Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia
Abstract
1. Introduction
2. The Brain and Obesity
2.1. Brain Mechanisms of Appetite Regulation and Food Consumption
2.1.1. Hypothalamic Appetite-Regulating System
2.1.2. The Mesocorticolimbic Reward System and Food Consumption
2.1.3. The Prefrontal Cortex and Food Consumption
2.2. Brain Abnormalities Implicated in Dysregulation of Energy Homeostasis and Obesity
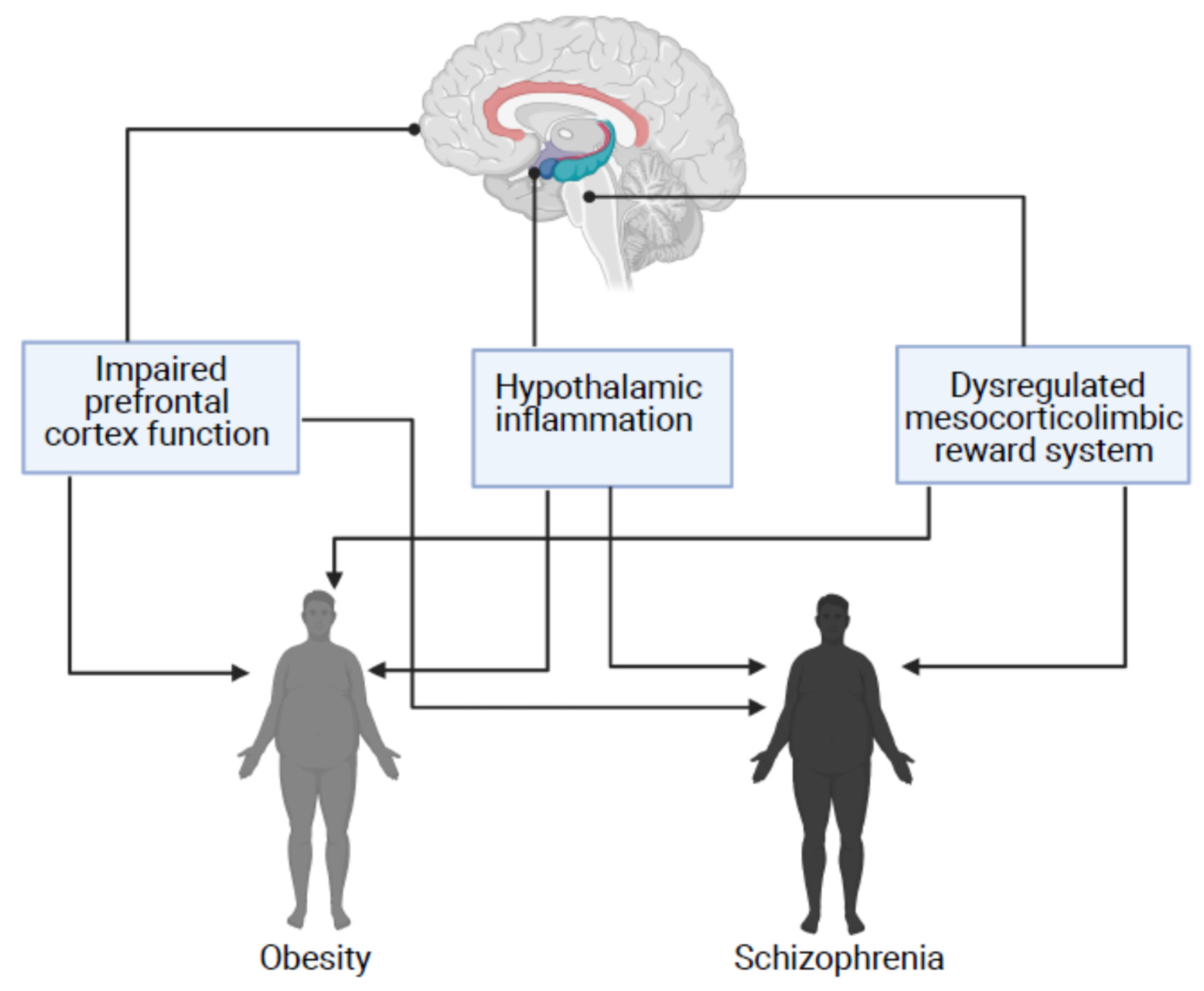
2.2.1. Hypothalamic Inflammation and Obesity
2.2.2. Dysregulated Mesocorticolimbic Reward System and Uncontrolled Food Consumption
2.2.3. Impaired Prefrontal Cortex Function and Food Consumption
2.3. Brain Abnormalities Implicated in Dysregulated Energy Homeostasis and Obesity Have Also Been Implicated in the Neurobiology of Schizophrenia
2.3.1. Hypothalamic Inflammation in Schizophrenia
2.3.2. Dysregulated Mesocorticolimbic Reward System in Patients with Schizophrenia
2.3.3. Impaired Prefrontal Cortex (PFC) Function in Patients with Schizophrenia
3. Repetitive Transcranial Magnetic Stimulation (rTMS) as a Potential Treatment for Obesity in Schizophrenia
3.1. The Basics of rTMS
3.2. Efficacy of rTMS for Reducing Food Craving, Food Consumption and Treating Obesity in Non-Psychiatric Samples
4. rTMS Effects That We Hypothesize to Be Relevant for Reducing Food Craving, Food Consumption and Inducing Weight Loss in Obese Patients with Schizophrenia
4.1. Anti-Inflammatory Effect of rTMS
4.2. Modulation of the Mesocorticolimbic Reward Circuitry by rTMS
4.3. Modulation of Pre-Frontal Cortex Function by rTMS
5. Deep TMS
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annamalai, A.; Kosir, U.; Tek, C. Prevalence of obesity and diabetes in patients with schizophrenia. World J. Diabetes 2017, 8, 390–396. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Kim, D.; Kim, S.W.; Kim, J.H.J.J.; Kim, J.H.J.J.; Nam, H.J.; Ryu, S.; Park, I.H.; An, S.K.; et al. Prevalence of metabolic syndrome in patients with schizophrenia in Korea: A multicenter nationwide cross-sectional study. Psychiatry Investig. 2017, 14, 44–50. [Google Scholar] [CrossRef]
- Laursen, T.M.; Wahlbeck, K.; Hällgren, J.; Westman, J.; Ösby, U.; Alinaghizadeh, H.; Gissler, M.; Nordentoft, M. Life Expectancy and Death by Diseases of the Circulatory System in Patients with Bipolar Disorder or Schizophrenia in the Nordic Countries. PLoS ONE 2013, 8, e67133. [Google Scholar] [CrossRef]
- Maciukiewicz, M.; Tiwari, A.K.; Zai, C.C.; Gorbovskaya, I.; Laughlin, C.P.; Nurmi, E.L.; Liebermann, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. Genome-wide association study on antipsychotic-induced weight gain in Europeans and African-Americans. Schizophr. Res. 2019, 212, 204–212. [Google Scholar] [CrossRef]
- Faulkner, G.; Cohn, T.; Remington, G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.H.; Kim, T.H.; Lim, S.H.; Kim, Y.; Lee, Y.A.; Song, S.W. The effects of repetitive transcranial magnetic stimulation on eating behaviors and body weight in obesity: A randomized controlled study. Brain Stimul. 2018, 11, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zilverstand, A.; Gui, W.; Li, H.J.; Zhou, X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimul. 2019, 12, 606–618. [Google Scholar] [CrossRef]
