Digital Phenotyping of Sensation Seeking: A Machine Learning Approach Using Gait Analysis
Abstract
1. Introduction
1.1. Related Work
1.2. Structure of the Paper
2. Methods
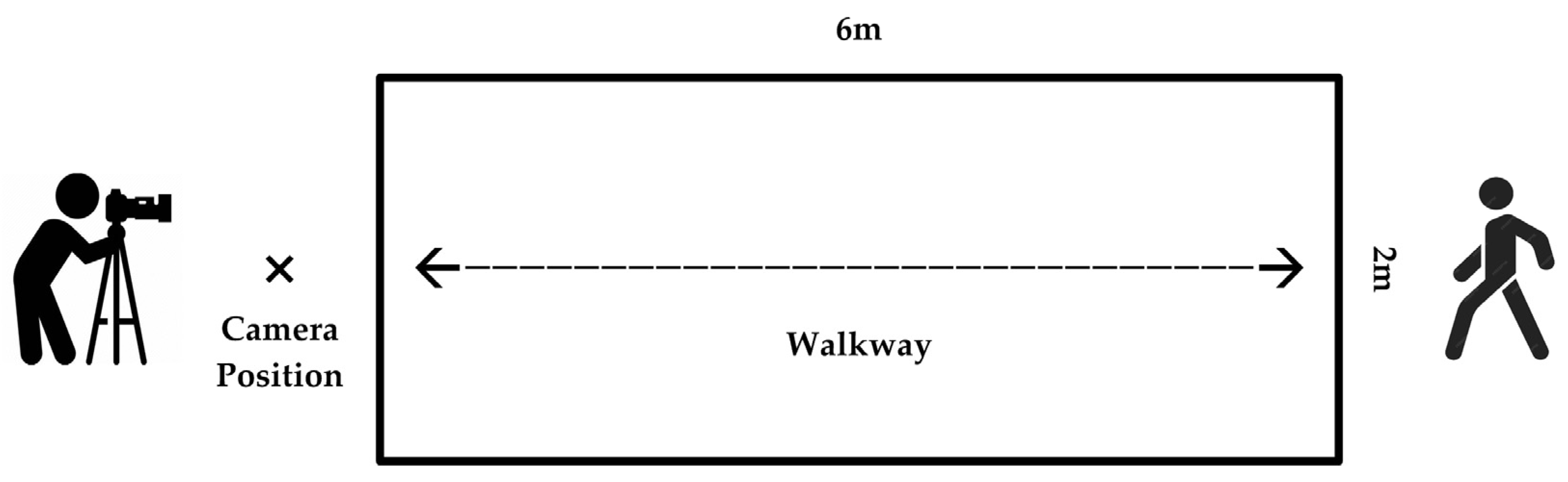
2.1. Data Collection
2.2. Data Preprocessing
2.2.1. Data Unification
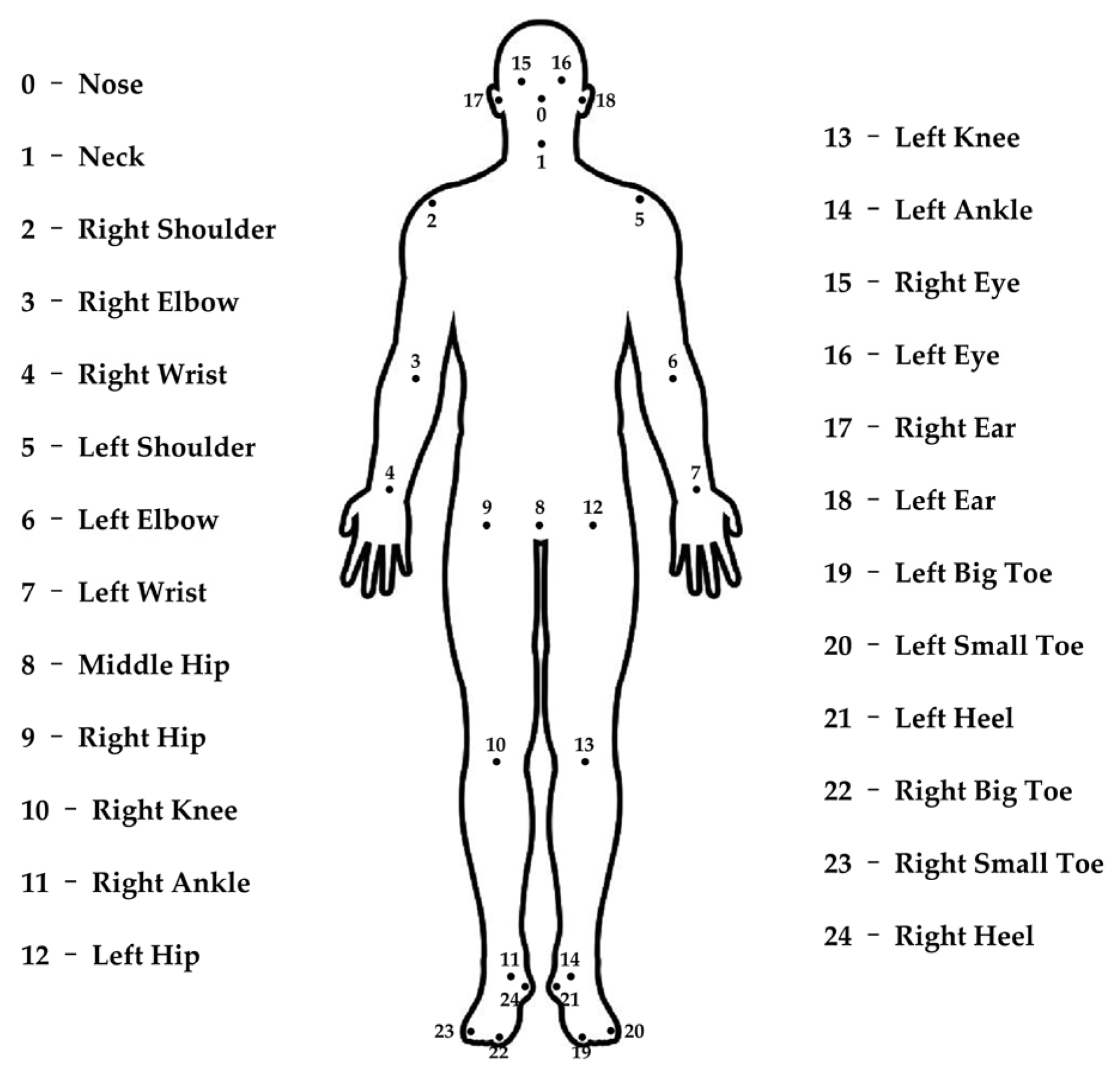
2.2.2. Skeletal Keypoints Extraction
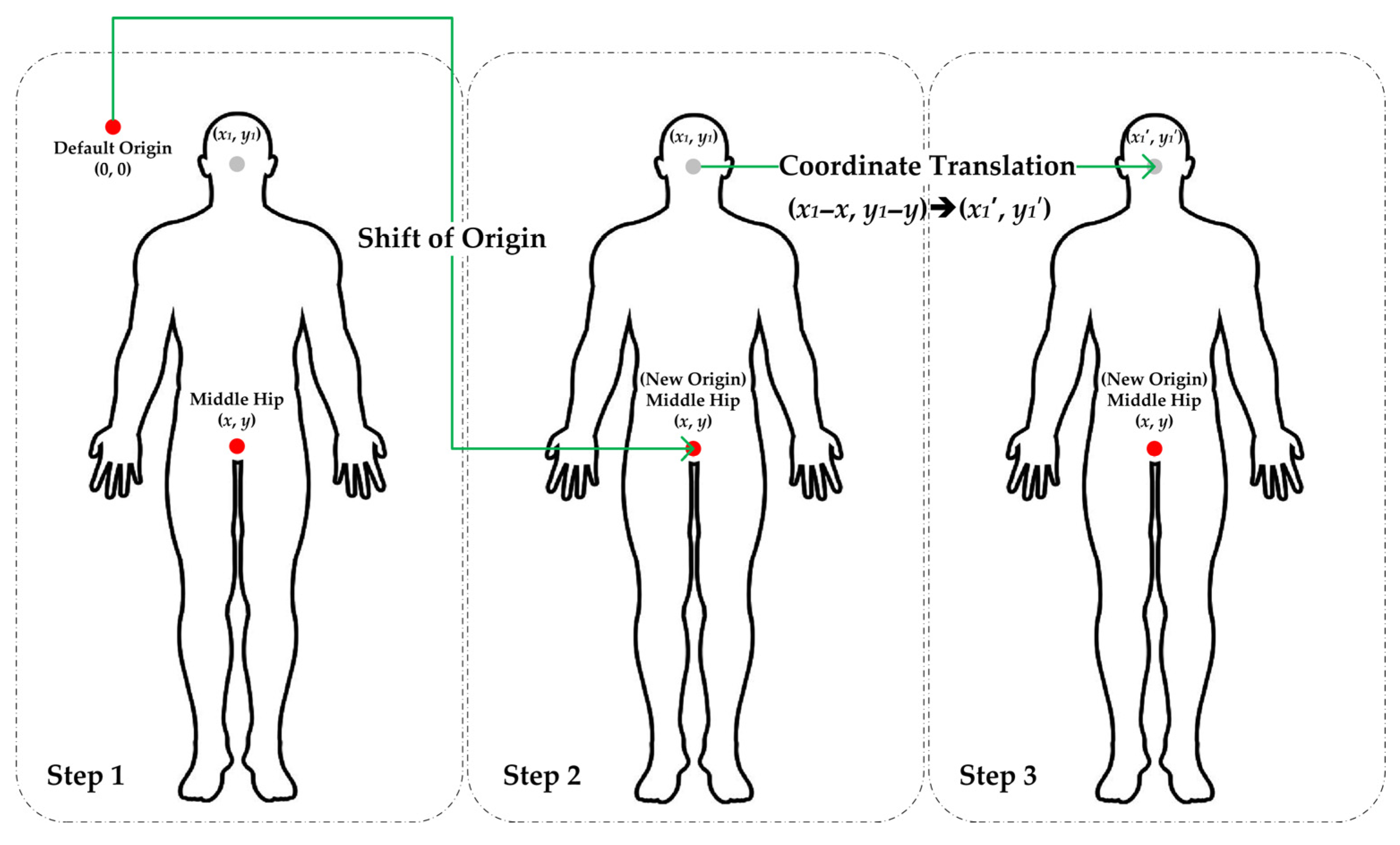
2.2.3. Coordinate Translation
2.2.4. Noise Removal
2.2.5. Feature Extraction
2.3. Data Modeling
3. Results
3.1. General Analysis
3.2. Feature Selection
3.3. Model Training
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adlou, B., Wadsworth, D., Grace, J. L., Kosek, J., Wilburn, C., & Weimar, W. (2025). Complex interplay between emotional states and gait parameters: A domain-specific investigation in healthy young adults. Experimental Brain Research, 243(4), 100. [Google Scholar] [CrossRef]
- Avanzino, L., Lagravinese, G., Abbruzzese, G., & Pelosin, E. (2018). Relationships between gait and emotion in Parkinson’s disease: A narrative review. Gait Posture, 65, 57–64. [Google Scholar] [CrossRef]
- Bishop, C. M. (2006). Pattern recognition and machine learning. Springer. [Google Scholar]
- Bogdan, S. R., Măirean, C., & Havârneanu, C.-E. (2016). A meta-analysis of the association between anger and aggressive driving. Transportation Research Part F: Traffic Psychology and Behaviour, 42, 350–364. [Google Scholar] [CrossRef]
- Boyle, G. J., & Helmes, E. (2009). Methods of personality assessment. In P. J. Corr, & G. Matthews (Eds.), The Cambridge handbook of personality psychology (pp. 110–126). Cambridge University Press. [Google Scholar]
- Cao, Z., Hidalgo, G., Simon, T., Wei, S., & Sheikh, Y. (2021). OpenPose: Realtime multi-person 2D pose estimation using part affinity fields. IEEE Transactions on Pattern Analysis and Machine Intelligence, 43(1), 172–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. J., & Lee, Y. J. (2024). Classification of gait variation under mental workload in big five personalities. Gait Posture, 113, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X., Li, F., Nydegger, L., Gong, J., Ren, Y., Dinaj-Koci, V., Sun, H., & Stanton, B. (2013). Brief sensation seeking scale for Chinese—Cultural adaptation and psychometric assessment. Personality and Individual Differences, 54(5), 604–609. [Google Scholar] [CrossRef]
