From Fear to Hopelessness: The Buffering Effect of Patient-Centered Communication in a Sample of Oncological Patients during COVID-19
Abstract
1. Introduction
2. Method
2.1. Sample Size Determination
2.2. Participants
2.3. Measures: (Development of) the Structured Interview
2.4. Procedure
2.5. Statistical Analyses
3. Results
3.1. Psychometric Properties of the Structured Interview
3.2. Preliminary Analysis
3.3. Sequential Multiple Mediation Model
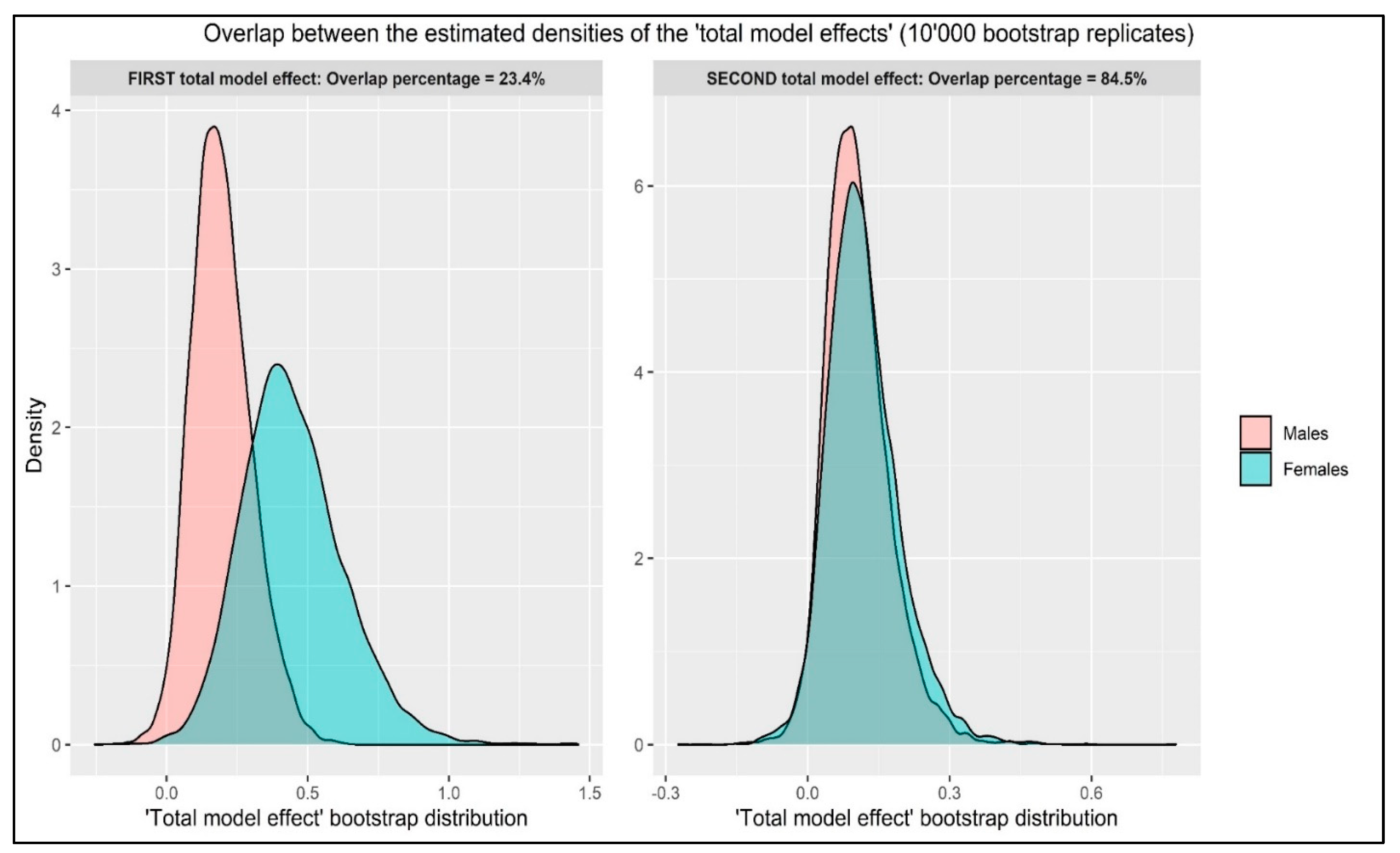
3.4. Overlapping the Total Model Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 46; WHO Press: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 June 2021).
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 June 2021).
- Casagrande, M.; Favieri, F.; Tambelli, R.; Forte, G. The enemy who sealed the world: Effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020, 75, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gebbia, V.; Piazza, D.; Valerio, M.R.; Borsellino, N.; Firenze, A. Patients with Cancer and COVID-19: A WhatsApp Messenger-Based Survey of Patients’ Queries, Needs, Fears, and Actions Taken. JCO Glob. Oncol. 2020, 6, 722–729. [Google Scholar] [CrossRef]
- Rossi, A.; Panzeri, A.; Pietrabissa, G.; Manzoni, G.M.; Castelnuovo, G.; Mannarini, S. The Anxiety-Buffer Hypothesis in the Time of COVID-19: When Self-Esteem Protects from the Impact of Loneliness and Fear on Anxiety and Depression. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Parola, A.; Rossi, A.; Tessitore, F.; Troisi, G.; Mannarini, S. Mental Health Through the COVID-19 Quarantine: A Growth Curve Analysis on Italian Young Adults. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Rossi Ferrario, S.; Panzeri, A.; Cerutti, P.; Sacco, D. The Psychological Experience and Intervention in Post-Acute COVID-19 Inpatients. Neuropsychiatr. Dis. Treat. 2021, 17, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Saricali, M.; Satici, S.A.; Satici, B.; Gocet-Tekin, E.; Griffiths, M.D. Fear of COVID-19, Mindfulness, Humor, and Hopelessness: A Multiple Mediation Analysis. Int. J. Ment. Health Addict. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pietrabissa, G.; Simpson, S. Psychological Consequences of Social Isolation During COVID-19 Outbreak. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Giusti, E.M.; Pedroli, E.; D’Aniello, G.E.; Stramba Badiale, C.; Pietrabissa, G.; Manna, C.; Stramba Badiale, M.; Riva, G.; Castelnuovo, G.; Molinari, E. The Psychological Impact of the COVID-19 Outbreak on Health Professionals: A Cross-Sectional Study. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Ministero della Salute. COVID-19—Situazione nel Mondo. 2021. Available online: https://www.reteccp.org/primepage/2020/virus20/Covid-nel-mondo.pdf (accessed on 1 June 2021).
