Effect of Confinement on Anxiety Symptoms and Sleep Quality during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Assessments
2.2.1. Anxiety
2.2.2. Sleep Quality
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 94; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Perea Tinajero, G.; Bąk, A. To Lock or Not to Lock? Mexico Case. Hist. Philos. Life Sci. 2021, 43, 1–5. [Google Scholar]
- Sher, L. COVID-19, Anxiety, Sleep Disturbances and Suicide. Sleep Med. 2020, 70, 124. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Domenico, M.D.; Uddin, L.Q.; Cauda, V.; Grossmann, I.; Lacasa, L.; Grancini, G.; Mahmoudi, M.; Rezaei, N. Correction to: COVID-19 and Its Global Economic Impact. In Coronavirus Disease-COVID-19; Springer: Berlin/Heidelberg, Germany, 2021; p. C1. [Google Scholar]
- Trógolo, M.A.; Moretti, L.S.; Medrano, L.A. A Nationwide Cross-Sectional Study of Workers’ Mental Health During the COVID-19 Pandemic: Impact of Changes in Working Conditions, Financial Hardships, Psychological Detachment from Work and Work-Family Interface. BMC Psychol. 2022, 10, 73. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Talevi, D.; Socci, V.; Carai, M.; Carnaghi, G.; Faleri, S.; Trebbi, E.; Bernardo, A.D.; Capelli, F.; Pacitti, F. Mental Health Outcomes of the CoViD-19 Pandemic. Riv. Psichiatr. 2020, 55, 137–144. [Google Scholar]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors During the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic Among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Mohammad, D.; Qureshi, M.F.H.; Abbas, M.Z.; Aleem, S. Prevalence, Psychological Responses and Associated Correlates of Depression, Anxiety and Stress in a Global Population, During the Coronavirus Disease (COVID-19) Pandemic. Community Ment. Health J. 2021, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, L.C.L.; Florio, M.A.D.; Leyes, C.A.; Fong, S.B.; Rigalli, A.; Godoy, J.C. Levels and Predictors of Depression, Anxiety, and Suicidal Risk During COVID-19 Pandemic in Argentina: The Impacts of Quarantine Extensions on Mental Health State. Psychol. Health Med. 2022, 27, 13–29. [Google Scholar] [CrossRef]
- Tollos, I.; Theodorakopoulou, A.; Christodoulou, G.N. Stress and Pathophysiological Mechanisms for the Development of Psychosomatic Disease. Psychiatrike 2021, 32, 148–156. [Google Scholar] [CrossRef]
- McMakin, D.L.; Alfano, C.A. Sleep and Anxiety in Late Childhood and Early Adolescence. Curr. Opin. Psychiatry 2015, 28, 483. [Google Scholar] [CrossRef]
- Gould, C.E.; Spira, A.P.; Liou-Johnson, V.; Cassidy-Eagle, E.; Kawai, M.; Mashal, N.; O’Hara, R.; Beaudreau, S.A. Association of Anxiety Symptom Clusters with Sleep Quality and Daytime Sleepiness. J. Gerontol. Ser. B 2018, 73, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Maya, R.; Paola, E. Efecto de La Ansiedad En El Ciclo Sueño-Vigilia. Bachelor’s Thesis, Benemérita Universidad Autónoma de Puebla, Puebla, Mexico, 2021. [Google Scholar]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.; Yang, H.; Elhai, J.; Asmundson, G.J. Anxiety Regarding Contracting COVID-19 Related to Interoceptive Anxiety Sensations: The Moderating Role of Disgust Propensity and Sensitivity. J. Anxiety Disord. 2020, 73, 102233. