Abstract
Numerous psychoneuroimmune factors participate in complex bodily reactions to psychological stress, and some of them can be easily and non-invasively measured in saliva (cortisol, alpha-amylase, proinflammatory cytokines). Cortisol plays a crucial role in the stress response; thus, stressful events (academic examinations, cardiac surgery, dental procedures) are accompanied by an increase in cortisol levels. (A correlation between cortisol blood levels and salivary values has already been confirmed, particularly during stress). Academic stress is defined as everyday stress among students that has an impact on aspects of their psychological and physiological well-being. For example, exams are considered one of the most acute stressful experiences for students. The strength of the association between academic self-efficacy, psychological stress, and anxiety depends on a variety of factors: the type of academic challenge (e.g., oral exam), the presence of an audience, etc. Higher stress levels were predominantly recorded among younger students, primarily regarding their academic tasks and concerns (grades, exams, competing with peers for grades, fear of failing the academic year, etc.). The measurement of stress levels during academic stress can improve our understanding of the character and influence of stressful events in populations of students, preventing adverse reactions to long-term stress, such as a decreased immune response and increased anxiety.
Keywords:
stress; psychological factors; psychological disturbances; students; saliva; biomarkers; cortisol; amylase; HPA axis 1. Introduction
Due to the influence of various stressful stimuli, psychological stress alters the homeostasis of the organism. Consequently, the organism reacts, and the sympathetic-adrenal-medullary (SAM) system and the hypothalamic-pituitary-adrenal (HPA) axis are activated, producing and releasing specific hormones [1,2]. Therefore, the psychological response to emotional stress also modulates the functions of the immune system, the autonomic nervous system (ANS), levels of hypothalamic and pituitary hormones, neuropeptides, cytokines, and other factors involved in this network [3,4].
The stress response results in changes at the molecular level of the whole body (Figure 1) [5,6,7,8,9]. Thus, short-term and long-term effects of stress are associated with changes/alterations in HPA axis functioning, which alters glucocorticoid levels and may influence different health outcomes [10]. The stress response includes the connection between the central nervous system (CNS) and the immune system, with bidirectional connections. In addition, adaptation to stress is a very important mechanism in the body’s response to stress. Thus, the effectiveness of the reaction to stress depends on the type, intensity, and duration of stress as well as the characteristics of the person [11].
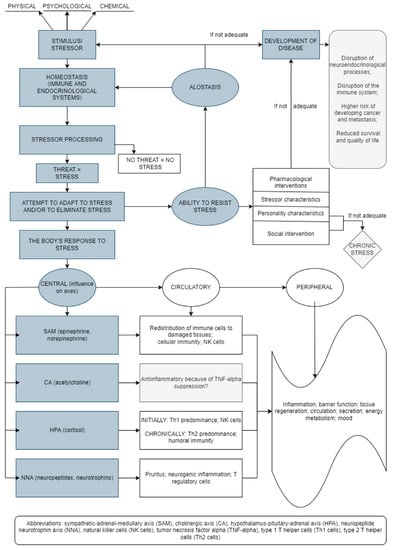
Figure 1.
Stress-induced bodily reactions and factors involved in this network (an original scheme based on current literature data).
The aim of this narrative review is to present current data on psychological stress among graduate students and the possibilities for its assessment using salivary biomarkers. Research findings are based on articles published in English, available through the PubMed database and other prominent sources of research literature.
2. Physiology of Acute and Chronic Stress Reactions and the HPA Axis
A stress reaction implies greater activity of the HPA axis. The organism’s response to acute stress involves a complex process/network which is mediated by the HPA axis and involves changes in psychological and social factors. In addition to acute stress, chronic psychological stress also activates the HPA axis, which causes elevated glucocorticoid levels [12]. It is well-known that this release of stress-associated glucocorticoids can impair memory and cognitive functions [1,13,14,15]. Chronic stress can also weaken the immune response (as confirmed by the determination of antibody responses to vaccines) and can cause or contribute to different diseases such as cardiovascular, endocrine, gastrointestinal diseases, and others [12]. The research literature indicates that acute stress can also change levels of different immune factors, but by increasing them [16]. Increased cortisol levels lead to hyperactivity of the HPA axis, while disorders of the HPA axis present an increased risk for developing various diseases and conditions [17,18]. However, research on the impact of naturally stressful events on diseases has given contradictory results about the increased risk of developing certain diseases [10].
The peripheral and central nervous systems are important factors in a complex network of reactions involved in the body’s response to stress [11]. The brain receives and processes various neurosensory impulses (cortical, limbic, visual, somatosensory, nociceptive, visceral, etc.), including signals coming from the blood (hormones, cytokines, mediators). During psychological stress, the crucial role belongs to the sympathetic nervous system, mostly to the ANS, which controls the functions of the internal organs via the sympathetic and parasympathetic nervous systems [11]. The acute stress response includes, firstly, the registration of the stressful stimulus, which is transmitted from the cerebral cortex to the nucleus of the brainstem locus coeruleus/norepinephrine (LC/NA) and pons, whose neurons stimulate (via receptors) the release of catecholamines from the adrenal medulla directly into circulation. This release of catecholamines affects the body by making an individual more alert and cautious, activating defensive behavior patterns, usually with higher aggression, stimulating the cardiovascular and respiratory systems and inhibiting the gastrointestinal system. Consequently, the increase in catecholamine levels also results in increased levels of plasma glucose and fatty acids. These reactions are crucial for survival and protection of the organism during stressful periods [11].
Stressors stimulate the hypothalamus to secrete corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which leads to the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary lobe. This is followed by activation of adrenal gland cells, which produce and release glucocorticoids. Additionally, HPA axis activation is crucial for the body’s catabolic processes and for supplying the organism with energy. Increased plasma cortisol concentration stimulates the liver to perform the process of gluconeogenesis and causes insulin resistance in peripheral tissues [19]. Consequently, glucocorticoids are crucial in controlling the duration of the stress response. Activation of the stress system causes clinical manifestations that include physiological reactions (oxygen and nutrients are directed to the CNS and other body systems) and behavioral reactions (e.g., increased excitement, vigilance, caution, focus, euphoria, or dysphoria, etc.) [11]. Clearly, multiple systems are involved in the adaptation of the body to new circumstances, and numerous processes (e.g., lipolysis and gluconeogenesis) take place to supply the body with energy at such times. At the same time, the activities of other systems (gastrointestinal, reproductive, and immune systems) are inhibited [11].