- Ferrulli, A.; Macrì, C.; Terruzzi, I.; Massarini, S.; Ambrogi, F.; Adamo, M.; Milani, V.; Luzi, L. Weight loss induced by deep transcranial magnetic stimulation in obesity: A randomized, double-blind, sham-controlled study. Diabetes, Obes. Metab. 2019, 21, 1849–1860. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.H.; Kim, T.H.; Lim, S.H.; Kim, Y.; Eun, Y.M.; Lee, Y.A. The effects of repetitive transcranial magnetic stimulation on body weight and food consumption in obese adults: A randomized controlled study. Brain Stimul. 2019, 12, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Haley, A.P. Obesity and the Brain: Another Brain-Body Versus Body-Brain Conundrum. Psychosom. Med. 2020, 82, 258–260. [Google Scholar] [CrossRef]
- Quarta, C.; Fioramonti, X.; Cota, D. POMC Neurons Dysfunction in Diet-induced Metabolic Disease: Hallmark or Mechanism of Disease? Neuroscience 2020, 447, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Francke, P.; Tiedemann, L.J.; Menz, M.M.; Beck, J.; Büchel, C.; Brassen, S. Mesolimbic white matter connectivity mediates the preference for sweet food. Sci. Rep. 2019, 9, 4349. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Reichelt, A.C.; Hall, P.A. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn. Sci. 2019, 23, 349–361. [Google Scholar] [CrossRef]
- Di Bonaventura, E.M.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Di Bonaventura, M.V.M.; Cifani, C. The melanocortin system behind the dysfunctional eating behaviors. Nutrients 2020, 12, 3502. [Google Scholar] [CrossRef]
- Subramaniapillai, M.; Mcintyre, R.S. A review of the neurobiology of obesity and the available pharmacotherapies. CNS Spectr. 2017, 22, 29–38. [Google Scholar] [CrossRef]
- Dickson, S.L.; Chowen, J.A. Neuroscience of obesity. Neuroscience 2020, 447, 1–2. [Google Scholar] [CrossRef]
- Castillo-Armengol, J.; Fajas, L.; Lopez-Mejia, I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019. [Google Scholar] [CrossRef]
- Manceau, R.; Majeur, D.; Alquier, T. Neuronal control of peripheral nutrient partitioning. Diabetologia 2020, 63, 673–682. [Google Scholar] [CrossRef]
- Taber, K.H.; Black, D.N.; Porrino, L.J.; Hurley, R.A. Neuroanatomy of dopamine: Reward and addiction. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 1–4. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Ballard, I.C.; Murty, V.P.; McKell Carter, R.; Macinnes, J.J.; Huettel, S.A.; Alison Adcock, R. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J. Neurosci. 2011, 31, 10340–10346. [Google Scholar] [CrossRef]
- Cassidy, R.M.; Tong, Q. Hunger and satiety gauge reward sensitivity. Front. Endocrinol. 2017, 8, 104. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S. Neural vulnerability factors that increase risk for future weight gain. Psychol. Bull. 2016, 142, 447–471. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; García-García, I.; Dagher, A. Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. Eur. J. Neurosci. 2019, 50, 2430–2445. [Google Scholar] [CrossRef]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharm. Res. 2019, 42, 383–392. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Puig, J.; Blasco, G.; Daunis-I-Estadella, J.; Molina, X.; Xifra, G.; Ricart, W.; Pedraza, S.; Fernández-Aranda, F.; Fernández-Real, J.M. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J. Clin. Endocrinol. Metab. 2015, 100, E276–E281. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & inflammation: The linking mechanism & the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Chen, P.S.; Kuo, Y.M.; Tzeng, S.F. Intermittent peripheral exposure to lipopolysaccharide induces exploratory behavior in mice and regulates brain glial activity in obese mice. J. Neuroinflammation 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.E.; Unger, E.K.; Cheung, C.C.; Xu, A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E697–E706. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- de Weijer, B.A.; van de Giessen, E.; van Amelsvoort, T.A.; Boot, E.; Braak, B.; Janssen, I.M.; van de Laar, A.; Fliers, E.; Serlie, M.J.; Booij, J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Wang, G.J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Stice, E.; Burger, K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 2019, 68, 38–53. [Google Scholar] [CrossRef]
- de Decker, A.; Sioen, I.; Verbeken, S.; Braet, C.; Michels, N.; de Henauw, S. Associations of reward sensitivity with food consumption, activity pattern, and BMI in children. Appetite 2016, 100, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.M.; Eastman, A.; Ugwoaba, U.A.; Demos, K.E.; Lillis, J.; Wing, R.R. Food reward sensitivity, impulsivity, and weight change during and after a 3-month weight loss program. PLoS ONE 2020, 15, e0243530. [Google Scholar] [CrossRef]
- Rösch, S.A.; Schmidt, R.; Lührs, M.; Ehlis, A.C.; Hesse, S.; Hilbert, A. Evidence of fnirs-based prefrontal cortex hypoactivity in obesity and binge-eating disorder. Brain Sci. 2021, 11, 19. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Lowe, C.J.; Staines, W.R.; Mannochio, F.; Hall, P.A. The neurocognitive mechanisms underlying food cravings and snack food consumption. A combined continuous theta burst stimulation (cTBS) and EEG study. Neuroimage 2018, 177, 45–58. [Google Scholar] [CrossRef]
- Lowe, C.J.; Hall, P.A.; Staines, W.R. The effects of continuous theta burst stimulation to the left dorsolateral prefrontal cortex on executive function, food cravings, and snack food consumption. Psychosom. Med. 2014, 76, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Pannacciulli, N.; Del Parigi, A.; Chen, K.; Le, D.S.N.T.; Reiman, E.M.; Tataranni, P.A. Brain abnormalities in human obesity: A voxel-based morphometric study. Neuroimage 2006, 31, 1419–1425. [Google Scholar] [CrossRef]
- Widge, A.S.; Heilbronner, S.R.; Hayden, B.Y. Prefrontal cortex and cognitive control: New insights from human electrophysiology. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, L.E.; Murdaugh, D.L.; Cox, J.E.; Cook, E.W.; Weller, R.E. Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain Imaging Behav. 2013, 7, 116–128. [Google Scholar] [CrossRef]
- Steward, T.; Miranda-Olivos, R.; Soriano-Mas, C.; Fernández-Aranda, F. Neuroendocrinological mechanisms underlying impulsive and compulsive behaviors in obesity: A narrative review of fMRI studies. Rev. Endocr. Metab. Disord. 2019, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Trépanier, M.O.; Hopperton, K.E.; Mizrahi, R.; Mechawar, N.; Bazinet, R.P. Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Mol. Psychiatry 2016, 21, 1009–1026. [Google Scholar] [CrossRef]