- Alizadehsani, R.; Alizadeh Sani, Z.; Behjati, M.; Roshanzamir, Z.; Hussain, S.; Abedini, N.; Hasanzadeh, F.; Khosravi, A.; Shoeibi, A.; Roshanzamir, M.; et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J. Med. Virol. 2021, 93, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Jing, X.; Wang, Y.; Miao, M.; Tao, L.; Zhou, Z.; Xie, Y.; Huang, Y.; Lei, J.; et al. Mortality risk of COVID-19 in elderly males with comorbidities: A multi-country study. Aging 2021, 13, 27–60. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Mazzitelli, M.; Serapide, F.; Pelle, M.C.; Tassone, B.; Arrighi, E.; Perri, G.; Fusco, P.; Scaglione, V.; Davoli, C.; et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci. Rep. 2020, 10, 20834. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, A.; Komici, K.; Cerutti, P.; Sacco, D.; Pistono, M.; Ferrario, S.R. Gender differences and long-term outcome of over 75 elderlies in cardiac rehabilitation: Highlighting the role of psychological and physical factors through a secondary analysis of a cohort study. Eur. J. Phys. Rehabil. Med. 2021, 57, 288–297. [Google Scholar] [CrossRef]
- Balestroni, G.; Panzeri, A.; Omarini, P.; Cerutti, P.; Sacco, D.; Giordano, A.; Pistono, M.; Komici, K.; Rossi Ferrario, S. Psychophysical health of great elder inpatients in cardiac rehabilitation: A retrospective cohort study. Eur. J. Phys. Rehabil. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.K.; Jakobsen, L.H.; Hollensberg, L.; Ryg, J.; Midttun, M.; Frederiksen, H.; Glenthøj, A.; Kodahl, A.R.; Secher-Johnsen, J.; Nielsen, L.K.; et al. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19–A retrospective cohort study. Arch. Gerontol. Geriatr. 2021, 94, 104335. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Palmieri, L.; Vanacore, N.; Giuliano, M.; Brusaferro, S.; The Italian National Institute of Health COVID-19 mortality group. Nonrespiratory Complications and Obesity in Patients Dying with COVID-19 in Italy. Obesity 2021, 29, 20–23. [Google Scholar] [CrossRef]
- Dietz, W.; Santos-Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity 2020, 28, 1005. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, R.; Castelnuovo, G.; Musetti, A.; Varallo, G.; Spatola, C.A.M.; Riboni, F.V.; Usubini, A.G.; Tosolin, F.; Manzoni, G.M.; Capodaglio, P.; et al. ACTonHEALTH study protocol: Promoting psychological flexibility with activity tracker and mHealth tools to foster healthful lifestyle for obesity and other chronic health conditions. Trials 2018, 19, 659. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, Y.; Wei, Y.; Hu, Y.; Wang, F.; Li, G.; Liu, K.; Du, R.; Wang, C.-Y.; Zhu, W. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J. Med. Virol. 2020, 92, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef]
- Rath, D.; Petersen-Uribe, Á.; Avdiu, A.; Witzel, K.; Jaeger, P.; Zdanyte, M.; Heinzmann, D.; Tavlaki, E.; Müller, K.; Gawaz, M.P. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin. Res. Cardiol. 2020, 109, 1491–1499. [Google Scholar] [CrossRef]
- Santoso, A.; Pranata, R.; Wibowo, A.; Al-Farabi, M.J.; Huang, I.; Antariksa, B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am. J. Emerg. Med. 2021, 44, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Masetti, C.; Generali, E.; Colapietro, F.; Voza, A.; Cecconi, M.; Messina, A.; Omodei, P.; Angelini, C.; Ciccarelli, M.; Badalamenti, S.; et al. High mortality in COVID-19 patients with mild respiratory disease. Eur. J. Clin. Investig. 2020, 50, e13314. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Patiño, A.; Arrieta, O.; Pino, L.E.; Rolfo, C.; Ricaurte, L.; Recondo, G.; Zatarain-Barron, Z.-L.; Corrales, L.; Martín, C.; Barrón, F.; et al. Mortality and Advanced Support Requirement for Patients with Cancer with COVID-19: A Mathematical Dynamic Model for Latin America. JCO Glob. Oncol. 2020, 6, 752–760. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: Cancer mortality could rise at least 20% because of pandemic, study finds. BMJ 2020, 369, m1735. [Google Scholar] [CrossRef]
- Curigliano, G. Cancer Patients and Risk of Mortality for COVID-19. Cancer Cell 2020, 38, 161–163. [Google Scholar] [CrossRef]
- Assaad, S.; Avrillon, V.; Fournier, M.-L.; Mastroianni, B.; Russias, B.; Swalduz, A.; Cassier, P.; Eberst, L.; Steineur, M.-P.; Kazes, M.; et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur. J. Cancer 2020, 135, 251–259. [Google Scholar] [CrossRef]
- Schade, E.C.; Elkaddoum, R.; Kourie, H.R. The psychological challenges for oncological patients in times of COVID-19 pandemic: Telemedicine, a solution? Future Oncol. 2020, 16, 2265–2268. [Google Scholar] [CrossRef]
- Tsamakis, K.; Gavriatopoulou, M.; Schizas, D.; Stravodimou, A.; Mougkou, A.; Tsiptsios, D.; Sioulas, V.; Spartalis, E.; Sioulas, A.D.; Tsamakis, C.; et al. Oncology during the COVID-19 pandemic: Challenges, dilemmas and the psychosocial impact on cancer patients (Review). Oncol. Lett. 2020, 20, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, S.; Zhu, J.; Shi, J.; Dai, K.; Chen, X. Clinical characteristics and outcomes of cancer patients with COVID-19. J. Med Virol. 2020, 92, 2067–2073. [Google Scholar] [CrossRef]
- Islam, J.Y.; Vidot, D.C.; Camacho-Rivera, M. Evaluating Mental Health–Related Symptoms Among Cancer Survivors During the COVID-19 Pandemic: An Analysis of the COVID Impact Survey. JCO Oncol. Pract. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, Z.; Ma, Z.; Mao, Y.; Li, X.; Wilson, A.; Qin, H.; Ou, J.; Peng, K.; Zhou, F.; et al. Epidemiology of mental health problems among patients with cancer during COVID-19 pandemic. Transl. Psychiatry 2020, 10, 263. [Google Scholar] [CrossRef]
