Theory of Mind Development in Deaf and Hard-of-Hearing Individuals: A Systematic Review
Abstract
1. Introduction
1.1. Theory of Mind
1.2. ToM Development in Typically Hearing Individuals
1.3. Explanations of ToM Development
1.4. ToM Development in DHH Individuals
2. Research Questions of the Systematic Review
3. Method
3.1. Eligibility Criteria
3.2. Information Sources and Search Strategy
3.3. Selection and Data Collection Process
4. Results
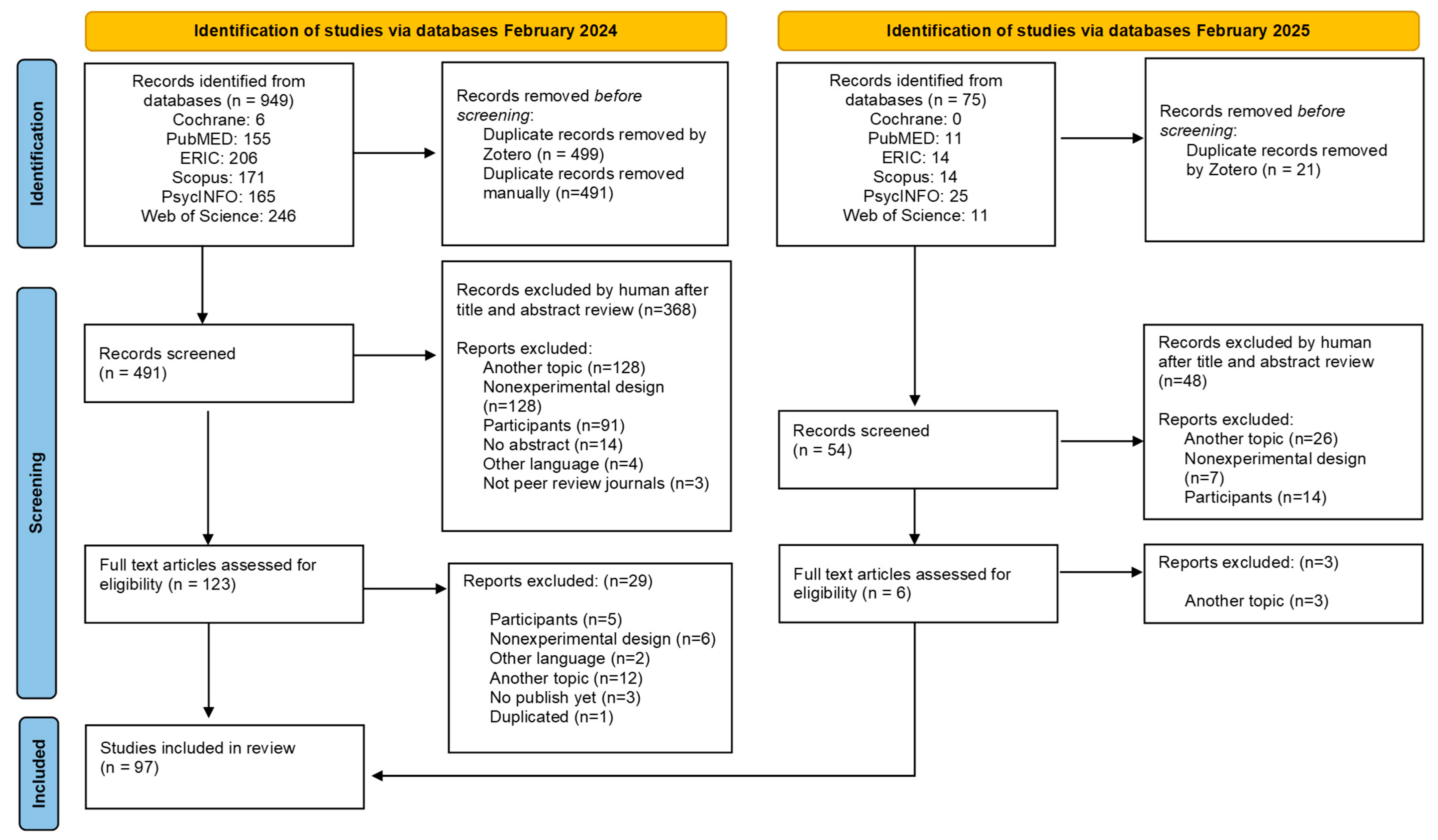
4.1. Search Results
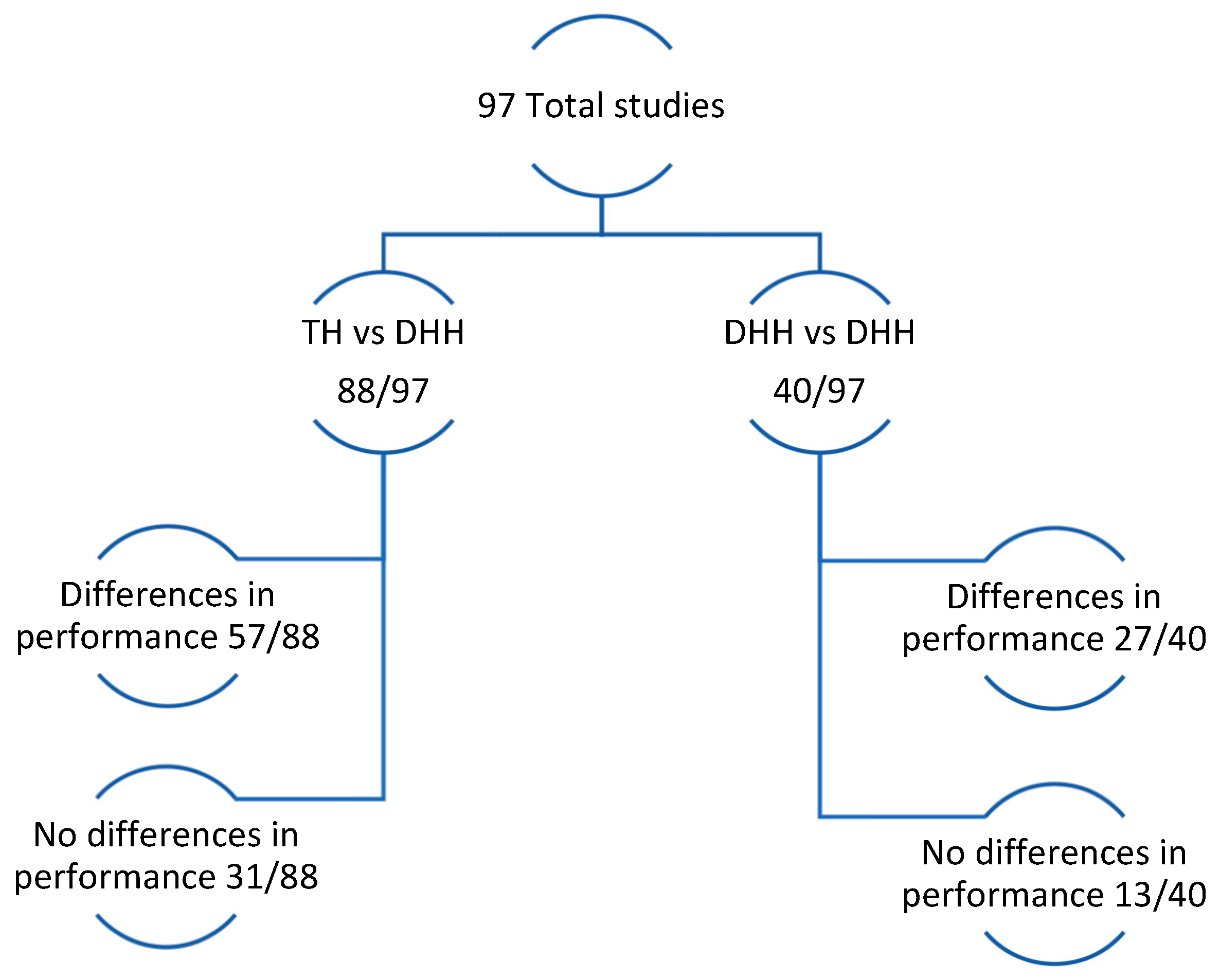
- Research Question 1: Do DHH individuals (Population), compared to a control sample of TH and/or among themselves (Comparator) in an assessment of ToM (Intervention) have differentiated results (Outcomes)?
