Abstract
Subtle loss of functionality in healthy older adults is considered one of the most important predictors of cognitive decline. Neurocognitive interventions are increasingly being used, from a preventive maintenance approach to functional capacity. This study evaluates the effectiveness of different neurocognitive approaches on the functionality of healthy older adults. In this systematic review (CRD42023473944), an extensive search was conducted for articles published in the last 10 years (2013–2023) in the following databases: Medline, Scopus, and Web of Science. A total of 809 trials were identified, of which 18 were considered to be eligible for inclusion in the review. The data revealed heterogeneity in sample size, measures of functional assessment, neurocognitive interventions used, number of sessions, session duration, and time. Traditional cognitive stimulation is shown to have no significant functional benefit, while other less commonly used neurocognitive interventions, such as those based on everyday cognition, are associated with more significant benefits. Moreover, it is demonstrated that although the Instrumental Activities of Daily Living scale (IADL) is the most used test in similar studies, it is not sensitive enough to detect changes in functionality in healthy elderly individuals, with other tests such as the Timed Instrumental Activities of Daily Living (TIADL) being more advantageous. Therefore, a new guideline is proposed for its use in clinical practice and research, using homogeneous study protocols and neurocognitive interventions that allow for the transfer and generalization of results in daily life.
1. Introduction
Population aging is one of the greatest health achievements, with life expectancy now reaching 72.8 years in developed countries—seven years less in less developed countries—and projected to rise to 77.2 years by 2050. However, this demographic shift is not only a triumph but also poses sustainability challenges for health and social systems [1]. In response, the World Health Organization (WHO) has published the Global Report on Ageing and Health, which asserts that healthy aging is essential to enjoying a long life of high quality, emphasizing the maintenance of health, safety, and participation in daily activities that are key to adding years to life [2].
Healthy aging is described as the functional capacity that enables well-being and is determined by intrinsic capacity (the combination of physical and mental capacity) and the physical, social, and political environment. Therefore, a multi-factorial analysis of aging that comprehends the synergy between different components is essential [2,3]. Several studies [4,5] aim to define the domains of the construct that offer a framework for guiding clinical practice and research regarding intrinsic functional capacity.
Older people with altered cognitive function are more likely to lose functional capacity and develop dementia [6]. Loss of autonomy in basic (ADLs) and Instrumental Activities of Daily Living (IADLs) is so significant that it is the discriminator between cognitively healthy older adults and a diagnosis of mild cognitive impairment or dementia [7]. It has been demonstrated that subtle functional changes precede the diagnosis of dementia by 10 to 12 years [8]. Thus, participation in everyday activities could serve as a predictor of cognitive function and, therefore, a predictor of the presence or absence of cognitive decline. One factor to consider in the daily and cognitive functioning of older adults is the cognitive reserve hypothesis. This explains why some older adults are able to maintain their cognitive abilities despite the presence of age-related brain changes or illnesses.
Furthermore, an increasing number of experts advocate for a preventive cognitive approach aimed at healthy adult individuals. The main neurocognitive intervention proposals used are “cognitive training”, “cognitive rehabilitation”, and “cognitive stimulation” [9]. Although these concepts are becoming increasingly differentiated in scientific literature, they are used interchangeably in clinical practice and healthcare, leading to confusion. Cognitive training implements guided practice of routine tasks (either individually or in groups) that reflect specific cognitive functions such as memory, attention, or problem-solving, with the aim of improving or maintaining the functioning of a particular domain [10]. Cognitive rehabilitation is a patient-centered approach that aims to identify and address the individual needs and goals of people with already established cognitive impairments, with the ultimate objective of improving everyday functioning. Cognitive stimulation involves exposure to and participation in activities and materials that require a certain degree of cognitive processing. It is usually delivered in a group setting and is more flexible than cognitive training, as it does not have specific therapeutic goals and takes into account other concepts such as social participation and affectivity [11]. Cognitive stimulation is the most commonly used concept in clinical practice [12].