- Spicer, J.; Chamberlain, C.; Papa, S. Provision of cancer care during the COVID-19 pandemic. Nat. Rev. Clin. Oncol. 2020, 17, 329–331. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Garassino, M.C. Caring for Patients with Cancer During the COVID-19 Outbreak in Italy. JAMA Oncol. 2020, 6, 821–822. [Google Scholar] [CrossRef]
- Curigliano, G. How to Guarantee the Best of Care to Patients with Cancer During the COVID-19 Epidemic: The Italian Experience. Oncologist 2020, 25, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Hershman, D.L.; Basch, E. Oncology Practice During the COVID-19 Pandemic. JAMA 2020, 323, 2005–2006. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Formenti, S.C. Cancer and COVID-19—Pdeleterious effects of delaying radiotherapy. Nat. Rev. Clin. Oncol. 2020, 17, 332–334. [Google Scholar] [CrossRef]
- Panzeri, A.; Rossi Ferrario, S. Supporting rehabilitation patients with COVID-19 during the pandemic: Experiences from a technologybased psychological approach. In Proceedings of the CEUR Workshop Proceedings: Second Symposium on Psychology-Based Technologies—Psychobit, Naples, Italy, 28–29 September 2020; Gigliotta, O., Ponticorvo, M., Eds.; CEUR Workshop Proceedings: Aachen, Germany, 2020. [Google Scholar]
- Sigorski, D.; Sobczuk, P.; Osmola, M.; Kuć, K.; Walerzak, A.; Wilk, M.; Ciszewski, T.; Kopeć, S.; Hryń, K.; Rutkowski, P.; et al. Impact of COVID-19 on anxiety levels among patients with cancer actively treated with systemic therapy. ESMO Open 2020, 5, e000970. [Google Scholar] [CrossRef]
- Panzeri, A.; Rossi Ferrario, S.; Cerutti, P. Psychological Differences Among Healthcare Workers of a Rehabilitation Institute During the COVID-19 Pandemic: A Two-Step Study. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef]
- Jee, J.; Foote, M.B.; Lumish, M.; Stonestrom, A.J.; Wills, B.; Narendra, V.; Avutu, V.; Murciano-Goroff, Y.R.; Chan, J.E.; Derkach, A.; et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J. Clin. Oncol. 2020, 38, 3538–3546. [Google Scholar] [CrossRef]
- Panzeri, A.; Rossi Ferrario, S.; Vidotto, G. Interventions for Psychological Health of Stroke Caregivers: A Systematic Review. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef]
- Ratti, M.M.; Bertin, F.; Rossi, A.; Portaluppi, A.; Marconi, M.; Sarno, L.; Verusio, C. Evaluation OF psychological aspects of taking care cancer patients: A multicentre study on a sample of caregivers. Annals Oncol. 2017, 28, vi83. [Google Scholar] [CrossRef]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef]
- Van de Haar, J.; Hoes, L.R.; Coles, C.E.; Seamon, K.; Fröhling, S.; Jäger, D.; Valenza, F.; de Braud, F.; De Petris, L.; Bergh, J.; et al. Caring for patients with cancer in the COVID-19 era. Nat. Med. 2020, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Karaçin, C.; Bilgetekin, I.; Başal, F.; Oksuzoglu, B. How does COVID-19 fear and anxiety affect chemotherapy adherence in patients with cancer. Future Oncol. 2020. [Google Scholar] [CrossRef]
- Brothers, B.M.; Andersen, B.L. Hopelessness as a predictor of depressive symptoms for breast cancer patients coping with recurrence. Psycho-Oncol. 2009, 18, 267–275. [Google Scholar] [CrossRef]
- Hagerty, R.G.; Butow, P.N.; Ellis, P.M.; Lobb, E.A.; Pendlebury, S.C.; Leighl, N.; Leod, C.M.; Tattersall, M.H.N. Communicating with Realism and Hope: Incurable Cancer Patients’ Views on the Disclosure of Prognosis. J. Clin. Oncol. 2005, 23, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Sardell, A.N.; Trierweiler, S.J. Disclosing the cancer diagnosis. Procedures that influence patient hopefulness. Cancer 1993, 72, 3355–3365. [Google Scholar] [CrossRef]
- Beach, W.A.; Dozier, D.M. Fears, Uncertainties, and Hopes: Patient-Initiated Actions and Doctors’ Responses During Oncology Interviews. J. Health Commun. 2015, 20, 1243–1254. [Google Scholar] [CrossRef]
- Epstein, R.M.; Street, R.L. Patient-centered communication in cancer care: Promoting healing and reducing suffering. 2007. [Google Scholar] [CrossRef]
- Robinson, J.D.; Hoover, D.R.; Venetis, M.K.; Kearney, T.J.; Street, R.L., Jr. Consultations Between Patients with Breast Cancer and Surgeons: A Pathway from Patient-Centered Communication to Reduced Hopelessness. J. Clin. Oncol. 2013, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.L.; Buell, S.; Zettler, P.; White, M.; Ruston, D.C.; Lo, B. Patient-centered communication. J. Gen. Intern. Med. 2004, 19, 1069–1079. [Google Scholar] [CrossRef]
- Saha, S.; Beach, M.C. The impact of patient-centered communication on patients’ decision making and evaluations of physicians: A randomized study using video vignettes. Patient Educ. Couns. 2011, 84, 386–392. [Google Scholar] [CrossRef]
- Hashim, M.J. Patient-Centered Communication: Basic Skills. Am. Fam. Physician 2017, 95, 29–34. [Google Scholar] [PubMed]
- Mazor, K.M.; Beard, R.L.; Alexander, G.L.; Arora, N.K.; Firneno, C.; Gaglio, B.; Greene, S.M.; Lemay, C.A.; Robinson, B.E.; Roblin, D.W.; et al. Patients’ and family members’ views on patient-centered communication during cancer care. Psycho-Oncology 2013, 22, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Krupat, E.; Bell, R.A.; Kravitz, R.L.; Thom, D.; Azari, R. When physicians and patients think alike: Patient-centered beliefs and their impact on satisfaction and trust. (Original Research). J. Fam. Pract. 2001, 12, 1057. [Google Scholar]