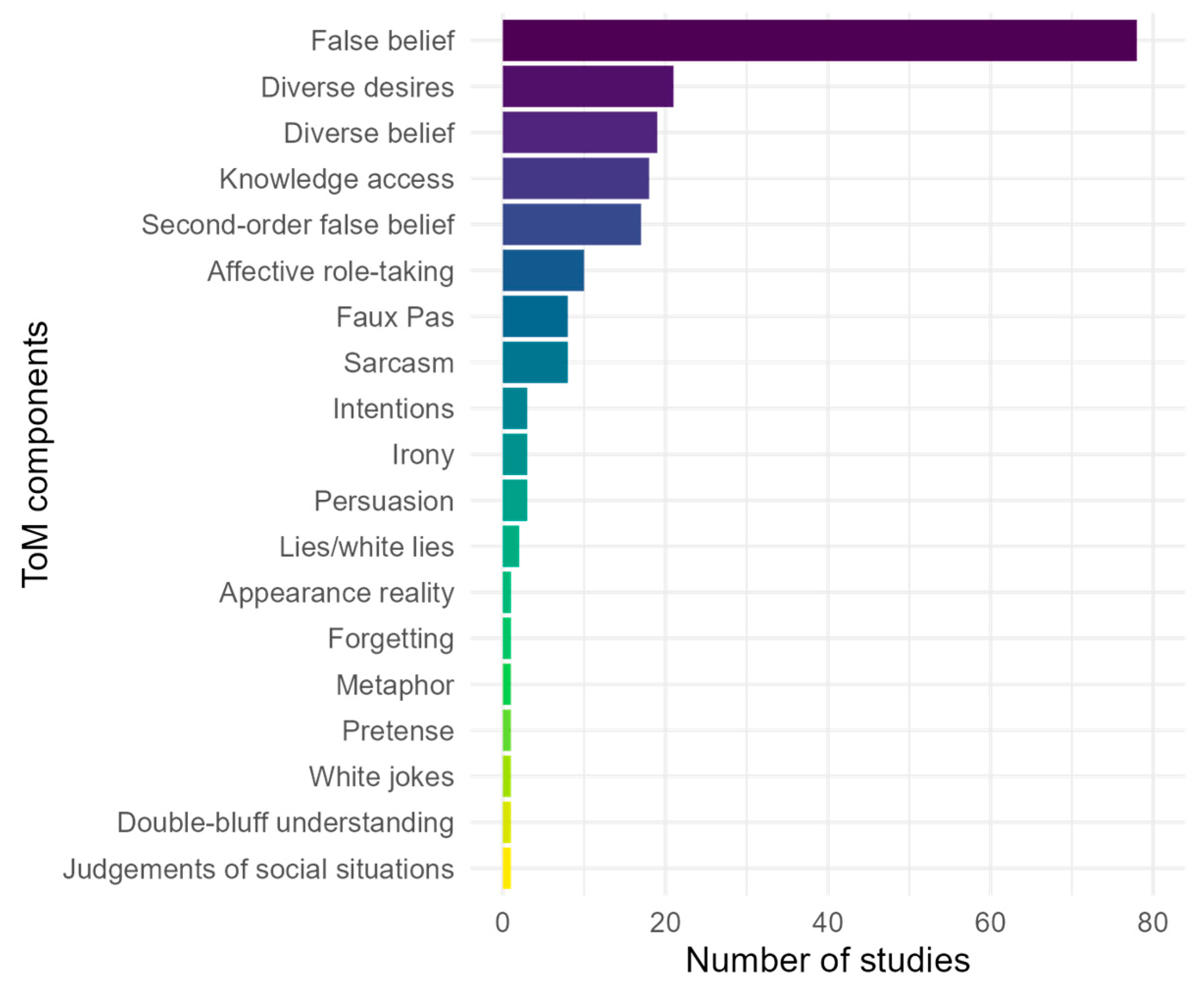
- Research Question 2: What ToM components have been studied in DHH individuals?
4.2. Risk of Bias Assessment
5. Discussion
5.1. ToM Task Performance of DHH Individuals
5.1.1. Quality of Early Interaction
5.1.2. Early Access to Language
5.1.3. Other Variables
5.2. ToM Components Assessed in DHH Individuals
5.3. Limitations of the Review Process
5.4. Conclusions and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Data Extraction
- AuthorsPublication year
- Sample size of typical hearing individualsSample size of typical deaf and hard-of-hearing individualsType of hearing devices used and bilateral/unilateralAge of hearing AccessCommunication modality and languageAge interval
- Risk of BiasToM component assessed and performanceToM taskMain resultsResults from comparing language modalityResults from comparing hearing devicesResults from comparing family hearing statusTask presentationOther conclusionsExecutive functions assessmentLanguage assessmentCommunication/interaction assessmentIQ assessmentOther measures
Appendix B
| Authors | Year | TH | DHH | HD | Family Hearing Status | Age Interval (Years) | ToM Component and Performance | TH vs. DHH | DHH vs. DHH | Quality Papers |
|---|---|---|---|---|---|---|---|---|---|---|
| (Peterson & Siegal, 1995) | 1995 | 0 | 26 | NR | 25 HF 1 DF | 8.1;13 | False belief | TH > DHH | - | *** |
| (Steeds et al., 1997) | 1997 | 0 | 22 | NR | HF | 5.8;12.4 | Diverse desires False belief | TH > DHH | - | *** |
| (Peterson & Siegal, 1998) | 1998 | Ex1—35 Ex2—47 | Ex1—30 Ex2—24 | NR | HF | DHH-5.9;11.10 TH-3.6;4.5 DHH-6.2;12.5 TH-3.6;4.5 | False belief | TH > DHH | - | *** |
| (Russell et al., 1998) | 1998 | 0 | 32 | NR | 30 HF 2 DF | DHH1-4.9;7.9 DHH2-8.9;12.6 DHH3-13.6;16.11 | False belief | TH > DHH | - | *** |
| (Peterson & Siegal, 1999) | 1999 | 21 | 59 | NR | 48 HF 11 DF | DHH-5.6;13.2 TH-3.11;4.4 | False belief | TH = DoDP & DoHP(oral) | DoHP(oral) & DoDP > DoHP(SL) | ** |
| (Courtin, 2000) | 2000 | 39 | 155 | NR | 99 HF 37 DF | DHH-5;8 TH-4;6 | False belief | TH = DoDP | DoDP > DoHP | *** |
| (Marschark et al., 2000) | 2000 | 15 | 15 | NR | HF | DHH-9.7;15.10 TH-10.6;15.5 | False belief (Mental state attribution) | TH > DHH | - | *** |
| (Rhys-Jones & Ellis, 2000) | 2000 | 40 | 34 | NR | 28 HF 6 DF | 6;11 | Comprehension and judgments of social situations | TH > DHH TH (11;16y) = DHH(11;16y) | - | ** |
| (Rieffe & Terwogt, 2000) | 2000 | 85 | 23 | NR | 22 HF 1 DF | 6;10 | Emotion recognition | TH = DHH | - | *** |
| (Figueras-Costa & Harris, 2001) | 2001 | 0 | 21 | 1 CI—NR 20 HA—NR | HF | DHH1-4.5;6.4 DHH2-6.7;11.9 | False belief | TH > DHH | - | ** |
| (Jackson, 2001) | 2001 | 48 | 50 | 5 CI—NR | 46 HF 4 Dfamily | Early SL-5.2;10.10 Late SL-4.10;8.3 SL & Oral-5;12.11 Oral-4.11;11.11 | False belief 2nd-order false belief | TH > DHH | Dfamily > Hfamily EarlyS L > LateSL & oral | *** |
| (Scott et al., 1999) | 1999 | 0 | 22 | NR | 20 HF 2 DF | DHH1-5.1;8.3 DHH2-9.1;12.10 | Diverse desires Diverse intentions | TH > DHH | - | *** |
| (Al-Hilawani et al., 2002) | 2002 | 44 | 28 | NR | NR | 8;11 | Cognitive ToM | TH = DHH | - | *** |
| (Lundy, 2002) | 2002 | 0 | 29 | 9 CI—NR 20 HA—NR | HF | 5;10 | False belief | TH > DHH | DHH(oral) = DHH(SL) | *** |
| (Peterson, 2002) Ex1 Ex2 | 2002 | 25 26 | 21 21 | NR | HF | DHH-6.8;12.6 TH-4.1;5.8 DHH-6.11;12.11 TH-3.10;5.1 | False belief | TH > DHH | - | *** |
| (Woolfe et al., 2002) Ex1 Ex2 | 2002 | 40 0 | 51 39 | NR | 32 HF 19 DF 21 HF 18 DF | DHH-4;8 TH-3;4 | False belief | - | DoDP > DoHP | *** |
| (Rieffe et al., 2003) | 2003 | 67 | 53 | NR | 52 HF 1 DF | DHH-9.2;11.8 TH-9;11.2 | Emotion recognition | TH > DHH | - | *** |
| (Woolfe et al., 2003) | 2003 | 0 | 20 + 20 siblings | NR | 7 Hsibling 13 Dsibling | Hsibling-4;8.6 Dsibling-4.2;16.3 | False belief | - | Dsibling = Hsigling | * |
| (Howley & Howe, 2004) Ex1 Ex2 | 2004 | 10 20 | 10 25 | NR 21 HA—NR | 4 HF 6 DF 20 HF 5 DF | 6.94;8.93 DHH1-5.08;8.42 DHH2-8.42;11.58 | Affective role-taking Perceptual role-taking | TH > DHH TH = DHH | DoHP = DoDP | *** |
| (Peterson, 2004) | 2004 | 17 | 26 | 13 CI—NR 13 HA—NR | HF | 4;12 | False belief | TH > DHH | DHH(bl school) = DHH(oral school) | *** |
| (Terwogt & Rieffe, 2004) | 2004 | 36 | 21 | NR | 20 HF 1 DF | DHH-10.5;12.4 TH-9.8;12.10 | Diverse desires False belief | TH > DHH | - | ** |
| (Courtin & Melot, 2005) | 2005 | 36 | 88 | NR (CI excluded) | 60 HF 28 DF | 5;7 | Appearance-Reality False belief | TH > DHH DoDP >TH | DoDP > DoHP | *** |
| (Peterson et al., 2005) | 2005 | 62 | 47 | NR | 36 HF 11 DF | 3.7;13.7 | Diverse beliefs Diverse desires Knowledge access Hidden emotion False Belief | TH = DHH TH > DoHP | DoDP > DoHP | ** |
| (Falkman & Hjelmquist, 2007) | 2007 | 10 | 10 | 4 CI—NR | 8 HF 2 DF (no SL) | 7.4;11.3 | False belief | TH > DHH | - | * |
| (A. M. González-Cuenca & Quintana García, 2006) | 2006 | 0 | 54 | NR | HF | 6;19 | False belief | TH > DHH | - | *** |
| (Macaulay & Ford, 2006) | 2006 | 0 | 10 | 10 CI—NR | HF | DHH-4.4;11.1 | False belief | TH > DHH | EarlyCI = LateCI | *** |
| (Moeller & Schick, 2006) | 2006 | 26 | 22 | 10 CI—NR 10 HA—BI | HF | DHH-4.25;9.92 TH-4.25;5.92 | False belief (verbal) False belief (Nonverbal) | TH > DHH TH = DHH | - | * |
| (Morgan & Kegl, 2006) | 2006 | 0 | 22 | NR | HF | Early SL-8;24 Late SL-12;39 | False belief | - | EarlySL > LateSL | * |
| (Peterson & Slaughter, 2006) Ex1 Ex2 | 2006 | 13 17 | 21 17 | NR | HF | 6;11 DHH-6;12 TH-4;5 | Knowledge access False belief | TH > DHH | - | ** |
| (Falkman et al., 2007) | 2007 | 10 | 10 | 3 CI—NR | HF | DHH-7;9.6 TH-8;9.6 | Diverse belief False belief | TH > DHH | Wide variability | *** |
| (A. M. González-Cuenca et al., 2007) | 2007 | 0 | 54 | No CI | HF | 6;19 14;19 | False belief | TH > DHH | - | *** |
| (Meristo et al., 2007) Ex1 Ex2 | 2007 | 105 26 | 97 61 | NR | 41 HF 56 DF 37 HF 24 DF | Late SL-6.5;12.9 Native SL-4.6;12.8 TH-3.5;10.8 7.4;16.1 | True belief False belief Emotion Recognition Diverse beliefs Diverse desires False belief 2nd-order false belief Moral reasoning | TH = DoDP(bl school) | DoDP(bl school) > DoDP & DoHP(bl school) | *** |
| (Schick et al., 2007) | 2007 | 42 | 176 | 33 CI—NR 53 HA—NR | 127 HF 49 DF | 4;8.38 | False belief FB Verbal FB Nonverbal Knowledge access | TH = DoDP | DoDP > DoHP | * |
| (A. M. González-Cuenca et al., 2008) | 2008 | 0 | 54 | No CI | HF | 6;19 | False belief 2nd-order false belief | TH > DHH | - | *** |
| (Meristo & Hjelmquist, 2009) | 2009 | 0 | 61 | NR | 37 HF 24 DF | 7.4;16.1 | Emotion Recognition Diverse beliefs Diverse desires False belief 2nd-order false belief Moral reasoning | - | EarlySL(bl school) > EarlySL(oral school) & LateSL(blschool) | *** |
| (Peterson & Wellman, 2009) | 2009 | 60 | 33 | 12 CI—NR | HF | DHH-5.10;13.6 TH-2.8;5.9 | Diverse beliefs Diverse desires False belief Knowledge access Hidden emotion Social pretending | TH > DHH | - | ** |
| (Peters et al., 2009) | 2009 | 0 | 30 | 30 CI—NR | HF | 3.1;12.0 | False belief | - | - | * |
| (Pyers & Senghas, 2009) | 2009 | 0 | 18 | NR | NR | EarlySL-26.8;28.7 LateSL-17.5;19.1 | False belief | - | EarlySL > LateSL | *** |
| (Hao et al., 2010) | 2010 | 32 | 53 | NR | 45 HF 8 DF | 21.5 mean age | Advanced ToM implicit Advanced ToM explicit | TH = DoDP TH > DHH | DoDP > DoHP | ** |
| (Torres & Rodríguez, 2011) | 2011 | 25 | 25 | 10 CI—NR 15 HA—NR | HF | DHH-4.17;16.5 TH-4.5;16.33 | Graphic False belief graphic 2nd-order false belief Standard False belief 2nd-order false belief | TH = DHH TH > DHH | - | *** |