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Doyle, W.J.; Alper, C.M.; Janicki-Deverts, D.; Turner, R.B. Sleep Habits and Susceptibility to the Common Cold. Arch. Intern. Med. 2009, 169, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Born, J. Sleep, Don’t Sneeze: Longer Sleep Reduces the Risk of Catching a Cold. Sleep 2015, 38, 1341–1342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prather, A.A.; Janicki-Deverts, D.; Hall, M.H.; Cohen, S. Behaviorally Assessed Sleep and Susceptibility to the Common Cold. Sleep 2015, 38, 1353–1359. [Google Scholar] [CrossRef]
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of Sleep Disturbance During COVID-19 Pandemic: A Systematic Review. Nat. Sci. Sleep 2021, 13, 933. [Google Scholar] [CrossRef]
- Idrissi, A.J.; Lamkaddem, A.; Benouajjit, A.; El Bouaazzaoui, M.B.; El Houari, F.; Alami, M.; Labyad, S.; Chahidi, A.; Benjelloun, M.; Rabhi, S.; et al. Sleep Quality and Mental Health in the Context of COVID-19 Pandemic and Lockdown in Morocco. Sleep Med. 2020, 74, 248–253. [Google Scholar] [CrossRef]
- Pieh, C.; Budimir, S.; Probst, T. The Effect of Age, Gender, Income, Work, and Physical Activity on Mental Health During Coronavirus Disease (COVID-19) Lockdown in Austria. J. Psychosom. Res. 2020, 136, 110186. [Google Scholar] [CrossRef]
- Kasper, S.; Wehr, T.A.; Bartko, J.J.; Gaist, P.A.; Rosenthal, N.E. Epidemiological Findings of Seasonal Changes in Mood and Behavior: A Telephone Survey of Montgomery County, Maryland. Arch. Gen. Psychiatry 1989, 46, 823–833. [Google Scholar]
- Goikolea, J.; Miralles, G.; Cabré, A.B.; Vieta, E.; Bulbena, A. Adaptación Española Del Cuestionario de Evaluación de Perfil Estacional (Seasonal Pattern Assessment Questionnaire, SPAQ) En Las Versiones de Adultos e Infanto-Juvenil. Actas Españolas De Psiquiatr. 2003, 31, 192–198. [Google Scholar]
- De Rudder, B. Grundriss Einer Meteorobiologie Des Menschen: Wetter-Und Jahreszeiteneinflüsse; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rosen, L.N.; Targum, S.D.; Terman, M.; Bryant, M.J.; Hoffman, H.; Kasper, S.F.; Hamovit, J.R.; Docherty, J.P.; Welch, B.; Rosenthal, N.E. Prevalence of Seasonal Affective Disorder at Four Latitudes. Psychiatry Res. 1990, 31, 131–144. [Google Scholar] [CrossRef]
- Ozaki, N.; Ono, Y.; Ito, A.; Rosenthal, N.E. Prevalence of Seasonal Difficulties in Mood and Behavior Among Japanese Civil Servants. Am. J. Psychiatry 1995, 152, 1225–1227. [Google Scholar]
- Sakamoto, K.; Kamo, T.; Nakadaira, S.; Tamura, A.; Takahashi, K. A Nationwide Survey of Seasonal Affective Disorder at 53 Outpatient University Clinics in Japan. Acta Psychiatr. Scand. 1993, 87, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Aravena, C.; Estrada-Goic, C.; Núñez-Espinosa, C. Sintomatología Depresiva y Calidad de Vida En Estudiantes de Medicina En Alta Latitud Sur. Rev. Médica De Chile 2021, 149, 357–365. [Google Scholar] [CrossRef]
- Gobierno de Chile. Instructivo Para Permisos de Desplazamiento, Actualizado al 09 de Julio de 2020. Plan de Acción Coronavirus COVID-19. Available online: https://cdn.digital.gob.cl/public_files/Campa%C3%B1as/Corona-Virus/documentos/Instructivo_Cuarentena_09072020.pdf (accessed on 15 August 2022).