Successful adaptation to stress activates the body’s mechanisms that control and inhibit stress reactions—in other words, they prevent an excessive response to stress. Without these mechanisms in place, the stress response would be too intense and prolonged, going beyond successful adjustment, thus potentially causing a pathological condition. There is a constant, dynamic balance between the stimulatory and inhibitory mechanisms of a stress reaction, but the mechanisms that inhibit stress reactions prevail during the recovery process. A proper response to stress involves timely calming or neutralization of stress effects. Catabolic processes predominate after a stressful situation, i.e., energy mobilization occurs due to the effects of stress hormones (catecholamines, cortisol, glucagon) [20]. During rest and recovery, anabolic processes predominate due to the influence of growth hormone and gonadal steroids important for healing and growth. Thus, to establish the homeostasis that each cell tries to achieve, this balance between the anabolic and catabolic processes is needed [11].
Psychological stress is associated with various diseases and conditions, including cardiovascular diseases (e.g., hypertension), diabetes, gastrointestinal disorders, increased susceptibility to infections, autoimmune disorders, malignant diseases, and others [21]. In addition, high levels of stress have an impact on mental health and can lead to drug abuse, reduced work efficiency, absenteeism, and other behaviors associated with poor mental or overall health [2,22,23]. Literature data illustrates how medical interventions (pharmacological, psychotherapeutic) try to reduce the impact of cortisol in response to stressful stimuli [10].
It is important to mention, here, that gender influences the stress response—psychoendocrinological studies have reported lower stress responses in women than in men [24,25,26]. In addition, a subjectively higher perception of stress in women, and their typical cognitive styles, likely contribute to their higher risk of developing psychological disorders and anxiety disorders [27]. Gender’s significant impact on the immune system has been confirmed by several studies that analyzed the acute stress response. Notably, the menstrual cycle affects endocrine and immune variations by altering the level of circulating cytokines or growth factors [28]. A blunting impact on the stress response may be caused by fluctuations of gonadal steroids during the menstrual cycle and may also be related to oral contraceptive use [27,29]. Thus, Helbig et al. [27] showed a stronger cortisol response in women in the luteal phase than in those in the follicular phase or in those taking oral contraceptives. Additionally, salivary secretion differs between the sexes and may be associated with variations in the secretion of gonadal steroids and ANS regulation of salivary glands [30].
3. Immunity during Stress: The Inflammatory Reaction and Stress
The impact that stressful events can have on the immune system is a significant factor that affects the body’s ability to defend itself against diseases. Stress reduces the activity of natural killer (NK) cells and the proliferation of lymphocytes to specific viral antigens or nonspecific mitogens of B- or T-lymphocytes [31,32]. Stress is also associated with lower values of secretory immunoglobulin A (s-IgA) [33].
The relationship between psychological stress and immune parameters (e.g., immune cells, enzymes, cytokines) could be important and so are analyzed using various diagnostic tools [28]. There has been a lot of research in this area because a better understanding of the relationship could reveal useful data on how psychological stress affects the body. There are a few important mediators/cytokines linking the CNS and the immune system: TNF-α, IL-1β, IFN-γ, nerve growth factor (NGF)-β, which can activate the HPA axis [34,35]. Additionally, cytokines IL-1β, TNF-α, and IL-6 are crucial and reliable peripheral biomarkers associated with depression [36]. In addition, studies have revealed that macrophage-derived cytokine migration inhibitory factor (MIF) plays an important role in stress-immune network/interactions. Thus, MIF has proinflammatory, metabolic and angiogenic effects and participates in the pathogenesis of many diseases/disorders (inflammatory diseases, autoimmune diseases, atherosclerosis) and physiologic processes (e.g., wound healing), oncogenesis, metabolic disorders, etc. Animal studies have shown that hypothalamic CRH stimulates the secretion of MIF and thus influences NK cell activities important for inflammatory processes [37]. A particularly meaningful immune factor is IL-1β, especially for immune signaling and induction of the inflammatory process mediated by NK cells; it is linked to inflammatory tissue destruction [24].
The results of studies on the influence of stress on serum IL-1β are contradictory, i.e., some reports found that IL-1β levels during a stressful period had increased while others found that they had decreased [19,38,39]. Most studies in this area assessed the stress response and confirmed that IL-1β responds to antigen stimulation (in vivo and in vitro), e.g., there is a stress-associated increase [24,40,41,42,43]. However, a few researchers, it should be noted, found no effect on IL-1β [44,45,46] and some even observed a decrease [47].
4. Salivary Biomarkers for Stress Assessment
Different types of stress/stressful events are associated with endogenous oxidative and inflammatory stress, key sources of different biomarkers. Inflammatory reactions generate free radicals that generate other inflammatory mediators, such as IL-6, TNF-α, IL-10 or IL-1β [45]. Such circumstances lead to the organism’s greater susceptibility to infection [33]. Therefore, inflammation has been identified as a major etiological factor of various diseases/conditions (e.g., myocardial infarction, cardiovascular diseases, metabolic syndrome, diabetes, cancer, rheumatoid arthritis, neuropsychiatric disorders, etc.) [4,48,49,50,51].
The complexity of stress mechanisms makes acute stress measurement difficult to quantify and interpret. Because of this, the use of many subjective and objective diagnostic methods has been suggested, including salivary stress biomarkers (objective) [52]. Indicators of endocrine stress-associated changes include classical stress biomarkers, such as levels of hormones like cortisol and epinephrine [53]. Along with the ANS and the immune system, there are other HPA axis-associated factors involved in the stress response that present potential stress biomarkers (cortisol, alpha-amylase, and proinflammatory cytokines) (Figure 2) [4,9]. According to literature data, the most valuable salivary markers of stress, potentially, are cortisol, salivary alpha-amylase (sAA), chromogranin A and lysozyme [54]. However, stress hormones (e.g., glucocorticoids), along with cytokines, could also be very useful since they mediate various conditions, including post-traumatic stress disorder (PTSD). They represent important homeostatic regulators but are also promising functional stress biomarkers and can therefore help in the development of new therapeutics [55].