| (Wellman et al., 2011) | 2011 | 61 | 31 | 19 CI—NR | HF | Chinese-3.1;3.11 English-3.1;5 Auslan-4.2;12.8 | Diverse desires Diverse beliefs Knowledge access False belief Hidden emotion | TH > DHH | - | *** |
| (P. A. de Villiers & de Villiers, 2012) | 2012 | 45 | 45 | 25 CI—NR 20 HA—NR | HF | DHH-4.6;7.11 TH-3.1;5.11 | False belief | TH > DHH | - | *** |
| (Ketelaar et al., 2012) | 2012 | 69 | 72 | 72 CIs 2/3 CI—UNI 1/3 CI—BI | HF | 1;6 | Intention Common-desire Uncommon-desire False belief | TH = DHH TH > DHH | - | ** |
| (Lecciso et al., 2012) | 2012 | 17 | 17 | 17 HA—NR | HF | 5.4;4.6 | False belief 2nd-order false belief Mentalistic understanding of non-literal communication Perceptual component of ToM | TH > DHH | - | ** |
| (Levrez et al., 2012) | 2012 | 12 | 12 | 5 CI—NR 7 HA—NR | HF | DHH-9.3;12.2 TH-6.7;8.3 | False belief | TH > DHH | - | ** |
| (Meristo et al., 2012) | 2012 | 10 | 10 | 5 CI—NR 5 HA—NR | HF | DHH-1.5;2.2 TH-1.7;2.3 | False belief True belief | TH > DHH TH = DHH | - | ** |
| (Peterson et al., 2012) | 2012 | 68 | 31 | NR | HF | 3;13 | Diverse beliefs Diverse desires False belief Hidden emotion Knowledge access Sarcasm Understanding | TH > DHH | - | *** |
| (Tomasuolo et al., 2012) | 2012 | 15 | 30 | 22 HA | 23 HF 7 DF | DHH-6.1;14.6 TH-6.6;13.9 | False belief | DHH(bl school) > TH TH > DHH(SL school) | DHH(blschool) > DHH(SLschool) | *** |
| (Macaulay & Ford, 2013) | 2013 | 47 | 48 | 21 CI—NR 27 HA—BI | 46 HF 2 DF | DHH-4;11 TH-3;5 | False belief 2nd-order false belief | TH > DHH | - | ** |
| (Ziv et al., 2013) | 2013 | 23 | 30 | 22 CI—UNI 8 HA—BI | 20 HF 10 DF | 5;7 | Emotion recognition False belief | TH = DHH(oral) TH > DHH(SL) | DHHCI > DHHNoCI | * |
| (Hao & Su, 2014) | 2014 | 25 | 22 | NR | HF | DHH-9;13.11 TH-4;6.11 | Emotion recognition False belief no eye-gaze cues False belief eye-gaze cues | TH = DHH TH > DHH TH = DHH | - | ** |
| (K. O’Reilly et al., 2014) Ex1 Ex2 | 2014 | 39 35 | 42 35 | 17 CI—NR | 32 HF 10 DF 27 HF 8 DF | 5;12 18;69 | False belief 2nd-order false belief Sarcasm | TH > DHH TH(old) = DoDP(old) TH(old) > DoHP(old) | DoDP > DoHP | ** |
| (Sundqvist et al., 2014) | 2014 | 18 | 16 | 14 CI—BI 2 CI—UNI | HF | DHH-4.25;9.5 TH-6;8 | Emotion recognition False belief 2nd-order false belief | TH = EarlyCI TH > LateCI TH > DHH | EarlyCI > LateCI | ** |
| (Jones et al., 2015) | 2015 | 46 | 27 | 14 CI—NR 13 HA—NR | HF 12—signers 15—No signers | DHH-6.7;12.1 TH1-4.5;5.9 TH2-6.1;11.6 | False belief (location) False belief (content) 2nd-order false belief | TH1 = DHH TH2 > DHH | DHH(oral) > DHH(SL) | ** |
| (Peterson, 2016) Ex1 Ex2 | 2016 | 31 21 | 30 35 | 12 CI—NR 18 CI—NR | HF | DHH-5.92;12.92 TH-4.25;10.50 DHH-5.67;13.33 TH-7.33;12.42 | Diverse beliefs Diverse desires Knowledge access False belief Hidden emotion Sarcasm Understanding | TH > DHH | - | *** |
| (Holmer et al., 2016) | 2016 | 0 | 13 | Unclear | 11 HF 2 DF | 7.3;14.10 | Diverse desires Diverse beliefs Knowledge access False belief Hidden emotion | TH > DHH | DoHP = DoDP | ** |
| (Lecciso et al., 2016) | 2016 | 47 | 47 | 4 CI—NR 19 HA—NR | 32 HF 15 DF | 15.9;28.1 | 2nd-order false belief Advanced ToM (misunderstanding of intention, persuasion, white lies, double buff) Mentalistic understanding of non-literal communication | TH = EarlySL TH > DHH(oral & LateSL) TH > DHH(EarlySL, LateSL &oral) | DoDP > DoHP | ** |
| (Meristo et al., 2016) | 2016 | 15 | 15 | 5 CI—BI 3 CI—UNI 7 HA—NR | HF | DHH-CI-3.11;8.5 DHH-HA-4;7.11 TH-4.1;8.11 | False belief spontaneous False belief elicited | TH > DHH TH = DHH CI TH > DHH HA | - | ** |
| (Peterson et al., 2016a) | 2016 | 21 | 36 | 18 CI—NR | HF | DHH-5.67;14.7 TH-7.33; 13.42 | Diverse beliefs Diverse desires Knowledge access False belief Hidden emotion Sarcasm Understanding | TH > DHH | - | *** |
| (Peterson et al., 2016b) | 2016 | 53 | 66 | 29 CI—NR | 54 HF 12 DF | DHH-5.00; 12.08 TH-6.00; 13.42 | Diverse beliefs Diverse desires knowledge access False belief Hidden emotion | TH = DoDP TH > DoHP | DoDP > DoHP | *** |
| (Fujino et al., 2017) | 2017 | 0 | 369 | NR | NR | 4;12 | False belief | TH > DHH | - | *** |
| (Hutchins et al., 2017) | 2017 | 0 | 12 | 11 CI—NR 1 HA—NR | HF | 5.2;11.1 | Early, basic, and advanced skills | TH > DHH | EarlyCI > LateCI | * |
| (Serra et al., 2017) | 2017 | 30 | 10 | NR | NR | DHH-4.5;9 TH-4.5;6 | Emotion recognition Emotion recognition (visual cues) | TH = DHH TH > DHH | - | *** |
| (Walker et al., 2017) | 2017 | 106 | 272 | 0 CI 134 HA—NR | HF | 5;6 | False belief 2nd-order False belief | TH > DHH TH = DHH | - | * |
| (Amraei et al., 2018) | 2018 | 0 | 30 | 30 CI—NR | 15 HF 15 DF | HF-5;8 DF-3;8 | False belief | - | DoDP > DoHP | *** |
| (Liu et al., 2018) | 2018 | 46 | 36 | 36 CI—NR | HF | 3.8;6.9 | Diverse desire False belief | TH > DHH | - | ** |
| (Marschark et al., 2019) | 2019 | 41 | 94 | 46 CI—UNI | 81 HF 9 DF | NR; college students | 2nd-order false belief Double-bluff understanding Sarcasm | TH > DHH | DoHP = DoDP | ** |
| (Peterson & Wellman, 2018a) | 2018 | 37 | 27 | NR | HF | 3;11 | Diverse beliefs Diverse desires Knowledge access False belief Hidden emotion Sarcasm Understanding | TH > DHH | - | *** |
| (Peterson et al., 2018) Ex1 | 2018 | 43 | 28 | NR | HF | DHH-5.25;11.17 TH1-7.25;10.17 TH2-3.83;5.00 | False belief Diverse beliefs Diverse desires Knowledge access False belief Hidden emotion Persuasion Sarcasm | TH > DHH | - | *** |
| (Peterson & Wellman, 2018b) | 2018 | 44 | 31 | 17 CI—NR | HF | DHH-5.83;12.42 TH-3.08;5.00 | False belief (prediction) False belief (explanation) | TH = DHH TH > DHH | - | ** |
| (Smogorzewska et al., 2018) | 2018 | 243 | 268 | 74 CI—NR 145 HA—NR | HF | DHH-5.11;10.11 TH-5.11;12.0 | Diverse desires Diverse beliefs False belief Knowledge access Content false belief Hidden emotion Faux pas | TH > DHH | - | *** |
| (Aslıer et al., 2020) | 2020 | 82 | 77 | 77 CI—UNI | HF | 3;9 | False belief | TH > DHH | EarlyHD > LateHD | * |
| (de Gracia et al., 2020) Ex1 Ex2 | 2020 | 150 0 | 59 42 | NR NR | HF 41 HF 1 DF | DHH-8;14.58 TH1-8.75;14.83 TH2-3.33; 7.92 DHH-15.25;22.17 TH1-8.75;14.83 TH2-3.33;7.92 | Diverse desires Diverse beliefs Knowledge access Content false belief Hidden emotion | TH > DHH | - | ** |
| (Edwards et al., 2021) | 2021 | 38 | 74 | 26 CI—NR 6 CI + HA | NR | 18;24 | Sarcasm Metaphor Inference making Tasks | TH > DHH | - | * |
| (Figueroa et al., 2020) | 2020 | 54 | 36 | 3 HA + CI 10 CI—BI 23 CI—UNI | HF | DHH-14.03;21 TH-13.5;18 | Affective ToM: False belief Cognitive ToM: False belief | TH = DHH TH > DHH | EarlyCI > LateCI BICI > UNICI | * |
| (A. González-Cuenca & Linero, 2020) | 2020 | 38 | 58 | 20 CI-UNI | HF | 10;19 | False belief 2nd-order belief Selfish and white lies Critical irony | TH > DHH | DHHCI = DHHNoCI | *** |
| (Meristo & Strid, 2020) | 2020 | 24 | 22 | 7 CI—BI 6 CI—UNI | 22 HF 24 DF | 1.5;8.9 | False belief | TH = DoDP TH > DoHP | DoDP > DoHP | *** |
| (Sidera et al., 2020) | 2020 | 75 | 54 | 22 CI—NR 30 HA—NR | 8 DF 24 Drelative | DHH-3.4;8.9 TH-3.2;8.9 | Emotion recognition (understanding pretend emotions) | TH > DHH | DHHCI = DHHHA | ** |
| (Yu et al., 2021) | 2020 | 0 | 84 | 12 CI—UNI 16 CI—BI 56 HA—BI | HF | 3;6 | Diverse desires Diverse beliefs Social pretend Knowledge access False belief | - | EarlyHD > LateHD | * |
| (Delkhah et al., 2021) | 2021 | 36 | 36 | 36 CI—NR | NR | 5;9 | Basic ToM skills Advanced ToM skills | TH > DHH | - | ** |
| (Panzeri et al., 2021) | 2021 | 56 | 28 | 20 CI—BI 8 CI + HA | HF | DHH-4;12 TH1-5;12 TH2-3;10 | Emotion recognition False belief 2nd-order false belief Irony | TH > DHH TH1 > DHH TH2 = DHH TH > DHH | - | * |