The scientific literature has determined that training specific cognitive domains is effective in improving concrete cognitive functions such as working memory [13], inhibition, cognitive flexibility, selective attention [14], processing speed [15], and quality of life [16]. However, the heterogeneity of intervention programs and assessment measures remains a problem. As Tardif et al. state in a review, there is no consensus in this regard. The Mini-Mental State Examination (MMSE) remains the most used test for assessing cognitive function, despite evidence indicating its lack of sensitivity to individual differences such as level of education, age, or high-functioning individuals. This study suggests that the MoCA test is more suitable for evaluating cognitive function [17]. In addition, the number of trials on the subject remains limited, with poor methodology and lacking participant follow-up [18]. The most commonly used test in scientific literature for assessing instrumental activities of daily living is the Lawton and Brody Index, which was published in 1969. The test directly asks the person or their primary caregiver about their abilities. This test evaluates a person’s ability to use the telephone, shop, prepare food, take care of the house, do laundry, use transportation, take medication responsibly, and manage money on a scale of 0 (total dependence) to 8 (total independence).
Other more novel approaches to the use of cognitive training are new technologies, such as computer-based cognitive interventions (CCIs) [19], as they can be beneficial for preservation or improvement in healthy people or in the initial stages of cognitive disease, adding motivation and ease of adaptation. Another example is the meta-analysis conducted by Son and colleagues [20], which confirmed among its most relevant results that cognitive training based on virtual reality is a useful tool for improving IADL performance and is therefore ecologically valid.
However, the major limitation of neurocognitive intervention studies remains the problem of transferring and generalizing results to everyday functioning [21]. A meta-analysis presented in 2017 by Kim et al. on cognitive stimulation, which identified 7354 articles, highlights among its results that only three of them measured ADLs and IADLs, and none of them showed any significant benefit, coinciding with other studies that state that the main difficulty in determining the impact of cognitive interventions on everyday functioning is that they do not include measures of functional outcomes [22].
Due to the aforementioned limitations, various neurocognitive intervention proposals are being considered, including those that draw on everyday cognition, a term that refers to the capacity for solving complex cognitive problems in real-world settings. While it is true that recent research has focused on the role of fundamental cognitive skills in everyday cognitive performance [23], as demonstrated by Farias et al., who identified episodic memory, executive function, and spatial skills as the primary determinants of functional everyday skills [24], an expanding body of research [25,26,27] proposes direct intervention for everyday cognition. The 2021 RCT carried out by Sánchez et al. indicates that a daily cognitive training program has more significant advantages (in terms of global cognitive performance and everyday cognition) compared to traditional cognitive training.
Based on the above, it is necessary to conduct a comprehensive review assessing the effectiveness of various neurocognitive interventions for maintaining or improving functionality in older adults without cognitive impairment. To date, no review or meta-analysis has explored this topic. Therefore, the aim of this review is to provide a useful tool that guides research and clinical practice in selecting a cognitive training method for healthy older adults to improve or maintain daily functionality and, thus, enhance quality of life.
2. Materials and Methods
2.1. Protocol and Registration
The present systematic review was conducted following the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, version 2020 [28]. For further details, please refer to Supplementary Materials File S1. The protocol was previously registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42023473944.
2.2. Eligibility Criteria
This research question was: “Which neurocognitive intervention approach is most effective in enhancing or maintaining functionality in healthy older adults?” The search and eligibility criteria were established through the PICO strategy [29]:
Population: The target population comprised individuals aged 60 years or older, without any diagnoses of neurodegenerative or psychiatric diseases, who participated in various neurocognitive intervention proposals.
Intervention: Any neurocognitive intervention program applied to healthy older adults as a preventive approach to functional capacity loss was eligible.
Comparison: The comparison group pertains to control groups that do not receive any intervention (passive) or receive a different treatment (active), such as usual treatments, educational talks, other interventions that differ from cognitive, etc.
Outcome: The outcome analyzed should include as one of this study variables the impact of the neurocognitive intervention on the improvement or maintenance of functioning. As there is no scientific consensus on the use of a specific questionnaire to assess functionality, it was decided to include all those standardized instruments that are frequently used in similar studies.
