Alexithymia as a Predictor of Arousal and Affect Dysregulations when Batterers with Attention Deficit Hyperactivity Disorder Cope with Acute Stress
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Psychological State Variables
2.4. Alexithymia Traits
2.5. Alcohol Assessment
2.6. Electrophysiological Recording
2.7. Data Analysis
3. Results
3.1. Participant Characteristics and Appraisal Scores
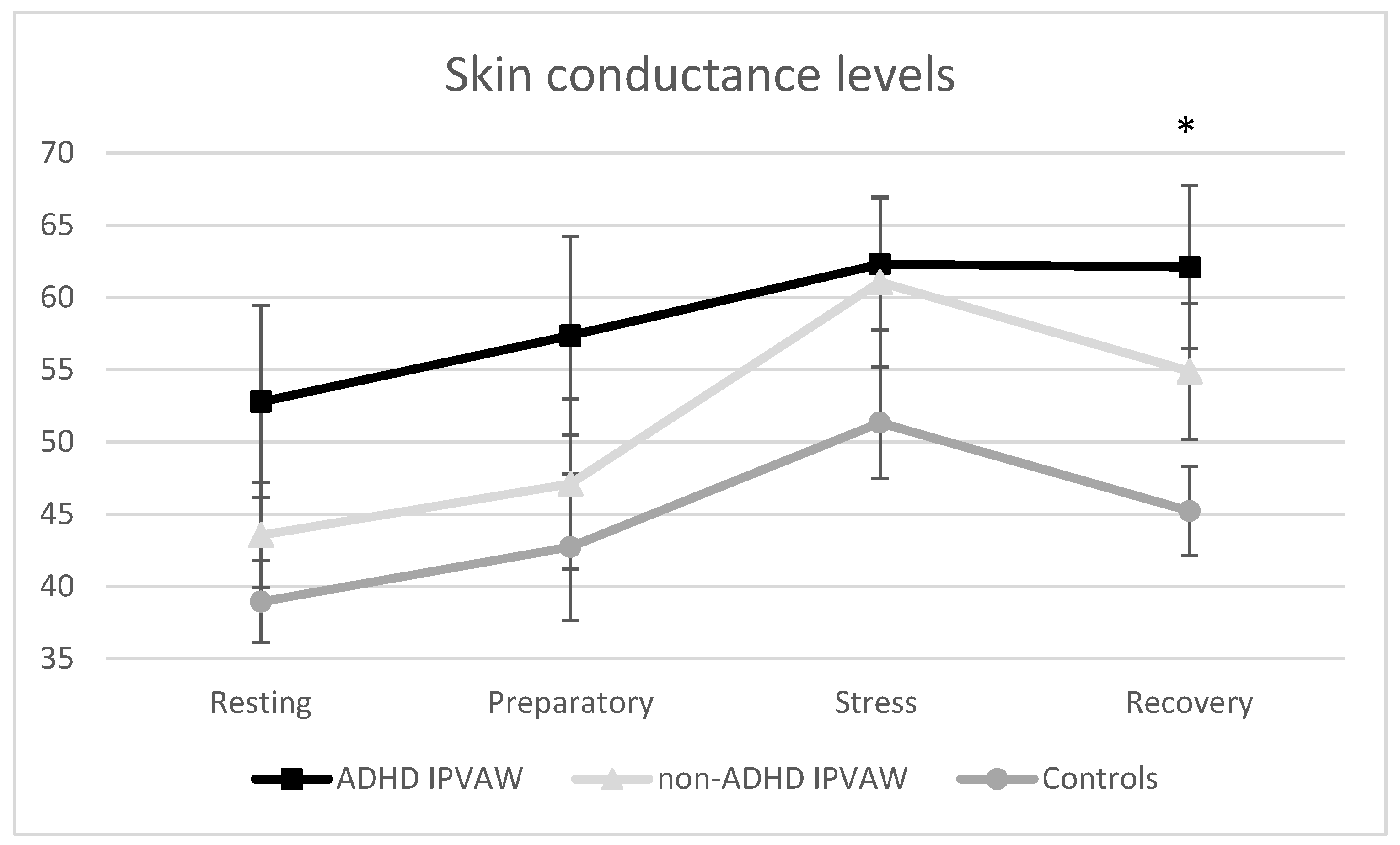
3.2. Stress Responses
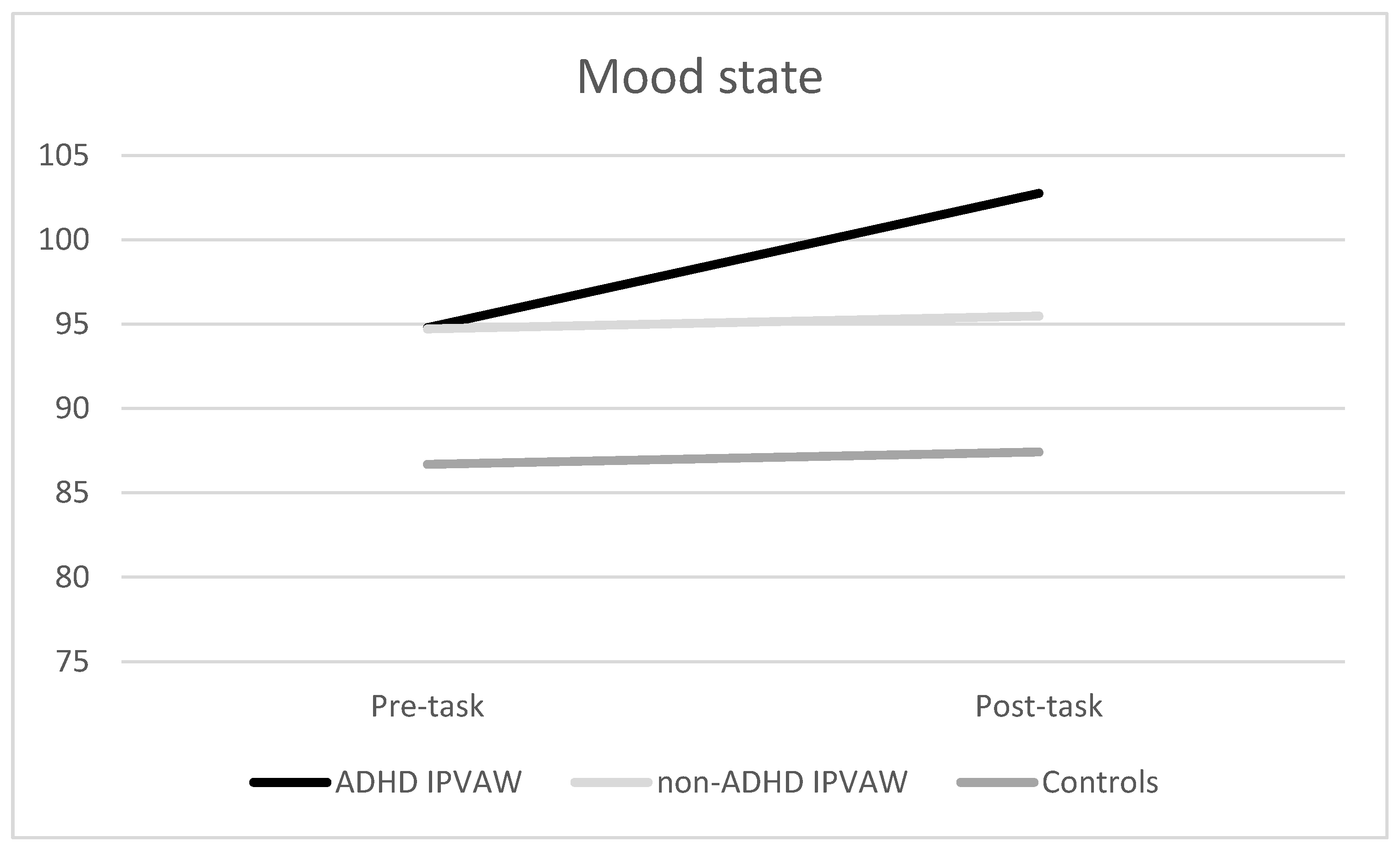
3.3. Psychological Responses to the Laboratory Acute Task
3.4. Alexithymia Traits (TAS-20 Total Score) as a Predictor of Electrodermal and Psychological Responses to the Laboratory Acute Task, Controlling for Group and Drug Misuse (Alcohol and Other Drugs)
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global and Regional Estimates of Violence against Women: Prevalence and Health Effects of Intimate Partner Violence and Non-Partner Sexual Violence; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Babcock, J.C.; E Green, C.; Robie, C. Does batterers’ treatment work? A meta-analytic review of domestic violence treatment. Clin. Psychol. Rev. 2004, 23, 1023–1053. [Google Scholar] [CrossRef]
- Pinto, L.A.; Sullivan, E.L.; Rosenbaum, A.; Wyngarden, N.; Umhau, J.C.; Miller, M.; Taft, C.T. Biological correlates of intimate partner violence perpetration. Aggress. Violent Behav. 2010, 15, 387–398. [Google Scholar] [CrossRef]
- Moya-Albiol, L.; Sarinana-Gonzalez, P.; Vitoria-Estruch, S.; Romero-Martinez, Á. Neurocriminology as an Emerging Applied Discipline. Vox Juris 2017, 33, 15. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Murciano-Martí, S.; Moya-Albiol, L. Is Sertraline a Good Pharmacological Strategy to Control Anger? Results of a Systematic Review. Behav. Sci. 2019, 9, 57. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Moya-Albiol, L. Neuropsychology of perpetrators of domestic violence: The role of traumatic brain injury and alcohol abuse and/or dependence. Revista de Neurología 2013, 57, 515–522. [Google Scholar]
- Buitelaar, N.J.L.; Posthumus, J.A.; Bijlenga, D.; Buitelaar, J.K. The Impact of ADHD Treatment on Intimate Partner Violence in a Forensic Psychiatry Setting. J. Atten. Disord. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wymbs, B.T.; Dawson, A.E.; Suhr, J.A.; Bunford, N.; Gidycz, C.A. ADHD Symptoms as Risk Factors for Intimate Partner Violence Perpetration and Victimization. J. Interpers. Violence 2016, 32, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Wymbs, B.T.; Dawson, A.E.; Egan, T.E.; Sacchetti, G.M. Rates of Intimate Partner Violence Perpetration and Victimization Among Adults With ADHD. J. Atten. Disord. 2016, 23, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Wymbs, B.T.; Walther, C.A.P.; Cheong, J.; Belendiuk, K.A.; Pedersen, S.L.; Gnagy, E.M.; Pelham, W.E.; Molina, B.S.G. Childhood ADHD Potentiates the Association Between Problematic Drinking and Intimate Partner Violence. J. Atten. Disord. 2014, 21, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D. Review: Electrodermal Responses: What Happens in the Brain. Neuroscience 2002, 8, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.C.; Boucsein, W.; Fowles, D.C.; Gruzelier, J. (Eds.) Progress in Electrodermal Research; Springer Science & Business Media: New York, NY, USA, 2012; Volume 249. [Google Scholar]
- Lim, C.L.; Rennie, C.; Barry, R.J.; Bahramali, H.; Lazzaro, I.; Manor, B.; Gordon, E. Decomposing skin conductance into tonic and phasic components. Int. J. Psychophysiol. 1997, 25, 97–109. [Google Scholar] [CrossRef]
- Du Rietz, E.; James, S.-N.; Banaschewski, T.; Brandeis, D.; Asherson, P.; Kuntsi, J. Autonomic arousal profiles in adolescents and young adults with ADHD as a function of recording context. Psychiatry Res. Neuroimaging 2019, 275, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.; McCarthy, R.; Selikowitz, M.; Macdonald, B.; Dupuy, F.E. Caffeine effects on resting-state electrodermal levels in AD/HD suggest an anomalous arousal mechanism. Boil. Psychol. 2012, 89, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, A.; Gerdes, A.; Mucha, R.F.; Weyers, P.; Lesch, K.-P.; Bähne, C.G.; Fallgatter, A.J.; Renner, T.; Warnke, A.; Romanos, M.; et al. Autonomic hypoactivity in boys with attention-deficit/hyperactivity disorder and the influence of methylphenidate. World J. Boil. Psychiatry 2013, 15, 56–65. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Katkin, E.S.; Strassberg, Z.; Snarr, J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J. Abnorm. Psychol. 2001, 110, 610–624. [Google Scholar] [CrossRef]
- Dupuy, F.E.; Clarke, A.; Barry, R.J.; Selikowitz, M.; McCarthy, R. EEG and electrodermal activity in girls with Attention-Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2014, 125, 491–499. [Google Scholar] [CrossRef]