| (Pluta et al., 2021) | 2021 | 94 | 45 | 29 CI—BI 7 CI—UNI 9 CI + HA | HF | DHH-3.25;8.25 TH-3.25;7.25 | False belief | TH(4;5y) > DHH(4;5y) TH(6;8) = DHH(6;8) | - | * |
| (Siriattakul et al., 2021) | 2021 | 0 | 200 | NR | 190 HF 10 DF | 8;15 | False belief | TH > DHH | DoDP > DoHP | *** |
| (Smogorzewska & Osterhaus, 2021) | 2021 | 237 | 234 | Unclear | NR | 7.5;9.5 | 2nd-order false belief Advanced ToM: Faux pas | TH > DHH | - | *** |
| (Blose & Schenkel, 2022) | 2022 | 144 | 104 | 23 CI—NR | 13 DF 24 Dsibling 67 Dfamily | 21 mean age | Affective ToM | TH > DoHP | DoDP > DoHP | *** |
| (Durrleman et al., 2022) | 2022 | 0 | 21 | 9 CI—BI 2 CI—UNI 5 CI—HA 5 HA—NR | HF | 5.9;11 | False belief | TH > DHH | - | *** |
| (Figueroa et al., 2022) | 2022 | 0 | 8 | 4 CI—BI 4 CI—UNI | HF | 10;14 | Diverse desires Diverse beliefs Knowledge access Content false belief Hidden emotion Cognitive ToM: False belief Affective ToM: Faux pas | - | EarlyCI > LateCI | * |
| (Smit et al., 2022) | 2022 | 34 | 14 | NR | 11 HF 3 DF | NR | Lies White joke Pretense Persuasion Appearance/reality misunderstanding Forgetting Double bluff Contrary emotions Irony | TH > DHH | - | *** |
| (Akkaya & Doğan, 2023) | 2023 | 100 | 100 | 33 HA—NR 68 CI—NR | NR | 4;5.9 | Emotion recognition False belief | TH = DHH TH (5y) = DHH (5y) TH (5.5y) > DHH (5.5y) | EarlyHD > LateHD | ** |
| (Choi & Jeong, 2023) | 2023 | 50 | 50 | 11 HA + CI 39 CI—BI | HF | 2;12 | Early skills Basic skills Advanced skills | TH = DHH TH > DHH | - | * |
| (Pluta et al., 2023) | 2023 | 52 | 39 | 39 CI—BI | HF | DHH-2.9;7.8 TH-3;5.8 | Early ToM Basic ToM Advanced ToM Combined ToM | TH > DHH | - | *** |
| (Tuohimaa et al., 2023) | 2023 | 45 | 86 | 22 CI—BI 19 HA—BI | HF | 4;4.9 | Contextual Inference with/without ToM demand: (Include feeling recognition and false belief) | TH > DHH | EarlyHD = LateHD | * |
| (Meristo et al., 2024) | 2024 | 18 | 12 | 8 CI—BI 5 CI—UNI | HF | 3.9;8.4 | False belief (verbal) False belief (Nonverbal) | TH = DHH TH > DHH | - | ** |
| (Remmel & Peters, 2009) | 2009 | 30 | 30 | 29 CI—UNI 1 CI—BI | HF | DHH-3.1;12 TH-4.5;6.4 | Diverse desires Diverse beliefs Knowledge access False belief Hidden emotion Nonverbal False belief Real apparent Emotion | TH = DHH | EarlyCI > LateCI | * |
| (Serrat et al., 2024) | 2024 | 25 | 22 | 7 CI—BI 2 CI—UNI 2 CI + HA 11 HA—BI | HF | DHH-8;12 TH-8;12 | False belief 2nd-order false belief | TH > DHH | - | ** |
| (Tuohimaa et al., 2025) | 2025 | 65 | 54 | 29 CI—BI 25 HA—BI | HF | 6.0;6.11 | Contextual Inference with/without ToM demand: (Include feeling recognition and false belief) | TH > DHH | DHHCI = DHHHA | * |
| (Chu, 2025) | 2025 | 53 | 57 | 31 CI—NR 26 HA—NR | HF | 4.6;5.75 | Diverse desires Diverse beliefs Knowledge access Content false belief Hidden emotion | TH = DHH TH = DHH HA TH > DHH CI TH > DHH | MHL > SHL | * |
References
- Akkaya, E., & Doğan, M. (2023). Emotion recognition and false belief in deaf or hard-of-hearing preschool children *. Journal of Deaf Studies and Deaf Education, 29(2), 134–144. [Google Scholar] [CrossRef]
- Al-Hilawani, Y. A., Easterbrooks, S. R., & Marchant, G. J. (2002). Metacognitive ability from a theory-of-mind perspective: A cross-cultural study of students with and without hearing loss *. American Annals of the Deaf, 147(4), 38–47. [Google Scholar] [CrossRef]
- Amraei, K., Azizi, M., Khoshkhabar, A., & Soori, H. (2018). The role of parental hearing status in theory of mind after cochlear implant surgery *. Indian Journal of Otology, 24(3), 157–161. [Google Scholar] [CrossRef]
- Aslıer, M., Aslıer, N. G. Y., Kirkim, G., & Güneri, E. A. (2020). The influence of age and language on developmental trajectory of theory of mind in children with cochlear implants *. International Journal of Pediatric Otorhinolaryngology, 135, 110127. [Google Scholar] [CrossRef] [PubMed]
- Aydın, U., & Özgeldi, M. (2020). Unpacking the roles of metacognition and theory of mind in Turkish undergraduate students’ academic achievement: A test of two mediation models. Croatian Journal of Education, 21(4), 1333–1398. [Google Scholar] [CrossRef]
- Baker, J. K., & Crnic, K. A. (2009). Thinking about feelings: Emotion focus in the parenting of children with early developmental risk. Journal of Intellectual Disability Research, 53(5), 450–462. [Google Scholar] [CrossRef]
- Baldwin, D. A. (2000). Interpersonal understanding fuels knowledge acquisition. Current Directions in Psychological Science, 9(2), 40–45. [Google Scholar] [CrossRef]
- Baron-Cohen, S., Leslie, A. M., & Frith, U. (1985). Does the autistic child have a “theory of mind”? Cognition, 21(1), 37–46. [Google Scholar] [CrossRef]
- Baron-Cohen, S., O’Riordan, M., Stone, V., Jones, R., & Plaisted, K. (1999). Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders, 29(5), 407–418. [Google Scholar] [CrossRef]
- Baron-Cohen, S., & Ring, H. (1994). A model of the mindreading system: Neuropsychological and neurobiological perspectives. In C. Lewis, & P. Mitchell (Eds.), Children’s early understanding of mind: Origins and development (pp. 183–207). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Beaudoin, C., Leblanc, É., Gagner, C., & Beauchamp, M. H. (2020). Systematic review and inventory of theory of mind measures for young children. Frontiers in Psychology, 10, 2905. [Google Scholar] [CrossRef]
- Benson, G., Abbeduto, L., Short, K., Nuccio, J. B., & Maas, F. (1993). Development of a theory of mind in individuals with mental retardation. American Journal of Mental Retardation, 98(3), 427–433. [Google Scholar]
- Bernier, A., Lapolice-Thériault, R., Matte-Gagné, C., & Cyr, C. (2023). Paternal mind-mindedness and children’s academic achievement: Investigating developmental processes. Developmental Psychology, 59(4), 758–769. [Google Scholar] [CrossRef] [PubMed]
- Blose, B. A., & Schenkel, L. S. (2022). Theory of mind and alexithymia in deaf and hard-of-hearing young adults *. Journal of Deaf Studies and Deaf Education, 27(2), 179–192. [Google Scholar] [CrossRef]
- Bosacki, S., & Astington, J. W. (1999). Theory of mind in preadolescence: Relations between social understanding and social competence. Social Development, 8(2), 237–255. [Google Scholar] [CrossRef]
- Callaghan, T., Rochat, P., Lillard, A., Claux, M. L., Odden, H., Itakura, S., Tapanya, S., & Singh, S. (2005). Synchrony in the onset of mental-state reasoning: Evidence from five cultures. Psychological Science, 16(5), 378–384. [Google Scholar] [CrossRef]
- Caputi, M., Lecce, S., Pagnin, A., & Banerjee, R. (2012). Longitudinal effects of theory of mind on later peer relations: The role of prosocial behavior. Developmental Psychology, 48(1), 257–270. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y. M., & Jeong, S. W. (2023). Theory of mind in children with cochlear implants: Comparison with age- and sex-matched children with normal hearing *. American Journal of Otolaryngology, 44(2), 103693. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y. (2025). Listening beyond words: Understanding theory of mind in hearing-impaired children with different hearing loss degree *. Speech, Language and Hearing, 28(1), 1–10. [Google Scholar] [CrossRef]
- Courtin, C. (2000). The impact of sign language on the cognitive development of deaf children: The case of theories of mind *. Journal of Deaf Studies and Deaf Education, 5(3), 266–276. [Google Scholar] [CrossRef] [PubMed]
- Courtin, C., & Melot, A.-M. (2005). Metacognitive development of deaf children: Lessons from the appearance-reality and false Belief tasks *. Developmental Science, 8(1), 16–25. [Google Scholar] [CrossRef]
