Lifelong Fitness in Ambulatory Children and Adolescents with Cerebral Palsy I: Key Ingredients for Bone and Muscle Health
Abstract
1. Current Physical Activity Guidelines for Children and Adolescents
2. Musculoskeletal (MSK) System
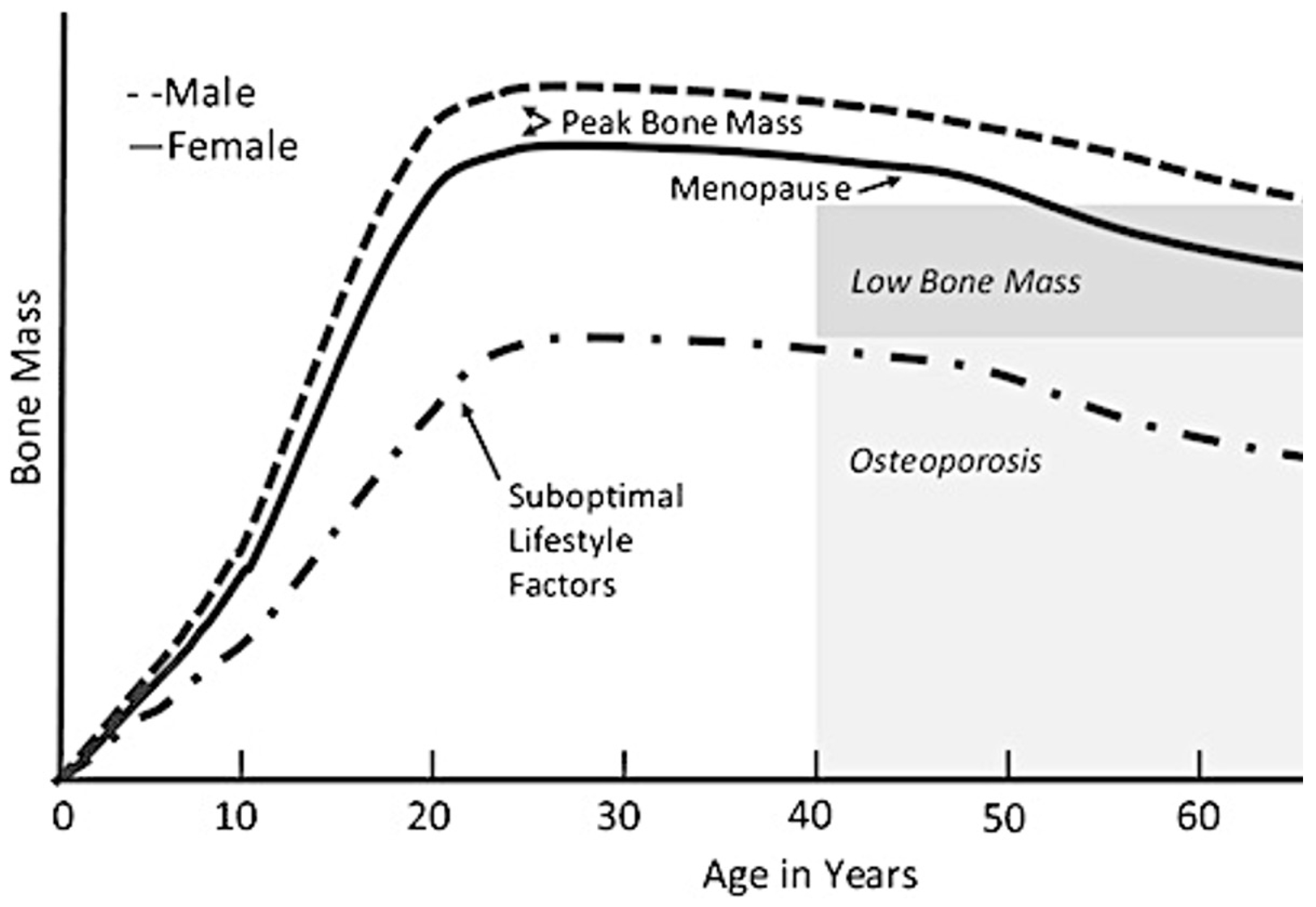
2.1. Skeletal System
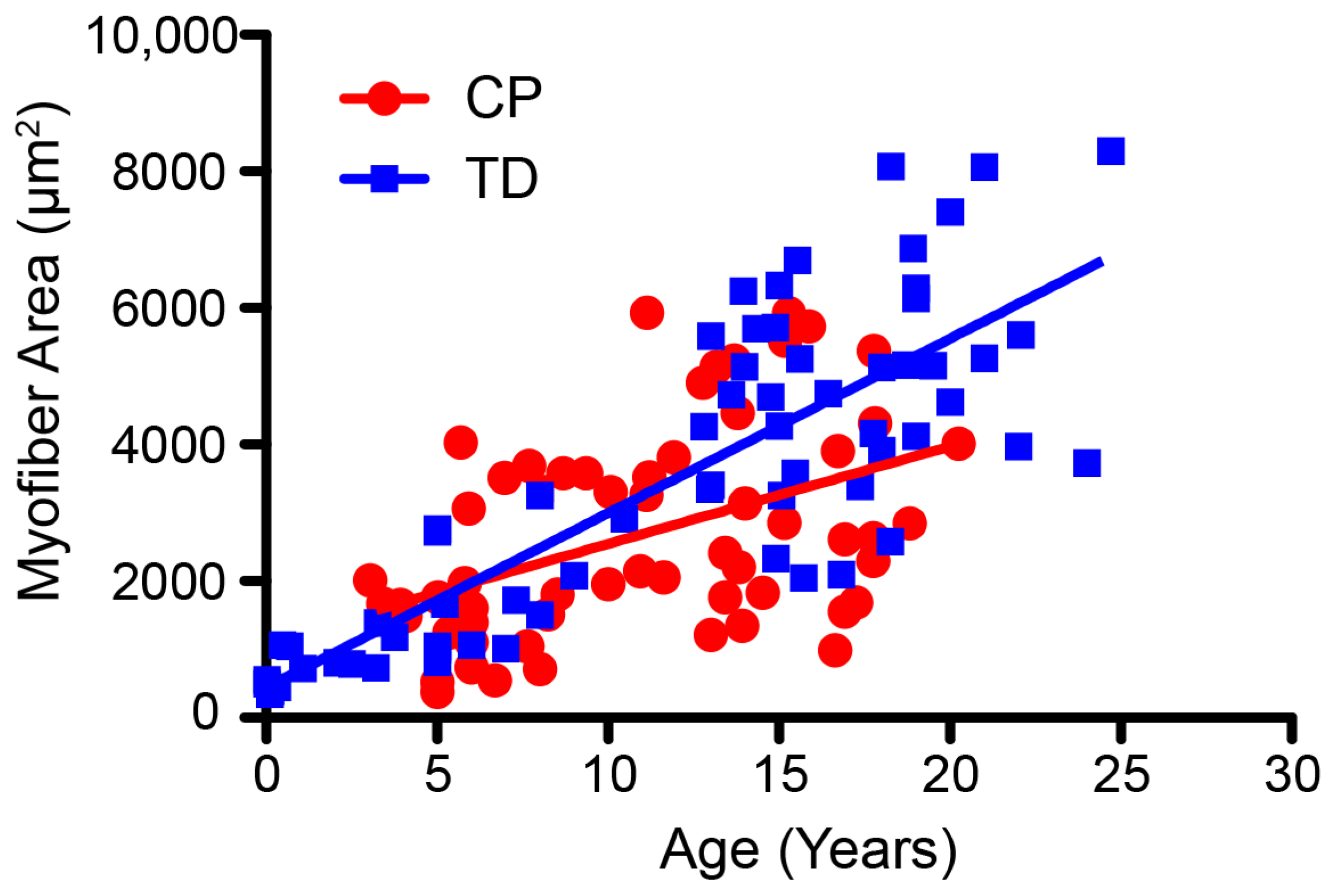
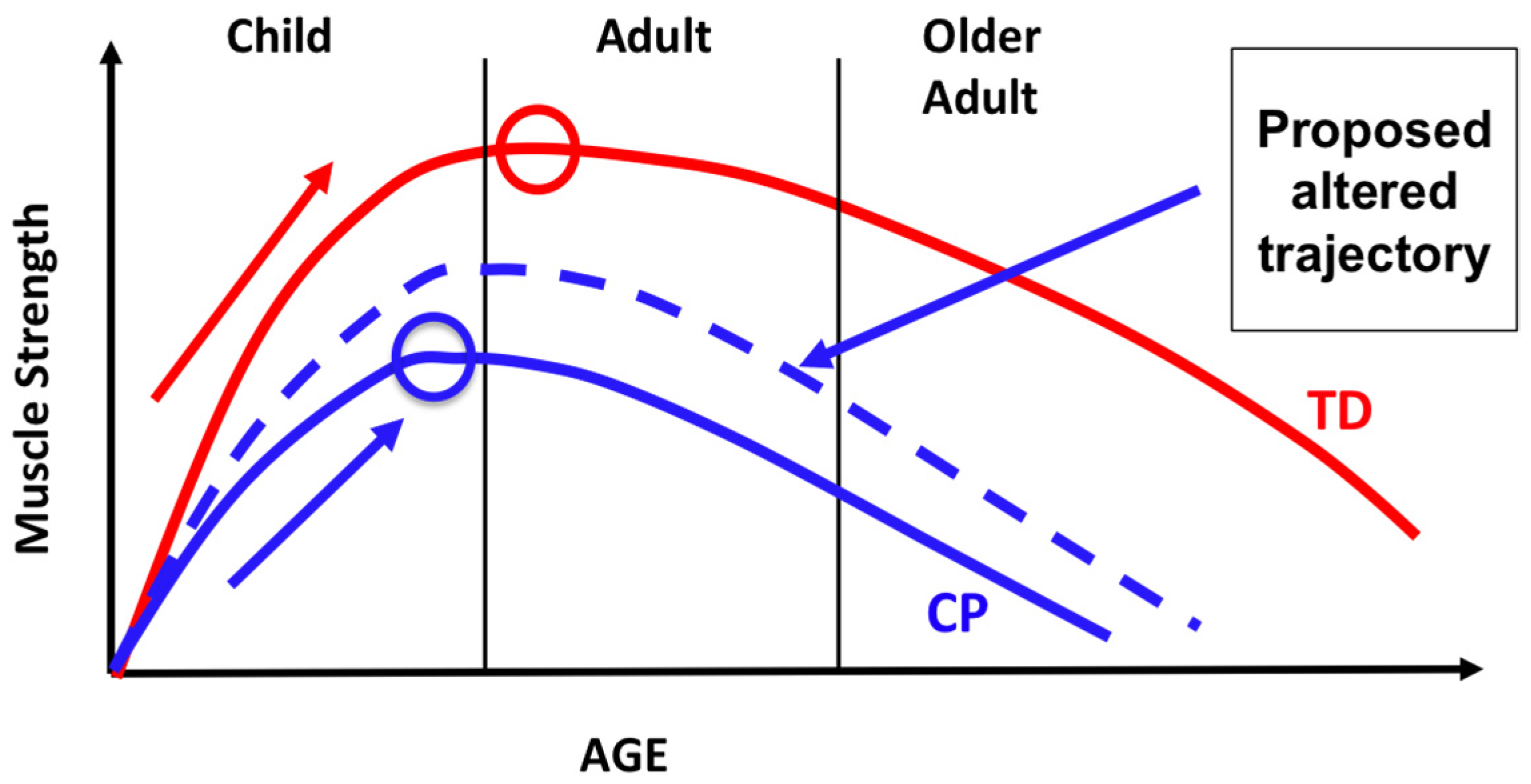
2.2. Muscular System
3. Building a Better Foundation for MSK Health in Children with CP
3.1. Critical Time Periods
3.2. Key Ingredients
3.2.1. Targeted Musculoskeletal Intervention
- Augmenting Bone Health. Sufficient physical activity that provides muscular stimulus and impact forces that target osteogenesis could prevent osteoporosis and reduce the risk of fracture. Yet, to ensure that the skeletal system of a person with CP can tolerate force-related and impact loading activities, pre-testing of bone density is required. Bone mineral content (BMC; g) and areal bone mineral density (BMD); g/cm2) are two clinical measures of bone health that can be assessed with dual X-ray absorptiometry (DXA). Knowing the patient’s bone health will ensure that precautions are taken to ensure a child at risk can begin to safely engage in physical activity. For children with low bone mass based on calculated Z-scores, it is still safe to exercise. For example, a child with a higher GMFCS level may have low bone mass, but if placed in a harness or assisted with the exercises, the individual can still benefit from the exercise while minimizing fall risk. Ultimately, fractures are primarily caused by an impact force from a fall or landing that exceeds the failure properties of the bone tissue [90]. Given the limited data in children on the risk of fracture during exercise and what the skeleton can tolerate, we can glean some insight from exercise interventions in osteoporotic women who are performing high-intensity exercise. In these studies, women with osteoporosis were able to handle high impact loads and did not fracture [91].
