Association Between Quantitative Electroencephalogram Frequency Composition and Post-Surgical Evolution in Pharmacoresistant Temporal Lobe Epilepsy Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Ethical Considerations
2.4. Post-Operative Follow-Up
2.5. Surgical Procedure and Resection Size
2.6. Histopathological Examination
2.7. Data Analysis
3. Results
3.1. Demographic Profile and Pathology
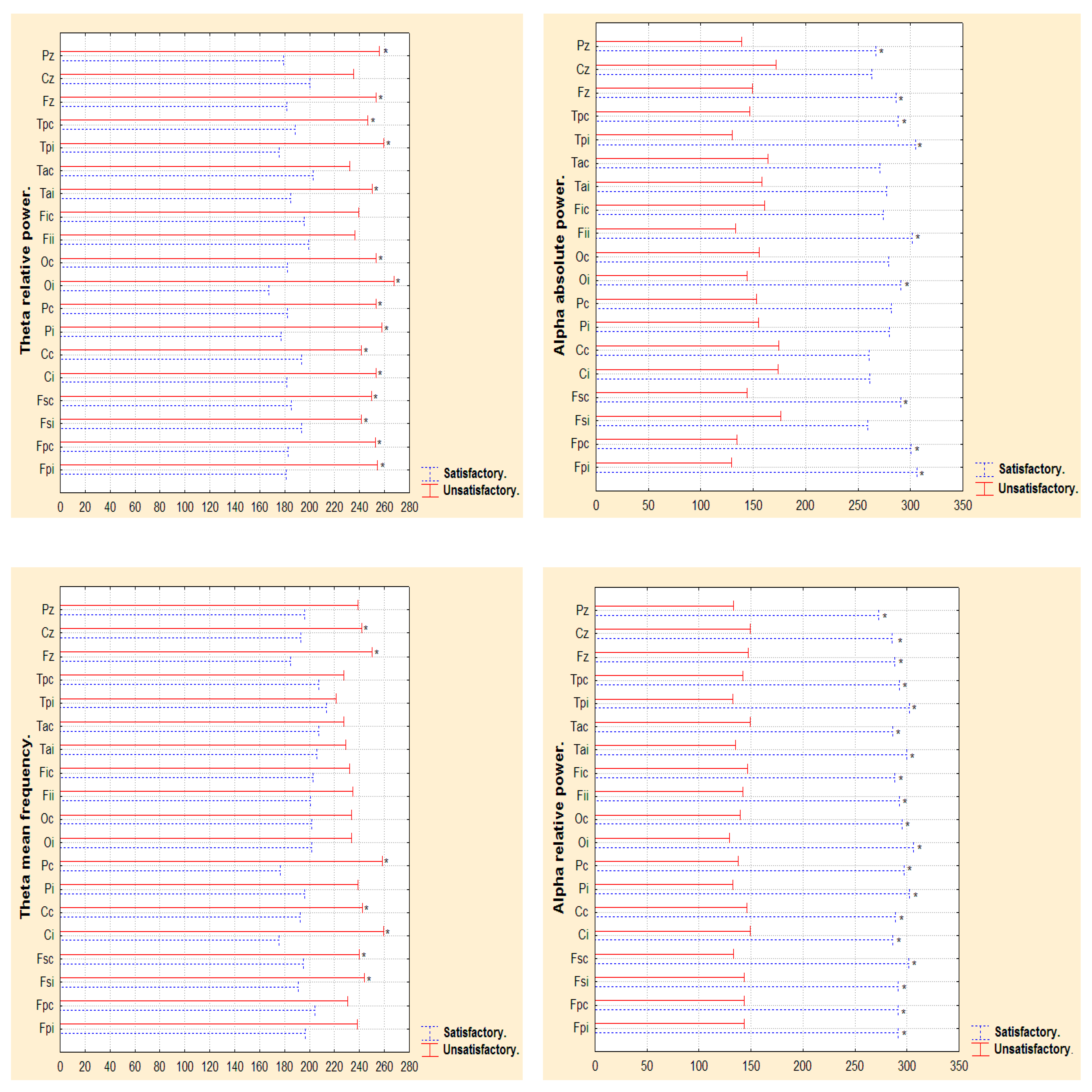
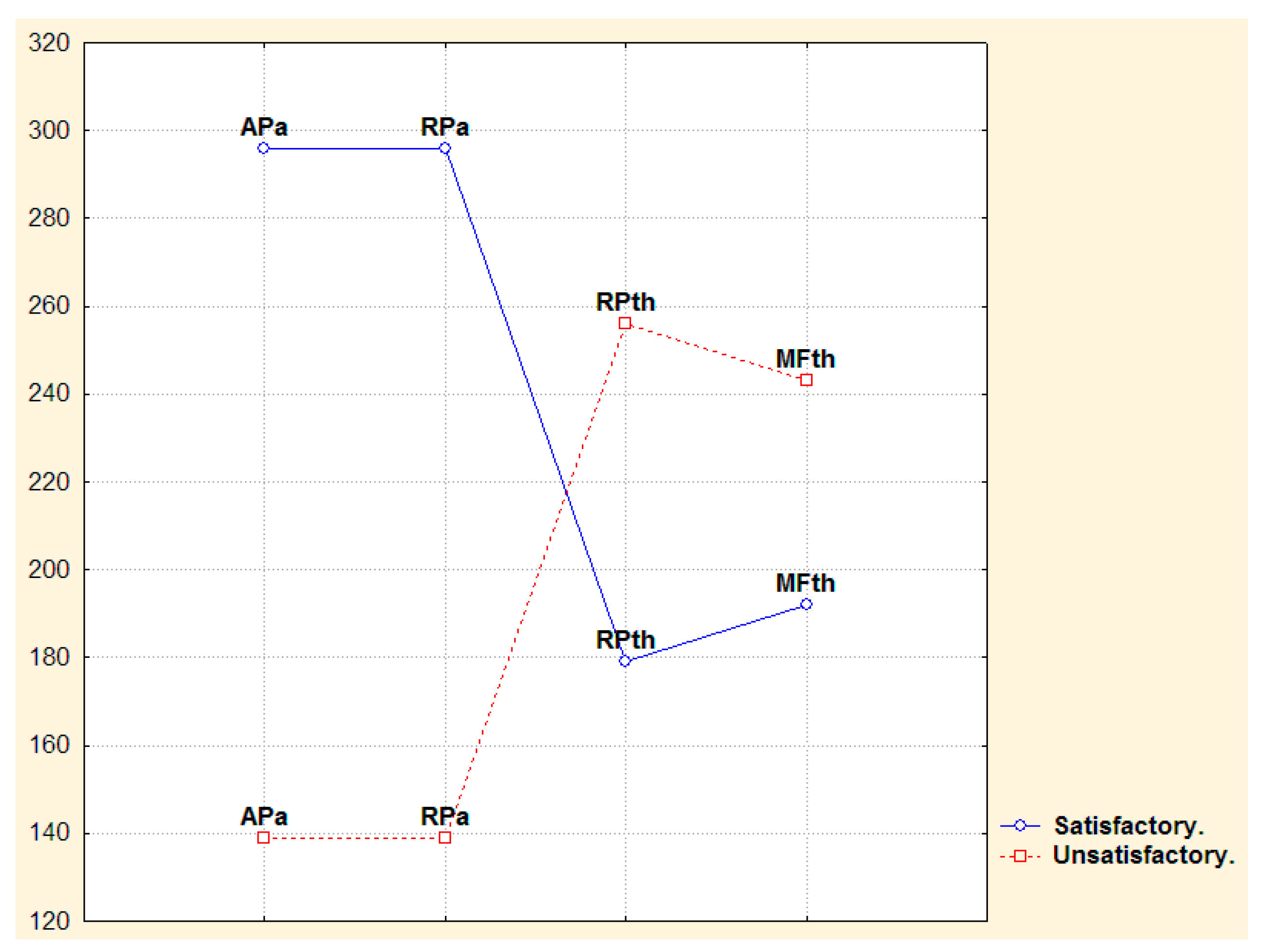
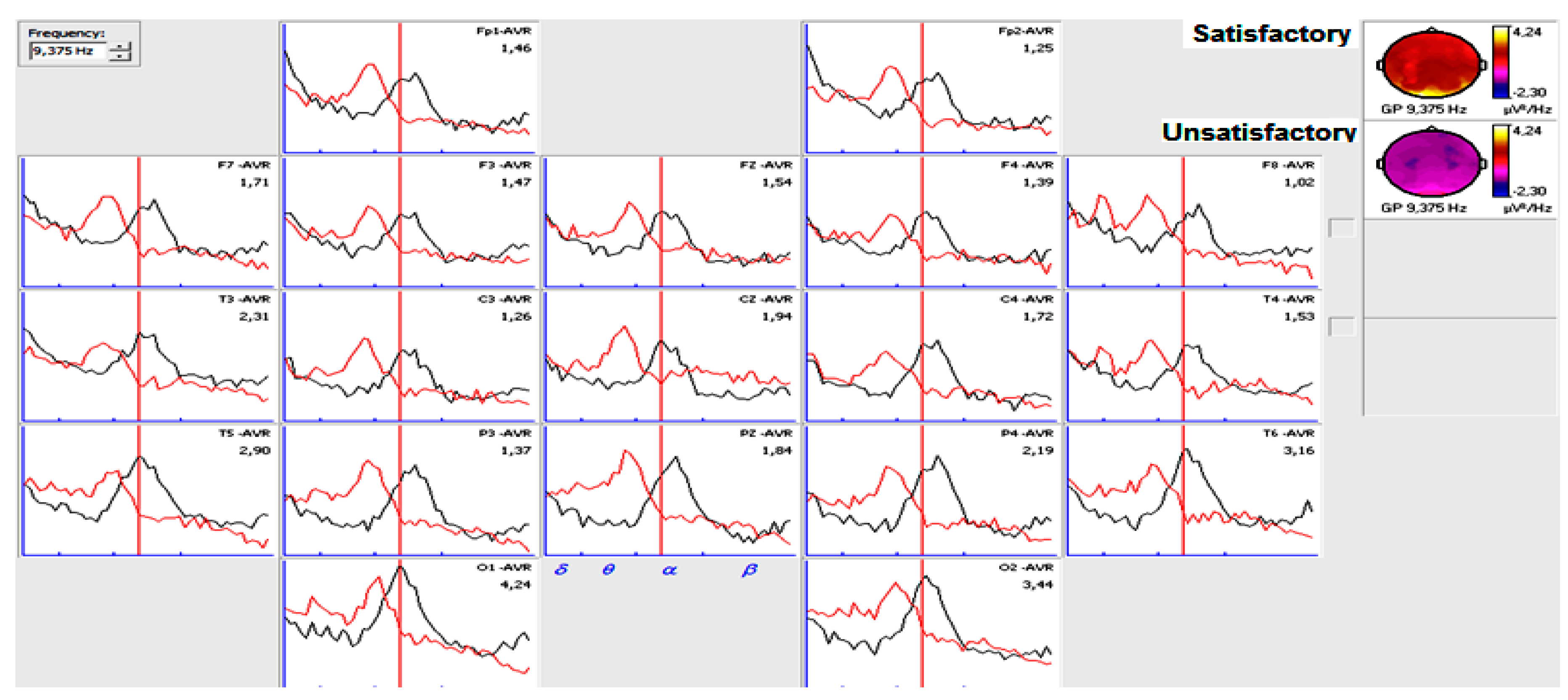
3.2. Narrow- and Broad-Band QEEGC in Pre-surgical Patients with Pharmacoresistance TLE
3.3. Narrow- and Broad-Band QEEGC Changes from Pre-surgical to Post-surgical Phase in Patients with Pharmacoresistance TLE
3.4. Narrow- and Broad-Band QEEGC Differences in Post-surgical Patients with Pharmacoresistance TLE
3.5. Predictive Value of QEEGC After Surgery for Seizure Recurrence in Pharmacoresistance TLE Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| AaP (µv2 Hz) | Electrodes.Wilcoxon p value. Pre-surgical vs Post-surgical phase. | ||||||||||||||||||
| Satisfactory | Fpi 0.49 | Fpc 0.49 | Fsi 0.73 | Fsc 0.31 | Ci 0.17 | Cc 0.73 | Pi 0.75 | Pc 0.73 | Oi 0.49 | Oc 0.39 | Fii 0.01 | Fic 0.86 | Tai 0.01 | Tac 0.73 | Tpi 0.49 | Tpc 0.73 | Fz 0.39 | Cz 0.39 | Pz 0.86 |
| Unsatisfactory | Fpi 0.44 | Fpc 0.51 | Fsi 0.37 | Fsc 0.06 | Ci 0.02 | Cc 0.26 | Pi 0.44 | Pc 0.20 | Oi 0.59 | Oc 0.59 | Fii 0.95 | Fic 0.37 | Tai 0.01 | Tac 0.76 | Tpi 0.51 | Tpc 0.26 | Fz 0.67 | Cz 0.95 | Pz 0.066 |
| ThaP (µv2 Hz) | Electrodes.Wilcoxon p value. Pre-surgical vs Post-surgical phase. | ||||||||||||||||||
| Satisfactory | Fpi 0.86 | Fpc 1.00 | Fsi 0.44 | Fsc 0.61 | Ci 0.31 | Cc 0.86 | Pi 0.86 | Pc 0.73 | Oi 0.73 | Oc 0.86 | Fii 0.39 | Fic 0.49 | Tai 0.04 | Tac 0.75 | Tpi 0.39 | Tpc 0.86 | Fz 0.31 | Cz 0.12 | Pz 0.39 |
| Unsatisfactory | Fpi 0.67 | Fpc 0.31 | Fsi 0.31 | Fsc 0.59 | Ci 0.13 | Cc 0.31 | Pi 0.76 | Pc 0.95 | Oi 0.44 | Oc 0.51 | Fii 0.95 | Fic 0.37 | Tai 0.00 | Tac 0.26 | Tpi 0.76 | Tpc 0.51 | Fz 0.76 | Cz 0.31 | Pz 0.59 |
References