Scientific articles published in the last decade, from January 2013 to September 2023, in either English or Spanish were selected for analysis. Experimental clinical trials were accepted, which investigated how different proposals for neurocognitive intervention act to improve and/or maintain functionality in older adults without a diagnosis of neurodegenerative and/or psychiatric disease as one of this study variables. Exclusion criteria include pilot studies or studies without results, clinical trials without human subjects, studies with participants who were not older adults and/or did not have a clinical diagnosis of neurodegenerative and/or psychiatric disease, and studies without an objective measure of function. Studies will not be accepted if the experimental group does not receive neurocognitive intervention, either alone or combined with other pharmacological interventions (such as the use of supplements or additional medication) or non-pharmacological interventions (such as physical intervention).
2.3. Search Strategy
The search in the databases were conducted between July and September 2023. Medline (through Pubmed), Scopus, and Web of Science. Additionally, completed clinical trials of interest with published results were reviewed on ClinicalTrials.gov. Medical Subject Headings (MeSHs) terms used were: cognitive training, problem-solving, functional status, activities of daily living, elderly, and frail. To enhance the search sensitivity and address the ambiguity surrounding the topic in scientific literature, we incorporated additional keywords, namely functionality, cognitive stimulation, practical problem-solving, everyday problem-solving, everyday cognition, and older adults. These keywords were combined using the Boolean operators OR/AND to form the final search strategy. (“Cognitive training” OR “problem-solving” OR “cognitive stimulation” OR “practical problem solving” OR “everyday problem solving” OR “everyday cognition”) AND (“functional status” OR “activities of daily living” OR “functionality”) AND (“aged” OR “frail elderly” OR “older adults”). The search strategies in all online databases can be seen in Table 1.

Table 1.
Search Strategy in the Databases.
2.4. Selection and Data Collection Processes
Authors S.S.G. and C.S.G. independently applied a process of eligibility for studies based on titles and abstracts; disagreements were discussed; and a third reviewer, E.J.F.R., was consulted when there was a lack of consensus. S.S.G. retrieved the full texts of the selected articles. Data extraction was performed by S.S.G. following the following sequence: author and year, study title, study design, sample size, age (mean and standard deviation), test/s used for functional assessment and assessment phases, description of the intervention (experimental group), passive or active group (control group/s), sessions (number, time, and duration), outcomes of interest, and quality of the studies. The characteristics of the selected studies are shown in Table 2.

Table 2.
Characteristics of the selected studies.
2.5. Risk of Bias
The Physiotherapy Evidence Database (PEDro) scale [30] was applied to assess the risk of bias in the included studies. Two authors, S.S.G. and C.S.G., independently reviewed and scored the included articles according to this scale. The same authors then shared the scores and discussed them item by item. When no consensus was reached, a third author, E.J.F.R., was invited to rank them to make a final decision.
3. Results
3.1. Literature Search and Study Selection
The search yielded 809 articles for potential inclusion in the review. After removing pre-screened studies due to duplication (n = 246) and those flagged as illegible by automation tools (n = 1), the identification search resulted in 562 articles. Initial screening was carried out based on title and abstract, leaving 210 articles. Of these 210 articles retrieved for evaluation, 49 were discarded, resulting in 161 publications assessed for eligibility. Of these, 56 were not healthy older adults, 25 were not part of a clinical trial, 5 did not report outcomes, 15 did not measure function, 10 did not include neurocognitive interventions, 20 combined other physical treatments, 6 included pharmacological treatments, 5 combined other treatments, and 1 was retracted. Finally, 18 articles were selected for review. The previous selection process is outlined in the PRISMA flow diagram (Figure 1).
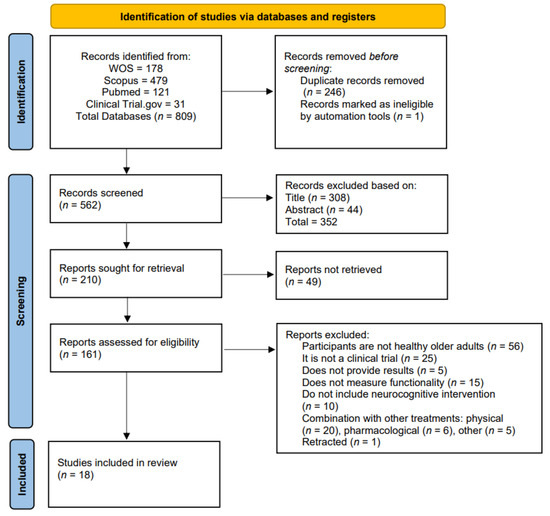
Figure 1.