- Iaboni, F.; Douglas, V.I.; Ditto, B. Psychophysiological response of ADHD children to reward and extinction. Psychophysiology 1997, 34, 116–123. [Google Scholar] [CrossRef]
- Hermens, D.F.; Williams, L.M.; Lazzaro, I.; Whitmont, S.; Melkonian, D.; Gordon, E. Sex differences in adult ADHD: A double dissociation in brain activity and autonomic arousal. Boil. Psychol. 2004, 66, 221–233. [Google Scholar] [CrossRef]
- Mayer, K.; Wyckoff, S.N.; Strehl, U. Underarousal in adult ADHD: How are peripheral and cortical arousal related? Clin. EEG Neurosci. 2016, 47, 171–179. [Google Scholar] [CrossRef]
- James, S.-N.; Cheung, C.H.; Rijsdijk, F.; Asherson, P.; Kuntsi, J. Modifiable Arousal in Attention-Deficit/Hyperactivity Disorder and Its Etiological Association with Fluctuating Reaction Times. Biol. Psychiatry 2016, 1, 539–547. [Google Scholar] [CrossRef]
- Donfrancesco, R.; Di Trani, M.; Gregori, P.; Auguanno, G.; Melegari, M.G.; Zaninotto, S.; Luby, J. Attention-deficit/hyperactivity disorder and alexithymia: A pilot study. Atten. Deficit Hyperact. Disord. 2013, 5, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Edel, M.-A.; Rudel, A.; Hubert, C.; Scheele, D.; Brüne, M.; Juckel, G.; Assion, H.-J. Alexithymia, emotion processing and social anxiety in adults with ADHD. Eur. J. Med Res. 2010, 15, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Allah-Gholilo, K.; Abolghasemi, A.; Dehghan, H.; Imani, H. The Association of Alexithymia and Sense of Coherence with Life Satisfaction in Attention Deficit Hyperactivity Disorder. Zahedan J. Res. Med Sci. 2015, 17. [Google Scholar] [CrossRef]
- Edel, M.-A.; Edel, S.; Kruger, M.; Assion, H.-J.; Juckel, G.; Brüne, M. Attachment, recalled parental rearing, and ADHD symptoms predict emotion processing and alexithymia in adults with ADHD. Ann. Gen. Psychiatry 2015, 14, 43. [Google Scholar] [CrossRef][Green Version]
- Matuszak, J.; Miller, G.; Kemmelmeier, M.; Mason, N. A Pilot Study of the Impact of Stimulant Pharmacotherapy in College Students with ADHD on Alexithymia and Psychological Mindedness. Open J. Med. Psychol. 2013, 2, 139–142. [Google Scholar] [CrossRef][Green Version]
- Romero-Martínez, Á.; Lila, M.; Williams, R.; Gonzalez-Bono, E.; Moya-Albiol, L. Skin conductance rises in preparation and recovery to psychosocial stress and its relationship with impulsivity and testosterone in intimate partner violence perpetrators. Int. J. Psychophysiol. 2013, 90, 329–333. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Nunes-Costa, R.; Lila, M.; Gonzalez-Bono, E.; Moya-Albiol, L. Cardiovascular reactivity to a marital conflict version of the Trier social stress test in intimate partner violence perpetrators. Stress 2014, 17, 321–327. [Google Scholar] [CrossRef]