- Tonini, C.; Beghi, E.; Berg, A.T.; Bogliun, G.; Giordano, L.; Newton, R.W.; Tetto, A.; Vitelli, E.; Vitezic, D.; Wiebe, S. Predictors of epilepsy surgery outcome: A meta-analysis. Epilepsy Res. 2004, 62, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Armonk, C.; Radtke, R.A.; Friedman, A.H.; Dawson, D.V. Predictors of outcome of epilepsy surgery: Multivariate analysis with validation. Epilepsia 1996, 37, 814–821. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; So, E.L.; Silbert, P.L.; Jack, C.R.; Cascino, G.D.; Sharbrough, F.W.; O’brien, P.C. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: A multivariate study. Neurology 1998, 51, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Cascino, G.D.; Kelly, P.J.; Sharbrough, F.W.; Hulihan, J.F.; Hirschorn, K.A.; Trenerry, M.R. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: Predictive factors and electroencephalographic results. Epilepsia 1992, 33, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.L.; Boleaga, B.; Brito, F.; Jiménez, F.; Gordillo, J.L.; Velasco, F.; Velasco, M. Absolute and relative predictor values of some non-invasive and invasive studies for the outcome of anterior temporal lobectomy. Arch. Med. Res. 2000, 31, 62–74. [Google Scholar] [CrossRef]
- Tuunainen, A.; Nousiainen, U.; Mervaala, E.; Pilke, A.; Vapalahti, M.; Leinonen, E.; Paljärvi, L.; Riekkinen, P. Postoperative EEG and electrocorticography: Relation to clinical outcome in patients with temporal lobe surgery. Epilepsia 1994, 35, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.; Luders, H.; Dinner, D.S.; Morris, H.H.; Wyllie, E.; Murphy, D. Significance of sharp waves in routine EEGs after epilepsy surgery. Epilepsia 1992, 33, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.; Berg, A.; Spencer, S.S. EEG and seizure outcome after epilepsy surgery. Epilepsia 1995, 36, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, J.M.; Marsan, C.A.; Mutsuga, N. Temporal-lobe seizures with additional foci treated by resection. J. Neurosurg. 1975, 43, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Groppel, G.; Aull-Watschinger, S.; Baumgartner, C. Temporal evolution and prognostic significance of postoperative spikes after selective amygdala-hippocampectomy. J. Clin. Neurophysiol. 2003, 20, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S.; Nasreddine, W.; Passaro, E.; Beydoun, A. Predictive value of early EEG after epilepsy surgery. J. Clin. Neurophysiol. 2005, 22, 410–414. [Google Scholar] [PubMed]