PRISMA 2020 flow diagram of systematic reviews.
The quality of the selected studies, as assessed by the PEDro scale, indicates high quality, with an average score of 9.05 out of 11 across all articles.
3.2. Characteristics of Included Studies
In total, the 18 selected studies were published between 2014 and 2023. The total sample included 7131 individuals over the age of 60 without a clinical diagnosis of cognitive impairment. In some studies [31,32,33], older adults had subjective complaints of memory loss. The mean age of the included studies was 72 (±5) years. The studies presented widely varying figures regarding sample size, with an average size of 440 (±978). Among the studies, the smallest sample consisted of 17 participants, while the largest sample included 2912 individuals [31].
Regarding functional assessment, twelve tests were used to evaluate functionality within the selected studies. Timed Instrumental Activities of Daily Living (TIADL) [13,34,35,36], The Everyday Problem Test (EPT) [13,35,37], The Activities of Daily Living Questionnaire (ADL) [31], the Chinese version of the Observed Task of Daily Living (OTDL-C) [38], and the Clinical Dementia Rating-Sum of Boxes (CDR-SOB) [32] are all commonly used in assessing cognitive function in older adults. The IADL scale [31,39,40,41,42,43], ECB test [26,27], Barthel Index [42], OTDL tasks [37], Chula ADL index [44], EPCCE [25], and DAFS [25] were utilized. The most employed were the IADL scale and its timed version, the TIADL.
All studies included in the analysis conducted a pre- and post-intervention assessment, except for one study [37] that did not include a post-test assessment but instead conducted assessments at later time points (1–3, 5, and 10 years). Out of the included studies, five conducted follow-ups at 3 months, six at 6 months, one at 9 months, and four at 12 months. One study [35] conducted a pretest assessment, another assessment at 16 weeks, and a final assessment at the end of this study. One study [35] conducted a pretest assessment, another assessment at 16 weeks, and a final assessment at the end of this study.
Regarding the intervention of the experimental and control groups, there is significant variation between studies. The main cognitive interventions for the experimental group include cognitive stimulation programs [27,33,39,40,41,44], as well as specific programs focusing on cognitive domains such as attention [32,34], memory [33,36,37,38], and working memory [13,35], processing speed [13,34], reasoning [25,37,38], or problem-solving [39], multi-component cognitive intervention [31], and everyday cognition [26,27]. The control group had six articles with passive control, twelve with active control, and two without intervention in the control group. For the active control group, interventions consisted of alternative or habitual activities, puzzles, educational tasks, standard cognitive stimulation, memory exercises, music therapy, and mind-motor training.
On average, participants attended 28,25 (±35.24) sessions, with 5 sessions being the minimum and 144 being the maximum. Each session lasted between 30 and 120 min, with a duration ranging from 3 weeks to 9 months.
To conclude this section on functional outcomes, twelve studies show beneficial results for various neurocognitive interventions on functionality [13,25,26,27,31,34,35,36,37,38,39,41], while six studies find no significant differences [32,33,40,42,43,44].
4. Discussion
Active aging approaches are essential in preventing functional capacity loss and, consequently, dementia in older adults. The current systematic review aims to assess the efficacy of various neurocognitive intervention proposals on functionality. A total of 18 high-quality studies with 7131 participants were selected. The results are relevant for guiding clinical practice and research on the use of neurocognitive interventions in healthy older adults, from a preventive approach to maintaining functional status. The data suggest a high degree of heterogeneity between studies in terms of sample size, functional measures used, intervention sessions (number, timing, and duration), and outcomes.
4.1. Main Measures of Functional Assessment
The most commonly used scale is the Instrumental Activities of Daily Living (IADL) scale. This outcome is unsurprising as it remains the most frequently used in research, despite its demonstrated low sensitivity and biases related to gender, culture, and socio-economic level [45]. Moreover, studies have confirmed that there is no correlation between the most commonly used cognitive tests in older adults and the IADL scale [46].
