1. Introduction
The allegations and remarks raised by family and friends of patients that underwent surgery, such as “He’s never been the same since his operation” or “She’s lost all interest in the family since …”, have led Bedford [
1] to publish the first comprehensive report that describes cognitive decline after surgery in the elderly. Bedford has attributed the phenomenon mostly to anesthesia, and hypoxic intervals during anesthesia and surgery. Majority of studies since then [
2,
3,
4,
5,
6,
7] have focused on cardiac surgeries, especially cardiopulmonary bypass surgery, presumably due to the potential for hypoxic/hypoperfusion injury and microemboli-related ischemia during these types of surgeries. However, a large study published in 1998 [
8] established long-term cognitive dysfunction (detected at 3 months after surgery) in patients undergoing non-cardiac surgery (major abdominal and orthopedic surgeries), and concluded that the occurrence of this dysfunction is not related to hypotension or hypoxemia, but to anesthesia and surgery, with advanced age being the strongest risk factor. Subsequent studies have also determined that this complication, now termed postoperative cognitive dysfunction (POCD), could occur after minor surgery [
9], and is not related to the depth of anesthesia [
10,
11]. Moreover, POCD has been shown to increase the risk of developing dementia 3–5 years after surgery [
12], and increase the risk of death in the first year following surgery [
13].
To distinguish it from postoperative delirium, which occurs in the first few days following surgery, POCD is defined as persistent cognitive impairments (lasting for more than one week) after surgery [
14]. Also, the acute and transient cognitive disturbances in delirium may involve fluctuating mental status, while POCD alterations are more subtle, and involve memory, concentration, language comprehension, and a decreased ability to process information, without changes in the levels of consciousness [
15,
16,
17,
18].
The pathophysiology underlying POCD is not yet fully understood. Accumulating evidence point to the role of peripheral systemic inflammation (due to surgical trauma) in causing exaggerated inflammatory responses in the brain [
14], as shown by patient studies [
19,
20,
21] and animal studies, particularly in the hippocampus [
22,
23,
24]. Many of the immunological changes observed in POCD, including tau phosphorylation, cytokine-induced glutamatergic excitotoxicity, and most importantly, beta-amyloid accumulation, are similar to the changes found in Alzheimer’s disease [
22,
25,
26]. Therefore, both entities could be viewed as a disease continuum [
15].
Currently, there is no adequate treatment or prophylaxis for POCD [
14,
15], and management usually revolves around identifying risks, and non-pharmacological preventive measures [
27,
28,
29]. Memantine is an N-methyl-D-aspartate receptor (NMDAR) antagonist that is shown to improve cognitive function in transgenic animal models of Alzheimer’s disease [
30,
31,
32], and its use is widely supported by human clinical trials in treating moderate to severe Alzheimer’s disease [
33,
34]. Surprisingly, little research exists on the role of memantine in the management of POCD. The purpose of this study is to investigate the effects of memantine on a spectrum of cognitive functions in a mouse model of POCD.
4. Discussion
The purpose of this study is to examine the effect of memantine on a spectrum of cognitive functions postoperatively. Hovens and colleagues [
14] point to a translational gap in postoperative cognitive dysfunction (POCD) research. POCD is often reported by clinical studies without specifying what cognitive functions or brain structures are involved. Memory, concentration, language comprehension, and information processing are commonly implicated, but only few studies employ neurophysiological testing to make distinctions between these cognitive domains (see [
45,
46,
47] for examples). On the other hand, pre-clinical research using animal models mostly focused on memory and hippocampal injury, and neglected other aspects of cognition. Taking that into consideration, we attempted to investigate the effect of surgery on different aspects of cognition in mice. These include learning and memory, anxiety, locomotor function, depression, sociability and preference for social novelty.
With regards to body weight, mice that underwent surgery (PB and M groups) suffered from weight loss on postoperative days 1 and 3, but regained their weight on postoperative day 7. Weight loss in the first few days after surgery in POCD models is commonly reported [
48,
49], and it could be attributed to dehydration and fluid loss during surgery.