- Cho, K., Kim, M., Cho, Y., Hur, J. W., Kim, D. H., Park, S., Park, S., Jang, M., Lee, C. G., & Kwon, J. S. (2024). Digital phenotypes for early detection of internet gaming disorder in adolescent students: Explorative data-driven study. JMIR Mental Health, 11, e50259. [Google Scholar] [CrossRef]
- Crocamo, C., Cioni, R. M., Canestro, A., Nasti, C., Palpella, D., Piacenti, S., Bartoccetti, A., Re, M., Simonetti, V., Barattieri di San Pietro, C., Bulgheroni, M., Bartoli, F., & Carrà, G. (2025). Acoustic and natural language markers for bipolar disorder: A pilot, mhealth cross-sectional study. JMIR Formative Research, 9, e65555. [Google Scholar] [CrossRef]
- Deligianni, F., Guo, Y., & Y, G. (2019). From emotions to mood disorders: A survey on gait analysis methodology. IEEE Journal of Biomedical and Health Informatics, 23(6), 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Deng, X., & Zhang, L. (2020). Neural underpinnings of the relationships between sensation seeking and emotion regulation in adolescents. International Journal of Psychology, 55(5), 851–860. [Google Scholar] [CrossRef]
- Dlima, S. D., Shevade, S., Menezes, S. R., & Ganju, A. (2022). Digital phenotyping in health using machine learning approaches: Scoping review. JMIR Bioinformatics and Biotechnology, 3(1), e39618. [Google Scholar] [CrossRef]
- Elboim-Gabyzon, M., Pitluk, M., & Shuper Engelhard, E. (2022). The correlation between physical and emotional stabilities: A cross-sectional observational preliminary study. Annals of Medicine, 54(1), 1678–1685. [Google Scholar] [CrossRef]
- Fang, J., Wang, T., Li, C., Hu, X., Ngai, E., & Seet, B. (2019). Depression prevalence in postgraduate students and its association with gait abnormality. IEEE Access, 7, 174425–174437. [Google Scholar] [CrossRef]
- Freund, V. A., Schulenberg, J. E., & Maslowsky, J. (2022). Boredom by sensation-seeking interactions during adolescence: Associations with substance use, externalizing behavior, and internalizing symptoms in a US national sample. Prevention Sciences, 22(5), 555–566. [Google Scholar] [CrossRef]
