Abstract
Heart Rate Variability (HRV) Biofeedback (BFB) has been shown to improve autonomic balance and wellbeing in chronic diseases. As cardiac variability represents an index of cognitive and emotional regulation, HRV-BFB has been shown to lead to improvements in physiological and psychological adaptability and quality of life. However, knowledge of HRV-BFB in cancer patients is lacking, and available results are diversified according to methods and outcomes. The present paper undertakes a scoping review, exploring the use of HRV-BFB to modulate autonomic balance, cancer symptom management, and quality of life in cancer. This scoping review analyzes empirical evidence considering study designs, BFB methods, and psychophysiological outcomes. Research that focused on HRV-BFB effects in cancer patients was selected (79%). In addition, a systematic review and meta-analysis (31%) focusing on HRV, or BFB in chronic conditions, including cancer, were considered. The studies examined BFB treatment for thyroid, lung, brain or colon cancer, hematologic cancer, and survivors or terminal cancer patients. Retrieved studies reported physiological and psychological indices as primary outcomes: they included HRV values, sleep, pain, fatigue, depression, anxiety, and quality of life. Although the heterogeneity of publications makes it difficult to generalize the effectiveness of HRV-BFB, the training has been proven to improve cancer symptoms and well-being.
1. Introduction
Notwithstanding the scientific advancements achieved in cancer care, 19.3 million new cancer cases have been estimated, and 10 million cancer deaths in 2020 alone [1]. The global cancer burden is expected to be 28.4 million cases in 2040 [2,3]. Receiving an oncological diagnosis and dealing with treatments might be associated with psychological distress [3], depression, and anxiety [4]. However, as oncological prevention and health paths improve and treatments or therapeutic protocols evolve [5], more patients are living longer with a better quality of life (QoL) compared to the past [6]. This poses important challenges for the management of psychological and physical morbidities related to the disease and its treatments.
Biofeedback (BFB) is a technique that could be used to learn to control specific human body functions (i.e., heart rate, temperature, muscular tone, skin conductance, breathing). During BFB, the subject is connected to electrical sensors, that help people receive information about their bodies. The received feedback helps make subtle changes in the body (such as slowing down the heart rate or relaxing some muscles), achieving specific results (for example, reducing pain). These bio-signals provide audio-video feedback, and additional suggestions may be given by the biofeedback therapist if needed. Thanks to the received feedback, the patient increases his/her ability to change impaired bodily functions, with a positive effect on well-being, and experiences feelings of self-control, regulation, and efficacy [7]. Although other innovative treatments have been shown to improve relaxation and psychological symptoms in cancer conditions [8], evidence reports that BFB is an effective method that enhances self-competence by providing feedback on physiological signals from which cancer patients can benefit, increasing physiological self-regulation and psychological well-being [9,10,11,12]. Thus, BFB might be a valuable practice in cancer treatment, allowing better management of the psychological morbidities related to cancer, as well as pain and fatigue caused by the antitumor therapies, or other physical dysfunctions [13]. Such elements have been associated with altered homeostasis and autonomic balance [14]. Thus, treatments that focus on homeostasis and autonomic rehabilitation may show positive effects both on physiological and psychological cancer-related issues. HRV, which is often employed in BFB protocols, is a physiological phenomenon described as continuous fluctuations in the interval between consecutive heartbeats [normal-to-normal beats] [15]. HRV BFB protocols are developed according to experimental and clinical specific needs and may be heterogeneous in procedure and timeline. However, Leher et colleagues (2007; 2013) provide reliable guidelines for the rehabilitation of HRV index through biofeedback. The complex variability of heart rate is under the direct control of the central nervous system that receives and coordinates autonomic, endocrine, and behavioral responses. In line with this, HRV has been shown to be a valuable biomarker of general health, autonomic balance, adaptability and psychological well-being, both in healthy individuals and patients [16,17,18,19]. Autonomic balance is represented by an optimal interplay of sympathetic and parasympathetic influences on the sinus node, and it is associated with a higher survival rate and health status in both clinical and general populations [20]. Parasympathetic influences slow down the heart rate and are associated with increased vagal activity, digestion, resting, and social engagement. Instead of sympathetic activity fixes the beat and it is responsible for general mobilization [21]. The link between HRV and brain processes has been suggested in the neurovisceral integration model [22], which highlights the relationships between the autonomic nervous system and the attentional and affective systems through structural and functional networks, to explain cognitive and emotional regulation. In this context, HRV may be considered a key psychophysiological proxy, being an indicator of central-peripheral feedback mechanisms.