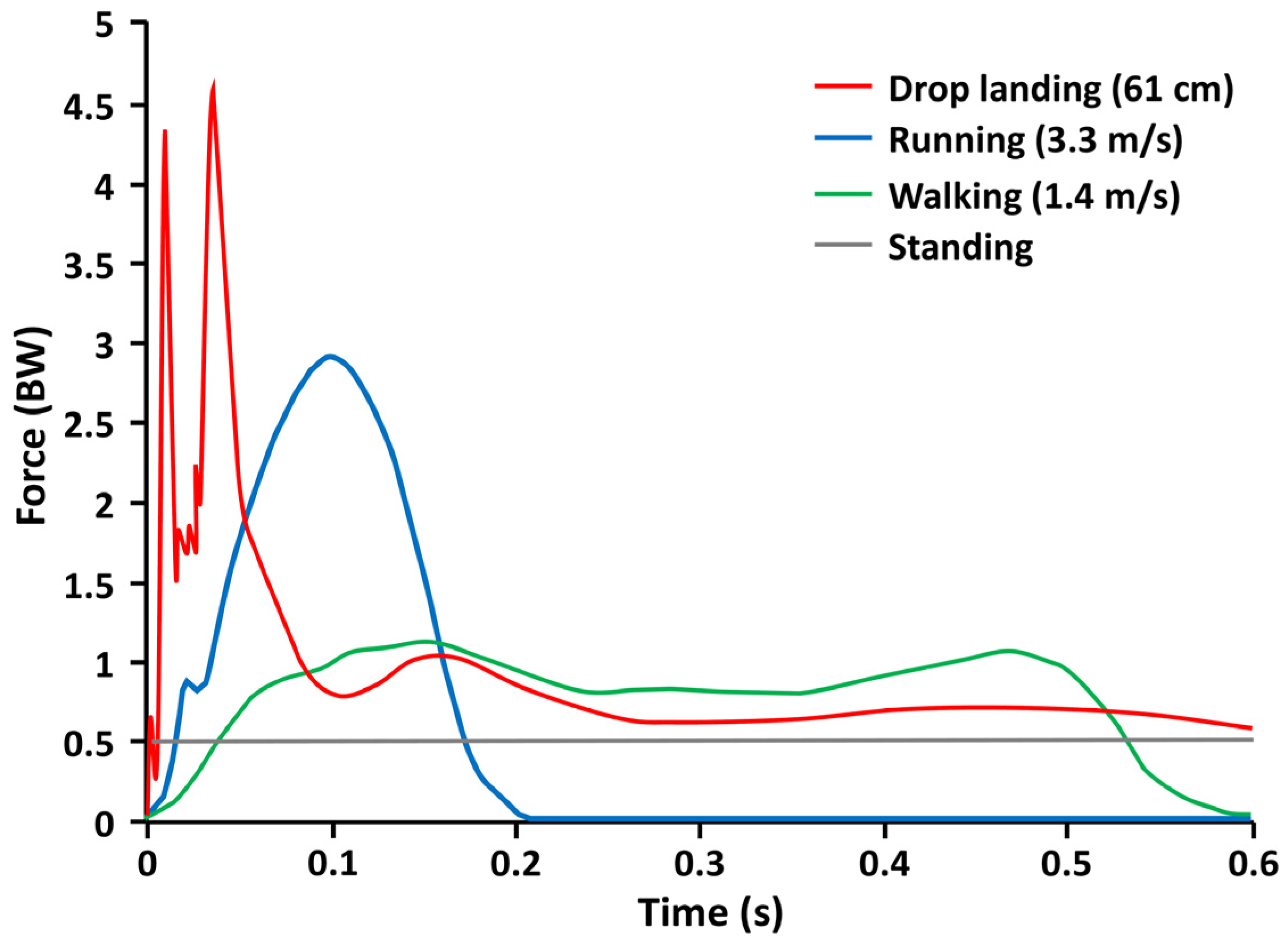
- Based on published and unpublished data [32,34] by Bauer et al. and Gunter et al. [31], GRFs per body weight (BW) for activities performed by a TD child are about 1.0 times BW for walking, 2.9 times BW for running, and 4.6 times BW for drop landing (see Figure 2). The GRFs per BW for activities performed by a child with CP are less widely known and may be strongly influenced by select impairments such as ligamentous laxity, joint deformity, body malalignment, inadequate passive and active range-of-motion, and insufficient eccentric muscular control. Quick, high-load tasks that a child with CP at GMFCS levels I–III may tolerate could include jumping rope, hopscotch, or jump downs off a bench. Individuals with mobility at GMFCS level III may require the use of a harness or walker for support and balance during loading tasks. Tasks with a low impact load, such as jumps on a mini-trampoline, may be safer, but they may not provide sufficient GRFs per BW to influence changes in bone mass and structure. It may be best to have a child participate in circuit training, which may include intermittent impact loading, allowing for periodic monitoring of safety and tolerance. For example, the sequence of a course could be: (1) hopscotch; (2) jump rope; (3) crawling through tubes; and (4) jump downs. Further study is needed to ascertain the types of loads safely tolerated by persons with CP at all levels of the GMFCS.
- Enhancing Muscle Performance. As reviewed, there are important age-related changes in the muscles of persons with CP that differ from those who are TD. Despite these differences, muscle hypertrophy, force production, and power can increase in children and adolescents with CP who undergo targeted training at a sufficient dosage [81,92]. Because muscle architecture can differentially adapt in response to different types of resistance training [81], the type of training and dosing essential to altering muscle function must be incorporated into programming (Section 3.2.2. Dosing Parameters).
- Strength training is recommended to build a strong muscular foundation, promote muscle hypertrophy, and provide a synergistic stimulus for bone health. Yet, the effects of traditional strength training have not been shown to carry over to activities such as gait and functional mobility in those with CP [93,94]. Power training, which involves training at moderate to high loads at a higher concentric velocity of movement, is recommended for better carryover to gait and functional activities. Targeted high-velocity training may not only increase muscle power but also induce muscle architectural adaptations, such as an increase in fascicle length and cross-sectional area, and promote a right-ward shift of the torque-angle curve, increasing torque production at higher velocity [81,83].
- Traditional resistive training equipment (i.e., free weights and isotonic machines) is readily available in most gyms and clinics and can easily be used for strength and power training. Basic bodyweight exercises can also be used, especially in very young children, and can be progressed to free weights, machines, or other loaded exercises. Other modifications for children at GMFCS level III may include the use of support walkers for balance and to encourage hands-free positioning in standing while promoting weightbearing through the lower limbs. Regardless of GMFCS level, the advantages of using weight machines are that the child can be supported and single or multiple joints can be isolated while preventing or discouraging compensatory patterns and unwanted movements. For example, an inclined leg press can be used to train and target multiple lower extremity muscle groups while safely supporting the trunk and body [95,96]. In this supported position, the muscles can be loaded in a safe manner to a greater extent than if the same movement was attempted as an upright standing squat.