- Di Gennaro, G.; Quarato, P.P.; Sebastiano, F.; Esposito, V.; Onorati, P.; Mascia, A.; Romanelli, P.; Grammaldo, L.G.; Falco, C.; Scoppetta, C.; et al. Postoperative EEG and seizure outcome in temporal lobe epilepsy surgery. Clin. Neurophysiol. 2004, 115, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Rakei, S.M.; Kamgarpour, A.; Taghipour, M.; Ashjazadeh, N.; Razmkon, A.; Zare, Z.; Bagheri, M.H. Outcome after temporal lobectomy in patients with medically-refractory mesial temporal epilepsy in Iran. J. Neurosurg. Sci. 2017, 61, 277–282. [Google Scholar] [PubMed]
- Perry, M.S.; Duchowny, M. Surgical versus medical treatment for refractory epilepsy: Outcomes beyond seizure control. Epilepsia 2013, 54, 2060–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Modur, P.N.; Barot, N.; Van Ness, P.C.; Agostini, M.A.; Ding, K.; Gupta, P.; Hays, R.; Mickey, B. Predictors of Postoperative Seizure Recurrence: A Longitudinal Study of Temporal and Extratemporal Resections. Epilepsy Res. Treat. 2016, 2016, 7982494. [Google Scholar] [CrossRef] [PubMed]
- Mikati, M.A.; Ataya, N.; El-Ferezli, J.; Shamseddine, A.; Rahi, A.; Herlopian, A.; Kurdi, R.; Bhar, S.; Hani, A.; Comair, Y.G. Epilepsy surgery in a developing country (Lebanon): Ten years experience and predictors of outcome. Epileptic Disord. 2012, 14, 267–274. [Google Scholar] [PubMed]
- Janszky, J.; Janszky, I.; Schulz, R.; Hoppe, M.; Behne, F.; Pannek, H.W.; Ebner, A. Temporal lobe epilepsy with hippocampal sclerosis: Predictors for long-term surgical outcome. Brain 2005, 128, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, E. The Normal EEG of the Waking Adult. In Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; p. 167. [Google Scholar]
- Morales Chacón, L.M.; Catasús, C.S.; Bender, J.E.; Bayard, J.B.; García, M.E.; García Maeso, I.; Lorigados, P.L.; Estupiñán, D.B.; Trápaga, Q.O.; Báez, M.M.; et al. A Neurofunctional Evaluation Strategy for Presurgical Selection of Temporal Lobe Epilepsy Patients. MEDICC Rev. 2009, 11, 29–35. [Google Scholar]
- Morales Chacón, L.M.; Garcia Maeso, I.; Baez Martin, M.M.; Bender del Busto, J.E.; García Navarro, M.E.; Quintanal Cordero, N.; Estupiñan Díaz, B.; Lorigados Pedre, L.; Valdés Yerena, R.; Gonzalez, J.; et al. Long-Term Electroclinical and Employment Follow up in Temporal Lobe Epilepsy Surgery. A Cuban Comprehensive Epilepsy Surgery Program. Article. Behavioral sciences. Behav. Sci. 2018, 8, 19. [Google Scholar]