- Sanz, J.; Navarro, M.E. Propiedades Psicométricas de Una Versión Española Del Inventario de Ansiedad de Beck (BAI) En Estudiantes Universitarios. Ansiedad Y Estrés 2003, 9, 59–84. [Google Scholar]
- Starosta, A.; LA, B. Beck Anxiety Inventory. In Encyclopedia of Clinical Neuropsychology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Jiménez-Genchi, A.; Monteverde-Maldonado, E.; Nenclares-Portocarrero, A.; Esquivel-Adame, G.; Vega-Pacheco, A. de la Confiabilidad y análisis Factorial de La Versión En Español Del íNdice de Calidad de Sueño de Pittsburgh En Pacientes Psiquiátricos. Gac. Médica De México 2008, 144, 491–496. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio; PBC: Boston, MA, USA, 2021. [Google Scholar]
- Rizopoulos, D. Ltm: An r Package for Latent Variable Modelling and Item Response Theory Analyses. J. Stat. Softw. 2006, 17, 1–25. [Google Scholar] [CrossRef]
- Suso-Ribera, C.; Martín-Brufau, R. How Much Support Is There for the Recommendations Made to the General Population During Confinement? A Study During the First Three Days of the Covid–19 Quarantine in Spain. Int. J. Environ. Res. Public Health 2020, 17, 4382. [Google Scholar] [CrossRef]
- Canet-Juric, L.; Andrés, M.L.; Del Valle, M.; López-Morales, H.; Poó, F.; Galli, J.I.; Yerro, M.; Urquijo, S. A Longitudinal Study on the Emotional Impact Cause by the COVID-19 Pandemic Quarantine on General Population. Front. Psychol. 2020, 11, 2431. [Google Scholar] [CrossRef]
- Gismero-González, E.; Bermejo-Toro, L.; Cagigal, V.; Roldán, A.; Martínez-Beltrán, M.J.; Halty, L. Emotional Impact of COVID-19 Lockdown Among the Spanish Population. Front. Psychol. 2020, 11, 616978. [Google Scholar] [CrossRef] [PubMed]