The relationship between the autonomic nervous system and cancer has been demonstrated in the literature [23,24]. For example, a study by Magnon and colleagues [25] showed that sympathetic influences were important at the first stages of tumorigenesis, while the parasympathetic fibers were affecting cancer progression at later metastatic phases of the disease. In line with this, another study showed a positive association between decreased HRV and severe pain in cancer patients [9]. Furthermore, research in the field of chronic pain assessed the relation between low HRV and psychological weakness (e.g., distress, cognitive or emotional dysregulation) [22,26]. Thus, several publications linked cardiac variability to health not only from a physiological perspective but also from a psychological point of view. In line with this, high HRV has been shown as a promising biomarker related to adaptability and well-being [27]. Specifically, in stressful conditions, HRV was associated with enhanced cognitive resilience, appropriate emotional regulation, and better modulation of cortisol, cardiovascular and inflammatory responses. To date, several studies have investigated the association between vagal nerve activity and the prediction of prognosis in cancer, highlighting the fact that HRV predicted patient survival [28,29]. Moreover, a systematic review asserted that valuable heterogeneous therapies for cancer patients (e.g., palliative care, relaxation therapies, music therapies, aerobic exercise, myofascial release sessions) had an effect on increasing HRV coherence or its absolute values [6]. HRV coherence is defined as a relatively harmonic (sine wave-like) signal with a very narrow and high-amplitude peak in the low-frequency domain of the power spectrum, with no major peaks in the other bands [30]. HRV coherence has been linked to favorable emotional states such as appreciation and compassion [31]. The increased HRV reported in the systematic review [6] is coherent with the positive outcome of selected therapies that would be associated with autonomic balance, health, and well-being, to reduce cancer-related fatigue and negative moods. However, given the limited amount of HRV data on cancer patients, the reported studies considered mixed cancer conditions; further studies focusing on specific cancer diagnoses are needed to validate the presented results.
Aim
The current scoping review aimed to provide a descriptive overview of the effectiveness of HRV BFB in cancer treatment. Specifically, the impact of HRV BFB on the management of cancer symptoms was investigated, evaluating physiological (autonomic balance, HRV coherence, HRV values, pain, sleep disorders) and psychological (pain, fatigue, distress, depression, cognitive and emotional regulation) aspects. Furthermore, sub-aims considered current evidence on the relation between (1) autonomic balance and cancer prognosis and (2) HRV BFB and chronic pain.
2. Methods
Since the research on the HRV-BFB in the cancer domain is quite heterogeneous, the method of a scoping review was chosen, to provide a more comprehensive and descriptive picture in investigating the effectiveness of HRV BFB. The effectiveness of HRV BFB considers either physiological or psychological symptoms. Indeed, the scoping review methodology permits the building of a knowledge synthesis of the main concepts and theories in a given field, where evidence is ambiguous and heterogeneous, as in the field of BFB training. The present scoping review is conducted following PRISMA guidelines for scoping reviews [32,33] and Munn’s guidance for authors [34]. The five steps were followed: (1) identification of the research question, (2) identification of the relevant studies, (3) study selection, (4) data mapping, (5) comparison, summary, and reporting of results [35].
2.1. Inclusion and Exclusion Criteria
A set of inclusion and exclusion criteria were established to drive the studies selection. Studies were eligible for inclusion if they conformed to the following criteria: (a) research within the field of health (nursing, psychology, and psychiatry); (b) HRV assessment or BFB training in cancer patients; (c) studies in English, Italian, or Spanish languages; (d) research published from January 2008 (when the Biofeedback Alliance and Nomenclature Task Force defined biofeedback) to the present; (e) experimental and quasi-experimental; observational; qualitative; mixed-method; systematic reviews; meta-analyses; scope reviews; overview articles; and narrative reviews. Conversely, studies were excluded if: (a) pathologies other than chronic pain or cancer were treated; (b) physiological indices other than HRV assessment or training were administered to patients; (c) researchers did not report quantitative analyses.
2.2. Literature Search Strategies
The search databases were EMBASE, ProQuest Central, PsycINFO, PubMed, and Scopus. Sources of unpublished studies and gray literature such as congress abstracts, clinical trials and current controlled trials were included. An adapted search strategy was used for gray literature to avoid an extremely high number of irrelevant results. The combination of keywords, labels, and synonyms was as follows: cancer (or tumor, neoplasms), HRV (or heart rate variability, heart rate, autonomic balance, vagal tone), and BFB (or biofeedback). A manual search was conducted in the selected books and chapters [36]. The reference list of all relevant studies was screened for additional studies. After the removal of duplicates, articles were selected in a two-step process by two reviewers working independently at each step: in step 1, two reviewers (GES and MM) screened independently all the titles and abstracts returned for potential relevance. Each reviewer assessed the article against the inclusion and exclusion criteria. The proposed abstracts included for full-text review were compared by the two reviewers.
Step 2 was undertaken for each abstract selected. The full version of the article was retrieved. Two reviewers (GES and MM) ran independent full-text analyses. Reasons for the exclusion of full-text articles were noted by each reviewer and provided as an appendix in the full review. The proposed full-text articles for the review were compared by the two reviewers until a final set was agreed upon by both. Where there was a disagreement between the two reviewers in either step, the final agreement was sought by mutual consensus with input from a third reviewer (SFMP). Please see Table A2 in Appendix B for further details.
2.3. Data Charting and Summarizing Data
Two reviewers (GES and MM) independently extracted study data. Data to be extracted from selected studies included information about the author(s), year and country of the study, study design, goals, sample characteristics, guidelines of intervention, BFB methodologies, and any other key findings related to the research questions. The methodology and statistical quality of the studies were not discriminative in the presented scoping review because the goals of this study were to identify gaps in the literature and to propose potential research questions for future systematic review [35].
3. Results
3.1. Selection of Sources of Evidence
A first search in the literature identified 6998 potential articles. The search string included the following words: (((“Heart Rate Variability *”) OR (“HRV*”) OR (“Autonomic*”) OR (“Vagal Tone*”) OR (“Heart Rate”)) AND ((“Cancer*”) OR (“Tumor*”) OR (“Carcinoma*”)) AND (“Biofeedback*”)).