- Engel, J., Jr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology 1993, 43, 1612–1617. [Google Scholar] [PubMed]
- Blumcke, I.; Muhlebner, A. Neuropathological work-up of focal cortical dysplasias using the new ILAE consensus classification system—Practical guideline article invited by the Euro-CNS Research Committee. Clin. Neuropathol. 2011, 30, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.R.; O’Brien, T.J.; Cascino, G.D.; So, E.L.; Radhakrishnan, K.; Silbert, P.; Marsh, W.R. Acute postoperative seizures following anterior temporal lobectomy for intractable partial epilepsy. J. Neurosurg. 1998, 89, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.; So, E.L.; Silbert, P.L.; Cascino, G.D.; Marsh, W.R.; Cha, R.H.; O’brien, P.C. Prognostic implications of seizure recurrence in the first year after anterior temporal lobectomy. Epilepsia 2003, 44, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.; Gotman, J.; Cendes, F.; Andermann, F. Focal intermittent delta activity in patients with mesiotemporal atrophy: A reliable marker of the epileptogenic focus. Epilepsia 1995, 36, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.X.; Chen, X.J.; Baldwin, M.; Yung, I.; Rose, S.; Frim, D.; Hawes-Ebersole, S.; Ebersole, J.S. Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 2011, 52, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenholz, D.M.; Jow, A.; Khan, O.I.; Bagic, A.; Sato, S.; Auh, S.; Kufta, C.; Inati, S.; Theodore, W.H. Preoperative prediction of temporal lobe epilepsy surgery outcome. Epilepsy Res. 2016, 127, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, E.H.; Zhang, D.X.; Mangan, P.; Fountain, N.; Rempe, D. Functional anatomy of limbic epilepsy: A proposal for central synchronization of a diffusely hyperexcitable network. EpilepsyRes. 1998, 32, 194–205. [Google Scholar] [CrossRef]
- Koutroumanidis, M.; Binnie, C.D.; Elwes, R.D.; Polkey, C.E.; Seed, P.; Alarcon, G.; Cox, T.; Barrington, S.; Marsden, P.; Maisey, M.N.; et al. Interictal regional slow activity in temporal lobe epilepsy correlates with lateral temporal hypometabolism as imaged with 18FDG PET: Neurophysiological and metabolic implications. J. Neurol. Neurosurg. Psychiatry 1998, 65, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.G. ILAE CommissionReport. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2004, 45, 695–714. [Google Scholar] [PubMed]