Learning and memory were assessed using the Morris water maze test. On postoperative days 1 and 7, mice that underwent surgery (PB-POD1 and PB-POD7) spent more time and travelled more distance to reach the platform, and spent less time in the disc zone in the probe test, compared to the groups that received anesthesia and analgesia without surgery (PA-POD1 and PA-POD7). Motor function was not affected, as all groups had similar speed of swimming. These findings suggest impaired learning and memory in mice following surgery. Several studies reported similar findings [
48,
50]. The duration of memory impairment following surgery varies in animal models of POCD. Most studies reported effects lasting between 1 and 7 days [
23,
24,
38,
51]. However, effects lasting for more than 1 week have also been reported [
24,
48,
52]. In our study, memory was not tested beyond one week and, therefore, it is not possible, based on these findings, to determine whether these deficits are persistent or not.
The abdominal surgery performed on mice in this study is considered minor surgery. The surgical model used [
38] was shown to lead to increased inflammatory changes in the hippocampus of aged mice (23–25 months old), but not in young adult mice (4–6 months old). Moreover, it did not result in significantly impaired performance in a reversal learning version of the Morris water maze. In our study, adult mice (aged 4–6 months) were tested. Interestingly, significant memory impairment was observed. This discrepancy could be attributed to the version of the Morris water maze test used. The standard Morris water maze, which tests spatial learning through visual cues with a probe test, is considered reflective of hippocampal function [
53,
54]. On the other hand, some variations of the Morris water maze, such as reversal tasks, are thought to be independent of hippocampal function [
54,
55]. Our findings support previous research, which have suggested that hippocampal-dependent memory is specifically susceptible to surgery-induced impairments [
23,
48,
51,
52,
56].
Anxiety may inhibit exploratory behavior in mice [