- Comer, A.L.; Carrier, M.; Tremblay, M.È.; Cruz-Martín, A. The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation. Front. Cell. Neurosci. 2020, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Schlaaff, K.; Dobrowolny, H.; Frodl, T.; Mawrin, C.; Gos, T.; Steiner, J.; Bogerts, B. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain. Behav. Immun. 2020, 88, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Van Beveren, N.J.M.; Ramsey, J.; Leweke, F.M.; Rothermundt, M.; Bogerts, B.; Steiner, J.; Guest, P.C.; Bahn, S. Identification of subgroups of Schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr. Bull. 2014, 40, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R. Neuropathology of Schizophrenia. Arch. Gen. Psychiatry 1982, 39, 1131–1139. [Google Scholar] [CrossRef]
- Kowalchuk, C.; Kanagasundaram, P.; Belsham, D.D.; Hahn, M.K. Antipsychotics differentially regulate insulin, energy sensing, and inflammation pathways in hypothalamic rat neurons. Psychoneuroendocrinology 2019, 104, 42–48. [Google Scholar] [CrossRef]
- He, M.; Huang, X.F.; Gao, G.; Zhou, T.; Li, W.; Hu, J.; Chen, J.; Li, J.; Sun, T. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology 2019, 104, 286–299. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, Y.; Sodhi, R.K.; Khare, P.; Bishnoi, M.; Kondepudi, K.K.; Medhi, B.; Kuhad, A. Role of TRPV1/TRPV3 channels in olanzapine-induced metabolic alteration: Possible involvement in hypothalamic energy-sensing, appetite regulation, inflammation and mesolimbic pathway. Toxicol. Appl. Pharmacol. 2020, 402, 115124. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- de Nijs, J.; Schnack, H.G.; Koevoets, M.G.J.C.; Kubota, M.; Kahn, R.S.; van Haren, N.E.M.; Cahn, W. Reward-related brain structures are smaller in patients with schizophrenia and comorbid metabolic syndrome. Acta Psychiatr. Scand. 2018, 138, 581–590. [Google Scholar] [CrossRef]
- Dipasquale, S.; Pariante, C.M.; Dazzan, P.; Aguglia, E.; McGuire, P.; Mondelli, V. The dietary pattern of patients with schizophrenia: A systematic review. J. Psychiatr. Res. 2013, 47, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Thornley, S.; Russell, B.; Kydd, R. Carbohydrate Reward and Psychosis: An Explanation For Neuroleptic Induced Weight Gain and Path to Improved Mental Health? Curr. Neuropharmacol. 2011, 9, 370–375. [Google Scholar] [CrossRef]
- Juckel, G. Inhibition of the reward system by antipsychotic treatment. Dialogues Clin. Neurosci. 2016, 18, 109–114. [Google Scholar] [CrossRef]
- Nielsen, M.O.; Rostrup, E.; Wulff, S.; Bak, N.; Broberg, B.V.; Lublin, H.; Kapur, S.; Glenthoj, B. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch. Gen. Psychiatry 2012, 69, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.Ø.; Rostrup, E.; Wulff, S.; Bak, N.; Lublin, H.; Kapur, S.; Glenthøj, B. Alterations of the brain reward system in antipsychotic nave schizophrenia patients. Biol. Psychiatry 2012, 71, 898–905. [Google Scholar] [CrossRef]
- Grimm, O.; Heinz, A.; Walter, H.; Kirsch, P.; Erk, S.; Haddad, L.; Plichta, M.M.; Romanczuk-Seiferth, N.; Pöhland, L.; Mohnke, S.; et al. Striatal response to reward anticipation evidence for a systems-level intermediate phenotype for schizophrenia. JAMA Psychiatry 2014, 71, 531–539. [Google Scholar] [CrossRef]