- Mueller, S.G.; Laxer, K.D.; Schuff, N.; Weiner, M.W. Voxel-based T2 relaxation rate measurements in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia 2007, 48, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Riederer, F.; Lanzenberger, R.; Kaya, M.; Prayer, D.; Serles, W.; Baumgartner, C. Network atrophy in temporal lobe epilepsy: A voxel-based morphometry study. Neurology 2008, 71, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.W. Diffusion Tensor Imaging in Temporal Lobe Epilepsy. Epilepsia 2011, 52 (Suppl. 4), 32–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Zhu, L.; Yu, X.; Jin, B.; Chen, C.; Wang, S.; Ding, M.; Zhang, M.; Chen, Z.; et al. Localized shape abnormalities in the thalamus and pallidum are associated with secondarily generalized seizures in mesial temporal lobe epilepsy. Epilepsy Behav. 2017, 70, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.S.; Mackay, C.E.; Barrick, T.R.; Wieshmann, U.C.; Howard, M.A.; Roberts, N. Voxel-based morphometric comparison of hippocampal and extra hippocampal abnormalities in patients with left and right hippocampal atrophy. NeuroImage 2002, 16, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, N.; Duchesne, S.; Janke, A.; Lerch, J.; Collins, D.L.; Bernasconi, A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobeepilepsy. NeuroImage 2004, 23, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Shang, H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobeepilepsy. Epilepsy Res. 2012, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.S.; Roberts, N. Voxel-based morphometry of temporal lobeepilepsy: An introduction and review of the literature. Epilepsia 2008, 49, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Santyr, B.G.; Lau, J.C.; Mirsattari, S.M.; Burneo, J.G.; Steven, D.A.; MacDougall, K.; Khan, A.R. Novel Connectivity Map Normalization Procedure for Improved Quantitative Investigation of Structural Thalamic Connectivity in Temporal Lobe Epilepsy. J. Magn. Resonance Imaging 2018, 48, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.I.; Stern, J.M.; Engelm, J., Jr.; Cohen, M.S. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 2002, 13, 2487–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazanova, O.M.; Vernon, D. Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 2014, 44, 94–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, W.G. The location of cerebral tumors by electroencephalography. Lancet 1936, 2, 305–308. [Google Scholar] [CrossRef]