- de Gracia, M. R. L., de Rosnay, M., Hawes, D., & Perez, M. V. T. (2020). Deafness and theory of mind performance: Associations among Filipino children, adolescents, and young adults *. Journal of Cognition and Development, 21(3), 326–347. [Google Scholar] [CrossRef]
- Delkhah, Z., Farmani, H. R., & Soleymani, Z. (2021). Language predictors of theory of mind in cochlear implant children compared to normal-hearing peers *. Auditory and Vestibular Research, 30(3), 200–208. [Google Scholar] [CrossRef]
- Dettman, S. J., Pinder, D., Briggs, R. J., Dowell, R. C., & Leigh, J. R. (2007). Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear and Hearing, 28(2 Suppl.), 11S–18S. [Google Scholar] [CrossRef]
- de Villiers, J. G., & de Villiers, P. A. (2014). The role of language in theory of mind development. Topics in Language Disorders, 34(4), 313–328. [Google Scholar] [CrossRef]
- de Villiers, J. G., Hobbs, K., & Hollebrandse, B. (2014). Recursive complements and propositional attitudes. In T. Roeper, & M. Speas (Eds.), Recursion: Complexity in cognition (pp. 183–202). Springer. [Google Scholar] [CrossRef]
- de Villiers, P. A., & de Villiers, J. G. (2012). Deception dissociates from false belief reasoning in deaf children: Implications for the implicit versus explicit theory of mind distinction *. The British Journal of Developmental Psychology, 30(Pt 1), 188–209. [Google Scholar] [CrossRef] [PubMed]
- Devine, R. T., & Hughes, C. (2019). Let’s talk: Parents’ mental talk (not mind-mindedness or mindreading capacity) predicts children’s false Belief understanding. Child Development, 90(4), 1236–1253. [Google Scholar] [CrossRef]
- Durrleman, S., Dumont, A., & Delage, H. (2022). Syntactic strategy training for theory of mind in deaf children *. Journal of Deaf Studies and Deaf Education, 27(1), 89–100. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L., Marschark, M., Kronenberger, W. G., Crowe, K., & Walton, D. (2021). Inferencing abilities of deaf college students: Foundations and implications for metaphor comprehension and theory of mind *. Journal of Developmental and Physical Disabilities, 33(2), 233–258. [Google Scholar] [CrossRef]
- Ensor, R., & Hughes, C. (2008). Content or connectedness? Mother-child talk and early social understanding. Child Development, 79(1), 201–216. [Google Scholar] [CrossRef]
- Faistauer, M., Silva, A. L., Dominguez, D. O. R., Bohn, R., Félix, T. M., Costa, S. S. D., & Rosito, L. P. S. (2022). Does universal newborn hearing screening impact the timing of deafness treatment? Jornal de Pediatria, 98(2), 147–154. [Google Scholar] [CrossRef] [PubMed]
- Falkman, K. W., & Hjelmquist, E. (2007). Do you see what I mean? Shared reference in non-native, early signing deaf children *. Journal of Deaf Studies and Deaf Education, 12(4), 460–472. [Google Scholar] [CrossRef]
- Falkman, K. W., Roos, C., & Hjelmquist, E. (2007). Mentalizing skills of non-native, early signers: A longitudinal perspective *. European Journal of Developmental Psychology, 4(2), 178–197. [Google Scholar] [CrossRef]
- Farrar, M. J., & Maag, L. (2002). Early language development and the emergence of a theory of mind. First Language, 22(2), 197–213. [Google Scholar] [CrossRef]
- Figueras-Costa, B., & Harris, P. (2001). Theory of mind development in deaf children: A nonverbal test of false-belief understanding *. The Journal of Deaf Studies and Deaf Education, 6(2), 92–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figueroa, M., Bayés, G., Darbra, S., & Silvestre, N. (2022). Reading and theory of mind during the primary-secondary educational transition: A multiple case study in pupils with a cochlear implant *. Reading Psychology, 44(5), 463–483. [Google Scholar] [CrossRef]
- Figueroa, M., Darbra, S., & Silvestre, N. (2020). Reading and theory of mind in adolescents with cochlear implant *. Journal of Deaf Studies and Deaf Education, 25(2), 212–223. [Google Scholar] [CrossRef]
- Fu, I. N., Chen, K. L., Liu, M. R., Jiang, D. R., Hsieh, C. L., & Lee, S. C. (2024). A systematic review of measures of theory of mind for children. Developmental Review, 67, 101061. [Google Scholar] [CrossRef]
- Fujino, H., Fukushima, K., & Fujiyoshi, A. (2017). Theory of mind and language development in Japanese children with hearing loss *. International Journal of Pediatric Otorhinolaryngology, 96, 77–83. [Google Scholar] [CrossRef]
- González-Cuenca, A., & Linero, M. J. (2020). Lies and irony understanding in deaf and hearing adolescents *. Journal of Deaf Studies and Deaf Education, 25(4), 517–529. [Google Scholar] [CrossRef]
- González Cuenca, A. M., Barajas Esteban, C., Linero Zamorano, M. J., & Quintana García, I. (2008). Deficiencia auditiva y teoría de la mente. Datos para la reflexión y la intervención *. Revista de Logopedia, Foniatría y Audiología, 28(2), 99–116. [Google Scholar] [CrossRef]
- González-Cuenca, A. M., & Quintana García, I. (2006). Deficiencia auditiva y teoría de la mente: El efecto del formato de la tarea sobre la comprensión de la falsa creencia en niños y adolescentes sordos *. Infancia y Aprendizaje, 29(4), 471–484. [Google Scholar] [CrossRef]
- González-Cuenca, A. M., Quintana-García, I., Barajas-Esteban, M. C., & Linero-Zamorano, M. J. (2007). The role of age and oral lexical competence in false belief understanding by children and adolescents with hearing loss *. The Volta Review, 107(2), 123–139. [Google Scholar] [CrossRef]
- Gopnik, A., & Slaughter, V. (1991). Young children’s understanding of changes in their mental states. Child Development, 62(1), 98–110. [Google Scholar] [CrossRef]
- Gordon, S. A., Waltzman, S. B., & Friedmann, D. R. (2022). Delayed cochlear implantation in congenitally deaf children: Identifying barriers for targeted interventions. International Journal of Pediatric Otorhinolaryngology, 155, 111086. [Google Scholar] [CrossRef]
- Hall, W. C., Smith, S. R., Sutter, E. J., DeWindt, L. A., & Dye, T. D. V. (2018). Considering parental hearing status as a social determinant of deaf population health: Insights from experiences of the “dinner table syndrome”. PLoS ONE, 13(9), e0202169. [Google Scholar] [CrossRef]
- Hao, J., & Su, Y. (2014). Deaf children’s use of clear visual cues in mindreading *. Research in Developmental Disabilities, 35(11), 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Hao, J., Su, Y., & Chan, R. C. (2010). Do deaf adults with limited language have advanced theory of mind *? Research in Developmental Disabilities, 31(6), 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Happé, F. G. (1994). An advanced test of theory of mind: Understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders, 24(2), 129–154. [Google Scholar] [CrossRef]
- Hollebrandse, B., van Hout, A., & Hendriks, P. (2014). Children’s first and second-order false-Belief reasoning in a verbal and a low-verbal task. Synthese, 191(3), 321–333. [Google Scholar] [CrossRef]
- Holmer, E., Heimann, M., & Rudner, M. (2016). Theory of mind and reading comprehension in deaf and hard-of-hearing signing children *. Frontiers in Psychology, 7, 854. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D., Dall, M., Sanduvete-Chaves, S., Saldaña, D., Chacón-Moscoso, S., & Fellinger, J. (2020). The impact of family environment on language development of children with cochlear implants: A systematic review and meta-analysis. Ear and Hearing, 41(5), 1077–1091. [Google Scholar] [CrossRef]
- Houston, D. M., Stewart, J., Moberly, A., Hollich, G., & Miyamoto, R. T. (2012). Word learning in deaf children with cochlear implants: Effects of early auditory experience. Developmental Science, 15(3), 448–461. [Google Scholar] [CrossRef] [PubMed]