- While there are selected types of equipment that provide precise measures of velocity and force, such as isokinetic equipment, these are not necessary for resistance training purposes. Power training can be feasibly conducted on most equipment by moving a constant load while decreasing the amount of time allowed to produce the concentric contraction (i.e., increasing the velocity). For example, a power leg press can be performed on an inclined leg press machine and would train and target the hip and knee extensors and ankle plantarflexors with a single exercise [95]. Typical verbal instructions include “Push, pull, or press as fast as possible” and “lower slow and controlled”, referring to the concentric and eccentric portions of the motion, respectively. Once a sufficient velocity is reached, the load should be increased. Instrumented versions can also be used to reliably measure power output while performing a power leg press [95]. Another example of equipment that can be used for power training is flywheel ergometers. The equipment can be in the mode of a bike, rower, or ski machine that couples resistance from the device with the speed of active motion while providing digital power output. In a randomized crossover study by Moreau and colleagues [97] in persons with CP, 7 to 24 years of age, power output in the upper extremities significantly increased after 15 training sessions using an upper extremity flywheel ergometer (Concept2 SkiErg™, Morrisville, VT, USA). Use of the device at home or in school strengthened adherence.
- Community-based training alternatives are also important for promoting mobility-based participation. RaceRunning (or Frame Running), which uses a three-wheeled running frame, is an example of how children within GMFCS levels I–IV can successfully engage in community-based sports programming if provided with adaptation [98]. Further, muscle hypertrophy and an increase in cardiorespiratory endurance were observed after a 12-week program across a wide age range (9 to 29 years) and mobility levels (GMFCS I–IV) [99]. Training alternatives to improve muscle and bone health while fostering engagement should continue to expand, allowing greater access to this type of programming in various settings.
- Despite the success of resistance training programs for persons with CP, there are some risks of pain and injury. However, no serious adverse events have been reported for resistance training interventions in children and adolescents with CP. A few studies have reported mild adverse events, such as joint or muscle soreness [100,101]. It is highly recommended that those participating in any resistive or power training program be supervised and monitored closely by a trained professional [80]. Safety and tolerance are key factors for all programs to augment muscle integrity and function.
3.2.2. Dosing Parameters
- Dosing for Bone. Dosing parameters used to guide interventions to improve bone health are often based on guidelines to increase peak bone mass and prevent osteoporosis [27,102], which may be an important consideration for those with CP given the risk factors. General guidelines for TD children have been advocated by Gunter et al. [31]. These guidelines have been framed within the dosing parameters of frequency and volume (Table 1). The authors propose that children engage in 40–60 min of daily weight-bearing activity to target hip structure and strength [103]. Based on their own findings, they recommend 10–15 min of jumping 3 times per week to augment bone mass and structure [33,104]. This frequency and volume equate to 100 jumps from a two-foot height with GRFs at least 3.5 BW and higher). Table 1 includes examples of bone-building exercises that could be performed in children across all GMFCS levels, with associated ideas for how to modify activities. Since tolerance to skeletal loading varies across the GMFCS spectrum, methods to augment bone health in persons with CP must consider the individual integrity of the skeletal system and monitor safety and tolerance throughout the training program.
- Dosing for Muscle. Recommended optimal dosing guidelines for progressive resistance training have been assembled as shown in Table 2, specific to muscle strengthening vs. power training [80]. Novice lifters should begin training at a lower intensity (percentage of 1RM) as described in Moreau [80] in more detail and then progress up to the optimal dosage provided in Table 2 in order to maximize muscle plasticity. For example, a novice may begin power training at 40% of 1RM and focus on form and speed, then progress to a higher percentage of 1RM after successful completion of the target reps at the higher concentric velocity. Of note is that intensity, volume, and speed differ between the two training paradigms. It is important that a 1RM test be performed to adequately dose the intensity of the intervention and the progression of the intensity throughout the intervention period. The safety, feasibility, and protocol for performing a 1RM in youth with CP have recently been published by Pontiff and Moreau [96]. Although a multiple repetition maximum test may be used to predict 1RM values, the prediction is less accurate for repetition ranges greater than 10 [105]. Regardless of what muscle performance parameter is being targeted, the recommended frequency for resistance training is 2 to 3 times per week on nonconsecutive days for a duration of 8 to 20 weeks (refer to Moreau, 2020 for more details) [80]. A recent review article by Moreau and Lieber [83] on resistance training interventions for youth with CP showed that if the optimal dosing guidelines were adhered to, then muscle plasticity was observed at the macroscopic structural level (i.e., increases in cross-sectional area, muscle thickness, volume, or fascicle lengths).
3.3. Maximizing Engagement and Addressing Barriers
4. Sustaining Gains
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Word Health Organization. Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 13 April 2021).
- American College of Sports Medicine. Physical Activity Guidelines. Available online: https://www.acsm.org/read-research/trending-topics-resource-pages/physical-activity-guidelines (accessed on 13 April 2021).
- Gunter, K.B.; Nader, P.A.; John, D.H. Physical activity levels and obesity status of Oregon Rural Elementary School children. Prev. Med. Rep. 2015, 2, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Bjornson, K.F.; Belza, B.; Kartin, D.; Logsdon, R.; McLaughlin, J.F. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys. Ther. 2007, 87, 248–257. [Google Scholar] [CrossRef]