A relevant finding is the association between this test and the results obtained in the selected studies, as four out of the six articles in which it was used did not show significant benefits in the main variable. The second most commonly used test was the timed version of the Instrumental Activities of Daily Living (TIADL), which showed significant effects on the primary variable in four out of five studies, making it a more sensitive measure of functionality.
4.2. Main Neurocognitive Interventions
Traditional cognitive stimulation remains the most utilized neurocognitive intervention. However, four out of the five studies that utilized it did not find significant benefits in functionality. This suggests, in agreement with similar studies [47], that cognitive stimulation does not provide benefits to the daily functioning of healthy older adults because the results do not transfer to everyday activities.
Among the selected articles, neurocognitive interventions that feature significant functionality benefits are those that train specific cognitive domains, such as working memory, processing speed, attention, executive functions, reasoning, or problem-solving. Additionally, interventions that incorporate everyday cognition have proven effective.
Regarding the intervention of specific cognitive domains, it makes sense for it to be beneficial in everyday functioning, as they are the foundations that enable and sustain proper performance. For example, the processing speed domain, which is related to autonomy in ADLs in various studies [48,49], or working memory.
Regarding other cognitive domains, there is more disagreement in the scientific literature on their relationship with daily performance in older adults. This is consistent with the findings of the current review, as one of the two studies that used specific neurocognitive attention interventions and one of the four studies that used memory techniques did not find significant benefits for functionality.
All studies that implemented the concept of everyday cognition in their interventions reported positive impacts on functionality. This neurocognitive approach requires individuals to utilize basic cognitive skills to resolve cognitively demanding problems, enabling autonomy in the instrumental activities of daily living. The challenges and daily activities faced by older individuals will be used as intervention material. Thus, this approach replaces specific cognitive function interventions, which have been shown in this review to not result in improved everyday functioning, with a neurocognitive intervention that uses the AIVD as both the means and the goal. Therefore, it is proposed that the most suitable neurocognitive intervention for achieving transfer and generalization of results to everyday life is everyday cognition.
4.3. Limitations
Due to the variability in the sessions, significant relationships with the obtained benefits have not been established, nor with the presence or type of follow-up. This confirms the need for more protocolized neurocognitive intervention modes. This prevents us from making a real comparison between the content and techniques of the different neurocognitive sessions. Regarding the high difference in the sample size, it makes it difficult to establish a real representation of this study population. This should be added to the deficits in studies we found in healthy elderly populations, as many studies that do meet the target population do not measure functionality as a study variable. This was frequently observed in the systematic review, as these two reasons were common causes for article exclusion.
One limitation of the review is that the selected studies were unable to control for all factors that may be associated with functional loss in healthy older adults, such as comorbidity, depression, or psychosocial and contextual factors that directly affect the variability of the results. Although it is true that all of them are aimed at healthy older people without cognitive impairment and that they describe and analyze sociodemographic variables such as age or education level, some are analyzed statistically.
These findings have significant implications for the early detection of cognitive decline and provide valuable information on potential targets for early intervention in healthy older adults.
5. Conclusions
This systematic review examines the most commonly used neurocognitive interventions currently utilized to prevent functional decline in healthy older adults. It is demonstrated that traditional cognitive stimulation has no positive effects on functionality and that new approaches should primarily aim for the transfer and generalization of results into the daily lives of healthy older individuals as a main quality of life indicator. This finding enables the direction of clinical practice and research towards new avenues, such as intervention based on everyday cognition. It lays the groundwork for future studies that should unify the use of updated assessments and interventions with current scientific literature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bs14020087/s1, File S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization and writing—original draft preparation, S.S.-G.; writing—review and editing, E.J.F.-R. and C.S.-G.; visualization, A.G.-M.; supervision L.P.-F.; project administration, F.J.B.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This study protocol is part of the Research Project on Active Aging with Preventive Physiotherapy PReGe, which has been funded by the Salamanca City Council. Project code: L9AB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Please contact the corresponding author to request data.
Acknowledgments
The authors would like to thank the University of Salamanca and the Salamanca City Council for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights. ST/ESA/SER.A/423. 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 5 June 2023).
