.01 | 0.01 | 0.01 |
| Insecta | Coleoptera | 79 | 8.04 | 1.53 | 8.06 | 924 | 10.63 | 6.14 | 7.79 |
| Insecta | Dermaptera | --- | --- | --- | --- | 1 | 0.01 | 0.01 | 0.00 |
| Insecta | Diptera | 120 | 0.68 | 2.32 | 0.68 | 338 | 1.76 | 2.25 | 1.29 |
| Insecta | Ephemeroptera | 76 | 0.03 | 1.47 | 0.03 | 244 | 0.14 | 1.62 | 0.10 |
| Insecta | Hemiptera | 45 | 1.07 | 0.87 | 1.07 | 139 | 3.00 | 0.92 | 2.20 |
| Insecta | Hymenoptera | 218 | 9.43 | 4.22 | 9.45 | 423 | 12.42 | 2.81 | 9.11 |
| Insecta | Lepidoptera | 4383 | 72.76 | 84.81 | 72.92 | 11,441 | 98.34 | 76.08 | 72.08 |
| Insecta | Mantodea | 1 | 0.17 | 0.02 | 0.17 | 1 | 0.03 | 0.01 | 0.02 |
| Insecta | Neuroptera | 31 | 0.13 | 0.60 | 0.13 | 91 | 0.25 | 0.61 | 0.19 |
| Insecta | Odonata | 63 | 4.80 | 1.22 | 4.81 | 194 | 7.31 | 1.29 | 5.36 |
| Insecta | Orthoptera | 2 | 0.10 | 0.04 | 0.10 | 1 | 0.01 | 0.01 | 0.00 |
| Insecta | Trichoptera | 131 | 0.28 | 2.53 | 0.28 | 1217 | 1.21 | 8.09 | 0.89 |
| Arachnida | Araneae | 16 | 0.55 | 0.31 | 0.55 | 22 | 0.59 | 0.15 | 0.43 |
| Gastropoda | Pulmonata | 3 | 1.74 | 0.06 | 1.74 | 1 | 0.73 | 0.01 | 0.53 |
| Total | 5168 | 99.77 | 100 | 100 | 15,038 | 136.43 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallottini, M.; Pagliarini, S.; Catasti, M.; Giontella, L.; La Porta, G.; Selvaggi, R.; Gaino, E.; Spacone, L.; Di Giulio, A.M.; Ali, A.; et al. Adult Chironomid (Chironomidae: Diptera) Positive Phototactic Behaviour—A Cue for Adult Population Management and Impact on Insect Biodiversity at Lake Trasimeno, Central Italy. Environments 2024, 11, 1. https://doi.org/10.3390/environments11010001
Pallottini M, Pagliarini S, Catasti M, Giontella L, La Porta G, Selvaggi R, Gaino E, Spacone L, Di Giulio AM, Ali A, et al. Adult Chironomid (Chironomidae: Diptera) Positive Phototactic Behaviour—A Cue for Adult Population Management and Impact on Insect Biodiversity at Lake Trasimeno, Central Italy. Environments. 2024; 11(1):1. https://doi.org/10.3390/environments11010001
Chicago/Turabian StylePallottini, Matteo, Sarah Pagliarini, Marianna Catasti, Leonardo Giontella, Gianandrea La Porta, Roberta Selvaggi, Elda Gaino, Leonardo Spacone, Alessandro Maria Di Giulio, Arshad Ali, and et al. 2024. "Adult Chironomid (Chironomidae: Diptera) Positive Phototactic Behaviour—A Cue for Adult Population Management and Impact on Insect Biodiversity at Lake Trasimeno, Central Italy" Environments 11, no. 1: 1. https://doi.org/10.3390/environments11010001
APA StylePallottini, M., Pagliarini, S., Catasti, M., Giontella, L., La Porta, G., Selvaggi, R., Gaino, E., Spacone, L., Di Giulio, A. M., Ali, A., & Goretti, E. (2024). Adult Chironomid (Chironomidae: Diptera) Positive Phototactic Behaviour—A Cue for Adult Population Management and Impact on Insect Biodiversity at Lake Trasimeno, Central Italy. Environments, 11(1), 1. https://doi.org/10.3390/environments11010001