- Bratteby Tollerz, L.U.; Forslund, A.H.; Olsson, R.M.; Lidstrom, H.; Holmback, U. Children with cerebral palsy do not achieve healthy physical activity levels. Acta Paediatr. 2015, 104, 1125–1129. [Google Scholar] [CrossRef]
- Maher, C.A.; Toohey, M.; Ferguson, M. Physical activity predicts quality of life and happiness in children and adolescents with cerebral palsy. Disabil. Rehabil. 2016, 38, 865–869. [Google Scholar] [CrossRef]
- Marker, A.M.; Steele, R.G.; Noser, A.E. Physical activity and health-related quality of life in children and adolescents: A systematic review and meta-analysis. Health Psychol. 2018, 37, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Van Wely, L.; Balemans, A.C.; Becher, J.G.; Dallmeijer, A.J. Physical activity stimulation program for children with cerebral palsy did not improve physical activity: A randomised trial. J. Physiother. 2014, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.V.; Kimbel, J.D.; Grant-Beuttler, M.; Sukal-Moulton, T.; Moreau, N.G.; Friel, K.M. Lifelong Fitness for Ambulatory Children and Adolescents with Cerebral Palsy II: Influencing the Trajectory. Behav. Sci. 2023. accepted. [Google Scholar]
- Peterson, M.D.; Ryan, J.M.; Hurvitz, E.A.; Mahmoudi, E. Chronic Conditions in Adults with Cerebral Palsy. JAMA 2015, 314, 2303–2305. [Google Scholar] [CrossRef]
- Ryan, J.M.; Peterson, M.D.; Ryan, N.; Smith, K.J.; O’Connell, N.E.; Liverani, S.; Anokye, N.; Victor, C.; Allen, E. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Peterson, M.D.; O’Connell, N.E.; Victor, C.; Liverani, S.; Anokye, N.; Ryan, J.M. Risk of Depression and Anxiety in Adults with Cerebral Palsy. JAMA Neurol. 2019, 76, 294–300. [Google Scholar] [CrossRef]
- Carlon, S.L.; Taylor, N.F.; Dodd, K.J.; Shields, N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: A systematic review. Disabil. Rehabil. 2013, 35, 647–655. [Google Scholar] [CrossRef]
- Maher, C.A.; Williams, M.T.; Olds, T.; Lane, A.E. Physical and sedentary activity in adolescents with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Keawutan, P.; Bell, K.L.; Oftedal, S.; Davies, P.S.; Ware, R.S.; Boyd, R.N. Habitual Physical Activity in Children with Cerebral Palsy Aged 4 to 5 Years Across All Functional Abilities. Pediatr. Phys. Ther. 2017, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Krakovsky, G.; Huth, M.M.; Lin, L.; Levin, R.S. Functional changes in children, adolescents, and young adults with cerebral palsy. Res. Dev. Disabil. 2007, 28, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lauruschkus, K.; Westbom, L.; Hallstrom, I.; Wagner, P.; Nordmark, E. Physical activity in a total population of children and adolescents with cerebral palsy. Res. Dev. Disabil. 2013, 34, 157–167. [Google Scholar] [CrossRef]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Barrett, R.; Lichtwark, G. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.A.; Read, F.; Lovatt Stern, J.; Lichtwark, G.; Boyd, R.N. Medial gastrocnemius muscle volume in ambulant children with unilateral and bilateral cerebral palsy aged 2 to 9 years. Dev. Med. Child Neurol. 2016, 58, 1146–1152. [Google Scholar] [CrossRef]
- Hanna, S.E.; Rosenbaum, P.L.; Bartlett, D.J.; Palisano, R.J.; Walter, S.D.; Avery, L.; Russell, D.J. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev. Med. Child Neurol. 2009, 51, 295–302. [Google Scholar] [CrossRef]
- Johnson, D.C.; Damiano, D.L.; Abel, M.F. The evolution of gait in childhood and adolescent cerebral palsy. J. Pediatr. Orthop. 1997, 17, 392–396. [Google Scholar] [CrossRef]
- Noble, J.J.; Fry, N.; Lewis, A.P.; Charles-Edwards, G.D.; Keevil, S.F.; Gough, M.; Shortland, A.P. Bone strength is related to muscle volume in ambulant individuals with bilateral spastic cerebral palsy. Bone 2014, 66, 251–255. [Google Scholar] [CrossRef]
- Noble, J.J.; Fry, N.R.; Lewis, A.P.; Keevil, S.F.; Gough, M.; Shortland, A.P. Lower limb muscle volumes in bilateral spastic cerebral palsy. Brain Dev. 2014, 36, 294–300. [Google Scholar] [CrossRef]
- Whitney, D.G.; Hurvitz, E.A.; Devlin, M.J.; Caird, M.S.; French, Z.P.; Ellenberg, E.C.; Peterson, M.D. Age trajectories of musculoskeletal morbidities in adults with cerebral palsy. Bone 2018, 114, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Herskind, A.; Ritterband-Rosenbaum, A.; Willerslev-Olsen, M.; Lorentzen, J.; Hanson, L.; Lichtwark, G.; Nielsen, J.B. Muscle growth is reduced in 15-month-old children with cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 485–491. [Google Scholar] [CrossRef]
- Warden, S.; Fuchs, R. Physical Activity to Promote Bone Health in Adolescents. In A Practical Approach to Adolescent Bone Health; Pitts, S., Gordon, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 53–76. [Google Scholar] [CrossRef]
- Warden, S.J.; Fuchs, R.K. Exercise and bone health: Optimising bone structure during growth is key, but all is not in vain during ageing. Br. J. Sports Med. 2009, 43, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Linden, C.; Alwis, G.; Ahlborg, H.; Gardsell, P.; Valdimarsson, O.; Stenevi-Lundgren, S.; Besjakov, J.; Karlsson, M.K. Exercise, bone mass and bone size in prepubertal boys: One-year data from the pediatric osteoporosis prevention study. Scand. J. Med. Sci. Sports 2007, 17, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Valdimarsson, O.; Sigurdsson, G.; Steingrímsdóttir, L.; Karlsson, M.K. Physical activity in the post-pubertal period is associated with maintenance of pre-pubertal high bone density—A 5-year follow-up. Scand. J. Med. Sci. Sports 2005, 15, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.B.; Almstedt, H.C.; Janz, K.F. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc. Sport Sci. Rev. 2012, 40, 13–21. [Google Scholar] [CrossRef]
- Bauer, J.; Fuchs, R.; Smith, G.; Snow, C. Quantifying Force Magnitude and Loading Rate from Drop Landings That Induce Osteogenesis. J. Appl. Biomech. 2001, 17, 142–152. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Bauer, J.J.; Snow, C.M. Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J. Bone Miner. Res. 2001, 16, 148–156. [Google Scholar] [CrossRef]
- Bauer, J. Ground reaction forces and rates of loading. 2001; unpublished work. [Google Scholar]
- O’Connell, N.E.; Smith, K.J.; Peterson, M.D.; Ryan, N.; Liverani, S.; Anokye, N.; Victor, C.; Ryan, J.M. Incidence of osteoarthritis, osteoporosis and inflammatory musculoskeletal diseases in adults with cerebral palsy: A population-based cohort study. Bone 2019, 125, 30–35. [Google Scholar] [CrossRef]