- Howley, M., & Howe, C. (2004). Social interaction and cognitive growth: An examination through the role-taking skills of deaf and hearing children *. British Journal of Developmental Psychology, 22(3), 219–243. [Google Scholar] [CrossRef]
- Hutchins, T. L., Allen, L., & Schefer, M. (2017). Using the theory of mind inventory to detect a broad range of theory of mind challenges in children with hearing loss: A pilot study *. Deafness & Education International, 19(1), 2–12. [Google Scholar] [CrossRef]
- Jackson, A. L. (2001). Language facility and theory of mind development in deaf children *. Journal of Deaf Studies and Deaf Education, 6(3), 161–176. [Google Scholar] [CrossRef]
- Johnson, A. S., Nelson, M., Rosa Lugo, L. I., Maul, T., & Pritchett, C. V. (2025). The effect of family structure on the cochlear implant experience of children. Perspectives of the ASHA Special Interest Groups, 10, 829–836. [Google Scholar] [CrossRef]
- Jones, A. C., Gutierrez, R., & Ludlow, A. K. (2015). Confronting the language barrier: Theory of mind in deaf children *. Journal of Communication Disorders, 56, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C., Morgan, G., Bannard, C., & Matthews, D. (2020). Early pragmatics in deaf and hard of hearing infants. Pediatrics, 146(Suppl. 3), S262–S269. [Google Scholar] [CrossRef]
- Ketelaar, L., Rieffe, C., Wiefferink, C. H., & Frijns, J. H. (2012). Does hearing lead to understanding? Theory of mind in toddlers and preschoolers with cochlear implants *. Journal of Pediatric Psychology, 37(9), 1041–1050. [Google Scholar] [CrossRef]
- Krysztofiak, M., & Pluta, A. (2021). Theory of mind development in deaf children with cochlear implants: Literature review. Journal of Hearing Science, 11(2), 9–18. [Google Scholar] [CrossRef]
- Lecciso, F., Levante, A., Baruffaldi, F., & Petrocchi, S. (2016). Theory of mind in deaf adults *. Cogent Psychology, 3(1), 1264127. [Google Scholar] [CrossRef]
- Lecciso, F., Petrocchi, S., & Marchetti, A. (2012). Hearing mothers and oral deaf children: An atypical relational context for theory of mind *. European Journal of Psychology of Education, 28(3), 903–922. [Google Scholar] [CrossRef]
- Lederberg, A. R., Schick, B., & Spencer, P. E. (2013). Language and literacy development of deaf and hard-of-hearing children: Successes and challenges. Developmental Psychology, 49(1), 15–30. [Google Scholar] [CrossRef] [PubMed]
- Levrez, C., Bourdin, B., Le Driant, B., D’Arc, B. F., & Vandromme, L. (2012). The impact of verbal capacity on theory of mind in deaf and hard of hearing children *. American Annals of the Deaf, 157(1), 66–77. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A. M., Hatrak, M., & Mayberry, R. I. (2014). Learning to look for language: Development of joint attention in young deaf children. Language Learning and Development, 10(1), 19–35. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A. M., Mitchiner, J., & Pontecorvo, E. (2022). Hearing parents learning American Sign Language with their deaf children: A mixed-methods survey. Applied Linguistics Review, 15(1), 309–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, M., Wu, L., Wu, W., Li, G., Cai, T., & Liu, J. (2018). The relationships among verbal ability, executive function, and theory of mind in young children with cochlear implants *. International Journal of Audiology, 57(12), 881–888. [Google Scholar] [CrossRef]
- Lundy, J. E. (2002). Age and language skills of deaf children in relation to theory of mind development *. Journal of Deaf Studies and Deaf Education, 7(1), 41–56. [Google Scholar] [CrossRef]
- Macaulay, C. E., & Ford, R. M. (2006). Language and theory-of-mind development in prelingually deafened children with cochlear implants: A preliminary investigation *. Cochlear Implants International, 7(1), 1–14. [Google Scholar] [CrossRef]
- Macaulay, C. E., & Ford, R. M. (2013). Family influences on the cognitive development of profoundly deaf children: Exploring the effects of socioeconomic status and siblings *. Journal of Deaf Studies and Deaf Education, 18(4), 545–562. [Google Scholar] [CrossRef]
- Marschark, M., Edwards, L., Peterson, C., Crowe, K., & Walton, D. (2019). Understanding theory of mind in deaf and hearing college students *. Journal of Deaf Studies and Deaf Education, 24(2), 104–118. [Google Scholar] [CrossRef] [PubMed]
- Marschark, M., Green, V., Hindmarsh, G., & Walker, S. (2000). Understanding theory of mind in children who are deaf *. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 41(8), 1067–1073. [Google Scholar] [CrossRef]
- McConkey Robbins, A., Koch, D. B., Osberger, M. J., Zimmerman-Phillips, S., & Kishon-Rabin, L. (2004). Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Archives of Otolaryngology–Head & Neck Surgery, 130(5), 570–574. [Google Scholar] [CrossRef]
- Meadow-Orlans, K. P., & Steinberg, A. G. (1993). Effects of infant hearing loss and maternal support on mother-infant interactions at 18 months. Journal of Applied Developmental Psychology, 14(3), 407–426. [Google Scholar] [CrossRef]
- Meltzoff, A. N. (1995). Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology, 31(5), 838–850. [Google Scholar] [CrossRef] [PubMed]
- Meristo, M., Falkman, K. W., Hjelmquist, E., Tedoldi, M., Surian, L., & Siegal, M. (2007). Language access and theory of mind reasoning: Evidence from deaf children in bilingual and oralist environments. Developmental Psychology, 43(5), 1156–1169. [Google Scholar] [CrossRef]
- Meristo, M., & Hjelmquist, E. (2009). Executive functions and theory-of-mind among deaf children: Different routes to understanding other minds *? Journal of Cognition and Development, 10(1–2), 67–91. [Google Scholar] [CrossRef]
- Meristo, M., Morgan, G., Geraci, A., Iozzi, L., Hjelmquist, E., Surian, L., & Siegal, M. (2012). Belief attribution in deaf and hearing infants *. Developmental Science, 15(5), 633–640. [Google Scholar] [CrossRef] [PubMed]
- Meristo, M., & Strid, K. (2020). Language first: Deaf children from deaf families spontaneously anticipate false beliefs *. Journal of Cognition and Development, 21, 622–630. [Google Scholar] [CrossRef]
- Meristo, M., Strid, K., & Hjelmquist, E. (2016). Early conversational environment enables spontaneous belief attribution in deaf children *. Cognition, 157, 139–145. [Google Scholar] [CrossRef]
- Meristo, M., Surian, L., & Strid, K. (2024). False belief understanding in deaf children: What are the difficulties *? Frontiers in Psychology, 15, 1238505. [Google Scholar] [CrossRef]
- Milligan, K., Astington, J. W., & Dack, L. A. (2007). Language and theory of mind: Meta-analysis of the relation between language ability and false-belief understanding. Child Development, 78(2), 622–646. [Google Scholar] [CrossRef]
- Mitchell, R. E., & Karchmer, M. A. (2004). When parents are deaf versus hard of hearing: Patterns of sign use and school placement of deaf and hard-of-hearing children. Journal of Deaf Studies and Deaf Education, 9(2), 133–152. [Google Scholar] [CrossRef]
- Moeller, M. P., & Schick, B. (2006). Relations between maternal input and theory of mind understanding in deaf children *. Child Development, 77(3), 751–766. [Google Scholar] [CrossRef]
- Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., Currie, M., Qureshi, R., Mattis, P., Lisy, K., & Mu, P. F. (2020). Systematic reviews of etiology and risk. In E. Aromataris, & Z. Munn (Eds.), JBI manual for evidence synthesis. JBI. Available online: https://synthesismanual.jbi.global (accessed on 27 July 2025).