After all duplicates and inappropriate titles and abstracts were removed, twenty-two full-text articles, a book chapter, and five abstracts were screened (by GES), in consultation with the second author (MM), for eligibility. Overall, nine studies that did not meet the inclusion criteria were excluded from the scoping review. After a three-part screening process, nineteen studies were considered. Specifically, eleven original research studies, four systematic reviews and meta-analyses, and four abstracts were included. Original research might be included or cited in the systematic reviews and meta-analysis publications. The majority of the studies (34%) were randomized control trials or cohort studies (40%) and there were also single case studies (13%). Finally, a non-experimental and a pilot study (13%) were considered. A total of 82% of included studies were conducted with adults, with 18% on children and adolescents. Selected publications (79%) included primary and secondary data.
The diagram of the selection of sources process is presented in Figure 1. Relevant data for each included source of evidence are reported in Table 1 (empirical studies) and Table 2 (theoretical studies).
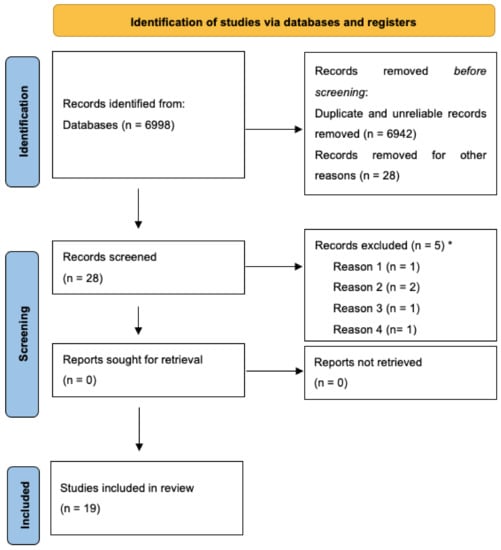
Figure 1.
PRISMA 2020 flow diagram for the review process. * Reason 1: BFB on other physiological signals (e.g., electromyography, temperature, skin conductance); reason 2: HRV assessment without BFB; reason 3: unclear primary or secondary outcomes; reason 4: sample of patients without cancer diagnosis. Reprinted with permission from Page et al. (2021). Copyright 2020 The PRISMA statement.

Table 1.
Empirical original research studies characteristics and results.

Table 2.
Theoretical Studies Characteristics and Results.
3.2. Synthesis of Results
Overall, the empirical and theoretical studies included in this review highlighted the potential effectiveness of HRV BFB interventions in cancer care. The BFB method was used alone or in combination with other treatments; included studies reported HRV BFB in association with (a) biofeedback of other physiological signals [37]; (b) physical exercise [38]; (c) resonant, paced, and belly breathing exercises [39,40,41]; (d) breakthrough pain treatments [42]. Further, considered studies investigated single assessments or full trainings on a daily–weekly basis with different duration, ranging from 10 s assessments [43] to 24 h measurements [42]. However, the most common duration for BFB trainings was between 5 and 60 min [40,44,45]. Most interventions [39,40,44,45,46,47,48,49] implicated hospital management of the treatment and the support of a BFB therapist, while others consisted of at-home self-training [41], and a minority were a mixed approach [50]. Specifically, home trainings were developed by implementing respiration and relaxation exercises on a deliverable device that monitored cardiac variability and obtained physiological data without the physical presence of patients.
The included interventions considered both single case studies [39] and large sample researches based on up to 272 cancer patients [45]. Selected studies targeted adult [38,41,42,45,46,47,48,51,52,53,54], adolescent [49], and child [39,40] cancer patients. According to the given information, although BFB training was extremely heterogeneous, there were no specific differences in BFB trainings based on the mean age of the included sample.
Explored outcomes reported pain, fatigue, sleep quality, depression, anxiety, HRV coherence, frequency HRV indexes, stress, and quality of life. Overall, a positive effect of HRV BFB on all these variables was observed. Specifically, cancer pain rates were lowered with the increase of HRV and according to self-report scoring [42,50,53], and so were fatigue scores [53]. Increased sleep efficiency, sleep duration and a general reduction of sleep symptoms, insomnia, and daytime impairments were registered after paced breathing and BFB training [41,47,50,53]. The levels of depression and anxiety were compared before and after HRV BFB trainings, reporting a significant reduction [48,53]. However, not all reported publications reached significant improvements in psychological aspects such as quality of life (QoL) and distress e.g., [47,49] Finally, HRV measurements and coherence scores (i.e., a measure of the degree of coherence in the heart rhythm pattern) were increased, following the BFB and respiration exercises in several studies [38,53], mirroring a better autonomic balance than in the pre-treatment condition. A synthesis of the most common outcomes extracted from the selected publications is reported in Appendix A.
4. Discussion
The current scoping review explored the use of HRV BFB in different types of cancer, exploring the impact both on physiological, physical, and psychological wellbeing. As previously reported, frequent cancer symptoms may include severe pain, fatigue, sleep and eating disorders, distress, depression, anxiety, and general psychological burden. Therefore, this population may particularly benefit from HRV BFB treatment [36]. Furthermore, a study conducted by De Couck and colleagues showed that HRV emerged as a potential variable inversely related to tumoral biomarkers, and that a low HRV was measured in cancer patients compared with healthy controls [55]. Evidence analyzed in the current scoping review suggested the potential effectiveness of HRV BFB on the management of physiological variables (HRV coherence and values to evaluate autonomic balance, sleep disorders), and psychological symptoms (depression, anxiety, distress, quality of life); pain and fatigue in cancer patients were explored, demonstrating a general positive effect of this treatment within the cancer population. The impact of HRV BFB was measured on primary and secondary outcomes of the included studies. Overall, included studies were conducted on thyroid cancer, lung cancer, brain cancer, colon cancer, hematologic patients, and in cancer survivors or terminal patients.