- Whitney, D.G.; Alford, A.I.; Devlin, M.J.; Caird, M.S.; Hurvitz, E.A.; Peterson, M.D. Adults with Cerebral Palsy have Higher Prevalence of Fracture Compared with Adults without Cerebral Palsy Independent of Osteoporosis and Cardiometabolic Diseases. J. Bone Miner. Res. 2019, 34, 1240–1247. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A.; Committee on Nutrition; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; Schwarzenberg, S.J. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Gannotti, M.E.; Liquori, B.M.; Thorpe, D.E.; Fuchs, R.K. Designing Exercise to Improve Bone Health Among Individuals with Cerebral Palsy. Pediatr. Phys. Ther. 2021, 33, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Trinh, A.; Wong, P.; Fahey, M.C.; Brown, J.; Strauss, B.J.; Ebeling, P.R.; Fuller, P.J.; Milat, F. Longitudinal changes in bone density in adolescents and young adults with cerebral palsy: A case for early intervention. Clin. Endocrinol. 2019, 91, 517–524. [Google Scholar] [CrossRef]
- Jesus, A.O.; Stevenson, R.D. Optimizing Nutrition and Bone Health in Children with Cerebral Palsy. Phys. Med. Rehabil. Clin. 2020, 31, 25–37. [Google Scholar] [CrossRef]
- Won, J.H.; Jung, S.H. Bone Mineral Density in Adults with Cerebral Palsy. Front. Neurol. 2021, 12, 733322. [Google Scholar] [CrossRef]
- Duran, I.; Katzmann, J.; Martakis, K.; Stark, C.; Semler, O.; Schoenau, E. Individualized evaluation of lumbar bone mineral density in children with cerebral palsy. Arch. Osteoporos. 2018, 13, 120. [Google Scholar] [CrossRef]
- Marciniak, C.; Gabet, J.; Lee, J.; Ma, M.; Brander, K.; Wysocki, N. Osteoporosis in adults with cerebral palsy: Feasibility of DXA screening and risk factors for low bone density. Osteoporos. Int. 2016, 27, 1477–1484. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Conaway, M.; Barrington, J.W.; Cuthill, S.L.; Worley, G.; Henderson, R.C. Fracture rate in children with cerebral palsy. Pediatr. Rehabil. 2006, 9, 396–403. [Google Scholar] [CrossRef]
- Verschuren, O.; Smorenburg, A.R.P.; Luiking, Y.; Bell, K.; Barber, L.; Peterson, M.D. Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: A narrative review of the literature. J. Cachexia Sarcopenia Muscle 2018, 9, 453–464. [Google Scholar] [CrossRef]
- Liquori, B.M.; Gannotti, M.E.; Thorpe, D.E.; Fuchs, R.K. Characteristics of Interventions to Improve Bone Health in Children with Cerebral Palsy: A Systematic Review. Pediatr. Phys. Ther. 2022, 34, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.C.; Gilbert, S.R.; Clement, M.E.; Abbas, A.; Worley, G.; Stevenson, R.D. Altered skeletal maturation in moderate to severe cerebral palsy. Dev. Med. Child. Neurol. 2005, 47, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Ke, J.Y.; Wang, C.J.; Wu, K.P.; Wu, C.Y.; Wong, A.M. Factors associated with bone density in different skeletal regions in children with cerebral palsy of various motor severities. Dev. Med. Child. Neurol. 2011, 53, 131–136. [Google Scholar] [CrossRef]
- Uddenfeldt Wort, U.; Nordmark, E.; Wagner, P.; Düppe, H.; Westbom, L. Fractures in children with cerebral palsy: A total population study. Dev. Med. Child. Neurol. 2013, 55, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Zabarte Fernández, J.M.; Ros Arnal, I.; Peña Segura, J.L.; García Romero, R.; Rodríguez Martínez, G. Bone health impairment in patients with cerebral palsy. Arch. Osteoporos. 2020, 15, 91. [Google Scholar] [CrossRef]
- Lieber, R.L.; Roberts, T.J.; Blemker, S.S.; Lee, S.S.M.; Herzog, W. Skeletal muscle mechanics, energetics and plasticity. J. Neuroeng. Rehabil. 2017, 14, 108. [Google Scholar] [CrossRef]
- Tveter, A.T.; Holm, I. Influence of thigh muscle strength and balance on hop length in one-legged hopping in children aged 7-12 years. Gait Posture 2010, 32, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Dayanidhi, S.; Dykstra, P.B.; Lyubasyuk, V.; McKay, B.R.; Chambers, H.G.; Lieber, R.L. Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J. Orthop. Res. 2015, 33, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, M.; Lorentzen, J.; Sinkjaer, T.; Nielsen, J.B. Passive muscle properties are altered in children with cerebral palsy before the age of 3 years and are difficult to distinguish clinically from spasticity. Dev. Med. Child Neurol. 2013, 55, 617–623. [Google Scholar] [CrossRef]
- Zogby, A.M.; Dayanidhi, S.; Chambers, H.G.; Schenk, S.; Lieber, R.L. Skeletal muscle fiber-type specific succinate dehydrogenase activity in cerebral palsy. Muscle Nerve 2017, 55, 122–124. [Google Scholar] [CrossRef]
- Boakes, J.L.; Foran, J.; Ward, S.R.; Lieber, R.L. Muscle adaptation by serial sarcomere addition 1 year after femoral lengthening. Clin. Orthop. Relat. Res. 2007, 456, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.E.; Goldspink, G. Longitudinal growth of striated muscle fibres. J. Cell. Sci. 1971, 9, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.E.; Goldspink, G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J. Anat. 1973, 116, 45–55. [Google Scholar] [PubMed]
- Mathewson, M.A.; Ward, S.R.; Chambers, H.G.; Lieber, R.L. High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J. Orthop. Res. 2015, 33, 33–39. [Google Scholar] [CrossRef]
- Smith, L.R.; Lee, K.S.; Ward, S.R.; Chambers, H.G.; Lieber, R.L. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 2011, 589, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Domenighetti, A.A.; Mathewson, M.A.; Pichika, R.; Sibley, L.A.; Zhao, L.; Chambers, H.G.; Lieber, R.L. Loss of myogenic potential and fusion capacity of muscle stem cells isolated from contractured muscle in children with cerebral palsy. Am. J. Physiol. Cell. Physiol. 2018, 315, C247–C257. [Google Scholar] [CrossRef]
- Sibley, L.A.; Broda, N.; Gross, W.R.; Menezes, A.F.; Embry, R.B.; Swaroop, V.T.; Chambers, H.G.; Schipma, M.J.; Lieber, R.L.; Domenighetti, A.A. Differential DNA methylation and transcriptional signatures characterize impairment of muscle stem cells in pediatric human muscle contractures after brain injury. FASEB J. 2021, 35, e21928. [Google Scholar] [CrossRef]
- Smith, L.R.; Chambers, H.G.; Lieber, R.L. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev. Med. Child Neurol. 2013, 55, 264–270. [Google Scholar] [CrossRef]
- Von Walden, F.; Gantelius, S.; Liu, C.; Borgstrom, H.; Bjork, L.; Gremark, O.; Stal, P.; Nader, G.A.; Ponte, N.E. Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro-inflammatory gene expression, and reduced rRNA synthesis. Muscle Nerve 2018, 58, 277–285. [Google Scholar] [CrossRef]
- Corvelyn, M.; De Beukelaer, N.; Duelen, R.; Deschrevel, J.; Van Campenhout, A.; Prinsen, S.; Gayan-Ramirez, G.; Maes, K.; Weide, G.; Desloovere, K.; et al. Muscle Microbiopsy to Delineate Stem Cell Involvement in Young Patients: A Novel Approach for Children with Cerebral Palsy. Front. Physiol. 2020, 11, 945. [Google Scholar] [CrossRef]