- Harris, N., Newby, J., & Klein, R. G. (2015). Competitiveness facets and sensation seeking as predictors of problem gambling among a sample of university student gamblers. Journal of Gambling Studies, 31(2), 385–396. [Google Scholar] [CrossRef]
- Kang, G. E., & Gross, M. M. (2016). The effect of emotion on movement smoothness during gait in healthy young adults. Journal of Biomechanics, 49(16), 4022–4027. [Google Scholar] [CrossRef]
- Kapetanovic, S., Zietz, S., Lansford, J. E., Bacchini, D., Bornstein, M. H., Chang, L., Deater-Deckard, K., Giunta, L. D., Dodge, K. A., Gurdal, S., Oburu, P., Junla, D., Pastorelli, C., Skinner, A. T., Sorbring, E., Tapanya, S., Steinberg, L., Tirado, L. M. U., Yotanyamaneewong, S., … Al-Hassan, S. M. (2023). Parenting, adolescent sensation seeking, and subsequent substance use: Moderation by adolescent temperament. Journal of Youth and Adolescence, 52(6), 1235–1254. [Google Scholar] [CrossRef]
- Klupp, S., Grob, A., & Möhring, W. (2023). Gait variability relates to prosocial, emotional and risk-taking behavior in typically developing children. Perceptual and Motor Skills, 130(1), 191–207. [Google Scholar] [CrossRef]
- Li, J., Zhou, Y., Ge, Y., & Qu, W. (2023). Sensation seeking predicts risky driving behavior: The mediating role of difficulties in emotion regulation. Risk Analysis, 43(9), 1871–1886. [Google Scholar] [CrossRef]
- Li, M., Wen, Y., Gao, X., Si, J., & Huang, H. (2022). Toward expedited impedance tuning of a robotic prosthesis for personalized gait assistance by reinforcement learning control. IEEE Transactions on Robotics, 38(1), 407–420. [Google Scholar] [CrossRef]
- Mason, R., Pearson, L. T., Megaritis, D., & Barry, G. (2025). A breakdown of the gait cycle: Biomechanics basics. In S. Stuart, & R. Morris (Eds.), Gait, Balance, and mobility analysis (pp. 141–154). Academic Press. [Google Scholar]
- Montero-Odasso, M., Verghese, J., Beauchet, O., & Hausdorff, J. M. (2012). Gait and cognition: A complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society, 60(11), 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Norbury, A., & Husain, M. (2015). Sensation-seeking: Dopaminergic modulation and risk for psychopathology. Behavioural Brain Research, 288, 79–93. [Google Scholar] [CrossRef]