In most cases, the studies analyzed highlighted the fact that HRV BFB showed positive results concerning pain levels, depression and anxiety, sleep disturbances and cognitive performance. Three randomized controlled studies [41,46,53] showed a significant increase in HRV values and improvements in sleep quality, fatigue, stress, and depression in patients that received BFB training, compared with controls. However, the BFB treatments markedly changed depending on training time, hole duration, and modalities between the studies, and the results should be interpreted cautiously. Similarly, Burch and colleagues (2020) found the greatest effect of the HRV BFB training on sleep symptoms and sleep-related daytime impairments. However, minor results in fatigue and distress scores were reported in this investigation. Furthermore, these results confirmed that increased HRV may facilitate homeostasis and cardiorespiratory synchronization leading to better sleep quality [55]. Some retrieved studies have investigated the procedure used to deliver the BFB at home [38,41,47] and in hospital settings [37,39,40,44,46,47,52,53]. For example, Hasuo and collaborators (2020) tested a program to improve sleep by implementing a home-based HRV BFB with a deliverable device. This approach led to significant results in sleep function and HRV, with patients reporting improvements in sleep induction disorder, nocturnal awakening and unrefreshing sleep, in only two weeks. The use of home-based BFB allowed patients to exercise daily and acquire technical respiration skills in a shortened period, compared with hospital interventions. This is in line with the active role in controlling lifestyle and decisions that patients should embrace [56,57]. Studies in which the BFB intervention was performed in hospital reported high rates of dropout and reduced feasibility of completing the purposed treatment [37]. Ozier and colleagues (2018) adopted a mixed method associated with hospital-training home exercises, and their method showed good patient adherence to the intervention. Generally, we argue that given the fact that cancer patients may particularly benefit from treatments that enhance QoL in a limited time, this could be a major advantage to home training or a mixed approach.
Selected cohort and case series studies reported improvement in physiological outcomes, depression, and anxiety [38,39,40,44,47]. In one of these [38], BFB was associated with physical exercise, and it brought improvements in physical capacity and muscle strength in association with a decrease in fatigue rates, although this last result did not reach statistical significance. As regards the psychological symptoms, results confirmed the effect of augmented cardiac variability on the reduction of depression and anxiety in the clinical population [16]. In the main, cancer patients who underwent HRV BFB intervention had lower scores for depression and dysphoric mood [47,53], and distress and anxiety levels [37,53].
Overall, the majority of the selected publications reported either physiological and/or psychological improvements: positive results were registered independently of the type and stage of cancer and were a confirmation of the possible beneficial effect of HRV BFB on psychophysiological adaptability. This is in line with the idea of HRV BFB as a possible therapy alone or combined with other treatments in the complex health path of cancer. The current scoping review also analyzed a set of theoretical studies on HRV suggesting it as a possible factor associated with cancer survival rate [43,58,59]. DeCouck and colleagues [43] reported the beneficial effects of vagal nerve stimulation on survival rate, measured in a noninvasive manner through the HRV index. Similarly, another group of studies highlighted the fact that that low vagus nerve activity was related to poor health outcomes, as well as vagus nerve stimulation slowing down tumor progression [60,61,62,63]. Furthermore, these studies showed that HRV significantly predicted tumor marker levels in different cancers, and that higher vagal activity predicted a better prognosis of cancer conditions. Zhou and colleagues [34], in a systematic review and meta-analysis pooled together the results of six studies with HRV measurements, demonstrating that survival was significantly longer in the higher HRV group than in the lower HRV group. However, the authors recommended caution in the interpretation of obtained results, given the common problem of heterogeneity of methods (HRV assessment guidelines and instruments) and samples (pancreatic cancer, breast cancer, lung cancer, and mixed cancer type, all metastatic). Overall, we might affirm that the considered theoretical studies report show links between cancer and low vagal influence and HRV [43,59] and they observe a positive association between high HRV and survivorship [58]. BFB represents a way of increasing cardiac variability. In line with the preliminary HRV BFB effectiveness on physiological and psychological outcomes shown by the reported publications, more attention should be given to HRV BFB, as cancer health paths could benefit from the treatment.
4.1. Implications and Suggestions for Research and Practice
The presented scoping review pointed out the need for additional original studies on HRV BFB to validate the presented results. Shared treatment standards (i.e., frequency, duration, and follow-ups) should be implemented in future investigations to adequately compare outcomes. Moreover, there is an evident need for additional studies to achieve empirical data on the feasibility and outcomes of HRV BFB, according to specific criteria such as type stage, the prognosis of cancer, age of patients, and previous or current presence of other treatments. Better statistical designs compared with currently available investigations are suggested, for example, larger sample sizes and dropout prevision should be implemented, to avoid inconsistent results.