- Dawson, M.E.; Schell, A.M.; Filion, D.L.; Cacioppo, J.T.; Tassinary, L.G.; Berntson, G. The Electrodermal System. In Handbook of Psychophysiology; Cambridge University Press (CUP): Cambridge, UK, 2016; pp. 217–243. [Google Scholar]
- Porges, S.W. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001, 42, 123–146. [Google Scholar] [CrossRef]
- Vitoria-Estruch, S.; Romero-Martínez, Á.; Lila, M.; Moya-Albiol, L. Could Alcohol Abuse Drive Intimate Partner Violence Perpetrators’ Psychophysiological Response to Acute Stress? Int. J. Environ. Res. Public Health 2018, 15, 2729. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Lila, M.; Moya-Albiol, L. Alexithymic traits are closely related to impulsivity and cognitive and empathic dysfunctions in intimate partner violence perpetrators: New targets for intervention. Appl. Neuropsychol. Adult 2019, 1–9. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Lila, M.; Moya-Albiol, L. The Importance of Considering Alexithymia during Initial Stages of Intimate Partner Violence Interventions to Design Adjuvant Treatments. Int. J. Environ. Res. Public Health 2019, 16, 3695. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, Á.; Lila, M.; Sariñana-González, P.; Gonzalez-Bono, E.; Moya-Albiol, L. High testosterone levels and sensitivity to acute stress in perpetrators of domestic violence with low cognitive flexibility and impairments in their emotional decoding process: A preliminary study. Aggress. Behav. 2013, 39, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.; Gracia, E.; Catalá-Miñana, A. Individualized motivational plans in batterer intervention programs: A randomized clinical trial. J. Consult. Clin. Psychol. 2018, 86, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Conners, K. The Conners’ Continuous Performance Test, 3rd ed.; Multi-Health Systems Incorporated: North Tonawanda, NY, USA, 2014. [Google Scholar]
- Páez, F.; Jiménez, A.; López, A.; Ariza, J.P.R.; Soto, H.O.; Nicolini, H. Estudio de validez de la traducción al castellano de la Escala de Impulsividad de Plutchik. Salud Ment. 1996, 19 (Suppl. 3), 10–12. [Google Scholar]