- OMS, 2020. Del Desarrollo Social y Económico, Una Vida Más Larga. Who. Int. Recuperado el 31 de Agosto de 2023. Available online: https://www.who.int/docs/default-source/decade-of-healthy-ageing/final-decade-proposal/decade-proposal-final-apr2020-es.pdf?sfvrsn=73137ef_4 (accessed on 1 July 2023).
- Informe Mundial Sobre el Envejecimiento y la Salud. Ginebra, Organización Mundial de la Salud. 2015. Available online: https://www.who.int/ageing/events/world-report-2015-launch/en/ (accessed on 3 July 2023).
- Waris, M.; Upadhyay, A.D.; Chatterjee, P.; Chakrawarty, A.; Kumar, P.; Dey, A.B. Establishment of Clinical Construct of Intrinsic Capacity in Older Adults and Its Prediction of Functional Decline. Clin. Interv. Aging 2022, 17, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Cooper, C.; Martin, F.C.; Reginster, J.Y.; Vellas, B.; Beard, J.R. Evidence for the Domains Supporting the Construct of Intrinsic Capacity. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wong, G.H.; Luo, H.; Tang, J.Y.; Xu, J.; Choy, J.C.; Lum, T.Y. Everyday cognitive functioning and global cognitive performance are differentially associated with physical frailty and chronological age in older Chinese men and women. Aging Ment. Health 2018, 22, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Lau, K.; Harvey, D.; Denny, K.G.; Barba, C.; Mefford, A.N. Early Functional Limitations in Cognitively Normal Older Adults Predict Diagnostic Conversion to Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2017, 65, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Mamalaki, E.; Charisis, S.; Anastasiou, C.A.; Ntanasi, E.; Georgiadi, K.; Balomenos, V.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; et al. The Longitudinal Association of Lifestyle with Cognitive Health and Dementia Risk: Findings from the HELIAD Study. Nutrients 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Puig, E.J.; Fleites, Z.F.; Pérez, Y.B.; Martínez, D.B.V. Efectos de la intervención neurocognitiva en adultos mayores. Una revision sistemática. MediSur 2021, 19, 877–886. [Google Scholar]
- Gómez-Soria, I.; Ferreira, C.; Oliván Blazquez, B.; Magallón Botaya, R.M.; Calatayud, E. Short-term memory, attention, and temporal orientation as predictors of the cognitive impairment in older adults: A cross-sectional observational study. PLoS ONE 2021, 16, e0261313. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.W.; So, Y.; Seo, J.; Kim, Y.J.; Park, J.H.; Lee, S.B.; Lee, J.J.; Jeong, H.G.; Kim, T.H.; et al. Cognitive Stimulation as a Therapeutic Modality for Dementia: A Meta-Analysis. Psychiatry Investig. 2017, 14, 626–639. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Dementia: A NICE-SCIE Guideline on Supporting People with Dementia and Their Carers in Health and Social Care; British Psychological Society: London, UK, 2007. [Google Scholar]
- Borella, E.; Cantarella, A.; Carretti, B.; De Lucia, A.; De Beni, R. Improving Everyday Functioning in the Old-Old with Working Memory Training. Am. J. Geriatr. Psychiatry 2019, 27, 975–983. [Google Scholar] [CrossRef]
- De Jesús Cruz-Peralta, M.; González-Celis, A.L. Intervenciones para mejorar la calidad de vida en adultos mayores: Revisión sistemática con preguntas PIO. Psicol. Salud 2023, 33, 415–426. [Google Scholar] [CrossRef]
- Nguyen, L.; Murphy, K.; Andrews, G. A Game a Day Keeps Cognitive Decline Away? A Systematic Review and Meta-Analysis of Commercially-Available Brain Training Programs in Healthy and Cognitively Impaired Older Adults. Neuropsychol. Rev. 2022, 32, 601–630. [Google Scholar] [CrossRef]