57], as well as performance in other cognitive tests. Anxiety-like behavior was assessed using the open field test and the elevated-plus maze. On postoperative day 1, sham-operated mice (PA-POD1) spent more time in the central area in the open field, and more time in the open arms of the elevated-plus maze with a higher number of entries to the open arms, compared to mice that underwent surgery (PB-POD1). On postoperative day 7, no significant differences were found in the open field test. However, sham-operated mice (PA-POD7) spent more time in the open arms of the elevated-plus maze with higher number of entries to the open arms compared to their surgical counterpart (PB-POD7). Collectively, these observations may reflect anxiety-like behavior and reduced interest in the environment. Several studies reported anxiety-like behavior following surgery [
48,
58,
59,
60], but mostly in the first two postoperative days. Because of that, anxiety in this context is often viewed as a transient response to acute illness or trauma [
48,
61,
62], rather than a persistent cognitive deficit related to POCD. In our experiment, however, anxiety was observed on days 1 and 7 postoperatively. It is unclear whether this anxiety reflects a sickness-response to trauma, or is related to inflammatory changes in the brain, as no inflammatory assays were performed.
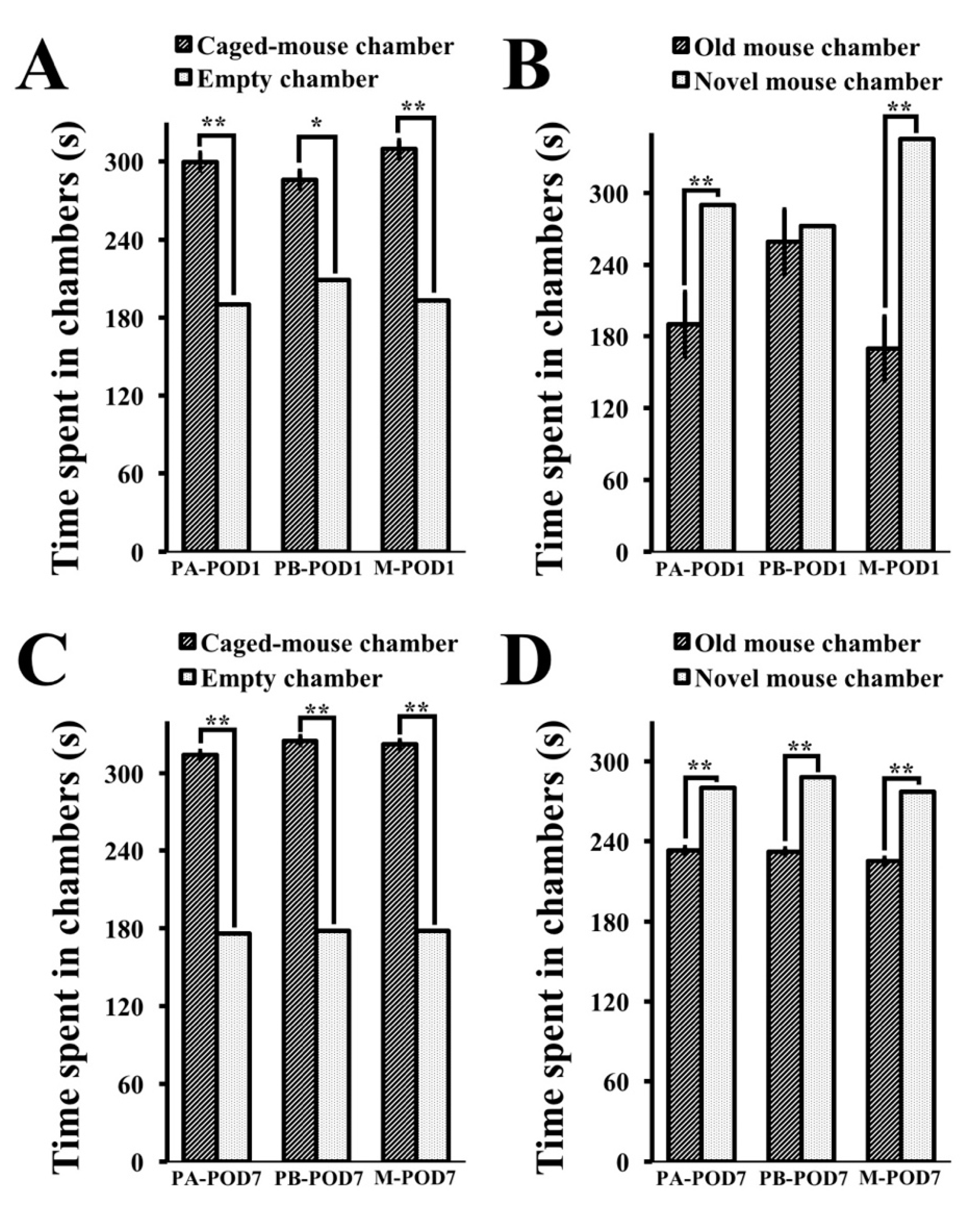
Decreased social activity following surgery has been reported previously by human studies [
58,
63], and animal models of peripheral inflammation [
64,
65] However, there is a lack of studies concerning sociability and social novelty following surgery in mice. In this study, sociability and preference for social novelty were assessed using the three-chamber test. Mice that underwent surgery (PB-POD1) exhibited normal sociability and no preference for social novelty on postoperative day 1. On postoperative day 7, operated mice (PB-POD7) showed normal sociability and preference for social novelty. Overall, these findings suggest normal social behavior in mice following surgery.