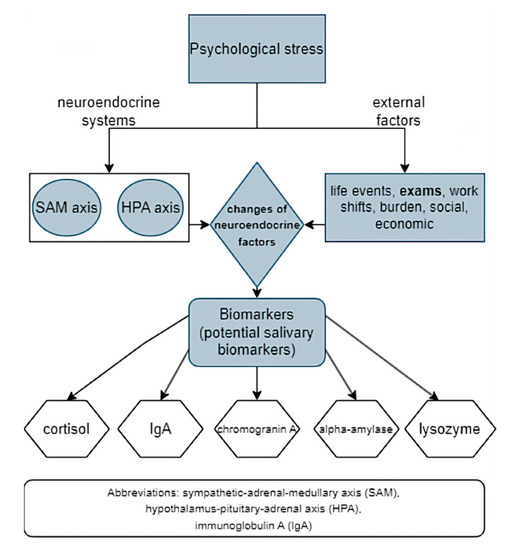
Figure 2.
Psychological stress reaction, changes in neuroendocrine factors and potential salivary biomarkers (an original scheme based on current literature data).
Stress induces several physiological and endocrine changes, disorders of functional parameters and changes in biochemical indicators [56]. This cascade affects the rapidly responding vital systems (primarily cardiovascular and CNS), but also other organ systems (hepatobiliary, pancreatic). Multisystem changes lead to disturbances of various stress-related biomarkers and factors which participate in this complex process [57]. When analyzing stress levels of persons without a psychiatric diagnosis, symptoms of anxiety and depression were associated with blunted/exaggerated cortisol responses to, and recovery from, stress, which may indicate an increased risk for unhealthy HPA axis dysregulation, allostatic load and disease [58]. Additionally, when analyzing HPA axis activation and cortisol levels of patients suffering from autoimmune diseases (systemic lupus erythematosus, Sjögren’s syndrome, and systemic sclerosis), changes in cortisol levels during the day were recorded (a higher area under the curve for cortisol levels was seen) [59]. Exaggerated stress-reactions have generally been viewed as maladaptive, with some evidence showing that individuals with exaggerated cardiovascular stress responses are at increased risk for developing cardiovascular disease due to various manifestations: hypertension, systemic atherosclerosis, coronary artery calcification, left ventricular hypertrophy, increased cardiovascular disease mortality, etc. [60]. Likewise, exaggerated cortisol reactivity was associated with coronary artery calcification and increased hypertension and cardiovascular disease risk. In healthy, older participants without a history/signs of coronary heart disease, there was an association between heightened cortisol reactivity and higher coronary artery calcification, which confirms that heightened HPA activity is a risk factor for coronary heart disease [61]. In addition, concerning stress and salivary cortisol in oral diseases, patients with oral lichen planus significantly exhibited higher stress, depression and anxiety scores compared to controls, with frequently increased cortisol levels (among 56.6% patients) and a positive correlation between psychological factors and salivary cortisol levels [62]. Stress-induced disorders of physiological, endocrine, immune, and metabolic functions lead to a complex cascade of oxidative, inflammatory, genomic, and proteomic responses responsible for excessive production of stress biomarkers [63,64]. Stress-induced reactions also include the participation of pro-inflammatory cytokines and oxidants of one generation that lead to/induce the formation of the second generation, which is followed by other factors such as reactive oxygen species (ROS) and inflammatory mediators [65]. Finally, complex disorders of the homeostatic mechanism cause alterations in protective defense mechanisms and result in different levels and types of stress responses, activating a genomic and proteomic response that expresses genes translated into proteins. It should be mentioned here that some metabolic, inflammatory, oxidative, genomic and proteomic factors can be used as stress biomarkers [63,64]. Thus, some biomarkers (cortisol, alpha-amylase, proinflammatory cytokines) have been established as stress biomarkers that reflect both SAM and HPA activity. Among various factors that belong to the neuroendocrine axis, cortisol plays a crucial role in the stress response [4]. Although new stress biomarkers are being investigated, recent studies have focused on cortisol of various origins [66]. It is well-known that increased cortisol levels are concomitant parameters during stressful events (academic examinations, cardiac surgery, dental procedures, etc.). According to the literature, there is a correlation between blood cortisol levels and salivary cortisol values, observed particularly during stress [67]. The HPA-axis regulates the secretion of cortisol, while the SAM regulates catecholamine secretion. After stress-induced activation of the HPA system, cortisol is secreted into the blood; thus, measuring serum and salivary cortisol levels can be a reliable indicator of stress. Salivary cortisol is mainly found in its unbound (free) form, and it accounts for about 70% of the total unbound cortisol in the organism [68]. Therefore, salivary cortisol is a useful biomarker of stressful conditions. However, the literature does not show uniform results regarding the association of stress and cortisol [54,69]. Another useful stress biomarker is salivary immunoglobulin A (s-IgA), which presents an antibody involved in the prevention of infectious diseases. Stress causes immune changes where s-IgA plays an important role as a biomarker of immune activation [70]. Salivary IgA levels are the first line of defense against various diseases and disorders such as upper respiratory tract disease, caries, and oral infections [16]. Previous studies have shown that acute stress causes activation of s-IgA, while chronic stress influences deleterious health consequences including decreased s-IgA levels [16,54,71]. Literature data shows that psychological stress is inversely related to IgA levels. Additionally, basal s-IgA levels are a potential indicator of health outcomes during stressful periods [16,54,72]. Recent studies have shown that an acute stress response causes a significant increase in salivary alpha-amylase (sAA) levels, and increased sAA concentrations have been reported as indicators and biomarkers of stressful situations [73]. According to the literature, salivary biomarkers are profoundly helpful in the diagnosis of various stress-related diseases including cancers, liver diseases, kidney diseases, neurological and cardiovascular diseases, psoriasis, systemic lupus erythematosus, rheumatoid arthritis [4,50]. Concerning potential influences on levels of salivary biomarkers, the impact of a higher body temperature and exercise on salivary biomarker levels indicates they significantly increase concentrations, i.e., significantly higher levels of cortisol, salivary alpha-amylase and total proteins [4,74]. Concerning s-IgA levels, much of the evidence indicated that mental imaging, relaxation, watching humorous videotapes, writing poetry, progressive muscle relaxation, and hypnosis are effective procedures for increasing s-IgA levels [16]. However, salivary biomarkers could be indicators of various biological processes (e.g., pathogenic or pharmacological responses) [4,53]. Concerning the use of biomarkers for measurements of stress levels among students (academic stress), there are various methods. Generally, questionnaires on stress are commonly used in practice for subjective data. The Scale for Assessing Academic Stress (SAAS), a 30-item self-reporting tool with “Yes” or “No” answers, measures students’ perceived stress [75]. The Perceived Stress Scale-10 (PSS-10) is also a frequently used scale to assess stress levels and evaluates the degree to which external demands appear to be higher than an individual’s perceived capability to handle the situation [76]. In contrast, objective