- Cheikh Ismail, L.; Mohamad, M.N.; Bataineh, M.F.; Ajab, A.; Al-Marzouqi, A.M.; Jarrar, A.H.; Abu Jamous, D.O.; Ali, H.I.; Al Sabbah, H.; Hasan, H.; et al. Impact of the Coronavirus Pandemic (COVID-19) Lockdown on Mental Health and Well-Being in the United Arab Emirates. Front. Psychiatry 2021, 12, 265. [Google Scholar] [CrossRef]
- Saunders, R.; Buckman, J.E.; Fonagy, P.; Fancourt, D. Understanding Different Trajectories of Mental Health Across the General Population During the COVID-19 Pandemic. Psychol. Med. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Graham, B.M. Why Are Women so Vulnerable to Anxiety, Trauma-Related and Stress-Related Disorders? The Potential Role of Sex Hormones. Lancet Psychiatry 2017, 4, 73–82. [Google Scholar] [CrossRef]
- Dagnino-Subiabre, A. Resilience to Stress and Social Touch. Curr. Opin. Behav. Sci. 2022, 43, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.A.D.B.; Martins, B.G.; Campos, L.A.; Marôco, J.; Saadiq, R.A.; Ruano, R. Early Psychological Impact of the COVID-19 Pandemic in Brazil: A National Survey. J. Clin. Med. 2020, 9, 2976. [Google Scholar] [CrossRef]
- Alexander, J.L.; Dennerstein, L.; Kotz, K.; Richardson, G. Women, Anxiety and Mood: A Review of Nomenclature, Comorbidity and Epidemiology. Expert Rev. Neurother. 2007, 7, S45–S58. [Google Scholar] [CrossRef]
- Hodes, G.E.; Epperson, C.N. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol. Psychiatry 2019, 86, 421–432. [Google Scholar] [CrossRef]
- Prater, K.E.; Hosanagar, A.; Klumpp, H.; Angstadt, M.; Phan, K.L. Aberrant Amygdala–Frontal Cortex Connectivity During Perception of Fearful Faces and at Rest in Generalized Social Anxiety Disorder. Depress. Anxiety 2013, 30, 234–241. [Google Scholar] [CrossRef]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021, 44, 227–240. [Google Scholar] [CrossRef]