- Dayanidhi, S.; Buckner, E.H.; Redmond, R.S.; Chambers, H.G.; Schenk, S.; Lieber, R.L. Skeletal muscle maximal mitochondrial activity in ambulatory children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- von Walden, F.; Vechetti, I.J., Jr.; Englund, D.; Figueiredo, V.C.; Fernandez-Gonzalo, R.; Murach, K.; Pingel, J.; McCarthy, J.J.; Stal, P.; Ponten, E. Reduced mitochondrial DNA and OXPHOS protein content in skeletal muscle of children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 1204–1212. [Google Scholar] [CrossRef]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 557–564. [Google Scholar] [CrossRef]
- Johnson, D.L.; Miller, F.; Subramanian, P.; Modlesky, C.M. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J. Pediatr. 2009, 154, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.G.; Singh, H.; Miller, F.; Barbe, M.F.; Slade, J.M.; Pohlig, R.T.; Modlesky, C.M. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone 2017, 94, 90–97. [Google Scholar] [CrossRef]
- Elder, G.C.; Kirk, J.; Stewart, G.; Cook, K.; Weir, D.; Marshall, A.; Leahey, L. Contributing factors to muscle weakness in children with cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Lampe, R.; Grassl, S.; Mitternacht, J.; Gerdesmeyer, L.; Gradinger, R. MRT-measurements of muscle volumes of the lower extremities of youths with spastic hemiplegia caused by cerebral palsy. Brain Dev. 2006, 28, 500–506. [Google Scholar] [CrossRef]
- Moreau, N.G.; Simpson, K.N.; Teefey, S.A.; Damiano, D.L. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys. Ther. 2010, 90, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, S.K.; Binder-Macleod, S.A.; Lee, S.C. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve 2005, 31, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Pontiff, M.R.T.; Connick, B.; Robertson, M.; Moreau, N.G. Age related changes in muscle size and strength across the lifespan in individuals with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 34–35. [Google Scholar]
- Andersson, C.; Mattsson, E. Adults with cerebral palsy: A survey describing problems, needs, and resources, with special emphasis on locomotion. Dev. Med. Child Neurol. 2001, 43, 76–82. [Google Scholar] [CrossRef]
- Jahnsen, R.; Villien, L.; Egeland, T.; Stanghelle, J.K.; Holm, I. Locomotion skills in adults with cerebral palsy. Clin. Rehabil. 2004, 18, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Molnar, G.E.; Lankasky, K. Medical and functional status of adults with cerebral palsy. Dev. Med. Child Neurol. 1995, 37, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Opheim, A.; Jahnsen, R.; Olsson, E.; Stanghelle, J.K. Walking function, pain, and fatigue in adults with cerebral palsy: A 7-year follow-up study. Dev. Med. Child Neurol. 2009, 51, 381–388. [Google Scholar] [CrossRef]
- Moreau, N.G. Muscle Performance in Children and Youth with Cerebral Palsy: Implications for Resistance Training. In Cerebral Palsy; Miller, F., Bachrach, S., Lennon, N., O’Neil, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 2629–2640. [Google Scholar] [CrossRef]
- Moreau, N.G.; Holthaus, K.; Marlow, N. Differential adaptations of muscle architecture to high-velocity versus traditional strength training in cerebral palsy. Neurorehabil. Neural Repair 2013, 27, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.G. Muscle structural adaptation in cerebral palsy and its relationship to function. In A Handbook of Pediatric Constraint-Induced Movement Therapy (P-CIMT): A Guide for Occupational and Physical Therapists, Researchers, and Clinicians; Ramey, S., Coker-Bolt, P., DeLuca, S., Eds.; American Occupational Therapy Association Press: Bethesda, MD, USA, 2013. [Google Scholar]
- Moreau, N.G.; Lieber, R.L. Effects of voluntary exercise on muscle structure and function in cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 700–708. [Google Scholar] [CrossRef]
- Gillett, J.G.; Boyd, R.N.; Carty, C.P.; Barber, L.A. The impact of strength training on skeletal muscle morphology and architecture in children and adolescents with spastic cerebral palsy: A systematic review. Res. Dev. Disabil. 2016, 56, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Faigenbaum, A.D.; Lloyd, R.S.; Myer, G.D. Youth resistance training: Past practices, new perspectives, and future directions. Pediatr. Exerc. Sci. 2013, 25, 591–604. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Bush, J.A.; McLoone, R.P.; Kreckel, M.C.; Farrell, A.; Ratamess, N.A.; Kang, J. Benefits of Strength and Skill-based Training During Primary School Physical Education. J. Strength Cond. Res. 2015, 29, 1255–1262. [Google Scholar] [CrossRef]
- Zwolski, C.; Quatman-Yates, C.; Paterno, M.V. Resistance Training in Youth: Laying the Foundation for Injury Prevention and Physical Literacy. Sports Health 2017, 9, 436–443. [Google Scholar] [CrossRef]
- Peterson, M.D.; Saltarelli, W.A.; Visich, P.S.; Gordon, P.M. Strength capacity and cardiometabolic risk clustering in adolescents. Pediatrics 2014, 133, e896–e903. [Google Scholar] [CrossRef]
- Peterson, M.D.; Zhang, P.; Saltarelli, W.A.; Visich, P.S.; Gordon, P.M. Low Muscle Strength Thresholds for the Detection of Cardiometabolic Risk in Adolescents. Am. J. Prev. Med. 2016, 50, 593–599. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Kersh, M.E.; Carballido-Gamio, J.; Thompson, W.R.; Keyak, J.H.; Warden, S.J. Physical Activity for Strengthening Fracture Prone Regions of the Proximal Femur. Curr. Osteoporos. Rep. 2017, 15, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women with Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- McNee, A.E.; Gough, M.; Morrissey, M.C.; Shortland, A.P. Increases in muscle volume after plantarflexor strength training in children with spastic cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 429–435. [Google Scholar] [CrossRef]
- Moreau, N.G.; Bodkin, A.W.; Bjornson, K.; Hobbs, A.; Soileau, M.; Lahasky, K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children with Cerebral Palsy: Systematic Review and Meta-analysis. Phys. Ther. 2016, 96, 1938–1954. [Google Scholar] [CrossRef]
- Ryan, J.M.; Cassidy, E.E.; Noorduyn, S.G.; O’Connell, N.E. Exercise interventions for cerebral palsy. Cochrane Database Syst. Rev. 2017, 6, CD011660. [Google Scholar] [CrossRef] [PubMed]
- Pontiff, M.; Li, L.; Moreau, N.G. Reliability, Validity and Minimal Detectable Change of a Power Leg Press Test in Individuals with Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2023; in press. [Google Scholar] [CrossRef]