indicators of stress are biomarkers analyzed primarily by blood or saliva. Since analysis of blood involves the invasive procedure of taking blood, salivary biomarkers provide a non-invasive, and thus advantageous, method of analysis. A few potential methods for analysis of salivary biomarkers exist: Enzyme-Linked Immuno Assays (ELISA), Radio Immuno Assays (RIA), as well as specific and sensitive techniques based on liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) [77,78]. The most commonly analyzed salivary biomarker is cortisol. It has been used for more than 30 years, including in studies on different populations, and it has been confirmed as a valid and sensitive method which exhibits parallelism, precision and accuracy [77]. Still, it should be taken into account that various factors can affect salivary cortisol results: sex hormone cycles, menstrual cycles, hormonal contraception usage, puberty, pregnancy, breastfeeding, menopause, etc. [68]. There are also other influences related to diagnostic procedures for salivary biomarkers; thus, although salivary cortisol concentration is not influenced by salivary flow rate and pH, this influence is possible during measurement of the salivary alpha-amylase activity [78]. In addition, there are some possible influences related to sampling procedures with saliva [79]. For example, procedures for collecting oral fluid (saliva) samples may differ, e.g., collecting may include non-stimulated and stimulated oral fluid samples [80]. This may influence the results, e.g., stimulation of the oral sample (saliva) allows for the collection of large sample volumes in a short time, limiting the variability of salivary pH [80].
5. Students and the Concept of Academic Stress
Academic stress is defined as everyday stress among students that has an impact on aspects of their psychological and physiological well-being [21]. Previous studies have noticed that younger students have higher stress levels than older students regarding their academic tasks and concerns (grades, exams, competing with peers for grades, fear of failing the academic year) [80]. Exams are considered one of the most acute stressful experiences for students [13].
Studies on stress reactions among students are common in the literature, and those that analyze the impact of academic stress on students’ reactions have provided useful data, as presented in Table 1, which is based on papers not older than 20 years [1,10,12,13,17,27,28,29,37,71,74,81,82,83,84]. According to a study by Bardi et al. [81], which monitored students during summer exams, students who obtained passing grades and had a better grade point average (GPA) also had a greater ability to remain calm during psychological stress and overall had higher levels of dehydroepiandrosterone (DHEA), a hormone that minimizes the negative effect of stress induced HPA axis activity. Other studies suggest that increased stress levels may be associated with an improved working memory and more pronounced emotional responses [81].

Table 1.
Recent significant studies, involving students as subjects, on salivary cortisol and other salivary biomarkers as indicators of academic stress.
Previous studies show that the strength of the association between academic self-efficacy, anxiety, and psychological stress depends on a variety of factors—the type of academic challenge (e.g., oral exam), the presence of an audience, etc. Although the results of various studies differ, some studies have shown a significant association between academic stress and a decreased immune response on the one hand and certain immune aspects on the other [85]. The heterogeneity of the results is likely due to the fact that not only the impact of academic stress was observed and that some of the studies were not conducted on humans [33]. Many studies on academic stress have been conducted with medical and dental students, possibly because many researchers are doctors who work with students. Previous research notes that medical students experience stress for multiple reasons, including adaptation to the medical environment, exposure to human suffering and death, the imbalance between effort invested in their work and the rewards received combined with the anxiety of having numerous exams and other academic challenges, etc. According to literature data, medical students’ NK cell activities on the first day of final exams were lower than the month prior to exams [11,86,87]. In addition, Kiecolt-Glaser et al. [87] compared Epstein–Barr virus-triggered lymphocyte proliferation in medical students on the first day of the academic year and the final exam period with the month prior to exams and one week after returning from summer vacation. They noted reduced proliferation in the majority of the seropositive students during final exams [87]. Concerning dental students, research results show that clinical work or in-hospital obligations cause the greatest stress [80]. The stress experienced by medical and dental students is associated with the fact that schools of medicine and schools of dental medicine are stressful environments for the majority of students. Their under-graduate programs are often associated with significant symptoms of stress because they are among the longest and most demanding programs of study [1,72,88]. These students are exposed to stress factors that are overwhelming for some of them and could be a reason some quit their studies [89]. Thus, we see that medical and dental students report increased anxiety, frequent depression, obsessive compulsive disorders, interpersonal sensitivity, and other psychological issues/disturbances. For dental students, examinations and clinical exercises are the most frequent causes of stress [88]. Many studies found that, in undergraduate dental students, gender did not affect any of the psychological variables analyzed (distress, emotional exhaustion, stressor intensity), but study results are not consistent [82]. Many studies have compared students’ skills and previous academic outcomes with students’ cognitive traits (the perception of self-efficacy, attributions of success and failure, self-reported coping styles, dysfunctional attitudes, and irrational beliefs) [81]. Dental students also face pressure to develop clinical competencies and interpersonal skills in a short period of time and are exposed to a number of adverse factors (physical position, noise, repetitive movements, high professional competitiveness, lack of time to relax and interact with friends and family, etc.) [30]. According to literature data, for dental students, exams/testing and grades caused the majority of their academic stress and that the big-gest social stressor was having a demanding role in their personal life, such as being a wife or husband [90,91]. The study also found that higher academic stress and total stress scores were noted in those who expected to graduate with great financial debt [90,91]. Finally, it is necessary to mention that the stress resulting from medical work (examinations and clinical competencies) can have a negative impact on mental health and learning [28]. However, a structured learning program can be beneficial for students because it reduces anxiety around exams and allows students to achieve better academic results.