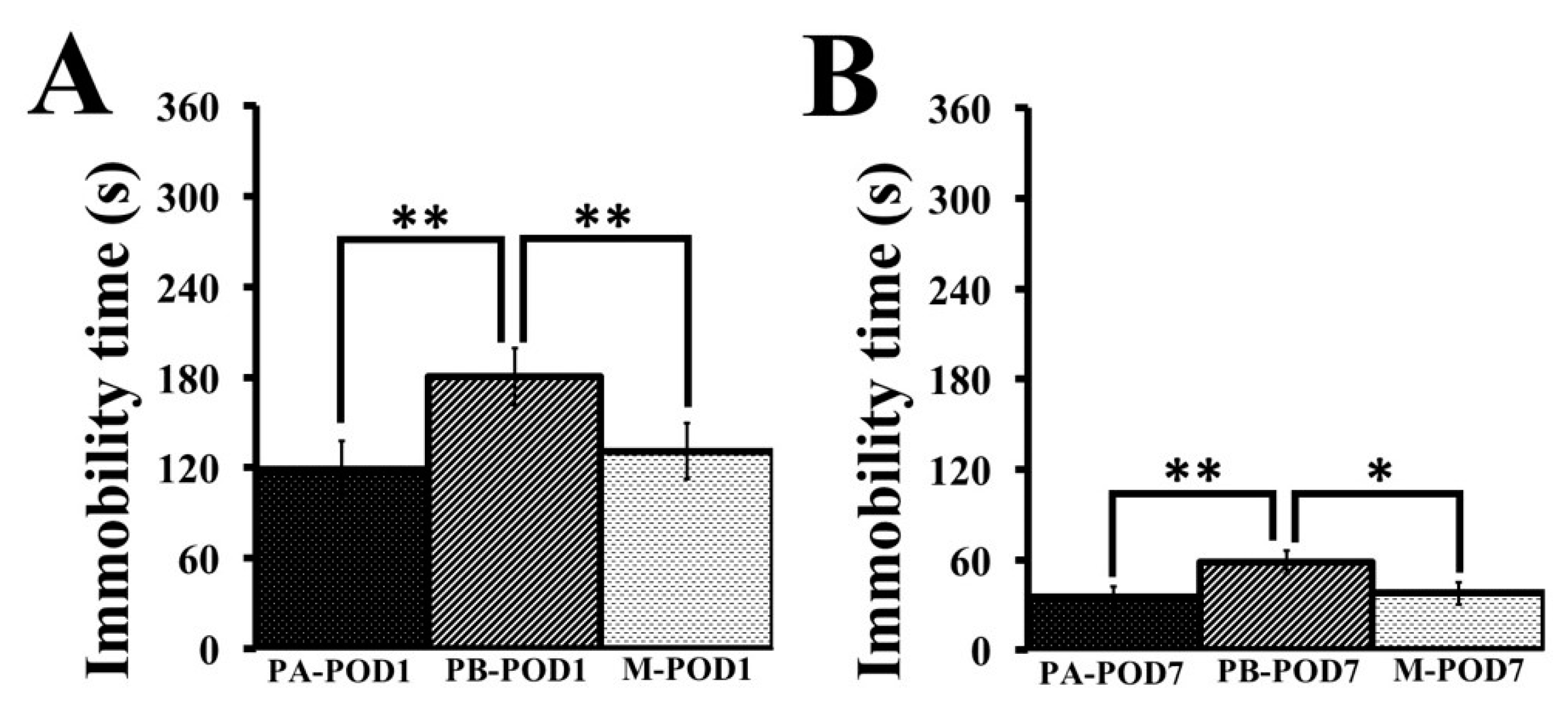
Depression was assessed using the tail-suspension test. Mice that underwent surgery (PB-POD1 and PB-POD7) showed depression-like behavior on postoperative days 1 and 7, as they had higher immobility times compared to their non-surgical counterparts (PA-POD1 and PA-POD7). Animal models of peripheral inflammation reported depression-like behavior, and attributed the phenomenon to a high turnover rate of brain serotonin as a result of an exaggerated elevation in inflammatory mediators in the brain [
66,
67]. On the contrary, depression was not shown to occur after surgery in humans [
8,
68,
69], but it is considered to be a risk factor for POCD [
47] and postoperative delirium [
70,
71].
As mentioned, the pathophysiology underlying POCD is not yet fully understood, and several etiological factors have been proposed. Hypocarbia due to hyperventilation during anesthesia was shown to prolong cognitive dysfunction after surgery [
72], but other studies failed to establish the link [
73,
74]. Prolonged hypotension in the perioperative period was thought to result in cerebral hypoperfusion, and subsequently cognitive decline. However, the effect was not shown to be important [
8,
75,
76,
77]. Cognitive decline is well-documented following cardiac surgery [
78,
79,
80], as it could be a source of microemboli (clots, fat, or air bubbles) that causes brain infarcts. However, POCD is often found following non-cardiac surgeries, and the presence of microemboli is not clearly linked to cognitive dysfunction [
17,
81,
82]. Sleep disturbances following surgery can affect cognitive function, but evidence for such an effect is scarce [
83]. Neurodegeneration can occur as a result of the use of volatile anesthetics, as shown by animal studies [
84,
85,
86], but several other studies failed to find a link [
17,
22,
23,
38,
87]. Other etiologies, including anticholinergic activity of medications routinely used in the perioperative period, and low intraoperative cerebral oxygenation, were also proposed, but the results were inconclusive [
15].