- Verheul, W.; Sanders, A.; Bensing, J. The effects of physicians’ affect-oriented communication style and raising expectations on analogue patients’ anxiety, affect and expectancies. Patient Educ. Couns. 2010, 80, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Schrooten, I.; de Jong, M.D.T. If You Could Read My Mind: The Role of Healthcare Providers’ Empathic and Communicative Competencies in Clients’ Satisfaction with Consultations. Health Commun. 2017, 32, 111–118. [Google Scholar] [CrossRef]
- Baer, L.; Weinstein, E. Improving oncology nurses’ communication skills for difficult conversations. Clin. J. Oncol. Nurs. 2013, 17 3, E45–E51. [Google Scholar] [CrossRef]
- Kim, S.S.; Kaplowitz, S.; Johnston, M.V. The Effects of Physician Empathy on Patient Satisfaction and Compliance. Eval. Health Prof. 2004, 27, 237–251. [Google Scholar] [CrossRef]
- Vliet, L.M.V.; Epstein, A.S. Current State of the Art and Science of Patient-Clinician Communication in Progressive Disease: Patients’ Need to Know and Need to Feel Known. J. Clin. Oncol. 2014, 32, 3474–3478. [Google Scholar] [CrossRef] [PubMed]
- Pollak, K.I.; Arnold, R.; Alexander, S.C.; Jeffreys, A.S.; Olsen, M.K.; Abernethy, A.P.; Rodriguez, K.L.; Tulsky, J.A. Do patient attributes predict oncologist empathic responses and patient perceptions of empathy? Support. Care Cancer 2010, 18, 1405–1411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zwingmann, J.; Baile, W.F.; Schmier, J.W.; Bernhard, J.; Keller, M. Effects of patient-centered communication on anxiety, negative affect, and trust in the physician in delivering a cancer diagnosis: A randomized, experimental study. Cancer 2017, 123, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, L.A.; Curbow, B.A.; Wingard, J.R.; McDonnell, K.; Somerfield, M.R. Can 40 seconds of compassion reduce patient anxiety? J. Clin. Oncol. 1999, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Hauk, H.; Bernhard, J.; McConnell, M.; Wohlfarth, B. Breaking bad news to cancer patients in times of COVID-19. Support. Care Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, R.; Pedersen, C.G.; Jensen, A.B.; Ehrnrooth, E.; Rossen, P.B.; von der Maase, H. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. Br. J. Cancer 2003, 88, 658–665. [Google Scholar] [CrossRef]
- Venetis, M.K.; Robinson, J.D.; Turkiewicz, K.L.; Allen, M. An evidence base for patient-centered cancer care: A meta-analysis of studies of observed communication between cancer specialists and their patients. Patient Educ. Couns. 2009, 77, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, N.; Prommer, E.; Sinclair, C.T.; Subbiah, I.M. Development of a Patient-Centered Framework for Oncology Clinicians to Address Uncertainty in Cancer Care During the COVID-19 Pandemic. Curr. Treat. Options Oncol. 2020, 21, 99. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling; The Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- Bentler, P.M.; Chou, C.H. Practical issues in structural modeling. Sociol. Methods Res. 1987, 16, 78–117. [Google Scholar] [CrossRef]
- Kelloway, E.K. Using Mplus for Structural Equation Modeling: A Researcher’s Guide; Sage Publications: New York, NY, USA, 2015. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics; Pearson: Harlow, UK, 2014. [Google Scholar]
- Pietrabissa, G.; Volpi, C.; Bottacchi, M.; Bertuzzi, V.; Guerrini Usubini, A.; Löffler-Stastka, H.; Prevendar, T.; Rapelli, G.; Cattivelli, R.; Castelnuovo, G.; et al. The Impact of Social Isolation during the COVID-19 Pandemic on Physical and Mental Health: The Lived Experience of Adolescents with Obesity and Their Caregivers. Int. J. Environ. Res. Public Health 2021, 18, 3026. [Google Scholar] [CrossRef] [PubMed]
- Milavic, B.; Padulo, J.; Grgantov, Z.; Milic, M.; Mannarini, S.; Manzoni, G.M.; Ardigo, L.P.; Rossi, A. Development and factorial validity of the Psychological Skills Inventory for Sports, Youth Version—Short Form: Assessment of the psychometric properties. PLoS ONE 2019, 14, e0220930. [Google Scholar] [CrossRef] [PubMed]
- Pietrabissa, G.; Rossi, A.; Borrello, M.; Manzoni, G.M.; Mannarini, S.; Castelnuovo, G.; Molinari, E. Development and Validation of a Self-Determination Theory-Based Measure of Motivation to Exercise and Diet in Children. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Pietrabissa, G.; Rossi, A.; Simpson, S.; Tagliagambe, A.; Bertuzzi, V.; Volpi, C.; Fava, G.; Manzoni, G.M.; Gravina, G.; Castelnuovo, G. Evaluation of the reliability and validity of the Italian version of the schema mode inventory for eating disorders: Short form for adults with dysfunctional eating behaviors. Eat. Weight Disord. 2020, 25, 553–565. [Google Scholar] [CrossRef]
- Simpson, S.; Pietrabissa, G.; Rossi, A.; Seychell, T.; Manzoni, G.M.; Munro, C.; Nesci, J.B.; Castelnuovo, G. Factorial Structure and Preliminary Validation of the Schema Mode Inventory for Eating Disorders (SMI-ED). Front. Psychol. 2018, 9, 600. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Rosseel, Y.; Oberski, D.; Byrnes, J.; Vanbrabant, L.; Savalei, V.; Merkle, E.; Hallquist, M.; Rhemtulla, M.; Katsikatsou, M.; Barendse, M. Package ‘lavaan’. 2015. [Google Scholar]
- Pastore, M.; Calcagnì, A. Measuring Distribution Similarities Between Samples: A Distribution-Free Overlapping Index. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2018. [Google Scholar]
- Robitzsch, A.; Kiefer, T.; Wu, M. TAM: Test. Analysis Modules 3.4-26. 2020. [Google Scholar]
- Iannone, R. DiagrammeR: Graph/Network Visualization, Version 1.0.0. 2018. Available online: http://visualizers.co/diagrammer/articles/graphviz-mermaid.html (accessed on 14 June 2021).