6. Benefits of, and Perspectives on, the Further Use of Salivary Biomarkers for Research Purposes and in Practice
There are many advantages of using saliva as a biofluid for diagnostic purposes: its collection is fast, easy, non-invasive and inexpensive [92]. Its use for analysis of salivary biomarkers should be based on previous study results, which have confirmed a strong relationship between common psychological disturbances (stress, depression or anxiety) and fluctuations in levels/concentrations of stress-related salivary biomarkers (cortisol, alpha-amylase, chromogranin A and lysozyme) [54]. Results obtained from these salivary biomarkers offer insight into individuals’ stress levels, anxiety or depression, primarily using salivary cortisol, immunoglobulin A (sIgA), salivary alpha-amylase, chromogranin A, lysozyme, melatonin, and fibroblast growth factor 2 (FGF-2). Furthermore, assessment of salivary cortisol and melatonin can be helpful for differentiating between stress and depression [54]. When interpreting salivary biomarker results, however, it must be noted that sampling protocols may alter the composition of the saliva, and thus the concentration of some of the most important stress-linked analytes. It should also be taken into account that levels of stress biomarkers are changed by certain physiological and metabolic conditions (e.g., cardiovascular diseases, oral cancers, oral lichen planus, and others); thus, results are not necessarily due to stress itself.
Despite any limitations to its use, evidence/data that saliva can reflect physiological or pathological states means that salivary biomarkers can serve as diagnostic or monitoring tools in many branches of healthcare, such as medicine, dentistry, and pharmacotherapy. Additionally, data on stress-related salivary biomarkers may help our understanding of the complex relationship between psychological and neuroimmune reactions to stress and the influence of psychological stress on the organism, including during academic stress.
In the future, more studies will likely be conducted in this area on measuring and monitoring academic and student stress with salivary biomarkers. This approach could be useful and applied in real life, for example, by helping design measures for the timely recognition/avoidance of developing psychological disturbances and (when possible) the development of psychiatric conditions/diseases. Such data could be helpful in identifying students who might need medical attention due to their perceived stress and who may develop distress-related mental health disturbances. It could also provide evidence-based support for specific public health measures for students to decrease the negative influences of emotional stress and similar disorders, which significantly affect academic performance and, later, their professional work.
7. Conclusions
The organism’s complex reactions to psychological stress, such as academic stress among students, involves various endocrine and immune factors, some of which can be easily measured in saliva and used as biomarkers of stress. According to previous studies, the measurement of stress levels by salivary biomarkers (salivary cortisol, sIgA, salivary alpha-amylase, chromogranin A, lysozyme, melatonin, and others) is a simple, non-invasive and reliable way of collecting samples and obtaining data on the influence of stressful events on students. Some of these biomarkers may also by useful for measuring anxiety or depression and can even help identify students who are prone to high stress associated with exams or studying, including those at high risk for experiencing specifically negative influences of stress on their ability/capacity to study. It could also help identify the students who need greater help coping with stress in general, thus reducing their risk for the development of conditions that are influenced by stress factors (e.g., cardiovascular, autoimmune, cancer). Such data could be a basis for the potential implementation of further anti-stress measures in practice and could lead to organizing better academic courses that allow students to learn more effectively or better manage the demands of academic work. Since students are prone to stress even after graduation, these results could help diminish the impact of stress on the organism during their transition to professional life as well. More research should be done in this field on larger samples of students to gather more specific data on stress-associated salivary biomarkers. This would provide better insight into the usefulness of their interpretation in practice.
Author Contributions
Conceptualization, L.L.-M.; writing—original draft preparation, B.Š. and L.L.-M.; writing—review and editing, M.V., A.G., M.C. and A.Š.; supervision, L.L.-M., B.Š. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ouda, S.; Alaki, S.; Safi, M.A.; Nadhreen, A.; Johani, K.A. Salivary Stress Biomarkers–Are They predictors of Academic Assessment Exams Stress. J. Clin. Exp. Pathol. 2016, 15, 276–279. [Google Scholar]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and Health: A Review of Psychobiological Processes. Annu. Rev. Psychol. 2020, 72, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.L.; Ain, T.S.; Gowhar, O. Effect of Academic Stress on Plaque and Gingival Health among Dental Students of Moradabad, India. J. Int. Acad. Periodontol. 2014, 16, 115–120. [Google Scholar] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Ader, R.; Cohen, N.; Felten, D. Psychoneuroimmunology: Interactions between the Nervous System and the Immune System. Lancet 1995, 345, 99–103. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Besedovsky, H.O.; Rey, A.D. Physiology of Psychoneuroimmunology: A Personal View. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Cvitanović, H.; Djaković, I.; Kuna, M.; Šešerko, A. The Influence of Psychological Stress on HPV Infection Manifestations and Carcinogenesis. Cell Physiol. Biochem. 2021, 55, 71–88. [Google Scholar]