- Mena, F.J.; De Paz, V.; Avilés, M.; Orantes, L. Educabilidad y Salud Mental de Universitarios Salvadoreños Durante La Pandemia Por Covid-19. Cienc. Y Educ. 2021, 5, 19–38. [Google Scholar] [CrossRef]
- Oliver, N.; Barber, X.; Roomp, K.; Roomp, K. The Covid19 Impact Survey: Assessing the Pulse of the COVID-19 Pandemic in Spain via 24 Questions. arXiv 2020, arXiv:2004.01014 2020. [Google Scholar]
- ElHafeez, S.A.; Cruz, M.M.E.; Gouda, S.; Nofal, M.; Fayed, A.; Ghazy, R.M.; Mekky, J. Sleep Quality and Anxiety Among Egyptian Population During Covid-19 Pandemic. Sleep Sci. 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Pachito, D.V.; Morihisa, R.; Carvalho, P.; Lobao, A.; Poyares, D. Sleep Quality in the Brazilian General Population: A Cross-Sectional Study. Sleep Epidemiol. 2022, 2, 100020. [Google Scholar] [CrossRef]
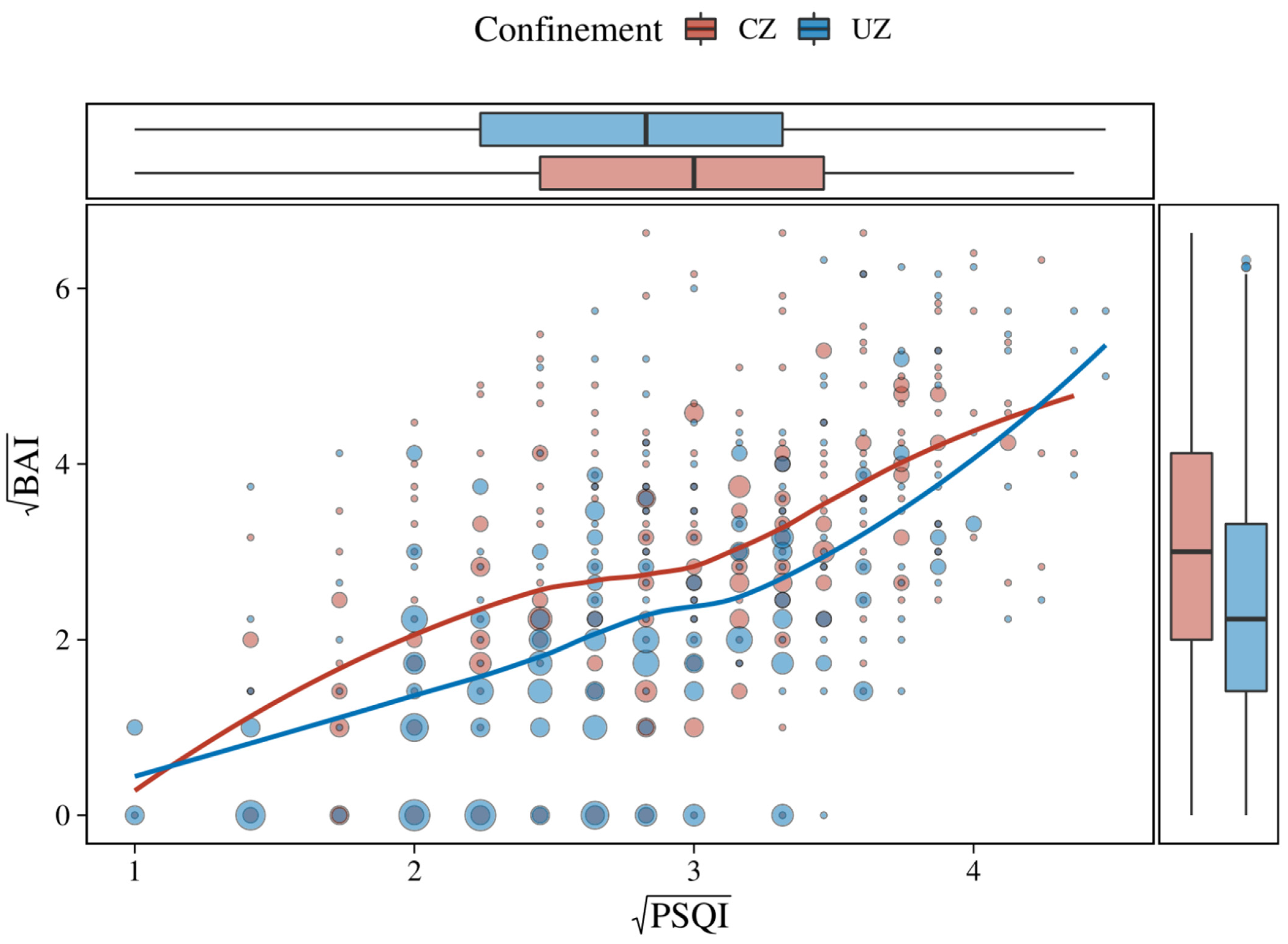
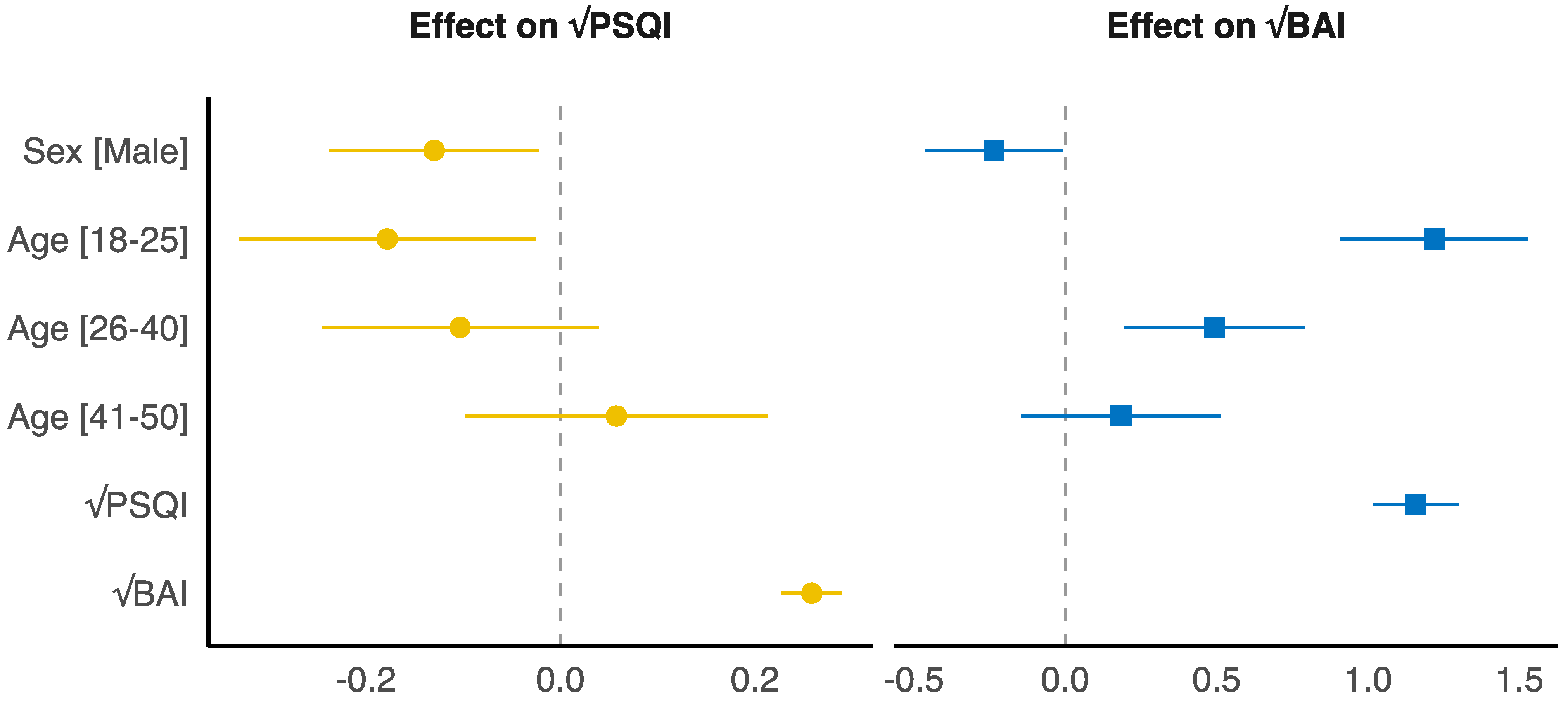
| Variable | Age Groups | |||||
|---|---|---|---|---|---|---|
| 18–25 Years n = 178 (28.8%) | 26–40 Years n = 212 (34.4%) | 41–50 Years n = 129 (20.9%) | >50 Years n = 98 (15.9%) | p Value | ||
| CZ | Sex | 0.814 | ||||
| Female | 113 (18%) | 63 (10%) | 28 (4%) | 21 (3%) | ||
| Male | 28 (4%) | 17 (3%) | 10 (2%) | 7 (1%) | ||
| BAI score | 13 (15) x | 9 (11.3) z | 7 (7) | 3.5 (6.3) | <0.001 | |
| PSQI score | 10 (5) | 8.5 (6) | 10 (6) | 8 (6.3) | 0.075 | |
| PZ | Sex | 0.808 | ||||
| Female | 25 (4%) | 94 (15%) | 71 (11%) | 49 (8%) | ||
| Male | 13 (2%) | 40 (6%) | 25 (4%) | 21 (3%) | ||
| BAI score | 9 (12) x | 4 (11) | 4.5 (8) | 4 (7) | 0.017 | |
| PSQI score | 7 (4) | 8 (6) | 8.5 (6) | 8 (5) | 0.519 | |
| Model | Parameter | SS | df | MS | F | p | 95% CI | SS | df | MS | F | p | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zone | 80.74 | 1 | 80.74 | 33.4 | <0.001 | 0.05 | [0.03, 1.00] | 3.72 | 1 | 3.72 | 7.34 | 0.007 | 0.01 | [0.00, 1.00] |