- Nielsen, M.O.; Rostrup, E.; Wulff, S.; Glenthøj, B.; Ebdrup, B.H. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry 2016, 73, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Callicott, J.H.; Bertolino, A.; Mattay, V.S.; Langheim, F.J.P.; Duyn, J.; Coppola, R.; Goldberg, T.E.; Weinberger, D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex 2000, 10, 1078–1092. [Google Scholar] [CrossRef]
- Ragland, J.D.; Yoon, J.; Minzenberg, M.J.; Carter, C.S. Neuroimaging of cognitive disability in schizophrenia: Search for a pathophysiological mechanism. Int. Rev. Psychiatry 2007, 19, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J.; Laird, A.R.; Thelen, S.; Carter, C.S.; Glahn, D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry 2009, 66, 811. [Google Scholar] [CrossRef]
- Orellana, G.; Slachevsky, A. Executive functioning in schizophrenia. Front. Psychiatry 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.W.; Carter, C.S. Event-Related fMRI Study of Context Processing in Dorsolateral Prefrontal Cortex of Patients with Schizophrenia. J. Abnorm. Psychol. 2003, 112, 689–697. [Google Scholar] [CrossRef]
- Robison, A.J.; Thakkar, K.N.; Diwadkar, V.A. Cognition and Reward Circuits in Schizophrenia: Synergistic, Not Separate. Biol. Psychiatry 2020, 87, 204–214. [Google Scholar] [CrossRef]
- Wang, H.; Lesh, T.A.; Maddock, R.J.; Fassbender, C.; Carter, C.S. Delay discounting abnormalities are seen in first-episode schizophrenia but not in bipolar disorder. Schizophr. Res. 2020, 216, 200–206. [Google Scholar] [CrossRef]
- Gold, J.M.; Waltz, J.A.; Prentice, K.J.; Morris, S.E.; Heerey, E.A. Reward processing in schizophrenia: A deficit in the representation of value. Schizophr. Bull. 2008, 34, 835–847. [Google Scholar] [CrossRef]
- George, M.S.; Lisanby, S.H.; Sackeim, H.A. Transcranial magnetic stimulation: Applications in neuropsychiatry. Arch. Gen. Psychiatry 1999, 56, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Burt, T.; Lisanby, S.H.; Sackeim, H.A. Neuropsychiatric applications of transcranial magnetic stimulation: A meta-analysis. Int. J. Neuropsychopharmacol. 2002, 5, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Amir, A.; Levkovitz, Y.; Zangen, A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J. Clin. Neurophysiol. 2007, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- McClintock, S.M.; Kallioniemi, E.; Martin, D.M.; Kim, J.U.; Weisenbach, S.L.; Abbott, C.C. A Critical Review and Synthesis of Clinical and Neurocognitive Effects of Noninvasive Neuromodulation Antidepressant Therapies. Focus 2019, 17, 18–29. [Google Scholar] [CrossRef]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J.; Cracco, J.B.; Rudell, A.; Eberle, L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1989, 74, 458–462. [Google Scholar] [CrossRef]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Storch, E.A.; Tendler, A.; Schneider, S.C.; Guzick, A.G.; La Buissonniere-Ariza, V.; Goodman, W.K. Moderators and predictors of response to deep transcranial magnetic stimulation for obsessive-compulsive disorder. J. Psychiatr. Res. 2020. [Google Scholar] [CrossRef]