- Pontiff, M.; Moreau, N.G. Safety and Feasibility of 1-Repetition Maximum (1-RM) Testing in Children and Adolescents with Bilateral Spastic Cerebral Palsy. Pediatr. Phys. Ther. 2022, 34, 472–478. [Google Scholar] [CrossRef]
- Colquitt, G.; Kiely, K.; Caciula, M.; Li, L.; Vogel, R.L.; Moreau, N.G. Community-Based Upper Extremity Power Training for Youth with Cerebral Palsy: A Pilot Study. Phys. Occup. Ther. Pediatr. 2020, 40, 31–46. [Google Scholar] [CrossRef]
- Sukal-Moulton, T.; Egan, T.; Johnson, L.; Lein, C.; Gaebler-Spira, D. Use of Frame Running for Adolescent Athletes with Movement Challenges: Study of Feasibility to Support Health and Participation. Front. Sports Act. Living 2022, 4, 830492. [Google Scholar] [CrossRef]
- Hjalmarsson, E.; Fernandez-Gonzalo, R.; Lidbeck, C.; Palmcrantz, A.; Jia, A.; Kvist, O.; Pontén, E.; von Walden, F. RaceRunning training improves stamina and promotes skeletal muscle hypertrophy in young individuals with cerebral palsy. BMC Musculoskelet. Disord. 2020, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Mockford, M.; Caulton, J.M. Systematic review of progressive strength training in children and adolescents with cerebral palsy who are ambulatory. Pediatr. Phys. Ther. 2008, 20, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, W.H. Meta-analysis of the effect of strengthening interventions in individuals with cerebral palsy. Res. Dev. Disabil. 2014, 35, 239–249. [Google Scholar] [CrossRef]
- Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; Goonathilake, M.R.; Waqar, S.; George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; et al. Exercise Prescription and the Minimum Dose for Bone Remodeling Needed to Prevent Osteoporosis in Postmenopausal Women: A Systematic Review. Cureus 2022, 14, e25993. [Google Scholar] [CrossRef]
- Janz, K.F.; Burns, T.L.; Levy, S.M.; Torner, J.C.; Willing, M.C.; Beck, T.J.; Gilmore, J.M.; Marshall, T.A. Everyday activity predicts bone geometry in children: The lowa bone development study. Med. Sci. Sports Exerc. 2004, 36, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.; Baxter-Jones, A.D.; Mirwald, R.L.; Almstedt, H.; Fuchs, R.K.; Durski, S.; Snow, C. Impact exercise increases BMC during growth: An 8-year longitudinal study. J. Bone Miner. Res. 2008, 23, 986–993. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Milliken, L.A.; Westcott, W.L. Maximal strength testing in healthy children. J. Strength Cond. Res. 2003, 17, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Wexler, L. School-Based Positive Youth Development: A Systematic Review of the Literature. J. Sch. Health 2017, 87, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.W.D.; McCauley, C.; Grant-Beuttler, M. Assessing Functional Outcomes in Children with Disabilities through a Community Dance Program. Pediatr. Phys. Ther. 2021, 34, 97–140. [Google Scholar]
- Wiart, L. How do we ensure sustainable physical activity options for people with disabilities? Dev. Med. Child Neurol. 2016, 58, 788. [Google Scholar] [CrossRef]
- Shields, N.; Synnot, A. Perceived barriers and facilitators to participation in physical activity for children with disability: A qualitative study. BMC Pediatr. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Roberts, R.; Bowman, G.; Crettenden, A. Barriers and facilitators to physical activity participation for children with physical disability: Comparing and contrasting the views of children, young people, and their clinicians. Disabil. Rehabil. 2019, 41, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, H.; Richardson, E.V.; Sandal, L.F.; Juul-Kristensen, B.; Troelsen, J. Fitness for all: How do non-disabled people respond to inclusive fitness centres? BMC Sports Sci. Med. Rehabil. 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, H.; Sandal, L.F.; Juhl, C.B.; Troelsen, J.; Juul-Kristensen, B. Barriers to, and Facilitators of, Exercising in Fitness Centres among Adults with and without Physical Disabilities: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 7341. [Google Scholar] [CrossRef]

| Intensity * | Volume | Skeletal Site † | Speed | Duration # | Rest |
|---|---|---|---|---|---|
| Body weight | 100 jumps of boxes of varying heights up to 24 inches. | Hip, spine | Controlled, landing with both feet | 3–6 mths | 15–30 sec between jumps |
| Body weight | 100 jump circuit (hopscotch, jump ups, skips, side jumps) from floor height. | Hip, spine | Controlled landing with both feet | 3–6 mths | 15–30 sec between jumps |
| Body weight | Jump roping, 5–10 min (~50 jumps/min) | Hip, spine | Controlled, landing with both feet | 3–6 mths |
| Parameter | Intensity | Volume | Speed | Frequency | Duration | Rest |
|---|---|---|---|---|---|---|
| Muscle strength | 70% to 85% of 1RM | 3 sets of 6 to 10 repetitions | Slow and controlled to moderate (concentric and eccentric) | 2–3 × per week (nonconsecutive days) | 8–20 weeks | 1–2 min. between sets; 48 h between sessions |
| Muscle power | 60% to 80% of 1RM | 3–6 sets of 1 to 6 repetitions | Concentric: As fast as possible Eccentric: Slow and controlled over 2–3 s | 2–3 × per week (nonconsecutive days) | 8–20 weeks | 1–2 min. between sets; 48 h between sessions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, N.G.; Friel, K.M.; Fuchs, R.K.; Dayanidhi, S.; Sukal-Moulton, T.; Grant-Beuttler, M.; Peterson, M.D.; Stevenson, R.D.; Duff, S.V. Lifelong Fitness in Ambulatory Children and Adolescents with Cerebral Palsy I: Key Ingredients for Bone and Muscle Health. Behav. Sci. 2023, 13, 539. https://doi.org/10.3390/bs13070539
Moreau NG, Friel KM, Fuchs RK, Dayanidhi S, Sukal-Moulton T, Grant-Beuttler M, Peterson MD, Stevenson RD, Duff SV. Lifelong Fitness in Ambulatory Children and Adolescents with Cerebral Palsy I: Key Ingredients for Bone and Muscle Health. Behavioral Sciences. 2023; 13(7):539. https://doi.org/10.3390/bs13070539
Chicago/Turabian StyleMoreau, Noelle G., Kathleen M. Friel, Robyn K. Fuchs, Sudarshan Dayanidhi, Theresa Sukal-Moulton, Marybeth Grant-Beuttler, Mark D. Peterson, Richard D. Stevenson, and Susan V. Duff. 2023. "Lifelong Fitness in Ambulatory Children and Adolescents with Cerebral Palsy I: Key Ingredients for Bone and Muscle Health" Behavioral Sciences 13, no. 7: 539. https://doi.org/10.3390/bs13070539
APA StyleMoreau, N. G., Friel, K. M., Fuchs, R. K., Dayanidhi, S., Sukal-Moulton, T., Grant-Beuttler, M., Peterson, M. D., Stevenson, R. D., & Duff, S. V. (2023). Lifelong Fitness in Ambulatory Children and Adolescents with Cerebral Palsy I: Key Ingredients for Bone and Muscle Health. Behavioral Sciences, 13(7), 539. https://doi.org/10.3390/bs13070539