| Residuals | 1486.69 | 615 | 2.42 | 312.24 | 615 | 0.51 | |||||||||
| 2 a,b | Sex | 42.54 | 1 | 42.54 | 17.97 | <0.001 | 0.03 | [0.01, 1.00] | 9.24 | 1 | 9.24 | 18.66 | <0.001 | 0.03 | [0.01, 1.00] |
| Zone | 71.22 | 1 | 71.22 | 30.08 | <0.001 | 0.05 | [0.02, 1.00] | 2.78 | 1 | 2.78 | 5.61 | 0.018 | 0.01 | [0.00, 1.00] | |
| Sex × Zone | 0.51 | 1 | 0.51 | 0.22 | 0.643 | 0.00 | [0.00, 1.00] | 0.04 | 1 | 0.04 | 0.08 | 0.774 | 0.00 | [0.00, 1.00] | |
| Residuals | 1453.16 | 613 | 2.37 | 303.91 | 613 | 0.50 | |||||||||
| 3 a | Sex | 42.54 | 1 | 42.54 | 19.31 | <0.001 | 0.03 | [0.01, 1.00] | 9.24 | 1 | 9.24 | 18.72 | <0.001 | 0.03 | [0.01, 1.00] |
| Age cat | 166.87 | 3 | 55.62 | 25.25 | <0.001 | 0.11 | [0.07, 1.00] | 3.80 | 3 | 1.27 | 2.57 | 0.054 | 0.01 | [0.00, 1.00] | |
| Zone | 11.83 | 1 | 11.83 | 5.37 | 0.021 | 0.01 | [0.00, 1.00] | 1.40 | 1 | 1.40 | 2.86 | 0.092 | 0.00 | [0.00, 1.00] | |
| Sex × Age cat | 2.37 | 3 | 0.79 | 0.36 | 0.782 | 0.00 | [0.00, 1.00] | 1.35 | 3 | 0.45 | 0.92 | 0.432 | 0.00 | [0.00, 1.00] | |
| Sex × Zone | 0.33 | 1 | 0.33 | 0.15 | 0.701 | 0.00 | [0.00, 1.00] | 0.02 | 1 | 0.02 | 0.04 | 0.833 | 0.00 | [0.00, 1.00] | |
| Age cat × Zone | 14.91 | 3 | 4.97 | 2.26 | 0.080 | 0.01 | [0.00, 1.00] | 3.03 | 3 | 1.01 | 2.05 | 0.105 | 0.01 | [0.00, 1.00] | |
| Residuals | 1328.59 | 604 | 2.20 | 297.12 | 604 | 0.49 | |||||||||
| Compared Levels | Difference | 95% CI | SE | t(613) | p † | Difference | 95% CI | SE | t(613) | p† | |||||
| Female CZ | Female PZ | 0.72 | [0.34, 1.10] | 0.14 | 4.98 | <0.001 | 0.13 | [−0.05, 0.30] | 0.07 | 1.91 | 0.225 | ||||
| Female CZ | Male CZ | 0.61 | [0.02, 1.19] | 0.22 | 2.76 | 0.030 | 0.24 | [−0.02, 0.51] | 0.10 | 2.42 | 0.075 | ||||