- Bond, T.G.; Fox, C.M. Applying the Rasch Model: Fundamental Measurement in the Human Sciences, 3rd ed.; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Mannarini, S. Assessing the rosenberg self-esteem scale dimensionality and items functioning in relation to self-efficacy and attachment styles. TPM Test. Psychom. Methodol. Appl. Psychol. 2010, 17, 229–242. [Google Scholar]
- Mannarini, S.; Boffo, M. The relevance of security: A latent domain of attachment relationships. Scand. J. Psychol. 2014, 55, 53–59. [Google Scholar] [CrossRef]
- Mannarini, S.; Boffo, M.; Bertucci, V.; Andrisani, A.; Ambrosini, G. A Rasch-based dimension of delivery experience: Spontaneous vs. medically assisted conception. J. Clin. Nurs. 2013, 22, 2404–2416. [Google Scholar] [CrossRef]
- Wright, B.; Linacre, J.; Gustafson, J.; Martin-Lof, P. Reasonable meansquare fit values. Rasch Meas. Trans. 1994, 8, 370–371. [Google Scholar] [CrossRef]
- Rossi Ferrario, S.; Panzeri, A.; Anselmi, P.; Vidotto, G. Development and Psychometric Properties of a Short Form of the Illness Denial Questionnaire. Psychol. Res. Behav. Manag. 2019, 12, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Devlieger, I.; Rosseel, Y. Factor score path analysis: An alternative for SEM? Methodology 2017, 13, 31–38. [Google Scholar] [CrossRef]
- Croon, M. Using predicted latent scores in general latent structure models. In Latent Variable and Latent Structure Modeling; Marcoulides, G., Moustaki, I., Eds.; Erlbaum: Mahwah, NJ, USA, 2002; pp. 195–223. [Google Scholar]
- Fritz, J.; Stochl, J.; Fried, E.I.; Goodyer, I.M.; van Borkulo, C.D.; Wilkinson, P.O.; van Harmelen, A.L. Unravelling the complex nature of resilience factors and their changes between early and later adolescence. BMC Med. 2019, 17, 203. [Google Scholar] [CrossRef]
- McNeish, D.; Wolf, M.G. Thinking twice about sum scores. Behav. Res. Methods 2020, 52, 2287–2305. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Marconi, M.; Bianchi, S.; Minelli, I.; Verusio, C. Il distress psicologico in un campione di pazienti oncologici: L’effetto di età e tipo di reparto. Ric. Psicol. 2020, 4, 1055–1074. [Google Scholar] [CrossRef]
- Frazier, P.A.; Tix, A.P.; Barron, K.E. Testing Moderator and Mediator Effects in Counseling Psychology Research. J. Couns. Psychol. 2004, 51, 115–134. [Google Scholar] [CrossRef]
- Weston, R.; Gore, P.A. A Brief Guide to Structural Equation Modeling. Couns. Psychol. 2006, 34, 719–751. [Google Scholar] [CrossRef]
- Iacobucci, D. Mediation Analysis; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- McDonald, R.P.; Ho, M.-H.R. Principles and practice in reporting structural equation analyses. Psychol. Methods 2002, 7, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Bollen, K.A. Structural Equations with Latent Variables; John Wiley & Sons, Inc.: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- MacKinnon, D.P. Introduction to Statistical Mediation Analysis; Routledge: New York, NY, USA, 2012. [Google Scholar]
- VanderWeele, T.; Vansteelandt, S. Mediation Analysis with Multiple Mediators. Epidemiol. Methods 2014, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, W.; von Eye, A. Direction of effects in mediation analysis. Psychol. Methods 2015, 20, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Gearhardt, A.N.; Castelnuovo, G.; Mannarini, S. Different methods of assessment, food addiction, emotional eating, and binge eating behaviors: Comparing the total model effects of sequential mediation analysis. In Proceedings of the CEUR Workshop Proceedings: Second Symposium on Psychology-Based Technologies—Psychobit, Naples, Italy, 28–29 September 202; Gigliotta, O., Ponticorvo, M., Eds.; CEUR Workshop Proceedings: Aachen, Germany, 2020. [Google Scholar]
- Wen, Z.; Fan, X. Monotonicity of effect sizes: Questioning kappa-squared as mediation effect size measure. Psychol. Methods 2015, 20, 193–203. [Google Scholar] [CrossRef]
- Huberty, C.J.; Lowman, L.L. Group overlap as a basis for effect size. Educ. Psychol. Meas. 2000, 60, 543–563. [Google Scholar] [CrossRef]