Based on currently available data, it seems that HRV BFB showed major effectiveness when hospital-based treatments were turned into home-based training through deliverable devices or at least associated with self-practice. Indeed, these modalities allowed patients to establish a time to achieve results, and prevented the potential issue of dropout. More studies embracing these BFB deliverable methodologies targeting specific cancer populations are encouraged.
4.2. Limitations
A rigorous approach to extract, search and evaluate the existing literature was adopted. However, the present scoping review had some limitations that should be considered. Firstly, retrieved studies showed a divergence of methods in physiological recordings, BFB instruments, BFB training (e.g., hospital and/or home-based), and follow-up evaluations. Secondly, given the inability to completely fulfill the research questions because of the lack of available sources, miscellaneous samples of cancer patients were included: mixed samples considered all cancer types (e.g., breast cancer, lung cancer, pancreatic cancer, mixed cancer), stages (I, II, III, IV) age ranges (from children to adulthood) and gender. In addition, included studies contained a lack of assessed control variables (e.g., physical activity, smoking habits, etc.), medium-to-high dropout rates, and missing data. Thirdly, the gray literature was included in the current scoping review to draft a better picture of the accruing evidence on HRV-BFB.
Differentiated studies according to cancer type and stage, and age and gender of patients, as well as larger sample sizes, should be encouraged for future studies. Similarly, common guidelines and standardized protocols in HRV measurements and BFB training should be available and followed by researchers and clinicians.
5. Conclusions
Evidence collected in the current scoping review revealed that HRV BFB might help to increase HRV coherence and values, thus improving both physiological symptoms (e.g., autonomic balance, general health condition, and sleep quality), psychological symptoms (e.g., fatigue, depression, anxiety) and pain, in cancer patients. Specifically, retrieved results showed an increase in autonomic balance, thus an increased HRV and/or HRV coherence, linked to physiological health and wellbeing. In addition, sleep quality and quantity seem to benefit from home-based HRV BFB training. Finally, selected studies showed promising results on reported fatigue, depressive and anxiety scores after treatment, compared with the previous condition. No differences between different diagnoses or grades of cancer were explored, given the need for further studies to explore the issue. In line with current results, clinicians might consider including HRV BFB either as home intervention or hospital-based training in the health path of oncological patients. Overall, this type of technique might be a challenging opportunity to better manage not only physical morbidities which are cancer-related but also psychological distress, improving health-related QoL along the cancer pathway. However, further high-quality studies are needed to establish reliable standards, as the heterogeneity of selected publications concerning method and sample makes it difficult to generalize results and assess the effectiveness of HRV BFB intervention.
Author Contributions
G.E.S. conceived the idea of the study G.E.S., M.M. and S.F.M.P. performed the literature review and the selection of papers. G.E.S. and M.M. wrote the first draft of the manuscript. G.P. supervised and revised all the steps of the manuscript. All the authors wrote, revised and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Glossary
| HRV | Heart Rate Variability |
| ANS | Autonomic Nervous System |
| HR | Heart Rate |
| HC | Health Controls |
| MS | Multiple Sclerosis |
| NFL | Neurofilament Light Chain |
| PC | Pancreatic Cancer |
| PNN50 | The proportion of NN50 divided by the total number of NN (R-R) intervals |
| QoL | Quality of Life |
| SD | Standard Deviation |
| SDNN | Standard Deviation Normal to Normal NN (R- R) intervals |
| SpO2 | Blood Oxygen Saturation |
| tcpO2 | Trans-cutaneous O2 Partial Pressure |
Appendix A

Table A1.
Frequencies of Reported Outcomes in Selected Empirical and Theoretical Studies.
Table A1.
Frequencies of Reported Outcomes in Selected Empirical and Theoretical Studies.
| Psychological and Physiological Variables | Number of Studies | Percentage of Studies |
|---|---|---|
| HRV (LF, LF/HF, PNN50, SDNN) | 5 | 32% |
| HRV coherence score | 3 | 16% |
| HR mean frequency | 3 | 16% |
| Respiration | 2 | 10% |
| Sleep Quality | 3 | 16% |
| Stress and Fatigue | 4 | 21% |
| Quality of Life | 4 | 21% |
| Anxiety | 2 | 10% |
| Depression | 3 | 16% |
Note: HRV = Heart Rate Variability; LF = Low Frequency; LF/HF = Low Frequency/High-Frequency Ratio; PNN50 = The proportion of NN50 divided by the total number of NN (R-R) intervals; SDNN = Standard Deviation Normal to Normal NN (R-R) intervals; HR = Heart Rate.
Appendix B

Table A2.
Procedure in Scoping Review Analysis and Authors’ Contribution.
Table A2.