- Onnela, J. P. (2020). Opportunities and challenges in the collection and analysis of digital phenotyping data. Neuropsychopharmacology, 46(1), 45–54. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F., Muir-Hunter, S. W., & Montero-Odasso, M. (2018). Do depressive symptoms affect balance in older adults with mild cognitive impairment? Results from the “gait and brain study”. Experimental Gerontology, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Salomon, A., Gazit, E., Ginis, P., Urazalinov, B., Takoi, H., Yamaguchi, T., Goda, S., Lander, D., Lacombe, J., Sinha, A. K., Nieuwboer, A., Kirsch, L. C., Holbrook, R., Manor, B., & Hausdorff, J. M. (2024). A machine learning contest enhances automated freezing of gait detection and reveals time-of-day effects. Nature Communications, 15(1), 4853. [Google Scholar] [CrossRef]
- Sapuram, V. R., Vrshek-Schallhorn, S., Hilt, L. M., & Stroud, C. B. (2021). Dopaminergic genetic variation in young adolescents: Associations with sensation-seeking. Research on Child and Adolescent Psychopathology, 49(10), 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Satchell, L., Morris, P., Mills, C., O’Reilly, L., Marshman, P., & Akehurst, L. (2017). Evidence of big five and aggressive personalities in gait biomechanics. Journal of Nonverbal Behavior, 41(1), 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sârbescu, P., & Rusu, A. (2021). Personality predictors of speeding: Anger-aggression and impulsive-sensation seeking. A systematic review and meta-analysis. Journal of Safety Research, 77, 86–98. [Google Scholar] [CrossRef]
- Stachl, C., Au, Q., Schoedel, R., Gosling, S. D., Harari, G. M., Buschek, D., Völkel, S. T., Schuwerk, T., Oldemeier, M., Ullmann, T., Hussmann, H., Bischl, B., & Bühner, M. (2020). Predicting personality from patterns of behavior collected with smartphones. Proceedings of the National Academy of Sciences of the United States of America, 117(30), 17680–17687. [Google Scholar] [CrossRef]
- Taylor, K. I., Staunton, H., Lipsmeier, F., Nobbs, D., & Lindemann, M. (2020). Outcome measures based on digital health technology sensor data: Data- and patient-centric approaches. NPJ Digital Medicine, 3, 97. [Google Scholar] [CrossRef]