- Aguirre, E.; Hoare, Z.; Streater, A.; Spector, A.; Woods, B.; Hoe, J.; Orrell, M. Cognitive stimulation therapy (CST) for people with dementia-who benefits most? Int. J. Geriatr. Psychiatry 2013, 28, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.; Simard, M. Cognitive stimulation programs in healthy elderly: A review. Int. J. Alzheimers Dis. 2011, 2011, 378934. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Kawashima, R. Improving cognitive function from children to old age: A systematic review of recent smart ageing intervention studies. Adv. Neurosci. 2014, 2014, 235479. [Google Scholar] [CrossRef]
- Zuschnegg, J.; Schoberer, D.; Häussl, A.; Herzog, S.A.; Russegger, S.; Ploder, K.; Fellner, M.; Hofmarcher-Holzhacker, M.M.; Roller-Wirnsberger, R.; Paletta, L.; et al. Effectiveness of computer-based interventions for community-dwelling people with cognitive decline: A systematic review with meta-analyses. BMC Geriatr. 2023, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Son, C.; Park, J.H. Ecological Effects of VR-Based Cognitive Training on ADL and IADL in MCI and AD patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15875. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 2013, 2013, CD003260. [Google Scholar] [CrossRef]
- Reijnders, J.; van Heugten, C.; van Boxtel, M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res. Rev. 2013, 12, 263–275. [Google Scholar] [CrossRef]
- Gamaldo, A.A.; Allaire, J.C. Daily Fluctuations in Everyday Cognition: Is It Meaningful? J. Aging Health 2016, 28, 834–849. [Google Scholar] [CrossRef]
- Farias, S.T.; Park, L.Q.; Harvey, D.J.; Simon, C.; Reed, B.R.; Carmichael, O.; Mungas, D. Everyday cognition in older adults: Associations with neuropsychological performance and structural brain imaging. J. Int. Neuropsychol. Soc. 2013, 19, 430–441. [Google Scholar] [CrossRef]
- Williams, K.; Herman, R.; Bontempo, D. Reasoning exercises in assisted living: A cluster randomized trial to improve reasoning and everyday problem solving. Clin. Interv. Aging 2014, 9, 981–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez, C.S.; Rodríguez, E.J.F. The effectiveness of a training programme in everyday cognition in healthy older adults: A randomised controlled trial. BMC Geriatr. 2021, 21, 79. [Google Scholar] [CrossRef]
- Rodríguez, E.J.F.; Gómez, C.S.; Pérez, M.L.M.; Iglesias, F.J.B.; Arenillas, J.I.C. Estudio aleatorio de un programa de entrenamiento de cognición cotidiana frente a estimulación cognitiva tradicional en adultos mayores. Gerokomos Rev. Soc. Esp. Enferm. Geriátr. Gerontol. 2018, 29, 65–71. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Pergunta, D. Estrategia PICO para la construcción de la pregunta de investigación y la búsqueda de evidencias. Rev. Lat.-Am. Enferm. 2007, 15, 3. [Google Scholar]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.H.; Tang, Y.L.; Chiu, M.J.; Wu, C.T.; Mao, H.F. A Multicomponent Cognitive Intervention May Improve Self-Reported Daily Function of Adults With Subjective Cognitive Decline. Am. J. Occup. Ther. 2023, 77, 7704205040. [Google Scholar] [CrossRef]
- Cheng, C.P.; Chiu-Wa Lam, L.; Cheng, S.T. The Effects of Integrated Attention Training for Older Chinese Adults With Subjective Cognitive Complaints: A Randomized Controlled Study. J. Appl. Gerontol. 2018, 37, 1195–1214. [Google Scholar] [CrossRef]
- Frankenmolen, N.L.; Overdorp, E.J.; Fasotti, L.; Claassen, J.A.H.R.; Kessels, R.P.C.; Oosterman, J.M. Memory Strategy Training in Older Adults with Subjective Memory Complaints: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 1110–1120. [Google Scholar] [CrossRef]
- Belchior, P.; Yam, A.; Thomas, K.R.; Bavelier, D.; Ball, K.K.; Mann, W.C.; Marsiske, M. Computer and Videogame Interventions for Older Adults’ Cognitive and Everyday Functioning. Games Health J. 2019, 8, 129–143. [Google Scholar] [CrossRef]