| Female CZ | Male PZ | 1.19 | [0.70, 1.69] | 0.19 | 6.38 | <0.001 | 0.41 | [0.18, 0.63] | 0.09 | 4.76 | <0.001 | ||||
| Female PZ | Male PZ | 0.48 | [−0.02, 0.97] | 0.19 | 2.56 | 0.053 | 0.28 | [0.06, 0.51] | 0.09 | 3.31 | 0.005 | ||||
| Male CZ | Female PZ | 0.11 | [−0.47, 0.69] | 0.22 | 0.49 | 0.961 | −0.12 | [−0.38, 0.15] | 0.10 | −1.18 | 0.642 | ||||
| Male CZ | Male PZ | 0.58 | [−0.08, 1.25] | 0.25 | 2.33 | 0.093 | 0.16 | [−0.14, 0.47] | 0.11 | 1.43 | 0.482 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Aravena, C.; Arriaza, K.; Castillo-Aguilar, M.; Flores, K.; Dagnino-Subiabre, A.; Estrada-Goic, C.; Núñez-Espinosa, C. Effect of Confinement on Anxiety Symptoms and Sleep Quality during the COVID-19 Pandemic. Behav. Sci. 2022, 12, 398. https://doi.org/10.3390/bs12100398
Alvarado-Aravena C, Arriaza K, Castillo-Aguilar M, Flores K, Dagnino-Subiabre A, Estrada-Goic C, Núñez-Espinosa C. Effect of Confinement on Anxiety Symptoms and Sleep Quality during the COVID-19 Pandemic. Behavioral Sciences. 2022; 12(10):398. https://doi.org/10.3390/bs12100398
Chicago/Turabian StyleAlvarado-Aravena, Caren, Karem Arriaza, Matías Castillo-Aguilar, Karen Flores, Alexies Dagnino-Subiabre, Claudia Estrada-Goic, and Cristian Núñez-Espinosa. 2022. "Effect of Confinement on Anxiety Symptoms and Sleep Quality during the COVID-19 Pandemic" Behavioral Sciences 12, no. 10: 398. https://doi.org/10.3390/bs12100398
APA StyleAlvarado-Aravena, C., Arriaza, K., Castillo-Aguilar, M., Flores, K., Dagnino-Subiabre, A., Estrada-Goic, C., & Núñez-Espinosa, C. (2022). Effect of Confinement on Anxiety Symptoms and Sleep Quality during the COVID-19 Pandemic. Behavioral Sciences, 12(10), 398. https://doi.org/10.3390/bs12100398