- Samawi, H.M.; Yin, J.; Rochani, H.; Panchal, V. Notes on the overlap measure as an alternative to the Youden index: How are they related? Stat. Med. 2017, 36, 4230–4240. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Padhy, S.K. Ethical dilemmas faced by health care workers during COVID-19 pandemic: Issues, implications and suggestions. Asian J. Psychiatry 2020, 51, 102116. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Tambelli, R.; Casagrande, M. COVID-19 Pandemic in the Italian Population: Validation of a Post-Traumatic Stress Disorder Questionnaire and Prevalence of PTSD Symptomatology. Int. J. Environ. Res. Public Health 2020, 17, 4151. [Google Scholar] [CrossRef] [PubMed]
- Mannarini, S.; Boffo, M. An implicit measure of associations with mental illness versus physical illness: Response latency decomposition and stimuli differential functioning in relation to IAT order of associative conditions and accuracy. PLoS ONE 2014, 9, e101911. [Google Scholar] [CrossRef]
- Webster, D.M.; Kruglanski, A.W. Individual differences in need for cognitive closure. J. Personal. Soc. Psychol. 1994, 67, 1049–1062. [Google Scholar] [CrossRef]
- Pierro, A.; Kruglanski, A.W. “Seizing and Freezing” on a Significant-Person Schema: Need for Closure and the Transference Effect in Social Judgment. Personal. Soc. Psychol. Bull. 2008, 34, 1492–1503. [Google Scholar] [CrossRef]
- Kruglanski, A.W.; Chen, X.; Pierro, A.; Mannetti, L.; Erb, H.-P.; Spiegel, S. Persuasion According to the Unimodel: Implications for Cancer Communication. J. Commun. 2006, 56, S105–S122. [Google Scholar] [CrossRef]
- Kruglanski, A.W.; Molinario, E.; Lemay, E.P. Coping with COVID-19-induced threats to self. Group Process. Intergroup Relat. 2021, 24, 284–289. [Google Scholar] [CrossRef]
- Kruglanski, A.W.; Peri, N.; Zakai, D. Interactive Effects of Need for Closure and Initial Confidence on Social Information Seeking. Soc. Cogn. 1991, 9, 127–148. [Google Scholar] [CrossRef]
- Kennifer, S.L.; Alexander, S.C.; Pollak, K.I.; Jeffreys, A.S.; Olsen, M.K.; Rodriguez, K.L.; Arnold, R.M.; Tulsky, J.A. Negative emotions in cancer care: Do oncologists’ responses depend on severity and type of emotion? Patient Educ. Couns. 2009, 76, 51–56. [Google Scholar] [CrossRef]
- Webster, D.M.; Kruglanski, A.W. Cognitive and Social Consequences of the Need for Cognitive Closure. Eur. Rev. Soc. Psychol. 1997, 8, 133–173. [Google Scholar] [CrossRef]
- Dean, M.; Street, R.L. A 3-stage model of patient-centered communication for addressing cancer patients’ emotional distress. Patient Educ. Couns. 2014, 94, 143–148. [Google Scholar] [CrossRef]
- Gattellari, M.; Butow, P.N.; Tattersall, M.H.; Dunn, S.M.; MacLeod, C.A. Misunderstanding in cancer patients: Why shoot the messenger? Ann. Oncol. 1999, 10, 39–46. [Google Scholar] [CrossRef]
- Weeks, J.C.; Cook, E.F.; O’Day, S.J.; Peterson, L.M.; Wenger, N.; Reding, D.; Harrell, F.E.; Kussin, P.; Dawson, N.V.; Connors, A.F., Jr.; et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998, 279, 1709–1714. [Google Scholar] [CrossRef]
- Pietrabissa, G.; Manzoni, G.M.; Rossi, A.; Castelnuovo, G. The MOTIV-HEART study: A prospective, randomized, single-blind pilot study of brief strategic therapy and motivational interviewing among cardiac rehabilitation patients. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef]
- Stark, D.; Kiely, M.; Smith, A.; Morley, S.; Selby, P.; House, A. Reassurance and the anxious cancer patient. Br. J. Cancer 2004, 91, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Lelorain, S. Discussing Prognosis with Empathy to Cancer Patients. Curr. Oncol. Rep. 2021, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Razavi, D.; Delvaux, N.; Marchal, S.; Durieux, J.F.; Farvacques, C.; Dubus, L.; Hogenraad, R. Does training increase the use of more emotionally laden words by nurses when talking with cancer patients? A randomised study. Br. J. Cancer 2002, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Polini, C.; Forte, G.; Favieri, F.; Boncompagni, I.; Casagrande, M. The Effectiveness of Psychological Treatments in Women with Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Mannarini, S.; Boffo, M.; Balottin, L. Beliefs about the patient’s role in the psychotherapeutic relationship: A latent trait perspective. TPM Test. Psychom. Methodol. Appl. Psychol. 2013, 20, 277–294. [Google Scholar] [CrossRef]