- Te Brinke, L. W., van der Cruijsen, R., Green, K. H., & Crone, E. A. (2022). Positive and negative risk-taking in adolescence and early adulthood: A citizen science study during the COVID-19 pandemic. Frontiers in Psychology, 13, 885692. [Google Scholar] [CrossRef]
- Weisenburger, R. L., Mullarkey, M. C., Labrada, J., Labrousse, D., Yang, M. Y., MacPherson, A. H., Hsu, K. J., Ugail, H., Shumake, J., & Beevers, C. G. (2024). Conversational assessment using artificial intelligence is as clinically useful as depression scales and preferred by users. Journal of Affective Disorders, 351, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Wen, H., Sobolev, M., Vitale, R., Kizer, J., Pollak, J. P., Muench, F., & Estrin, D. (2021). mPulse mobile sensing model for passive detection of impulsive behavior: Exploratory prediction study. JMIR Mental Health, 8(1), e25019. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y., Li, B., Chen, D., & Zhu, T. (2022). Reliability and validity analysis of personality assessment model based on gait video. Frontiers in Behavioral Neuroscience, 16, 901568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X., Qu, X., Tao, D., & Xue, H. (2019). The association between sensation seeking and driving outcomes: A systematic review and meta-analysis. Accident Analysis & Prevention, 123, 222–234. [Google Scholar] [CrossRef]
- Zhang, Y., Stewart, C., Ranjan, Y., Conde, P., Sankesara, H., Rashid, Z., Sun, S., Dobson, R. J. B., & Folarin, A. A. (2025). Large-scale digital phenotyping: Identifying depression and anxiety indicators in a general UK population with over 10,000 participants. Journal of Affective Disorders, 375, 412–422. [Google Scholar] [CrossRef]
- Zhao, Y., Xing, H., Wang, X., Tian, Y., Sun, T., Chen, M., Yeung, D. Y. L., Ho, S. M. Y., Wang, J., & Li, W. J. (2024). Wearable internet of things gait sensors for quantitative assessment of Myers–Briggs Type Indicator personality. Advanced Intelligent System, 6(3), 2300328. [Google Scholar] [CrossRef]
- Zuckerman, M. (1994). Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press. [Google Scholar]
| Features | Description |
|---|---|
| F1 | Mean coordinate value |
| F2 | Standard deviation of coordinates |
| F3 | Distribution skewness |