- Murphy, J.T.; Gloor, P.; Yamamoto, Y.L.; Feindel, W. A comparison of electroencephalography and brain scan in supratentorial tumors. N. Engl. J. Med. 1967, 276, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Gloor, P.; Ball, G.; Schaul, N. Brain lesions that produce delta waves in the EEG. Neurology 1977, 27, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.D.; Bilir, E.; Faught, R.E.; Kuzniecky, R.; Gilliam, F. Significance of interictal temporal lobe delta activity for localization of the primary epileptogenic region. Neurology 1999, 52, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, R.C.; Laxer, K.D.; Klein, G.; Sawrie, S.; Ende, G.; Hawkins, R.A.; Aassar, O.S.; Soohoo, K.; Wong, S.; Barbaro, N. In vivo hipocampal glucose metabolism in mesial temporal lobeepilepsy. Neurology 2001, 57, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Erbayat Altay, E.; Fessler, A.J.; Gallagher, M.; Attarian, H.P.; Dehdashti, F.; Vahle, V.J.; Ojemann, J.; Dowling, J.L.; Gilliam, F.G. Correlation of severity of FDG-PET hypometabolism and interictal regional delta slowing in temporal lobeepilepsy. Epilepsia 2005, 46, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.S.; Cross, J.H.; Shinnar, S.; Mathern, B.W. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008, 49, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, J.T.; Salamon, N.; Hauptman, J.S.; Velasco, T.R.; Hemb, M.; Wu, J.Y.; Sankar, R.; Donald, S.W.; Engel, J., Jr.; Fried, I.; et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: A critical review and the UCLA experience. Epilepsia 2009, 50, 1310–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panayiotopoulos, C.P. A Clinical Guide to Epileptic Syndromes and their Treatment. In Revised Second Edition Based on the ILAE Classifications and Practice Parameter Guidelines; Springer Healthcare Ltd.: London, UK, 2010; ISBN 978-1-84996-160-8. [Google Scholar]
- Clemens, B.; Menes, A. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006, 7, 190–199. [Google Scholar] [CrossRef] [PubMed]
- User’s Manual. Neuronic Quantitative Tomographic EEG, Version 6. Revised Edition: September 2010. Available online: Soportetecnico@cneuro.edu.cu owww.neuronicsa.com (accessed on 15 September 2018).