- Gaab, J.; Sonderegger, L.; Scherrer, S.; Ehlert, U. Psychoneuroendocrine Effects of Cognitive-Behavioral Stress Management in a Naturalistic Setting—A Randomized Controlled Trial. Psychoneuroendocrinology 2006, 31, 428–438. [Google Scholar] [CrossRef]
- Ivković, N.; Božović, Đ.; Račić, M.; Popović-Grubač, D.; Davidović, B. Biomarkers of Stress in Saliva. Acta Fac. Med. Naissensis 2015, 32, 91–99. [Google Scholar] [CrossRef]
- McGregor, B.A.; Murphy, K.M.; Albano, D.L.; Ceballos, R.M. Stress, Cortisol, and B Lymphocytes: A Novel Approach to Understanding Academic Stress and Immune Function. Stress 2016, 19, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Koh, D.; Mok, B.Y.Y.; Chia, S.-E.; Lim, L.-P. Salivary Biomarkers Associated with Academic Assessment Stress among Dental Undergraduates. J. Dent. Educ. 2003, 67, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, Glucocorticoids and Memory: Implications for Treating Fear-Related Disorders. Nat. Rev. Neurosci. 2016, 18, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Wolf, O.T.; Jentsch, V.L. Delayed Effects of Acute Stress on Cognitive Emotion Regulation. Psychoneuroendocrinology 2021, 125, 105101. [Google Scholar] [CrossRef]
- Benham, G.; Nash, M.R.; Baldwin, D.R. A Comparison of Changes in Secretory Immunoglobulin a Following a Stress-Inducing and Stress-Reducing Task. Stress Health 2009, 25, 81–90. [Google Scholar] [CrossRef]
- Lenaert, B.; Barry, T.J.; Schruers, K.; Vervliet, B.; Hermans, D. Emotional Attentional Control Predicts Changes in Diurnal Cortisol Secretion Following Exposure to a Prolonged Psychosocial Stressor. Psychoneuroendocrinology 2016, 63, 291–295. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. Hypothalamic-Pituitary-Adrenal Axis and Stress. Chapter 4: Sex Differences in Neurology and Psychiatry. In Handbook of Clinical Neurology; Lanzenberger, R.G.S., Kranz, G.S., Savic, I., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2020; Volume 175, pp. 55–64. [Google Scholar] [CrossRef]
- Lelou, E.; Corlu, A.; Nesseler, N.; Rauch, C.; Mallédant, Y.; Seguin, P.; Aninat, C. The Role of Catecholamines in Pathophysiological Liver Processes. Cells 2022, 11, 1021. [Google Scholar] [CrossRef]
- Jones, B.J.; Tan, T.; Bloom, S.R. Minireview: Glucagon in Stress and Energy Homeostasis. Endocrinology 2012, 153, 1049–1054. [Google Scholar] [CrossRef]
- Knowles, S.R.; Nelson, E.A.; Palombo, E.A. Investigating the Role of Perceived Stress on Bacterial Flora Activity and Salivary Cortisol Secretion: A Possible Mechanism Underlying Susceptibility to Illness. Biol. Psychol. 2008, 77, 132–137. [Google Scholar] [CrossRef]
- McKerrow, I.; Carney, P.A.; Caretta-Weyer, H.; Furnari, M.; Miller Juve, A. Trends in Medical Students’ Stress, Physical, and Emotional Health throughout Training. Med. Educ. Online 2020, 25, 1709278. [Google Scholar] [CrossRef] [PubMed]
- Lever-van Milligen, B.A.; Lamers, F.; Smit, J.H.; Penninx, B.W.J.H. Physiological Stress Markers, Mental Health and Objective Physical Function. J. Psychosom. Res. 2020, 133, 109996. [Google Scholar] [CrossRef] [PubMed]
- Deinzer, R.; Granrath, N.; Stuhl, H.; Twork, L.; Idel, H.; Waschul, B.; Herforth, A. Acute Stress Effects on Local IL-1β Responses to Pathogens in a Human in Vivo Model. Brain Behav. Immun. 2004, 18, 458–467. [Google Scholar] [CrossRef]
- Hamidovic, A.; Van Hedger, K.; Choi, S.H.; Flowers, S.; Wardle, M.; Childs, E. Quantitative Meta-Analysis of Heart Rate Variability Finds Reduced Parasympathetic Cardiac Tone in Women Compared to Men during Laboratory-Based Social Stress. Neurosci. Biobehav. Rev. 2020, 114, 194–200. [Google Scholar] [CrossRef]
- Sze, Y.; Brunton, P.J. Sex, Stress and Steroids. Eur. J. Neurosci. 2019, 52, 2487–2515. [Google Scholar] [CrossRef] [PubMed]
- Helbig, S.; Backhaus, J. Sex Differences in a Real Academic Stressor, Cognitive Appraisal and the Cortisol Response. Physiol. Behav. 2017, 179, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, Y.; Katsuura, S.; Kuwano, Y.; Tanahashi, T.; Rokutan, K. Circulating Cytokine Signatures in Healthy Medical Students Exposed to Academic Examination Stress. Psychophysiology 2012, 49, 991–997. [Google Scholar] [CrossRef]
- Schoofs, D.; Hartmann, R.; Wolf, O.T. Neuroendocrine Stress Responses to an Oral Academic Examination: No Strong Influence of Sex, Repeated Participation and Personality Traits. Stress 2008, 11, 52–61. [Google Scholar] [CrossRef]
- Sangiorgio, J.P.M.; Seixas, G.F.; De Paula Ramos, S.; Dezan-Garbelini, C.C. Salivary Levels of SIgA and Perceived Stress among Dental Students. J. Health Biol. Sci. 2017, 6, 9. [Google Scholar] [CrossRef]
- Cvitanović, H.; Milošević, M.; Bukvić-Bešlić, I.; Lugović-Mihić, L. Determination of Psychological Stress, Serum Immune Parameters, and Cortisol Levels in Patients with Human Papilloma Virus. Clin. Ther. 2020, 42, 783–799. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugović-Mihić, L. Stress-Induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Jemmott, J.B.; Magloire, K. Academic Stress, Social Support, and Secretory Immunoglobulin A. J. Pers. Soc. Psychol. 1988, 55, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Corr, E.M.; van Solingen, C.; Schlamp, F.; Brown, E.J.; Koelwyn, G.J.; Lee, A.H.; Shanley, L.C.; Spruill, T.M.; Bozal, F.; et al. Chronic Stress Primes Innate Immune Responses in Mice and Humans. Cell Rep. 2021, 36, 109595. [Google Scholar] [CrossRef]
