Delta Event-Related Oscillations Are Related to a History of Extreme Binge Drinking in Adolescence and Lifetime Suicide Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. ERP Recordings
2.3. ERO Analyses
2.4. Data Analyses
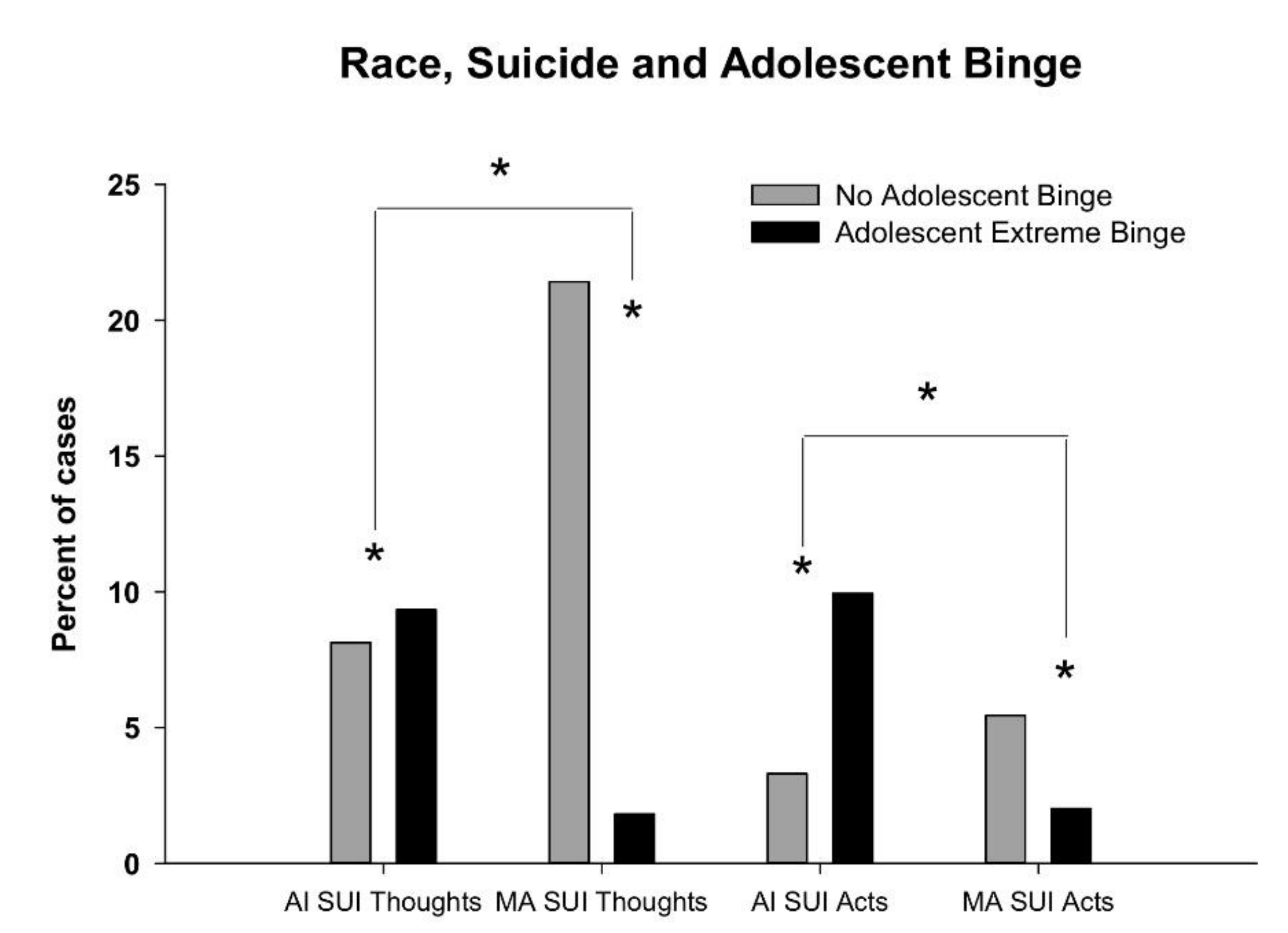
3. Results
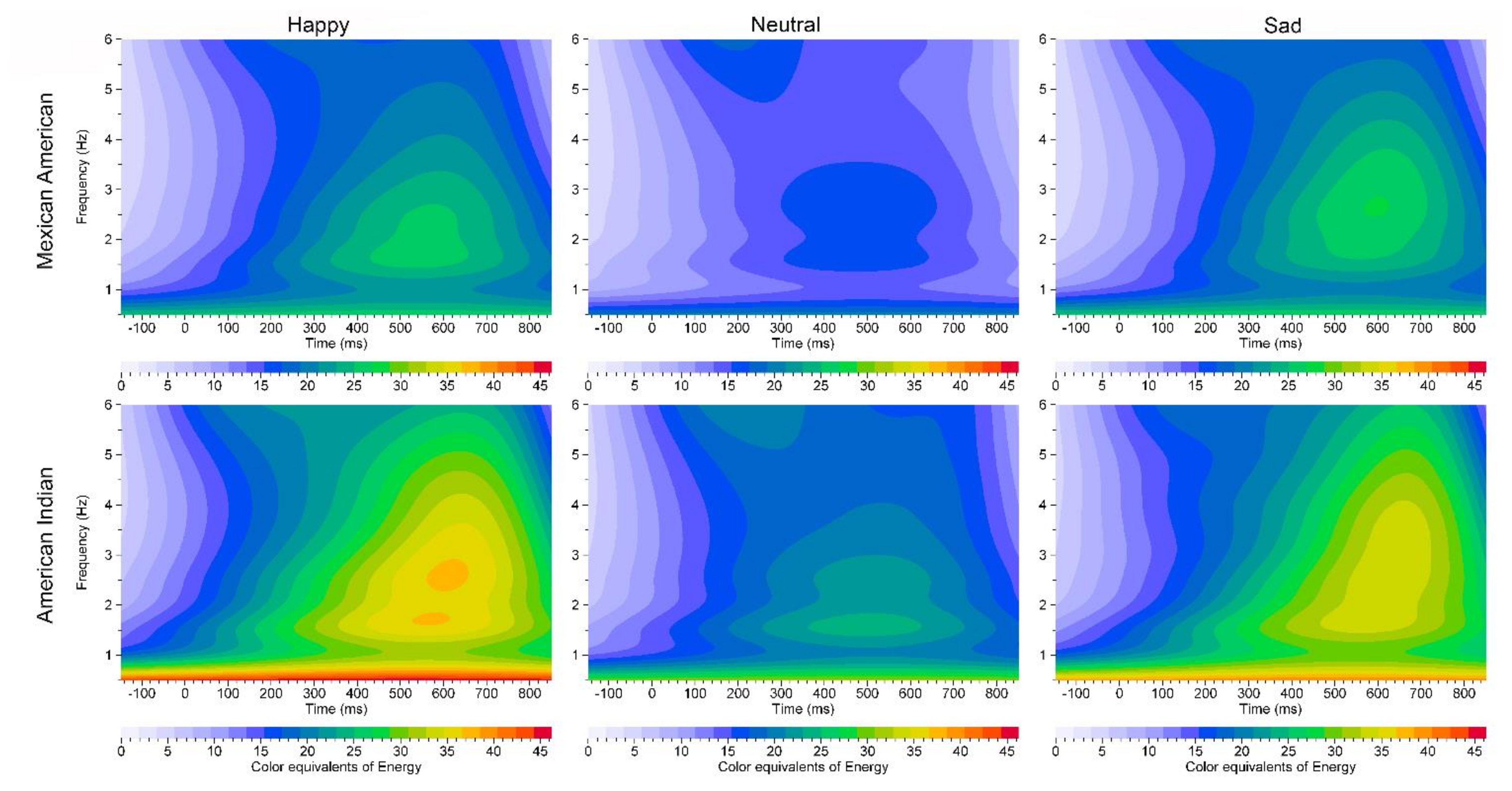
ERO analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brennan, P.L.; Schutte, K.K.; Moos, B.S.; Moos, R.H. Twenty-Year Alcohol-Consumption and Drinking-Problem Trajectories of Older Men and Women. J. Stud. Alcohol Drugs 2011, 72, 308–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Schuckit, M.A. Remarkable increases in alcohol use disorders. JAMA Psychiatry 2017, 74, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.D.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008; NIH Publication No. 09-7401; National Institute on Drug Abuse, U.S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2009.
- Centers for Disease, Control and Prevention. Vital signs: Binge drinking prevalence, frequency, and intensity among adults—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 14–19. [Google Scholar]