- Lee, D.J.; Elias, G.J.B.; Lozano, A.M. Neuromodulation for the treatment of eating disorders and obesity. Ther. Adv. Psychopharmacol. 2018, 8, 73–92. [Google Scholar] [CrossRef]
- Hall, P.A.; Vincent, C.M.; Burhan, A.M. Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: A review of methods, findings and controversies. Appetite 2018, 124, 78–88. [Google Scholar] [CrossRef]
- Antonelli, M.; Fattore, L.; Sestito, L.; Di Giuda, D.; Diana, M.; Addolorato, G. Transcranial Magnetic Stimulation: A review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addict. Behav. 2021, 114, 106760. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, M.; Dampil, O.A.; Damian, L.; Doquenia, M.L.; Redondo-Samin, D.C.; Woolbright, M.K. Efficacy of repetitive transcranial magnetic stimulation (Rtms) in inducing weight loss among obese filipino patients: A randomized controlled trial. J. ASEAN Fed. Endocr. Soc. 2020, 35, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Beros, J.; Bates, K.A.; Harvey, A.R.; Tang, A.D.; Rodger, J. Low intensity repetitive magnetic stimulation reduces expression of genes related to inflammation and calcium signalling in cultured mouse cortical astrocytes. Brain Stimul. 2021, 14, 183–191. [Google Scholar] [CrossRef]
- Aftanas, L.I.; Gevorgyan, M.M.; Zhanaeva, S.Y.; Dzemidovich, S.S.; Kulikova, K.I.; Al’perina, E.L.; Danilenko, K.V.; Idova, G.V. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on Neuroinflammation and Neuroplasticity in Patients with Parkinson’s Disease: A Placebo-Controlled Study. Bull. Exp. Biol. Med. 2018, 165, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sasso, V.; Bisicchia, E.; Latini, L.; Ghiglieri, V.; Cacace, F.; Carola, V.; Molinari, M.; Viscomi, M.T. Repetitive transcranial magnetic stimulation reduces remote apoptotic cell death and inflammation after focal brain injury. J. Neuroinflammation 2016, 13, 150. [Google Scholar] [CrossRef]
- Okada, K.; Matsunaga, K.; Yuhi, T.; Kuroda, E.; Yamashita, U.; Tsuji, S. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002, 957, 37–41. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Tian, Q.; Zhu, B.; Zhao, Z. Repetitive transcranial magnetic stimulation increases serum brain-derived neurotrophic factor and decreases interleukin-1β and tumor necrosis factor-α in elderly patients with refractory depression. J. Int. Med. Res. 2019, 47, 1848–1855. [Google Scholar] [CrossRef]
- Tian, L.; Sun, S.S.; Cui, L.B.; Wang, S.Q.; Peng, Z.W.; Tan, Q.R.; Hou, W.G.; Cai, M. Repetitive Transcranial Magnetic Stimulation Elicits Antidepressant- and Anxiolytic-like Effect via Nuclear Factor-E2-related Factor 2-mediated Anti-inflammation Mechanism in Rats. Neuroscience 2020, 429, 119–133. [Google Scholar] [CrossRef]
- Luan, D.; Zhao, M.-G.; Shi, Y.-C.; Li, L.; Cao, Y.-J.; Feng, H.-X.; Zhang, Z.-J. Mechanisms of repetitive transcranial magnetic stimulation for anti-depression: Evidence from preclinical studies. World J. Psychiatry 2020, 10, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Bourgon, J.; Ibáñez Alario, M.; Mayoral-van Son, J.; Gómez Revuelta, M.; Ayesa Arriola, R.; Juncal Ruiz, M.; Ortiz-García de la Foz, V.; Crespo Facorro, B. A 3-year prospective study on the metabolic effect of aripiprazole, quetiapine and ziprasidone: A pragmatic clinical trial in first episode psychosis patients. Eur. Neuropsychopharmacol. 2020, 39, 46–55. [Google Scholar] [CrossRef]