| Patient | Age at Surgery | Gender | Medical History | First Seizure Age | Epilepsy Duration | Histopathology | Affected Temporal Lobe | Engels Classification |
|---|---|---|---|---|---|---|---|---|
| 1 | 39 | m | ME | 29 | 10 | FCD IIIa | Right | IIA |
| 2 | 31 | m | FS | 1 | 30 | FCD IIIa | Left | IA |
| 3 | 33 | m | ME | 6 | 27 | FCD IIIa | Left | IA |
| 4 | 41 | f | N | 12 | 29 | CD | Left | IA |
| 5 | 37 | f | N | 29 | 8 | DP | Left | IA |
| 6 | 35 | f | FS | 1/5 | 35 | FCD IIIa | Right | IA |
| 7 | 29 | f | FS | 1 | 28 | DP | Right | IIIA |
| 8 | 52 | m | N | 27 | 25 | CD | Left | IA |
| 9 | 26 | f | FS | 1/2 | 26 | FCD IIIa | Left | IIA |
| 10 | 41 | f | N | 26 | 15 | FCD IIIa | Left | IIIA |
| 11 | 38 | f | PI | 20 | 18 | FCD IIIa | Left | IIIA |
| 12 | 26 | f | CCT | 15 | 11 | FCD IIIa | Right | IIA |
| 13 | 34 | f | FS | 5/7 | 34 | FCD IIIa | Left | IA |
| 14 | 36 | m | ME | 23 | 13 | CD | Left | IVA |
| 15 | 23 | f | N | 7 | 16 | FCD IIIa | Left | IIB |
| 16 | 35 | m | ME | 1 | 34 | FCD IIIa | Right | IA |
| 17 | 32 | f | N | 22 | 10 | FCD IIIa | Right | IA |
| 18 | 37 | f | N | 16 | 21 | CD | Right | IA |
| 19 | 29 | m | CCT | 18 | 12 | FCD IIIa | Right | IIIA |
| 20 | 21 | m | SR | 19 | 2 | FCD IIIb | Left | IA |
| 21 | 35 | f | PI | 14 | 21 | FCD IIIb | Left | IA |
| 22 | 32 | f | FS | 13 | 19 | FCD IIIb | Left | IIA |
| 23 | 25 | f | ME | 0.09 | 25 | FCD IIIa | Left | IA |
| 24 | 43 | m | FS | 1 | 42 | FCD IIIa | Right | IIA |
| 25 | 38 | m | N | 10 | 38 | FCD IIIa | Right | IA |
| 26 | 37 | m | FS | 10 | 27 | FCD IIIa | Right | IIB |
| 27 | 54 | m | N | 20 | 15 | FCD IIIa | Right | IA |
| 28 | 32 | m | N | 18 | 14 | FCD IIIc | Right | IIA |
| 29 | 26 | f | N | 5 | 42 | FCD IIIa | Left | IA |
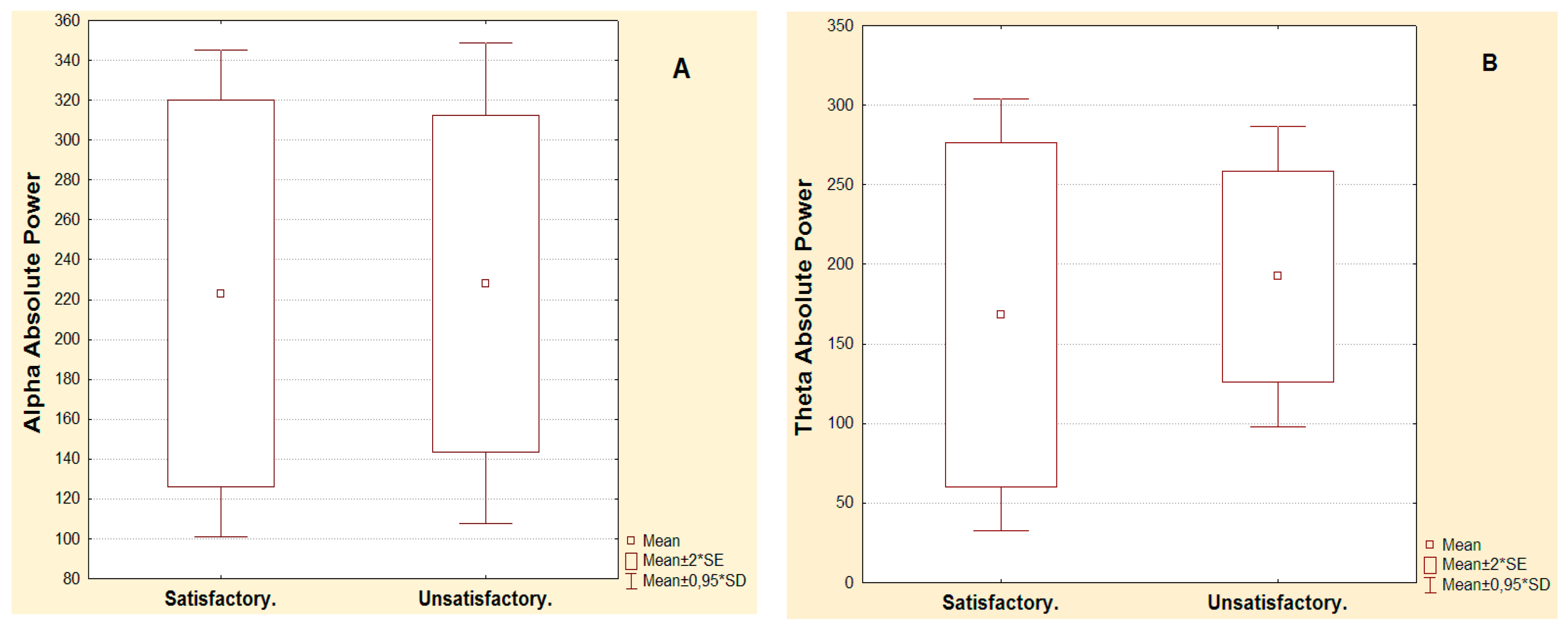
| Alpha Absolute Power µv2 Hz | Pre-surgical | Post-surgical | Significance Electrodes | p-Value Wilcoxon Test | Power Augmentation In Second Year | p-Value Mean comparison |
| Satisfactory post-surgical evolution in the second year | 6 | 9.6 | Frontal inferior ipsilateral | 0.017 | 12.1 | 0.001 |
| 5.2 | 13.7 | Temporal anterior ipsilateral | 0.017 | |||