Consistent evidence exists only for the role of postoperative peripheral inflammation in causing exaggerated neuroinflammatory responses in the brain, that manifest as cognitive decline. Severe systemic inflammation, as a result of trauma or infection, is known to affect the central nervous system (CNS) [
88,
89,
90], and surgery was shown to result in the systemic release of inflammatory mediators, such as cytokines, reactive oxygen species, and endothelins [
14,
91,
92,
93,
94,
95]. These changes lead to the activation of microglia in the CNS, which results in the release of cytokines and other inflammatory mediators that have been linked to cognitive dysfunction [
19,
20,
21,
23,
96,
97]. Moreover, the severity of the surgical intervention is correlated to the risk of cognitive dysfunction postoperatively, which supports the inflammatory hypothesis, as more severe procedures are related to higher magnitudes of immune activation [
14,
17,
23,
24,
98,
99,
100].
Interestingly, these neuroinflammatory responses also lead to neurodegenerative changes, such as tau phosphorylation, cytokine-induced glutamatergic excitotoxicity, synaptic impairment, and most importantly, beta-amyloid accumulation, which are also characteristic of Alzheimer’s disease [
22,
25,
26,
101,
102]. It comes as no surprise that POCD have been linked to an increased risk of developing dementia [
12] and, therefore, both entities can be viewed as manifestations of the same process [
15]. It should be noted that the absence of neurofibrillary tangles in POCD distinguishes it from Alzheimer’s disease. Therefore, cognitive deficits in POCD are thought to be specifically related to beta-amyloid accumulation [
25]. Beta-amyloid proteins are known to induce neuronal cell degeneration and apoptosis, especially in the hippocampus, resulting in memory impairment [
102]. Additionally, amyloid precursor protein (APP), whose proteolysis leads to the generation of beta-amyloid proteins and acts as an acute reactive protein during stress [
103,
104], was also found to be increased following surgery in mice [
25].
Management of POCD is centered around preventive measures. It involves identifying those who are at risk, and optimizing perioperative physical and mental health. Several measures have been shown to reduce the incidence of POCD [
18,
27,
28,
29,
105,
106,
107]. These include improving sleep hygiene in the perioperative period, having shorter periods of fasting before surgery, increasing the frequency of social contact with family and friends after surgery, optimization of nutritional status and hydration, selecting the least invasive procedures possible, and adequate pain control.
However, no effective pharmacological treatment exists. Research in this area focused mostly on anti-inflammatory medications, since neuroinflammation contributes to POCD significantly. For example, the administration of small doses of ketamine, which has an anti-inflammatory effect, was shown to reduce the incidence of POCD [
108]. Minocycline [
23,
109], a derivative of tetracycline, and berberine [
49], an isoquinoline alkaloid, were also shown to reduce neuroinflammation in mice. In Alzheimer’s disease, the use of non-steroidal anti-inflammatory medications has been shown to attenuate disease progression in the early stages [
110].
In the present study, we hypothesized that memantine, an NMDAR antagonist, that is shown to be effective in treating Alzheimer’s disease [
30,
31,
32,
33,
34], might have a protective effect against POCD. The release of pro-inflammatory cytokines in the CNS leads to an increase in the levels of glutamate, which increases NMDAR activity. That, in turn, increases Ca
2+ inflow through ion channels, which results in pathological over-excitation, and ultimately neuronal death. Furthermore, hyper-activation of NMDARs has also been associated with tau toxicity [
111]. As mentioned previously, both of these processes, glutamatergic excitotoxicity and tau phosphorylation, have been shown to occur in Alzheimer’s disease and in POCD [
25]. Memantine inhibits NMDARs by blocking the Ca
2+ ion channel, and thus it targets both of the pathological processes that are known to be involved in POCD. Previous research also showed that memantine reduces neuroinflamamtion in animal models and improves cognitive function [
112,
113,
114]. In our study, memantine improved the preference for social novelty, and had an anti-depressant-like effect, with no effect on memory or anxiety-like behavior on postoperative day 1. On day 7 postoperatively, memantine had a positive effect on memory and depression. In addition, memantine did not impact the body weight of treated mice. In humans, a randomized clinical trial conducted by Ghaffary and colleagues [
115] showed that the pre-operative administration of memantine protected patients from POCD following cardiac surgeries, and improved cognitive function after 3 months postoperatively. Collectively, these findings suggest that memantine has the potential to prevent POCD in humans.