- Terada, K.; Murata, A.; Toki, E.; Goto, S.; Yamakawa, H.; Setoguchi, S.; Watase, D.; Koga, M.; Takata, J.; Matsunaga, K.; et al. Atypical antipsychotic drug ziprasidone protects against rotenone-induced neurotoxicity: An in vitro study. Molecules 2020, 25, 4206. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Su, Y.; Guo, F.; Zhang, H.; Zhao, Y.; Huang, Q.; Xu, H. Deep rTMS Mitigates Behavioral and Neuropathologic Anomalies in Cuprizone-Exposed Mice Through Reducing Microglial Proinflammatory Cytokines. Front. Integr. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.H.; Hu, Y.; Sui, Y.F.; Peng, T.; Guo, T.C. Effects of Repetitive Transcranial Magnetic Stimulation on Astrocytes Proliferation and nNOS Expression in Neuropathic Pain Rats. Curr. Med. Sci. 2018, 38, 482–490. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Münch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef]
- Zhang, X.; Alnafisah, R.S.; Hamoud, A.R.A.; Shukla, R.; Wen, Z.; McCullumsmith, R.E.; O’Donovan, S.M. Role of Astrocytes in Major Neuropsychiatric Disorders. Neurochem. Res. 2021. [Google Scholar] [CrossRef]
- Keck, M.E.; Welt, T.; Müller, M.B.; Erhardt, A.; Ohl, F.; Toschi, N.; Holsboer, F.; Sillaber, I. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 2002, 43, 101–109. [Google Scholar] [CrossRef]
- Van Holstein, M.; Froböse, M.I.; O’Shea, J.; Aarts, E.; Cools, R. Controlling striatal function via anterior frontal cortex stimulation. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Zwanzger, P.; Mulert, C.; Rupprecht, R.; Möller, H.J.; Hegerl, U.; et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: Preliminary results of a dynamic [123I] IBZM SPECT study. J. Psychiatr. Res. 2006, 40, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Mulert, C.; Grossheinrich, N.; Rupprecht, R.; Möller, H.J.; Hegerl, U.; et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as d-amphetamine. Psychiatry Res. Neuroimaging 2007, 156, 251–255. [Google Scholar] [CrossRef]
- Bray, G.A. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Ann. Intern. Med. 1993, 119, 707–713. [Google Scholar] [CrossRef]
- Limongi, R.; Mackinley, M.; Dempster, K.; Khan, A.R.; Gati, J.S.; Palaniyappan, L. Frontal–striatal connectivity and positive symptoms of schizophrenia: Implications for the mechanistic basis of prefrontal rTMS. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271. [Google Scholar] [CrossRef]
- Li, X.; Yuan, X.; Kang, Y.; Pang, L.; Liu, Y.; Zhu, Q.; Lv, L.; Huang, X.F.; Song, X. A synergistic effect between family intervention and rTMS improves cognitive and negative symptoms in schizophrenia: A randomized controlled trial. J. Psychiatr. Res. 2020, 126, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Garza-Villarreal, E.A.; Alcala-Lozano, R.; Fernandez-Lozano, S.; Morelos-Santana, E.; Dávalos, A.; Villicaña, V.; Alcauter, S.; Castellanos, F.X.; Gonzalez-Olvera, J.J. Clinical and functional connectivity outcomes of 5-Hz repeated transcranial magnetic stimulation as an add-on treatment in cocaine use disorder: A double-blind randomized controlled trial. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, W.; Liang, Q.; Cao, X.; Lucas, M.V.; Yuan, T.F. Effect of Low-Frequency Repetitive Transcranial Magnetic Stimulation on Impulse Inhibition in Abstinent Patients with Methamphetamine Addiction: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200910. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.S.; Minichino, A.; Enticott, P.G.; Mazzarini, L.; Khan, N.; Antonacci, G.; Raccah, R.N.; Salviati, M.; Delle Chiaie, R.; Bersani, G.; et al. Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: A comprehensive review. Eur. Psychiatry 2013, 28, 30–39. [Google Scholar] [CrossRef]
- Tendler, A.; Barnea Ygael, N.; Roth, Y.; Zangen, A. Deep transcranial magnetic stimulation (dTMS)—Beyond depression. Expert Rev. Med. Devices 2016, 13, 987–1000. [Google Scholar] [CrossRef] [PubMed]

| Study | TMS Modality | Study Design | Inclusion Criteria | Treatment Allocation | Results |
|---|---|---|---|---|---|
| Kim et al. 2018 [6]. | HF rTMS (10Hz) to left DLPFC, 4 sessions/week for 2 weeks vs. sham TMS. | Randomized, sham-controlled, single-blind, parallel-group trial. | Male or female, between 18 and 65 years, BMI ≥ 25, no psychiatric illness | 30 randomized to active treatment and 30 to sham stimulation | Greater weight loss from baseline for active vs. sham stimulation (−1.35 ± 2.31 kg vs. 0.45 ± 1.28 kg; p = 0.002) |
| Kim et al. 2019 [9]. | HF rTMS (10Hz) to left DLPFC, 8 sessions/week for 4 weeks vs. sham TMS. | Randomized, sham-controlled, single-blind, parallel-group trial. | Male or female, between 18 and 70 years, BMI ≥ 25, no psychiatric illness | 21 randomized to active treatment and 22 to sham stimulation | Greater weight loss from baseline for active vs. sham stimulation (−2.75 ± 2.37 kg vs. 0.38 ± 1.0 kg; p < 0.01) |
| Ferrulli et al. 2019 [8]. | HF dTMS (18 Hz) vs. LF dTMS (1 Hz) or sham. | Randomized, sham-controlled, single-blind, parallel-group trial. | Male or female, between 22 and 65 years, BMI between 30–45, no psychiatric illness | 15 randomized to HF dTMS, 12 to LF dTMS and 12 to sham stimulation. | significant decrease in weight (−7.83 ± 2.28 kg; p = 0.0009) and BMI (−2.83 ± 0.83, p = 0.0009) for HF dTMS group. |
| Encarnacion et al. 2020 [83]. | HF rTMS (10Hz) to left DLPFC, 2 sessions/week for 2 weeks vs. sham TMS. | Randomized, sham-controlled, single-blind, parallel group trial, | Male or female, between 15 and 65 years, BMI ≥ 30, no psychiatric illness | 15 randomized to active treatment and 15 to sham stimulation | significant decrease in weight (−1.3 ± 1.3 kg; p =0.009) and BMI (0.6 ± 0.6, p =0.001) in the HF rTMS group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monem, R.G.; Okusaga, O.O. Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia. Behav. Sci. 2021, 11, 86. https://doi.org/10.3390/bs11060086
Monem RG, Okusaga OO. Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia. Behavioral Sciences. 2021; 11(6):86. https://doi.org/10.3390/bs11060086
Chicago/Turabian StyleMonem, Ramey G., and Olaoluwa O. Okusaga. 2021. "Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia" Behavioral Sciences 11, no. 6: 86. https://doi.org/10.3390/bs11060086
APA StyleMonem, R. G., & Okusaga, O. O. (2021). Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia. Behavioral Sciences, 11(6), 86. https://doi.org/10.3390/bs11060086