| Unsatisfactory post-surgical evolution in the second year | 7.3 | 12 | Temporal anterior ipsilateral | 0.010 | 6.1 | |
| 5.7 | 7.1 | Central ipsilateral | 0.028 | |||
| Theta Absolute Power µv2Hz | Pre-surgical | Post-surgical | Significance Electrodes | p-Value Wilcoxon Test | Power Augmentation In Second Year | p-Value Mean Comparison |
| Satisfactory post-surgical evolution in the second year | 5.9 | 14.8 | Temporal anterior ipsilateral | 0.042 | 8.9 | 0.700 |
| Unsatisfactory post-surgical evolution in the second year | 9.5 | 17.6 | Temporal anterior ipsilateral | 0.007 | 8.1 |
| Narrow-Band µv2Hz | Post-Surgical Evolution in the Second Year | Predicted | Percent Correct | Odds Ratio | P Value | |
|---|---|---|---|---|---|---|
| Satisfactory | Unsatisfactory | |||||
| Frequency value with maximum energy in occipital regions in the first six months | Satisfactory | 13 | 3 | 81.25 | 4.333 | 0.04 |
| Unsatisfactory | 6 | 6 | 50.0 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés Sedeño, R.R.; Morales Chacón, L.M.; Sánchez Coroneux, A. Association Between Quantitative Electroencephalogram Frequency Composition and Post-Surgical Evolution in Pharmacoresistant Temporal Lobe Epilepsy Patients. Behav. Sci. 2019, 9, 23. https://doi.org/10.3390/bs9030023
Valdés Sedeño RR, Morales Chacón LM, Sánchez Coroneux A. Association Between Quantitative Electroencephalogram Frequency Composition and Post-Surgical Evolution in Pharmacoresistant Temporal Lobe Epilepsy Patients. Behavioral Sciences. 2019; 9(3):23. https://doi.org/10.3390/bs9030023
Chicago/Turabian StyleValdés Sedeño, Raúl Roberto, Lilia María Morales Chacón, and Abel Sánchez Coroneux. 2019. "Association Between Quantitative Electroencephalogram Frequency Composition and Post-Surgical Evolution in Pharmacoresistant Temporal Lobe Epilepsy Patients" Behavioral Sciences 9, no. 3: 23. https://doi.org/10.3390/bs9030023
APA StyleValdés Sedeño, R. R., Morales Chacón, L. M., & Sánchez Coroneux, A. (2019). Association Between Quantitative Electroencephalogram Frequency Composition and Post-Surgical Evolution in Pharmacoresistant Temporal Lobe Epilepsy Patients. Behavioral Sciences, 9(3), 23. https://doi.org/10.3390/bs9030023