| F4 | Distribution kurtosis |
| F5 | Sum of squared coordinate values |
| F6 | Cumulative sum of absolute differences between consecutive observations |
| Statistically Significant Features | r |
|---|---|
| J0_X_F2 | −0.16 * |
| J1_X_F2 | −0.15 * |
| J2_X_F2 | −0.14 * |
| J2_Y_F4 | 0.16 * |
| J5_X_F2 | −0.17 ** |
| J8_X_F2 | −0.17 ** |
| J10_X_F2 | −0.16 * |
| J12_X_F2 | −0.15 * |
| J13_X_F2 | −0.14 * |
| J15_X_F2 | −0.14 * |
| J16_X_F2 | −0.17 * |
| J17_Y_F6 | −0.16 * |
| J18_X_F2 | −0.17 ** |
| J20_X_F6 | 0.14 * |
| J24_X_F2 | −0.13 * |
| Selected Gait Features | Standardized β | Tolerance | VIF |
|---|---|---|---|
| J1_Y_F3 | −0.12 | 0.92 | 1.09 |
| J2_X_F2 | −0.09 | 0.49 | 2.06 |
| J2_X_F4 | 0.11 | 0.88 | 1.13 |
| J2_Y_F4 | 0.09 | 0.90 | 1.12 |
| J4_Y_F3 | −0.04 | 0.91 | 1.10 |
| J5_X_F2 | −0.12 | 0.18 | 5.51 |
| J5_X_F3 | 0.09 | 0.79 | 1.27 |
| J6_Y_F3 | 0.10 | 0.74 | 1.35 |
| J6_Y_F4 | 0.05 | 0.72 | 1.38 |
| J10_X_F6 | −0.08 | 0.81 | 1.23 |
| J12_X_F6 | −0.05 | 0.73 | 1.38 |
| J14_X_F4 | −0.15 | 0.91 | 1.09 |
| J17_Y_F4 | 0.06 | 0.82 | 1.22 |
| J17_Y_F6 | −0.07 | 0.80 | 1.25 |
| J18_X_F2 | −0.06 | 0.18 | 5.69 |
| J19_X_F3 | −0.11 | 0.92 | 1.09 |
| J20_X_F6 | 0.21 | 0.84 | 1.19 |
| J22_X_F4 | −0.08 | 0.92 | 1.09 |
| ML Models | CC (r) | MAE | RMSE | R2 |
|---|---|---|---|---|
| SMO Regression | 0.60 | 3.50 | 4.59 | 0.26 |
| Multilayer Perceptron | 0.46 | 3.73 | 4.73 | 0.22 |
| Bagging | 0.53 | 3.51 | 4.58 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Yang, K. Digital Phenotyping of Sensation Seeking: A Machine Learning Approach Using Gait Analysis. Behav. Sci. 2025, 15, 1222. https://doi.org/10.3390/bs15091222
Li A, Yang K. Digital Phenotyping of Sensation Seeking: A Machine Learning Approach Using Gait Analysis. Behavioral Sciences. 2025; 15(9):1222. https://doi.org/10.3390/bs15091222
Chicago/Turabian StyleLi, Ang, and Keyu Yang. 2025. "Digital Phenotyping of Sensation Seeking: A Machine Learning Approach Using Gait Analysis" Behavioral Sciences 15, no. 9: 1222. https://doi.org/10.3390/bs15091222
APA StyleLi, A., & Yang, K. (2025). Digital Phenotyping of Sensation Seeking: A Machine Learning Approach Using Gait Analysis. Behavioral Sciences, 15(9), 1222. https://doi.org/10.3390/bs15091222