- Bentler, P.M.; Yuan, K.-H. Structural Equation Modeling with Small Samples: Test Statistics. Multivar. Behav. Res. 1999, 34, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Roter, D.L.; Frankel, R.M.; Hall, J.A.; Sluyter, D. The expression of emotion through nonverbal behavior in medical visits. J. Gen. Intern. Med. 2006, 21, 28–34. [Google Scholar] [CrossRef]
- Fiedler, K.; Schott, M.; Meiser, T. What mediation analysis can (not) do. J. Exp. Soc. Psychol. 2011, 47, 1231–1236. [Google Scholar] [CrossRef]
| Infit | Outfit | Xsi (SE) | |
|---|---|---|---|
| Fear of COVID-19 | |||
| Considering your oncological disease, are you afraid of COVID-19? | 0.726 | 0.501 | 1.180 (0.328) |
| Considering your oncological disease, are you anxious about COVID-19? | 0.788 | 0.620 | 0.138 (0.319) |
| Considering your oncological disease, are you preoccupied with COVID-19? | 0.851 | 0.680 | 0.966 (0.326) |
| Empathic communication | |||
| Was the doctor empathetic in communicating the management of cancer care during this period? | 0.998 | 0.983 | −0.577 (0.288) |
| Was the doctor reassuring in communicating the management of cancer care during this period? | 0.876 | 0.667 | −1.439 (0.302) |
| Was the doctor warm in communicating the management of cancer care during this period? | 0.984 | 0.823 | −1.439 (0.302) |
| Clarity of information | |||
| Was the doctor precise in communicating information relating to the management of cancer care during this period? | 0.792 | 0.612 | −1.544 (0.311) |
| Was the doctor explicit in communicating information relating to the management of cancer care during this period? | 0.911 | 0.783 | −1.742 (0.317) |
| Was the doctor clear in communicating information relating to the management of cancer care during this period? | 1.072 | 1.241 | −2.162 (0.332) |
| Hopelessness | |||
| Considering your oncological disease, did the future seem hopeless to you? | 0.953 | 0.848 | 2.024 (0.329) |
| Considering your oncological disease, did the future seem difficult to face? | 0.863 | 0.772 | 1.312 (0.310) |
| Considering your oncological disease, did the future seem more negative than positive to you? | 0.935 | 0.768 | 1.917 (0.326) |
| Fear of COVID-19 | Empathic Communication | Clarity of Information | Hopelessness | Age | |
|---|---|---|---|---|---|
| Fear of COVID-19 | - | ||||
| Empathic communication | −0.691 *** | - | |||
| Clarity of information | −0.475 *** | 0.658 *** | - | ||
| Hopelessness | 0.689 *** | −0.651 *** | −0.583 *** | - | |
| Age | 0.176 § | −0.177 § | −0.122 § | 0.169 § | - |
| External Variable | Dependent Variable | Β * | β (SE) | 95%CI (L U) | z-Value | p-Value |
|---|---|---|---|---|---|---|
| Civil status | Fear of COVID-19 | −0.164 | −0.628 (0.395) | (−1.403; 0.146) | −1.590 | p = 0.112 |
| Empathic communication | 0.126 | 0.365 (0.316) | (−0.254; 0.985) | 1.155 | p = 0.248 | |
| Clarity of information | −0.063 | −0.184 (0.336) | (−0.842; 0.474) | −0.549 | p = 0.583 | |
| Hopelessness | 0.013 | 0.041 (0.412) | (−0.767; 0.848) | 0.099 | p = 0.921 | |
| Work status | Fear of COVID−19 | 0.242 | 0.603 (0.246) | (0.121; 1.085) | 2.450 | p = 0.014 |
| Empathic communication | −0.108 | −0.205 (0.189) | (−0.576; 0.167) | −1.080 | p = 0.280 | |
| Clarity of information | −0.071 | −0.136 (0.165) | (−0.459; 0.186) | −0.829 | p = 0.407 | |
| Hopelessness | 0.112 | 0.228 (0.213) | (−0.189; 0.645) | 1.073 | p = 0.283 | |
| Education | Fear of COVID−19 | −0.068 | −0.253 (0.365) | (−0.968; 0.461) | −0.695 | p = 0.487 |
| Empathic communication | 0.233 | 0.659 (0.289) | (0.092; 1.226) | 2.278 | p = 0.023 | |
| Clarity of information | 0.279 | 0.800 (0.288) | (0.234; 1.365) | 2.772 | p = 0.006 | |
| Hopelessness | −0.121 | −0.369 (0.337) | (−1.028; 0.291) | −1.096 | p = 0.273 | |
| Type of cancer | Fear of COVID−19 | −0.116 | −0.227 (0.208) | (−0.634; 0.180) | −1.093 | p = 0.274 |
| Empathic communication | 0.047 | 0.070 (0.156) | (−0.235; 0.375) | 0.450 | p = 0.652 | |
| Clarity of information | 0.072 | 0.109 (0.151) | (−0.187; 0.404) | 0.719 | p = 0.472 | |