Procedure in Scoping Review Analysis and Authors’ Contribution.
| Scoping Review Guidelines * | Empirical Stages |
|---|---|
| Research question | All authors agreed on the aim to explore current evidence about HRV BFB Physiological and Psychological Outcomes in Cancer Patients |
| Relevant studies | GES and MM contributed to literature selection to identify articles on the topic of interest on selected scientific databases: all articles, abstracts and relevant grey literature. No systematic or scoping review emerged |
| Study selection | GES, MM and SFMP examined selected studies, removed duplicates and exluded publication according to the following criteria: 1) BFB on other physiological signals (e.g., Electromyografy, temperature, skin conductance) 2) HRV assessment without BFB 3) Unclear primary or secondary outcomes 4) Sample of patients without cancer diagnosis |
| Data mapping | All authors excluded a full systematic review method because of lack of homogeneous studies (HRV measure, BFB procedure, cacncer diagnosis); scoping review was a valid method for mapping HRV BFB evidences in cancer populations |
| Results organization (comparison, summary, and report) | GES organized the literature information in an Excel file. Raws were composed by selected publications. Columns indicates: author(s) and year, title, aim(s), BFB methods and procedure if available, sample characteristics, type and grade of cancer, primary and secondary outcomes. MM and SFMP revised the file |
| Article writing | All authors contributed to the writing process, to the article revision and tables design |
Note: HRV = Heart Rate Variability; BFB = Biofeedback. * Arksey H, O’Malley L. Scoping studies: Towards a methodological framework.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. Late-stage cancer detection in the USA is costing lives. Lancet 2010, 376, 1873. [Google Scholar] [CrossRef]
- Montgomery, M.; McCrone, S.H. Psychological distress associated with the diagnostic phase for suspected breast cancer: Systematic review. J. Adv. Nurs. 2010, 66, 2372–2390. [Google Scholar] [CrossRef] [PubMed]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Nave, O. A mathematical model for treatment using chemo-immunotherapy. Heliyon 2022, 8, e09288. [Google Scholar] [CrossRef]
- Palma, S.; Keilani, M.; Hasenoehrl, T.; Crevenna, R. Impact of supportive therapy modalities on heart rate variability in cancer patients—A systematic review. Disabil. Rehabil. 2018, 42, 36–43. [Google Scholar] [CrossRef]
- Crevenna, R. From neuromuscular electrical stimulation and biofeedback-assisted exercise up to triathlon competitions—regular physical activity for cancer patients in Austria. Eur. Rev. Aging Phys. Act. 2013, 10, 53–55. [Google Scholar] [CrossRef][Green Version]
- Pizzoli, S.F.M.; Mazzocco, K.; Triberti, S.; Monzani, D.; Raya, M.L.A.; Pravettoni, G. User-centered virtual reality for promoting relaxation: An innovative approach. Front Psychol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Crevenna, R. Biofeedback in medicine with a focus on cancer rehabilitation. Wien. Klin. Wochenschr. 2022, 134, 1–2. [Google Scholar] [CrossRef]
- Kelly, D.L.; Dickinson, K.; Hsiao, C.P.; Lukkahatai, N.; Gonzalez-Marrero, V.; McCabe, M.; Saligan, L.N. Biological Basis for the Clustering of Symptoms. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2016; Volume 32, pp. 351–360. [Google Scholar]
- Wood, L.J.; Weymann, K. Inflammation and neural signaling: Etiologic mechanisms of the cancer treatment-related symptom cluster. Curr. Opin. Supportive Palliat. Care 2013, 7, 54. [Google Scholar] [CrossRef]
- Rausa, M.; Spada, G.E.; Patron, E.; Pierangeli, G.; Palomba, D. Do catastrophizing and autonomic-reduced flexibility mediate pain outcomes in chronic headache? Neurol. Sci. 2021, 43, 3283–3295. [Google Scholar] [CrossRef]
- Cho, H.-M.; Kim, H.; Yoo, R.; Kim, G.; Kye, B.-H. Effect of Biofeedback Therapy during Temporary Stoma Period in Rectal Cancer Patients: A Prospective Randomized Trial. J. Clin. Med. 2021, 10, 5172. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Ellis, R.; Hillecke, T.; Thayer, J. Heart rate variability and experimentally induced pain in healthy adults: A systematic review. Eur. J. Pain 2014, 18, 301–314. [Google Scholar] [CrossRef]
- Malik, M. Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Lin, I.M.; Fan, S.Y.; Yen, C.F.; Yeh, Y.C.; Tang, T.C.; Huang, M.F.; Liu, T.L.; Wang, P.W.; Lin, H.C.; Tsai, H.Y.; et al. Heart Rate Variability Biofeedback Increased Autonomic Activation and Improved Symptoms of Depression and Insomnia among Patients with Major Depression Disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 222. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6478078/ (accessed on 2 August 2022). [CrossRef]
- Force, T. Society E, North T, Society A. Guidelines Heart rate variability. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Porges, S.W. Autonomic regulation and attention. In Attention and Information Processing in Infants and Adults: Perspectives from Human and Animal Research; Psychology Press: London, UK, 1992. [Google Scholar]
- Friedman, B.H.; Thayer, J.F. Anxiety and autonomic flexibility: A cardiovascular approach. Biol. Psychol. 1998, 47, 243–263. [Google Scholar] [CrossRef]
- Brook, R.D.; Julius, S. Autonomic imbalance, hypertension, and cardiovascular risk. Am. J. Hypertens. 2000, 13, 112S–122S. [Google Scholar] [CrossRef]
- Gibbons, C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019, 160, 407–418. [Google Scholar]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Walsh, D.; Nelson, K.A. Autonomic nervous system dysfunction in advanced cancer. Support. Care Cancer 2002, 10, 523–528. [Google Scholar] [CrossRef]
- Strasser, F.; Palmer, J.L.; Schover, L.R.; Yusuf, S.W.; Pisters, K.; Vassilopoulou-Sellin, R.; DeGracia, B.; Willey, J.S.; Bruera, E. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male with advanced center: A pilot study. Cancer 2006, 107, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Perna, G.; Riva, A.; Defillo, A.; Sangiorgio, E.; Nobile, M.; Caldirola, D. Heart rate variability: Can it serve as a marker of mental health resilience? Special Section on “Translational and Neuroscience Studies in Affective Disorders”. J. Affect. Disord. 2020, 263, 754–761. [Google Scholar] [CrossRef]
- Chiang, J.-K.; Koo, M.; Kuo, T.B.; Fu, C.-H. Association Between Cardiovascular Autonomic Functions and Time to Death in Patients With Terminal Hepatocellular Carcinoma. J. Pain Symptom Manag. 2010, 39, 673–679. [Google Scholar] [CrossRef]
- Hoffmann, J.; Grimm, W.; Menz, V.; Wied, M.; Sprenger, A.; Arnold, R.; Maisch, B. Prognostic Value of Heart Rate Variability Analysis in Patients with Carcinoid Syndrome. Digestion 2001, 63, 35–42. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The Coherent Heart Heart-Brain Interactions, Psychophysiological Coherence, and the Emergence of System-Wide Order. Integral Rev. Transdiscipl. Transcult. J. New Thought Res. Prax. 2009, 5. Available online: http://www.integral-review.org/issues/vol_5_no_2_mccraty_et_al_the_coherent_heart.pdf (accessed on 2 August 2022).