- Morgan, G., & Kegl, J. (2006). Nicaraguan Sign Language and theory of mind: The issue of critical periods and abilities *. Journal of Child Psychology and Psychiatry, 47(8), 811–819. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G., Meristo, M., Mann, W., Hjelmquist, E., Surian, L., & Siegal, M. (2014). Mental state language and quality of conversational experience in deaf and hearing children. Cognition, 132(2), 169–180. [Google Scholar] [CrossRef]
- O’Reilly, J., & Peterson, C. C. (2014). Theory of mind at home: Linking authoritative and authoritarian parenting styles to children’s social understanding *. Early Child Development and Care, 184(12), 1934–1947. [Google Scholar] [CrossRef]
- O’Reilly, K., Peterson, C. C., & Wellman, H. M. (2014). Sarcasm and advanced theory of mind understanding in children and adults with prelingual deafness. Developmental Psychology, 50(7), 1862–1877. [Google Scholar] [CrossRef]
- Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan—A web and mobile app for systematic reviews. Systematic Reviews, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., A Akl, E., E Brennan, S., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. [Google Scholar] [CrossRef]
- Panzeri, F., Cavicchiolo, S., Giustolisi, B., Di Berardino, F., Ajmone, P. F., Vizziello, P., Donnini, V., & Zanetti, D. (2021). Irony comprehension in children with cochlear implants: The role of language competence, theory of mind, and prosody recognition *. Journal of Speech, Language, and Hearing Research, 64(8), 3212–3229. [Google Scholar] [CrossRef]
- Perner, J., Leekam, S., & Wimmer, H. (1987). Three-year-olds’ difficulty with false Belief: The case for a conceptual deficit. British Journal of Developmental Psychology, 5(2), 125–137. [Google Scholar] [CrossRef]
- Perner, J., & Wimmer, H. (1985). “John thinks that Mary thinks that …”: Attribution of second-order Beliefs by 5- to 10-year-old children. Journal of Experimental Child Psychology, 39(3), 437–471. [Google Scholar] [CrossRef]
- Peters, K., Remmel, E., & Richards, D. (2009). Language, mental state vocabulary, and false belief understanding in children with cochlear implants *. Language, Speech, and Hearing Services in Schools, 40(3), 245–255. [Google Scholar] [CrossRef]
- Peterson, C. C. (2002). Drawing insight from pictures: The development of concepts of false drawing and false belief in children with deafness, normal hearing, and autism *. Child Development, 73(5), 1442–1459. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C. C. (2004). Theory-of-mind development in oral deaf children with cochlear implants or conventional hearing aids *. Journal of Child Psychology and Psychiatry and Allied Disciplines, 45(6), 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C. C. (2016). Empathy and theory of mind in deaf and hearing children *. Journal of Deaf Studies and Deaf Education, 21(2), 141–147. [Google Scholar] [CrossRef]
- Peterson, C. C., O’Reilly, K., & Wellman, H. M. (2016a). Deaf and hearing children’s development of theory of mind, peer popularity, and leadership during middle childhood *. Journal of Experimental Child Psychology, 149, 146–158. [Google Scholar] [CrossRef]
- Peterson, C. C., & Siegal, M. (1995). Deafness, conversation and theory of mind *. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 36(3), 459–474. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C. C., & Siegal, M. (1998). Changing focus on the representational mind: Deaf, autistic and normal children’s concepts of false photos, false drawings and false beliefs *. British Journal of Developmental Psychology, 16(3), 301–320. [Google Scholar] [CrossRef]
- Peterson, C. C., & Siegal, M. (1999). Representing inner worlds: Theory of mind in autistic, deaf, and normal hearing children *. Psychological Science, 10(2), 126–129. [Google Scholar] [CrossRef]
- Peterson, C. C., & Slaughter, V. P. (2006). Telling the story of theory of mind: Deaf and hearing children’s narratives and mental state understanding *. British Journal of Developmental Psychology, 24(1), 7–31. [Google Scholar] [CrossRef]
- Peterson, C. C., Slaughter, V., Moore, C., & Wellman, H. M. (2016b). Peer social skills and theory of mind in children with autism, deafness, or typical development *. Developmental Psychology, 52(1), 46–57. [Google Scholar] [CrossRef]
- Peterson, C. C., Slaughter, V., & Wellman, H. M. (2018). Nimble negotiators: How theory of mind (ToM) interconnects with persuasion skills in children with and without ToM delay *. Developmental Psychology, 54(3), 494–509. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C. C., & Wellman, H. M. (2009). From fancy to reason: Scaling deaf and hearing children’s understanding of theory of mind and pretence *. The British Journal of Developmental Psychology, 27(Pt 2), 297–310. [Google Scholar] [CrossRef]
- Peterson, C. C., & Wellman, H. M. (2018a). Longitudinal theory of mind (ToM) development from preschool to adolescence with and without ToM delay *. Child Development, 90(6), 1917–1934. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C. C., & Wellman, H. M. (2018b). Explaining the unpredictable: The development of causal theories of mind in deaf and hearing children *. Child Development, 90(6), e654–e674. [Google Scholar] [CrossRef]
- Peterson, C. C., Wellman, H. M., & Liu, D. (2005). Steps in theory-of-mind development for children with deafness or autism *. Child Development, 76(2), 502–517. [Google Scholar] [CrossRef]
- Peterson, C. C., Wellman, H. M., & Slaughter, V. (2012). The mind behind the message: Advancing theory-of-mind scales for typically developing children, and those with deafness, autism, or Asperger syndrome *. Child Development, 83(2), 469–485. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A., Krysztofiak, M., Zgoda, M., Wysocka, J., Golec, K., Gajos, K., Dołyk, T., Wolak, T., & Haman, M. (2023). Theory of mind and parental mental-state talk in children with Cis *. Journal of Deaf Studies and Deaf Education, 28(3), 288–299. [Google Scholar] [CrossRef]
- Pluta, A., Krysztofiak, M., Zgoda, M., Wysocka, J., Golec, K., Wójcik, J., Włodarczyk, E., & Haman, M. (2021). False belief understanding in deaf children with cochlear implants *. Journal of Deaf Studies and Deaf Education, 26(4), 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pons, F., Harris, P. L., & de Rosnay, M. (2004). Emotion comprehension between 3 and 11 years: Developmental periods and hierarchical organization. European Journal of Developmental Psychology, 1(2), 127–152. [Google Scholar] [CrossRef]
- Pontecorvo, E., Mitchiner, J., & Lieberman, A. M. (2024). Hearing parents as sign language learners: Describing and evaluating the ASL skills of parents learning ASL with their deaf children. Journal of Multilingual and Multicultural Development, 45, 1–18. [Google Scholar] [CrossRef]
- Poulin-Dubois, D., & Yott, J. (2018). Probing the depth of infants’ theory of mind: Disunity in performance across paradigms. Developmental Science, 21(4), e12600. [Google Scholar] [CrossRef]
- Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences, 1(4), 515–526. [Google Scholar] [CrossRef]
- Pyers, J. E., & Senghas, A. (2009). Language promotes false-Belief understanding: Evidence from learners of a new sign language *. Psychological Science, 20(7), 805–812. [Google Scholar] [CrossRef]
- Remmel, E., & Peters, K. (2009). Theory of mind and language in children with cochlear implants *. Journal of Deaf Studies and Deaf Education, 14(2), 218–236. [Google Scholar] [CrossRef]
- Rhys-Jones, S. L., & Ellis, H. D. (2000). Theory of mind: Deaf and hearing children’s comprehension of picture stories and judgments of social situations *. Journal of Deaf Studies and Deaf Education, 5(3), 248–265. [Google Scholar] [CrossRef][Green Version]
- Rieffe, C., & Terwogt, M. M. (2000). Deaf children’s understanding of emotions: Desires take precedence *. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 41(5), 601–608. [Google Scholar] [CrossRef]
- Rieffe, C., Terwogt, M. M., & Smit, C. (2003). Deaf children on the causes of emotions *. Educational Psychology: An International Journal of Experimental Educational Psychology, 23(2), 159–168. [Google Scholar] [CrossRef]
- Ruffman, T., Slade, L., & Crowe, E. (2002). The relation between children’s and mothers’ mental state language and theory-of-mind understanding. Child Development, 73(3), 734–751. [Google Scholar] [CrossRef] [PubMed]
- Russell, P. A., Hosie, J. A., Gray, C. D., Scott, C., Hunter, N., Banks, J. S., & Macaulay, M. C. (1998). The development of theory of mind in deaf children *. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 39(6), 903. [Google Scholar] [CrossRef]
- Saif-Ur-Rahman, K. M., Hasan, M., Hossain, S., Anwar, I., Hirakawa, Y., & Yatsuya, H. (2022). Prioritization and sequential exclusion of articles in systematic reviews. Campbell Systematic Reviews, 18(2), e1229. [Google Scholar] [CrossRef]
- Sanju, H. K., Jain, T., & Kumar, P. (2022). Is early cochlear implantation leads to better speech and language outcomes? Indian Journal of Otolaryngology and Head & Neck Surgery, 74(Suppl. 3), 3906–3910. [Google Scholar] [CrossRef]
- Schick, B., de Villiers, P., de Villiers, J., & Hoffmeister, R. (2007). Language and theory of mind: A study of deaf children *. Child Development, 78(2), 376–396. [Google Scholar] [CrossRef]
- Scott, C., Russell, P. A., Gray, C. D., Hosie, J. A., & Hunter, N. (1999). The interpretation of line of regard by prelingually deaf children *. Social Development, 8(1), 65–84. [Google Scholar] [CrossRef]
- Seidenfeld, A. M., Johnson, S. R., Cavadel, E. W., & Izard, C. E. (2014). Theory of mind predicts emotion knowledge development in Head Start children. Early Education and Development, 25(7), 933–948. [Google Scholar] [CrossRef]