| Hopelessness | −0.137 | −0.219 (0.156) | (−0.524; 0.086) | −1.405 | p = 0.160 |
| Path | β * | β (SE) | 95%CI (L U) | z-Value | p-Value | R2 | |
|---|---|---|---|---|---|---|---|
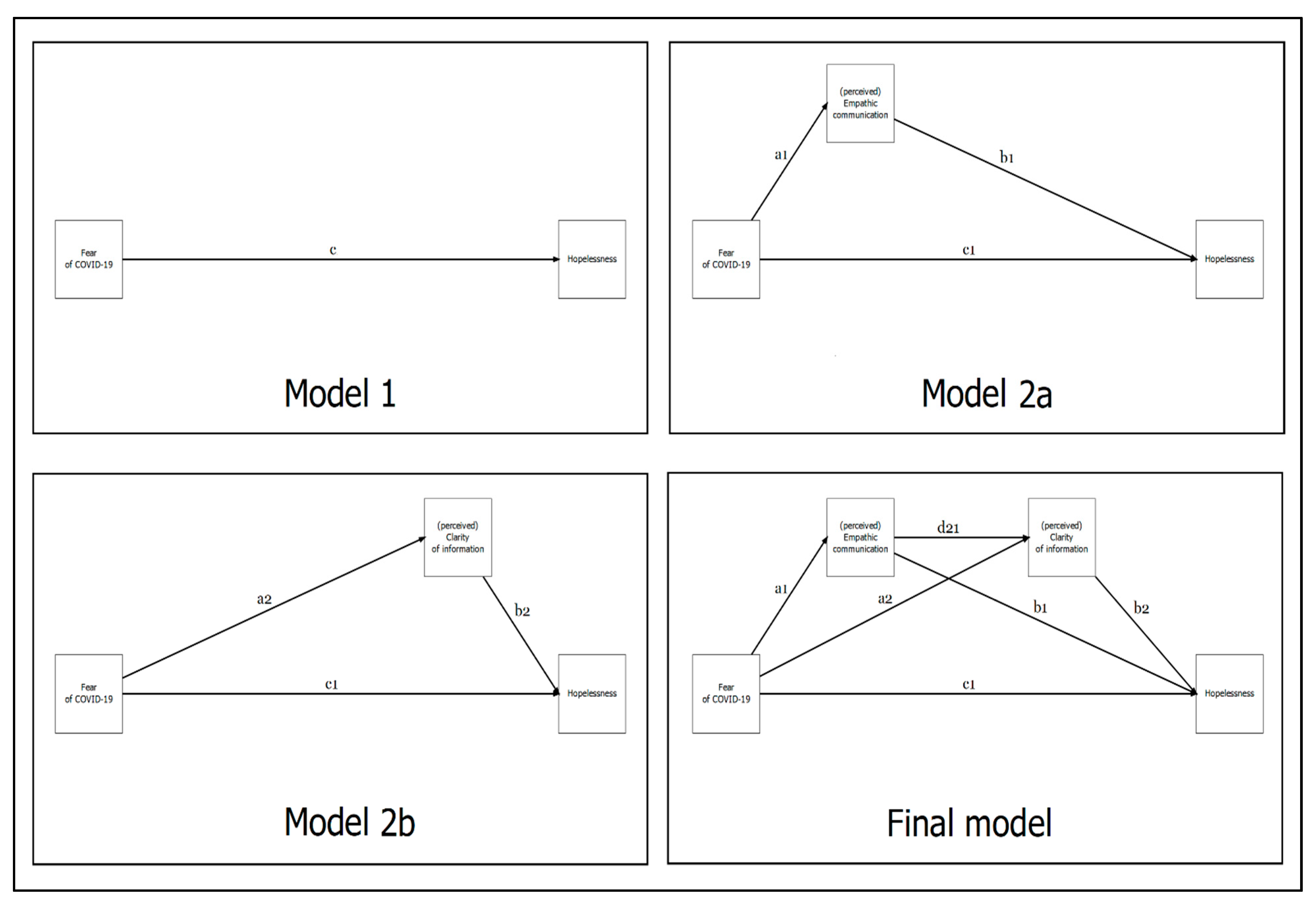
| Model 1 | |||||||
| Fear of COVID-19 (X) → Hopelessness (Y) | (c) | 0.689 | 0.563 (0.058) | (0.448; 0.676) | 9.625 | p < 0.001 | 0.475 |
| Model 2a | |||||||
| Fear of COVID-19 (X) → Empathic communication (M1) | (a1) | −0.691 | −0.524 (0.061) | −0.639 −0.401 | −8.585 | p < 0.001 | 0.478 |
| Empathic communication (M1) → Hopelessness (Y) | (b1) | −0.335 | −0.361 (0.135) | −0.621 −0.089 | −2.666 | p = 0.008 | 0.534 |
| Fear of COVID-19 (X) → Hopelessness (Y) | (c1) | 0.458 | 0.374 (0.100) | 0.183 0.576 | 3.723 | p < 0.001 | |
| Indirect effect of X on Y via M1 | (a1*b1) | 0.231 | 0.189 (0.076) | 0.046 0.350 | 2.478 | p = 0.013 | |
| Total effect X on Y | 0.689 | 0.563 (0.058) | 0.447 0.677 | 9.640 | p < 0.001 | ||
| Model 2b | |||||||
| Fear of COVID-19 (X) → Clarity of information (M2) | (a2) | −0.475 | −0.365 (0.076) | −0.512 −0.214 | −4.802 | p < 0.001 | 0.225 |
| Clarity of information (M2) → Hopelessness (Y) | (b2) | −0.330 | −0.350 (0.093) | −0.543 −0.177 | −3.756 | p < 0.001 | 0.559 |
| Fear of COVID-19 (X) → Hopelessness (Y) | (c1) | 0.533 | 0.435 (0.074) | 0.281 0.571 | 5.883 | p < 0.001 | |
| Indirect effect of X on Y via M2 | (a2*b2) | 0.156 | 0.128 (0.050) | 0.050 0.244 | 2.566 | p = 0.010 | |
| Total effect X on Y | 0.689 | 0.563 (0.058) | 0.445 0.675 | 9.673 | p < 0.001 |
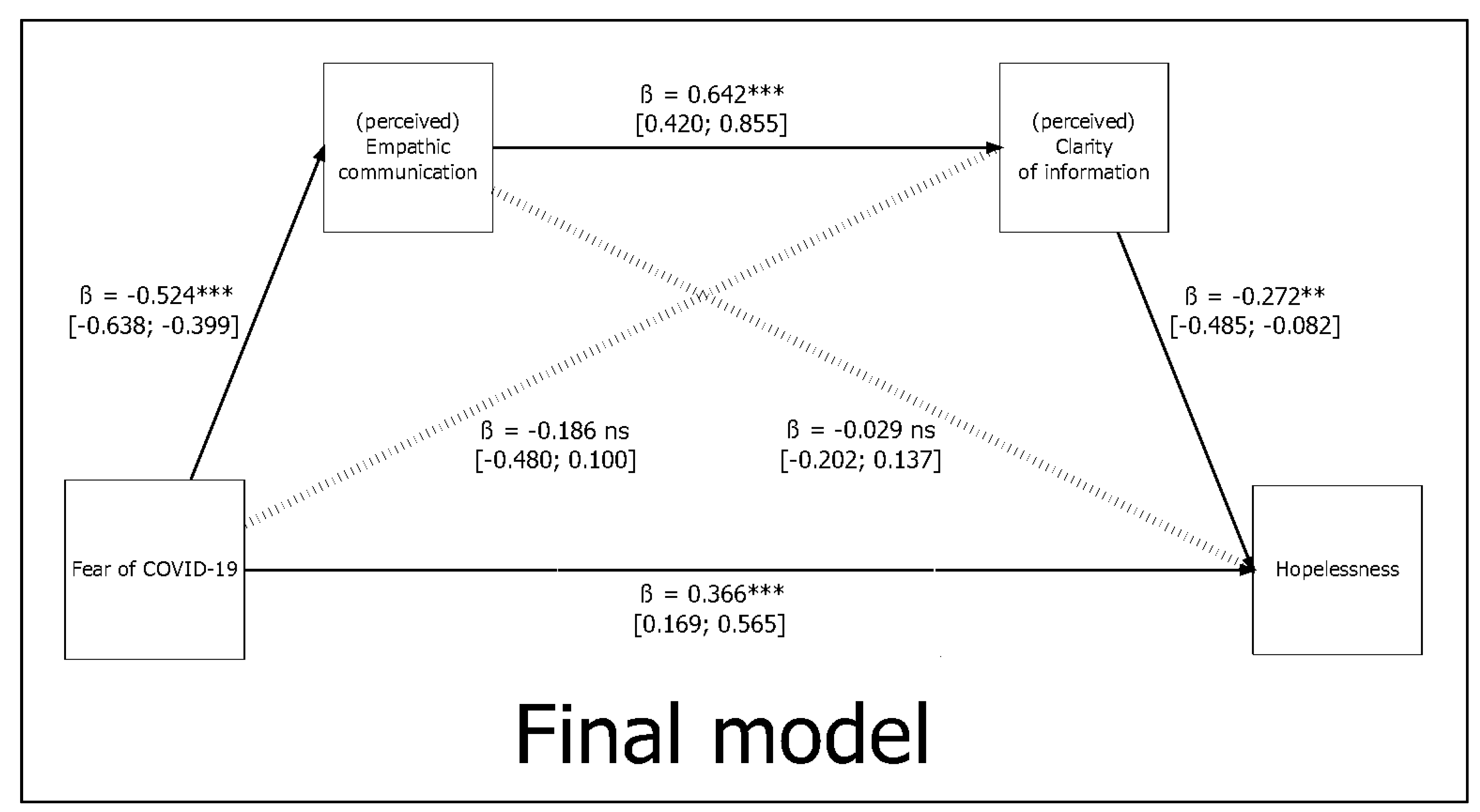
| Path | β * | β (SE) | 95%CI (L U) | z-Value | p-Value | R2 | |
|---|---|---|---|---|---|---|---|
| Fear of COVID-19 (X) → Empathic communication (M1) | (a1) | −0.691 | −0.524 (0.061) | (−0.638; −0.399) | −8.584 | p < 0.001 | 0.478 |
| Fear of COVID-19 (X) → Clarity of information (M2) | (a2) | −0.037 | −0.029 (0.086) | (−0.202; 0.137) | −0.333 | p = 0.739 | 0.438 |
| Empathic communication (M1) → Clarity of information (M2) | (d21) | 0.632 | 0.642 (0.110) | (0.420; 0.855) | 5.830 | p < 0.001 | |
| Empathic communication (M1) → Hopelessness (Y) | (b1) | −0.173 | −0.186 (0.148) | (−0.480; 0.100) | −1.262 | p = 0.207 | |
| Clarity of information (M2) → Hopelessness (Y) | (b2) | −0.256 | −0.272 (0.101) | (−0.485; −0.082) | −2.692 | p = 0.007 | |
| Fear of COVID−19 (X) → Hopelessness (Y) | (c1) | 0.448 | 0.366 (0.101) | (0.169; 0.565) | 3.626 | p < 0.001 | 0.571 |
| Indirect effect of X on Y via M1 | (a1*b1) | 0.119 | 0.098 (0.079) | (−0.055; 0.263) | 1.228 | p = 0.219 | |
| Indirect effect of X on Y via M2 | (a2*b2) | −0.010 | 0.008 (0.027) | (−0.033; 0.075) | 0.291 | p = 0.771 | |
| Indirect effect of X on Y via M1 and M2 | (a1*d21*b2) | 0.112 | 0.091 (0.038) | (0.027; 0.176) | 2.410 | p = 0.016 | |
| Total indirect effect | 0.241 | 0.197 (0.078) | (0.056; 0.362) | 2.523 | p = 0.012 | ||
| Total effect X on Y | 0.689 | 0.563 (0.059) | (0.447; 0.678) | 9.564 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, A.A.; Marconi, M.; Taccini, F.; Verusio, C.; Mannarini, S. From Fear to Hopelessness: The Buffering Effect of Patient-Centered Communication in a Sample of Oncological Patients during COVID-19. Behav. Sci. 2021, 11, 87. https://doi.org/10.3390/bs11060087
Rossi AA, Marconi M, Taccini F, Verusio C, Mannarini S. From Fear to Hopelessness: The Buffering Effect of Patient-Centered Communication in a Sample of Oncological Patients during COVID-19. Behavioral Sciences. 2021; 11(6):87. https://doi.org/10.3390/bs11060087
Chicago/Turabian StyleRossi, Alessandro Alberto, Maria Marconi, Federica Taccini, Claudio Verusio, and Stefania Mannarini. 2021. "From Fear to Hopelessness: The Buffering Effect of Patient-Centered Communication in a Sample of Oncological Patients during COVID-19" Behavioral Sciences 11, no. 6: 87. https://doi.org/10.3390/bs11060087
APA StyleRossi, A. A., Marconi, M., Taccini, F., Verusio, C., & Mannarini, S. (2021). From Fear to Hopelessness: The Buffering Effect of Patient-Centered Communication in a Sample of Oncological Patients during COVID-19. Behavioral Sciences, 11(6), 87. https://doi.org/10.3390/bs11060087