- Cantarella, A.; Borella, E.; Carretti, B.; Kliegel, M.; de Beni, R. Benefits in tasks related to everyday life competences after a working memory training in older adults. Int. J. Geriatr. Psychiatry 2017, 32, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.S.; Rendell, P.G.; Hering, A.; Kliegel, M.; Bidelman, G.M.; Craik, F.I. Cognitive and neural plasticity in older adults’ prospective memory following training with the Virtual Week computer game. Front. Hum. Neurosci. 2015, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.Y.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014, 62, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, Y.; Deng, W.; Sun, S. The Effects of Cognitive Training on Cognitive Abilities and Everyday Function: A 10-Week Randomized Controlled Trial. Int. J. Aging Hum. Dev. 2018, 86, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.; Owen, A.; Hampshire, A.; Grahn, J.; Stenton, R.; Dajani, S.; Burns, A.; Howard, R.; Williams, N.; Williams, G.; et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Yoon, J.S.; Charness, N.; Boot, W.R.; Roque, N.A.; Andringa, R.; Harrell, E.R.; Lewis, K.G.; Vitale, T. Relative effectiveness of general versus specific cognitive training for aging adults. Psychol. Aging 2022, 37, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Balaji, G.K.; Sahana, A.; Karthikbabu, S. Effects of Cognitive Versus Mind-Motor Training on Cognition and Functional Skills in the Community-Dwelling Older Adults. Indian J. Psychol. Med. 2021, 43, 300–305. [Google Scholar] [CrossRef]
- Gómez-Soria, I.; Ferreira, C.; Oliván-Blázquez, B.; Aguilar-Latorre, A.; Calatayud, E. Effects of cognitive stimulation program on cognition and mood in older adults, stratified by cognitive levels: A randomized controlled trial. Arch. Gerontol. Geriatr. 2023, 110, 104984. [Google Scholar] [CrossRef]
- Gamito, P.; Oliveira, J.; Alves, C.; Santos, N.; Coelho, C.; Brito, R. Virtual reality-based cognitive stimulation to improve cognitive functioning in community elderly: A controlled study. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 150–156. [Google Scholar] [CrossRef]
- Srisuwan, P.; Nakawiro, D.; Chansirikarnjana, S.; Kuha, O.; Chaikongthong, P.; Suwannagoot, T. Effects of a Group-Based 8-Week Multicomponent Cognitive Training on Cognition, Mood and Activities of Daily Living among Healthy Older Adults: A One-Year Follow-Up of a Randomized Controlled Trial. J. Prev. Alzheimers Dis. 2020, 7, 112–121. [Google Scholar] [CrossRef]
- Echeverría, A.; Astorga, C.; Fernández, C.; Salgado, M.; Villalobos Dintrans, P. Funcionalidad y personas mayores: ¿dónde estamos y hacia dónde ir? Rev. Panam. Salud Publica 2022, 46, e34. [Google Scholar] [CrossRef]
- Abut, P.Á.; Scharovsky, D.; Brugger, R.; Ayala, M.; Aleman, A.; Sánchez, M.; Gonorazky, S.E.; Latini, M.F. Correlación entre las actividades de la vida diaria y los tests de detección de demencia en nuestra población. Neurol. Argent. 2012, 4, 112–117. [Google Scholar] [CrossRef]
- Calatayud, E.; Plo, F.; Muro, C. Análisis del efecto de un programa de estimulación cognitiva en personas con envejecimiento normal en Atención Primaria: Ensayo clínico aleatorizado [Analysis of the effect of a program of cognitive stimulation in elderly people with normal aging in primary care: Randomized clinical trial]. Aten. Primaria 2020, 52, 38–46. [Google Scholar] [CrossRef]
- Aul, C.; Brau, J.M.; Sugarman, A.; DeGutis, J.M.; Germine, L.T.; Esterman, M.; McGlinchey, R.E.; Fortenbaugh, F.C. The functional relevance of visuospatial processing speed across the lifespan. Cogn. Res. Princ. Implic. 2023, 8, 51. [Google Scholar] [CrossRef]
- Owsley, C.; Sloane, M.; McGwin, G., Jr.; Ball, K. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology 2002, 48, 254–265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).