- Plutchik, R.; Van Praag, H.M. The measurement of suicidality and impulsivity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1989, 13, 23–24. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Moya-Albiol, L. Reduced cardiovascular activation following chronic stress in caregivers of people with anorexia nervosa. Stress 2017, 20, 390–397. [Google Scholar] [CrossRef]
- Romero-Martínez, Á.; Vitoria-Estruch, S.; Moya-Albiol, L. Emotional and autonomic dysregulation in abstinent alcoholic men: An idiosyncratic profile? Alcohol 2019, 77, 155–162. [Google Scholar] [CrossRef]
- Miguel-Tobal, J.; Casado, M.; Cano-Vindel, A.; Spielberger, C. Inventario de Expresión de Ira Estado-Rasgo (STAXI-2); TEA Ediciones: Madrid, Spain, 2001. [Google Scholar]
- Spielberger, C.D. Manual for the State-Trait Anger Expression Inventory-2; Psychological Assessment Resources Odessa: Lutz, FL, USA, 1999. [Google Scholar]
- De Arcos, F.A.; García, A.V.; Jiménez, Á.L.; Pareja, M.M.; Juárez, E.G.; Sánchez, F.A. Respuesta emocional ante estímulos afectivos en sujetos adictos a opiáceos bajo consumo controlado en el P.E.P.S.A. Adicciones 2008, 20, 27. [Google Scholar] [CrossRef]
- Bagby, R.; Parker, J.D.; Taylor, G.J. The twenty-item Toronto Alexithymia scale—I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Martínez-Sánchez, F. Adaptación española de la escala de Alexitimia de Toronto (TAS-20). Clínica y Salud 1996, 1, 19–32. [Google Scholar]
- Guillamón, M.C.; Solé, A.G.; Farran, J.C. Test para la identificación de transtornos por uso de alcohol (AUDIT): Traducción y validación del AUDIT al catalán y castellano. Adicciones 1999, 11, 337. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Vazsonyi, A.T.; Flannery, D.J.; DeLisi, M. Introduction: The Cambridge Handbook of Violent Behavior and Aggression; Cambridge University Press (CUP): Cambridge, UK, 2018. [Google Scholar]
- Samur, D.; Tops, M.; Schlinkert, C.; Quirin, M.; Cuijpers, P.; Koole, S.L. Four decades of research on alexithymia: Moving toward clinical applications. Front. Psychol. 2013, 4, 861. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.A.; Koven, N.S. The Relation of Alexithymic Traits to Affective Theory of Mind. Am. J. Psychol. 2015, 128, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Hsu, C.-H.; Locke, D.; Ritenbaugh, C.; Stonnington, C.M. Role of theory of mind in emotional awareness and alexithymia: Implications for conceptualization and measurement. Conscious. Cogn. 2015, 33, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, Á.; Lila, M.; Gracia, E.; Moya-Albiol, L. Improving empathy with motivational strategies in batterer intervention programmes: Results of a randomized controlled trial. Br. J. Clin. Psychol. 2018, 58, 125–139. [Google Scholar]