- Mössner, R.; Mikova, O.; Koutsilieri, E.; Saoud, M.; Ehlis, A.-C.; Müller, N.; Fallgatter, A.J.; Riederer, P. Consensus Paper of the WFSBP Task Force on Biological Markers: Biological Markers in Depression. World J. Biol. Psychiatry 2007, 8, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, S.; Kamezaki, Y.; Tominaga, K.; Masuda, K.; Nishida, K.; Yamamoto, Y.; Takeo, K.; Yamagishi, N.; Tanahashi, T.; Kawai, T. High-Throughput Screening of Brief Naturalistic Stress-Responsive Cytokines in University Students Taking Examinations. Int. J. Psychophysiol. 2010, 77, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular Links between Endocrine, Nervous and Immune System during Chronic Stress. Brain Behav. 2020, 11, e01960. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Nemeth, D.P.; Liu, X.; Witcher, K.G.; O’Neil, S.M.; Oliver, B.; Bray, C.E.; Sheridan, J.F.; Godbout, J.P.; Quan, N. Interleukin-1 Receptor on Hippocampal Neurons Drives Social Withdrawal and Cognitive Deficits after Chronic Social Stress. Mol. Psychiatry 2020, 26, 4770–4782. [Google Scholar] [CrossRef]
- Slavish, D.C.; Graham-Engeland, J.E.; Smyth, J.M.; Engeland, C.G. Salivary Markers of Inflammation in Response to Acute Stress. Brain Behav. Immun. 2015, 44, 253–269. [Google Scholar] [CrossRef]
- Auer, B.J.; Calvi, J.L.; Jordan, N.M.; Schrader, D.; Byrd-Craven, J. Communication and Social Interaction Anxiety Enhance Interleukin-1 Beta and Cortisol Reactivity during High-Stakes Public Speaking. Psychoneuroendocrinology 2018, 94, 83–90. [Google Scholar] [CrossRef]
- Buzgoova, K.; Balagova, L.; Marko, M.; Kapsdorfer, D.; Riecansky, I.; Jezova, D. Higher Perceived Stress Is Associated with Lower Cortisol Concentrations but Higher Salivary Interleukin-1beta in Socially Evaluated Cold Pressor Test. Stress 2019, 23, 1–8. [Google Scholar] [CrossRef]
- Gassen, J.; Makhanova, A.; Maner, J.K.; Plant, E.A.; Eckel, L.A.; Nikonova, L.; Prokosch, M.L.; Boehm, G.W.; Hill, S.E. Experimentally-Induced Inflammation Predicts Present Focus. Adapt. Hum. Behav. Physiol. 2019, 5, 148–163. [Google Scholar] [CrossRef]
- Lacey, K.; Zaharia, M.D.; Griffiths, J.; Ravindran, A.V.; Merali, Z.; Anisman, H. A Prospective Study of Neuroendocrine and Immune Alterations Associated with the Stress of an Oral Academic Examination among Graduate Students. Psychoneuroendocrinology 2000, 25, 339–356. [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C.; Graham-Engeland, J.E. The Effect of Acute Stress on Salivary Markers of Inflammation: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2020, 88, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.E.; Stanton, C.H.; Slavich, G.M.; Joormann, J. Executive Control, Cytokine Reactivity to Social Stress, and Depressive Symptoms: Testing the Social Signal Transduction Theory of Depression. Stress 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Shields, G.S.; Dunn, T.M.; Trainor, B.C.; Yonelinas, A.P. Determining the Biological Associates of Acute Cold Pressor Post-Encoding Stress Effects on Human Memory: The Role of Salivary Interleukin-1β. Brain Behav. Immun. 2019, 81, 178–187. [Google Scholar] [CrossRef]
- Gohel, V.; Jones, J.A.; Wehler, C.J. Salivary Biomarkers and Cardiovascular Disease: A Systematic Review. Clin. Chem. Lab. Med. 2018, 56, 1432–1442. [Google Scholar] [CrossRef]
- Chauhan, A.; Yadav, S.S.; Dwivedi, P.; Lal, N.; Usman, K.; Khattri, S. Correlation of Serum and Salivary Cytokines Level with Clinical Parameters in Metabolic Syndrome with Periodontitis. J. Clin. Lab. Anal. 2016, 30, 649–655. [Google Scholar] [CrossRef]
- Silvestre-Rangil, J.; Bagan, L.; Silvestre, F.; Martinez-Herrera, M.; Bagan, J. Periodontal, Salivary and IL-6 Status in Rheumatoid Arthritis Patients. A Cross-Sectional Study. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, 595–600. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Kryscio, R.J.; Campbell, C.; Kinane, D.F.; McDevitt, J.; Christodoulides, N.; Floriano, P.N.; Miller, C.S. Salivary and Serum Adiponectin and C-Reactive Protein Levels in Acute Myocardial Infarction Related to Body Mass Index and Oral Health. J. Periodontal. Res. 2016, 52, 419–427. [Google Scholar] [CrossRef]
- Weinstock, C.; Konig, D.; Harnischmacher, R.; Keul, J.; Berg, A.; Northoff, H. Effect of Exhaustive Exercise Stress on the Cytokine Response. Med. Sci. Sports Exerc. 1997, 29, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.E.; Dimov, R.K.; Boyanov, N.B.; Zografos, K.G.; Larentzakis, A.V.; Marinov, B.I. Feasibility of a New Wearable Device to Estimate Acute Stress in Novices during High-Fidelity Surgical Simulation. Folia Med. (Plovdiv) 2019, 61, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Norrholm, S.D.; Jovanovic, T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol. Psychiatry 2015, 78, 344–353. [Google Scholar] [CrossRef]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef]
- Hefnawy, A.; Helal, M.A.Y.; Sabek, A.; Shousha, S. Clinical, Behavioral and Biochemical Alterations due to Shearing Stress in Ossimi Sheep. J. Vet. Med. Sci. 2018, 80, 1281–1286. [Google Scholar] [CrossRef]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between Symptoms of Depression and Anxiety and Cortisol Responses to and Recovery from Acute Stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [CrossRef]
- Montero-López, E.; Santos-Ruiz, A.; González, R.; Navarrete-Navarrete, N.; Ortego-Centeno, N.; Martínez-Augustín, O.; Rodríguez-Blázquez, M.; Peralta-Ramírez, M.I. Analyses of Hair and Salivary Cortisol for Evaluating Hypothalamic-pituitary-adrenal Axis Activation in Patients with Autoimmune Disease. Stress 2017, 20, 541–548. [Google Scholar] [CrossRef]
- Bibbey, A.; Carroll, D.; Ginty, A.T.; Phillips, A.C. Cardiovascular and Cortisol Reactions to Acute Psychological Stress under Conditions of High versus Low Social Evaluative Threat. Psychosom. Med. 2015, 77, 599–608. [Google Scholar] [CrossRef]
- Hamer, M.; O’Donnell, K.; Lahiri, A.; Steptoe, A. Salivary Cortisol Responses to Mental Stress are Associated with Coronary Artery Calcification in Healthy Men and Women. Eur. Heart J. 2010, 31, 424–429. [Google Scholar] [CrossRef]