- Serra, A., Spinato, G., Cocuzza, S., Licciardello, L., Pavone, P., & Maiolino, L. (2017). Adaptive psychological structure in childhood hearing impairment: Audiological correlations *. Acta Otorhinolaryngologica Italica, 37(3), 175–179. [Google Scholar] [CrossRef]
- Serrat, E., Amadó, A., Durrleman, S., Intxaustegi, A., & Sidera, F. (2024). Expressive syntax matters for second-order false belief: A study with hearing-impaired children *. Frontiers in Communication, 9, 1401576. [Google Scholar] [CrossRef]
- Shahaeian, A., Peterson, C. C., Slaughter, V., & Wellman, H. M. (2011). Culture and the sequence of steps in theory of mind development. Developmental Psychology, 47(5), 1239–1247. [Google Scholar] [CrossRef]
- Sidera, F., Morgan, G., & Serrat, E. (2020). Understanding pretend emotions in children who are deaf and hard of hearing *. Journal of Deaf Studies and Deaf Education, 25(2), 141–152. [Google Scholar] [CrossRef]
- Siriattakul, P., Suttiwan, P., Slaughter, V., & Peterson, C. C. (2021). Theory of Mind (ToM) development in Thai deaf children *. Journal of Deaf Studies and Deaf Education, 26(2), 241–250. [Google Scholar] [CrossRef]
- Smit, L., Knoors, H., Rabeling-Keus, I., Verhoeven, L., & Vissers, C. (2022). Measuring theory of mind in adolescents with language and communication problems: An ecological perspective *. Frontiers in Psychology, 13, 761434. [Google Scholar] [CrossRef]
- Smogorzewska, J., & Osterhaus, C. (2021). Advanced theory of mind in children with mild intellectual disability and deaf or hard of hearing children: A two-year longitudinal study in middle childhood *. The British Journal of Developmental Psychology, 39(4), 603–624. [Google Scholar] [CrossRef]
- Smogorzewska, J., Szumski, G., & Grygiel, P. (2018). Same or different? Theory of mind among children with and without disabilities *. PLOS ONE, 13(10), e0202553. [Google Scholar] [CrossRef]
- Sobel, D. M., & Austerweil, J. L. (2016). Coding choices affect the analyses of a false belief measure. Cognitive Development, 40, 9–23. [Google Scholar] [CrossRef]
- Spencer, P., & Lederberg, A. (1997). Different modes, different models: Communication and language of young deaf children and their mothers. In L. Adamson, & M. Romski (Eds.), Research on communication and language disorders: Contributions to theories of language development (pp. 203–230). Paul Brookes. [Google Scholar]
- Steeds, L., Rowe, K., & Dowker, A. (1997). Deaf children’s understanding of beliefs and desires *. Journal of Deaf Studies and Deaf Education, 2(3), 185–195. [Google Scholar] [CrossRef]
- Sundqvist, A., Lyxell, B., Jönsson, R., & Heimann, M. (2014). Understanding minds: Early cochlear implantation and the development of theory of mind in children with profound hearing impairment *. International journal of pediatric otorhinolaryngology, 78(3), 537–543. [Google Scholar] [CrossRef]
- Terwogt, M. M., & Rieffe, C. (2004). Deaf children’s use of beliefs and desires in negotiation *. Journal of Deaf Studies and Deaf Education, 9(1), 27–38. [Google Scholar] [CrossRef][Green Version]
- Tomasello, M. (2018). How children come to understand false beliefs: A shared intentionality account. Proceedings of the National Academy of Sciences, 115(34), 8491–8498. [Google Scholar] [CrossRef]
- Tomasuolo, E., Valeri, G., Di Renzo, A., Pasqualetti, P., & Volterra, V. (2012). Deaf children attending different school environments: Sign language abilities and theory of mind *. Journal of Deaf Studies and Deaf Education, 18(1), 12–29. [Google Scholar] [CrossRef]
- Torres, J., & Rodríguez, I. R. (2011). La comprensión de falsa creencia en niños y adolescentes sordos: Tareas gráficas versus clásicas *. Journal for the Study of Education and Development, 34(1), 31–47. [Google Scholar] [CrossRef]
- Tuohimaa, K., Loukusa, S., Löppönen, H., Aarnisalo, A. A., Dietz, A., Hyvärinen, A., Laitakari, J., Rimmanen, S., Salonen, J., Sivonen, V., Tennilä, T., Tsupari, T., Vikman, S., Virokannas, N., Hautala, J., Tolonen, A. K., Välimaa, T., & Kunnari, S. (2025). Factors associated with social-pragmatic understanding in deaf and hard of hearing and typically hearing 6-year-old children *. Journal of Speech, Language, and Hearing Research, 68(2), 808–826. [Google Scholar] [CrossRef] [PubMed]
- Tuohimaa, K., Loukusa, S., Löppönen, H., Välimaa, T., & Kunnari, S. (2023). Development of social-pragmatic understanding in children with congenital hearing loss and typical hearing between the ages of 4 and 6 years *. Journal of Speech, Language, and Hearing Research, 66(7), 2503–2520. [Google Scholar] [CrossRef]
- Vaish, A., Hepach, R., & Grossmann, T. (2018). Desire understanding in 2-year-old children: An eye-tracking study. Infant Behavior & Development, 52, 22–31. [Google Scholar] [CrossRef]
- Walker, E. A., Ambrose, S. E., Oleson, J., & Moeller, M. P. (2017). False belief development in children who are hard of hearing compared with peers with normal hearing *. Journal of Speech, Language, and Hearing Research, 60(12), 3487–3506. [Google Scholar] [CrossRef]
- Wellman, H. M., Cross, D., & Watson, J. (2001). Meta-analysis of theory-of-mind development: The truth about false belief *. Child Development, 72(3), 655–684. [Google Scholar] [CrossRef] [PubMed]
- Wellman, H. M., Fang, F., & Peterson, C. C. (2011). Sequential progressions in a theory-of-mind scale: Longitudinal perspectives *. Child Development, 82(3), 780–792. [Google Scholar] [CrossRef]
- Wellman, H. M., & Liu, D. (2004). Scaling of theory-of-mind tasks. Child Development, 75(2), 523–541. [Google Scholar] [CrossRef]
- Wellman, H. M., & Woolley, J. D. (1990). From simple desires to ordinary beliefs: The early development of everyday psychology. Cognition, 35(3), 245–275. [Google Scholar] [CrossRef] [PubMed]
- Westby, C., & Robinson, L. (2014). A developmental perspective for promoting theory of mind. Topics in Language Disorders, 34(4), 362–382. [Google Scholar] [CrossRef]
- Wille, B., Van Lierde, K., & Van Herreweghe, M. (2019). Parental strategies used in communication with their deaf infants. Child Language Teaching and Therapy, 35(2), 165–183. [Google Scholar] [CrossRef]
- Wimmer, H., & Perner, J. (1983). Beliefs about Beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition, 13(1), 103–128. [Google Scholar] [CrossRef]
- Woolfe, T., Want, S. C., & Siegal, M. (2002). Signposts to development: Theory of mind in deaf children *. Child Development, 73(3), 768–778. [Google Scholar] [CrossRef]
- Woolfe, T., Want, S. C., & Siegal, M. (2003). Siblings and Theory of Mind in deaf native signing children *. Journal of Deaf Studies and Deaf Education, 8(3), 340–347. [Google Scholar] [CrossRef]
- Yoshinaga-Itano, C., Sedey, A. L., Mason, C. A., Wiggin, M., & Chung, W. (2020). Early intervention, parent talk, and pragmatic language in children with hearing loss. Pediatrics, 146(3), 270–277. [Google Scholar] [CrossRef]
- Yoshinaga-Itano, C., Sedey, A. L., Wiggin, M., & Chung, W. (2017). Early hearing detection and vocabulary of children with hearing loss. Pediatrics, 140(2), e20162964. [Google Scholar] [CrossRef]
- Yu, C. L., Stanzione, C. M., Wellman, H. M., & Lederberg, A. R. (2021). Theory-of-mind development in young deaf children with early hearing provisions *. Psychological Science, 32(1), 109–119. [Google Scholar] [CrossRef] [PubMed]
- Yu, C. L., & Wellman, H. M. (2023). Where do differences in theory of mind development come from? An agent-based model of social interaction and theory of mind. Frontiers in Developmental Psychology, 1, 1237033. [Google Scholar] [CrossRef]
- Ziv, M., Most, T., & Cohen, S. (2013). Understanding of emotions and false beliefs among hearing children versus deaf children *. Journal of Deaf Studies and Deaf Education, 18(2), 161–174. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria | Priority |
|---|---|---|
| Target population: DHH prelingual individuals with no developmental comorbidity. Any or no amplification Any modality of communication | Participants: No DHH participants in the study Presence of developmental comorbidity | 7 |
| Papers measuring ToM performance during ToM assessment | Another topic: Topics not concerned to the main question. Experiment does not assess ToM or participants ToM task performance Papers that assess cognitive skills or social skills possibly related to ToM but not ToM abilities | 6 |
| Only papers published in peer-reviewed journals | Not peer-reviewed articles No abstract available Duplication | 4 1 2 |
| Papers with experimental research, quantitative results. Cross-sectional studies, between-groups design, and longitudinal studies | Nonexperimental design: Opinion papers, case studies, literature or systematic review. Treatment/Training studies will be included if pre-treatment/training TOM results are included | 5 |
| Papers written in English or Spanish | Languages other than English or Spanish | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, L.; Figueroa, M.; de Diego-Lázaro, B.; Balboa-Castells, R.; Morgan, G. Theory of Mind Development in Deaf and Hard-of-Hearing Individuals: A Systematic Review. Behav. Sci. 2025, 15, 1065. https://doi.org/10.3390/bs15081065
Martín L, Figueroa M, de Diego-Lázaro B, Balboa-Castells R, Morgan G. Theory of Mind Development in Deaf and Hard-of-Hearing Individuals: A Systematic Review. Behavioral Sciences. 2025; 15(8):1065. https://doi.org/10.3390/bs15081065
Chicago/Turabian StyleMartín, Leire, Mario Figueroa, Beatriz de Diego-Lázaro, Raquel Balboa-Castells, and Gary Morgan. 2025. "Theory of Mind Development in Deaf and Hard-of-Hearing Individuals: A Systematic Review" Behavioral Sciences 15, no. 8: 1065. https://doi.org/10.3390/bs15081065
APA StyleMartín, L., Figueroa, M., de Diego-Lázaro, B., Balboa-Castells, R., & Morgan, G. (2025). Theory of Mind Development in Deaf and Hard-of-Hearing Individuals: A Systematic Review. Behavioral Sciences, 15(8), 1065. https://doi.org/10.3390/bs15081065