| ADHD-IPVAW (n = 19) | Non-ADHD IPVAW (n = 17) | Controls (n = 19) | F ANOVA/ Chi-Square | ||
|---|---|---|---|---|---|
| Age (years) | 39.53 ± 11.62 | 45.29 ± 12.24 | 43.21 ± 11.57 | 1.11 | |
| BMI | 25.21 ± 2.87 | 25.12 ± 2.33 | 25.80 ± 2.60 | 0.38 | |
| Nationality | 1.99 | ||||
| Spanish | 79% | 94% | 89% | ||
| Other | 21% | 6% | 11% | ||
| Marital status | 0.47 | ||||
| Married | 32% | 35% | 42% | ||
| Single/Divorced/Widowed | 68% | 65% | 58% | ||
| Level of education | 1.39 | ||||
| Primary/lower secondary | 63% | 53% | 53% | ||
| Upper secondary | 26% | 24% | 32% | ||
| University | 11% | 23% | 15% | ||
| Employment status | 0.99 | ||||
| Employed | 42% | 47% | 58% | ||
| Unemployed | 58% | 53% | 42% | ||
| TAS-20 | 69.58 ± 18.08 | 46.17 ± 20.49 | 40.32 ± 20.74 | 11.55 *** | |
| Alcohol misuse (AUDIT) Cut-off (>8) | Yes, low Yes, high No | 32% 16% 52% | 24% 29% 47% | 32% 16% 53% | 1.42 |
| Drug misuse | Yes No | 53% 47% | 35% 65% | - 100% | 13.22 *** |
| Violence against others | Yes No | 68% 32% | 30% 70% | - | 5.46 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Martínez, Á.; Lila, M.; Moya-Albiol, L. Alexithymia as a Predictor of Arousal and Affect Dysregulations when Batterers with Attention Deficit Hyperactivity Disorder Cope with Acute Stress. Behav. Sci. 2020, 10, 70. https://doi.org/10.3390/bs10040070
Romero-Martínez Á, Lila M, Moya-Albiol L. Alexithymia as a Predictor of Arousal and Affect Dysregulations when Batterers with Attention Deficit Hyperactivity Disorder Cope with Acute Stress. Behavioral Sciences. 2020; 10(4):70. https://doi.org/10.3390/bs10040070
Chicago/Turabian StyleRomero-Martínez, Ángel, Marisol Lila, and Luis Moya-Albiol. 2020. "Alexithymia as a Predictor of Arousal and Affect Dysregulations when Batterers with Attention Deficit Hyperactivity Disorder Cope with Acute Stress" Behavioral Sciences 10, no. 4: 70. https://doi.org/10.3390/bs10040070
APA StyleRomero-Martínez, Á., Lila, M., & Moya-Albiol, L. (2020). Alexithymia as a Predictor of Arousal and Affect Dysregulations when Batterers with Attention Deficit Hyperactivity Disorder Cope with Acute Stress. Behavioral Sciences, 10(4), 70. https://doi.org/10.3390/bs10040070