- Ginsberg, J.P.; Jennings, W.; Dorn, B.; Balagué, N.; Hagman, M.; Mccraty, R. New Frontiers in Heart Rate Variability and Social Coherence Research: Techniques, Technologies, and Implications for Improving Group Dynamics and Outcomes. Front. Public Health 2017, 5, 267. Available online: https://www.frontiersin.org/ (accessed on 2 August 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, 1–11. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Gulati, A.; Puttanniah, V.; Bruel, B.M.; Rosenberg, W.S.; Hung, J.C. Essentials of Interventional Cancer Pain Management; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Greenberg, B.R.; Grossman, E.F.; Bolwell, G.; Reynard, A.K.; Pennell, N.A.; Moravec, C.S.; McKee, M.G. Biofeedback Assisted Stress Management in Patients with Lung Cancer: A Feasibility Study. Appl. Psychophysiol. Biofeedback 2015, 40, 201–208. [Google Scholar] [CrossRef]
- Fournié, C.; Verkindt, C.; Dalleau, G.; Bouscaren, N.; Mohr, C.; Zunic, P.; Cabrera, Q. Rehabilitation Program Combining Physical Exercise and Heart Rate Variability Biofeedback in Hematologic Patients: A Feasibility Study. Support. Care Cancer 2022, 30, 2009–2016. [Google Scholar] [CrossRef]
- Murguía, M.A.S.; Rico, A.P.; Sastrías, J.M.F. Uso de biofeedback de variabilidad de la frecuencia cardiaca durante la radioterapia como mǸtodo de distracciòn cognitiva y autorregulaciòn en un paciente pediǭtrico: Informe de caso. Psicooncologìa 2017, 14, 255–265. [Google Scholar] [CrossRef]
- Shockey, D.P.; Menzies, V.; Glick, D.F.; Taylor, A.G.; Boitnott, A.; Rovnyak, V. Preprocedural Distress in Children with Cancer: An Intervention Using Biofeedback and Relaxation. J. Pediatr. Oncol. Nurs. 2013, 30, 129–138. [Google Scholar] [CrossRef]
- Hasuo, H.; Kanbara, K.; Shizuma, H.; Morita, Y.; Fukunaga, M. Short-term efficacy of home-based heart rate variability biofeedback on sleep disturbance in patients with incurable cancer: A randomised open-label study. BMJ Support. Palliat. Care 2020, 1–9. [Google Scholar] [CrossRef]
- Masel, E.K.; Huber, P.; Engler, T.; Watzke, H.H. Heart rate variability during treatment of breakthrough pain in patients with advanced cancer: A pilot study. J. Pain Res. 2016, 9, 1215–1220. [Google Scholar] [CrossRef]
- De Couck, M.; Caers, R.; Spiegel, D.; Gidron, Y. The Role of the Vagus Nerve in Cancer Prognosis: A Systematic and a Comprehensive Review. J. Oncol. 2018, 2018, 1236787. [Google Scholar] [CrossRef]
- Groff, D.G.; Battaglini, C.; Sipe, C.; Peppercorn, J.; Anderson, M.; Hackney, A.C. “Finding a New Normal:” Using Recreation Therapy to Improve the Well-Being of Women with Breast Cancer. Annu. Ther. Recreat. 2010, 18, 40–52. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2010459919&site=ehost-live (accessed on 3 August 2022).
- Gidron, Y.; De Couck, M.; De Leeuw, I.; Blase, K.; Vanacker, L. The Effects of Heart-Rate Variability Biofeedback on Levels of the Tumor Marker CEA in Metastatic Colon Cancer: A Pilot Controlled Study. Psychosom. Med. 2017, 79, A18. Available online: https://researchportal.vub.be/en/publications/the-effects-of-heart-rate-variability-biofeedback-on-levels-of-th (accessed on 3 August 2022).