- Shah, B.; Ashok, L.; Sujatha, G.P. Evaluation of Salivary Cortisol and Psychological Factors in Patients with Oral Lichen Planus. Indian J. Dent. Res. 2009, 20, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, V.; Sessa, F.; Valenzano, A.; Salerno, M.; Bitetti, I.; Precenzano, F.; Marotta, R.; Lavano, F.; Lavano, S.M.; et al. Sympathetic, Metabolic Adaptations, and Oxidative Stress in Autism Spectrum Disorders: How far from Physiology? Front. Physiol. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- van der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-Wide Analysis of Cysteine Oxidation Reveals Metabolic Sensitivity to Redox Stress. Nat. Commun. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Whongsiri, P.; Phoyen, S.; Boonla, C. Oxidative Stress in Urothelial Carcinogenesis: Measurements of Protein Carbonylation and Intracellular Production of Reactive Oxygen Species. Methods Mol. Biol. 2017, 1655, 109–117. [Google Scholar]
- Egawa, M.; Haze, S.; Gozu, Y.; Hosoi, J.; Onodera, T.; Tojo, Y.; Katsuyama, M.; Hara, Y.; Katagiri, C.; Inoue, N.; et al. Evaluation of Psychological Stress in Confined Environments Using Salivary, Skin, and Facial Image Parameters. Sci. Rep. 2018, 8, 8264. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef]
- Meštrović-Štefekov, J.; Novak-Bilić, G.; Kuna, M.; Pap, N.; Lugović-Mihić, L. Psychological Stress in Patients with Atopic Dermatitis. Acta Dermatovenerol. Croat. 2018, 26, 297–303. [Google Scholar]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological Biomarkers of Chronic Stress: A Systematic Review. Int. J. Health Sci. 2021, 15, 46. [Google Scholar]
- Jung, J.I.; Son, J.S.; Kim, Y.O.; Chae, C.H.; Kim, C.W.; Park, H.O.; Lee, J.H.; Shin, Y.H.; Ha, J.C. Changes of Depression and Job Stress in Workers after Merger without Downsizing. Ann. Occup. Environ. Med. 2018, 30, 54. [Google Scholar] [CrossRef]
- Viena, T.D.; Banks, J.B.; Barbu, I.M.; Schulman, A.H.; Tartar, J.L. Differential Effects of Mild Chronic Stress on Cortisol and S-IgA Responses to an Acute Stressor. Biol. Psychol. 2012, 91, 307–311. [Google Scholar] [CrossRef]
- Mocci, F.; Bullitta, M.A. Perception of Stress in the Nursing Profession: Study of the Behavior of s-IgA. G. Ital. Med. Lav. Ergon. 2006, 28, 219–221. [Google Scholar] [PubMed]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Suh, M. Salivary Cortisol Profile under Different Stressful Situations in Female College Students. J. Neurosci. Nurs. 2018, 50, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, S.; Al Omar, Z.; Binnwejim, M.S.; Alhamdan, F.; Aldrees, A.; Al-Bawardi, A.; Alsohim, M.; Alhabeeb, M. Perception of Academic Stress Among Health Science Preparatory Program students in Two Saudi Universities. Adv. Med. Educ. Pract. 2018, 12, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Manzar, M.D.; Alghadir, A.H.; Salahuddin, M.; Abdul Hameed, U. Psychometric Analysis of the Perceived Stress Scale Among Healthy University Students. Neuropsychiatr. Dis. Treat. 2020, 16, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Meštrović-Štefekov, J.; Lugović-Mihić, L.; Hanžek, M.; Bešlić, I.; Japundžić, I.; Karlović, D. Salivary Cortisol Values and Personality Features of Atopic Dermatitis Patients: A Prospective Study. Dermatitis 2022, 33, 341–348. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of Salivary α-amylase and Cortisol in Psoriatic Subjects Undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The Effect of Sampling Procedures on the Urate and Lactate Concentration in Oral Fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Alzahem, A.M.; van der Molen, H.T.; de Boer, B.J. Effect of Year of Study on Stress Levels in Male Undergraduate Dental Students. Adv. Med. Educ. Pract. 2013, 4, 217–222. [Google Scholar] [CrossRef]
- Bardi, M.; Koone, T.; Mewaldt, S.; O’Connor, K. Behavioral and Physiological Correlates of Stress Related to Examination Performance in College Chemistry Students. Stress 2011, 14, 557–566. [Google Scholar] [CrossRef]
- Pani, S.C.; Al Askar, A.M.; Al Mohrij, S.I.; Al Ohali, T.A. Evaluation of Stress in Final-Year Saudi Dental Students Using Salivary Cortisol as a Biomarker. J. Dent. Educ. 2011, 75, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Cipra, C.; Müller-Hilke, B. Testing Anxiety in Undergraduate Medical Students and Its Correlation with Different Learning Approaches. PLoS ONE 2019, 14, e0210130. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, T.; Lichtenfeld, S.; Becker, S.; Minkley, N. Stress Experience and Performance during an Oral Exam: The Role of Self-Efficacy, Threat Appraisals, Anxiety, and Cortisol. Anxiety Stress Coping 2018, 32, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.F.; Honart, A.W.; Robinson, A.; Jones, A.-A.; Squires, A. Thriving in Scrubs: A Qualitative Study of Resident Resilience. Reprod. Health 2018, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-Induced Immune Dysfunction: Implications for Health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Speicher, C.E.; Holliday, J.E.; Glaser, R. Stress and the Transformation of Lymphocytes by Epstein-Barr Virus. J. Behav. Med. 1984, 7, 1–12. [Google Scholar] [CrossRef]
- Manolova, M.S.; Stefanova, V.P.; Manchorova-Veleva, N.A.; Panayotov, I.V.; Levallois, B.; Tramini, P.; Orti, V. A Five-Year Comparative Study of Perceived Stress among Dental Students at Two European Faculties. Folia Med. 2019, 61, 134–142. [Google Scholar] [CrossRef]
- McClelland, D.C.; Ross, G.; Patel, V. The Effect of an Academic Examination on Salivary Norepinephrine and Immunoglobulin Levels. J. Human Stress 1985, 11, 52–59. [Google Scholar] [CrossRef]
- Humphris, G.; Blinkhorn, A.; Freeman, R.; Gorter, R.; Hoad-Reddick, G.; Murtomaa, H.; O’Sullivan, R.; Splieth, C. Psychological Stress in Undergraduate Dental Students: Baseline Results from Seven European Dental Schools. Eur. J. Dent. Educ. 2002, 6, 22–29. [Google Scholar] [CrossRef]
- Muirhead, V.; Locker, D. Canadian Dental Students Perceptions of Stress and Social Support. Eur. J. Dent. Educ. 2008, 12, 144–148. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva Diagnostics—Current Views and Directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).