- Burch, J.B.; Ginsberg, J.P.; McLain, A.C.; Franco, R.; Stokes, S.; Susko, K.; Hendry, W.; Crowley, E.; Christ, A.; Hanna, J.; et al. Symptom Management Among Cancer Survivors: Randomized Pilot Intervention Trial of Heart Rate Variability Biofeedback. Appl. Psychophysiol. Biofeedback 2020, 45, 99–108. [Google Scholar] [CrossRef]
- Ozier, D.; Linden, W. Heart Variability Biofeedback as Supplementary Care for Brain Cancer: A Feasibility Study. J. Altern. Complement. Med. 2018, 24, 852–853. [Google Scholar] [CrossRef]
- Chen, C.-M.; Jou, S.-T.; Hung, G.-Y. The Effects of the Resilience Intervention on Adolescents and Young Adults with Cancer. In Proceedings of the 30th International Nursing Research Congress: Theory-to-Practice: Catalyzing Collaborations to Connect Globally, Calgary, AB, Canada, 25–29 July 2019; Available online: https://urlsand.esvalabs.com/?u=http%3A%2F%2Fhdl.handle.net%2F10755%2F17762&e=52c209d0&h=7cb5da25&f=y&p=n (accessed on 3 August 2022).
- Fournié, C.; Chouchou, F.; Dalleau, G.; Caderby, T.; Cabrera, Q.; Verkindt, C. Heart rate variability biofeedback in chronic disease management: A systematic review. Complement. Ther. Med. 2021, 60, 102750. [Google Scholar] [CrossRef]
- Gidron, Y.; De Couck, M.; Reynders, T.; Marechal, R.; Engelborghs, S.; D’Hooghe, M. Stronger Correlations between Neurophysiological and Peripheral Disease Biomarkers Predict Better Prognosis in Two Severe Diseases. J. Clin. Med. 2020, 9, 26. [Google Scholar] [CrossRef]
- Hunakova, L.; Zvarik, M.; Majerova, K.; Mestanik, M.; Bella, V.; Tonhajzerova, I. Cardiovagal regulation and transcutaneous pO2 in breast cancer patients—A pilot study. Neoplasma 2013, 60, 607–616. [Google Scholar] [CrossRef]
- Cosentino, C.; Sgromo, D.; Merisio, C.; Berretta, R.; Pruneti, C. Psychophysiological Adjustment to Ovarian Cancer: Preliminary Study on Italian Women Condition. Appl. Psychophysiol. Biofeedback 2018, 43, 161–168. [Google Scholar] [CrossRef]
- O’Rourke, M.A.; Franco, R.A.; Sofge, J.; Ginsberg, J.; Susko, K.; Crowley, E.; Anderson, A.; Christ, A.; Hanna, J.; Hendry, W.; et al. Use of heart rate variability (HRV) biofeedback for symptom management among cancer survivors. J. Clin. Oncol. 2017, 35, 10099. [Google Scholar] [CrossRef]
- De Couck, M.; Van Brummelen, D.; Schallier, D.; De Greve, J.; Gidron, Y. The relationship between vagal nerve activity and clinical outcomes in prostate and non-small cell lung cancer patients. Oncol. Rep. 2013, 30, 2435–2441. [Google Scholar] [CrossRef]
- Jerath, R.; Harden, K.; Crawford, M.; Barnes, V.A.; Jensen, M. Role of cardiorespiratory synchronization and sleep physiology: Effects on membrane potential in the restorative functions of sleep. Sleep Med. 2014, 15, 279–288. [Google Scholar] [CrossRef]
- Oliveri, S.; Renzi, C.; Masiero, M.; Pravettoni, G. Living at Risk: Factors That Affect the Experience of Direct-to-Consumer Genetic Testing. Mayo Clin. Proc. 2015, 90, 1323–1326. [Google Scholar] [CrossRef]
- Oliveri, S.; Scotto, L.; Ongaro, G.; Triberti, S.; Guiddi, P.; Pravettoni, G. “You do not get cancer by chance”: Communicating the role of environmental causes in cancer diseases and the risk of a “guilt rhetoric”. Psycho-Oncology 2019, 28, 2422–2424. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, Z.; Zhang, L.; Zhou, S.; Wang, J.; Wang, B.; Fu, W. Heart rate variability in the prediction of survival in patients with cancer: A systematic review and meta-analysis. J. Psychosom. Res. 2016, 89, 20–25. [Google Scholar] [CrossRef]
- Arab, C.; Dias, D.P.M.; Barbosa, R.T.D.A.; de Carvalho, T.D.; Valenti, V.E.; Crocetta, T.B.; Ferreira, M.; de Abreu, L.C.; Ferreira, C. Heart rate variability measure in breast cancer patients and survivors: A systematic review. Psychoneuroendocrinology 2016, 68, 57–68. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Van Westerloo, D.J.; Giebelen, I.A.; Florquin, S.; Bruno, M.J.; LaRosa, G.J.; Ulloa, L.; Tracey, K.J.; van der Poll, T. The Vagus Nerve and Nicotinic Receptors Modulate Experimental Pancreatitis Severity in Mice. Gastroenterology 2006, 130, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.H.O.; Moeller, S.; Lücke, C.; Lam, A.; Braun, N.; Philipsen, A. Vagus Nerve Stimulation (VNS) and Other Augmentation Strategies for Therapy-Resistant Depression (TRD): Review of the Evidence and Clinical Advice for Use. Front. Neurosci. 2018, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Chaudhry, H.; Williams, K.A.; Christo, P.J. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr. Pain Headache Rep. 2015, 19, 54. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).