- Patrick, M.E.; Schulenberg, J.E.; Martz, M.E.; Maggs, J.L.; O’Malley, P.M.; Johnston, L.D. Extreme binge drinking among 12th-grade students in the United States. JAMA Pediatr. 2013, 167, 1019. [Google Scholar] [CrossRef] [Green Version]
- White, A.M.; Kraus, C.L.; Swartzwelder, H.S. Many college freshmen drink at levels far beyond the binge threshold. Alcohol. Clin. Exp. Res. 2006, 30, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Terry-McElrath, Y.M. High-intensity drinking by underage young adults in the United States. Addiction 2016, 112, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Terry-McElrath, Y.M.; Kloska, D.D.; Schulenberg, J.E. High-intensity drinking among young adults in the United States: Prevalence, frequency, and developmental change. Alcohol. Clin. Exp. Res. 2016, 40, 1905–1912. [Google Scholar] [CrossRef] [Green Version]
- Dawson, D.A.; Goldstein, R.B.; Chou, S.P.; Ruan, W.J.; Grant, B.F. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res. 2008, 32, 2149–2160. [Google Scholar] [CrossRef]
- Miller, J.W.; Naimi, T.S.; Brewer, R.D.; Jones, S.E. Binge drinking and associated health risk behaviors among high school students. Pediatrics 2007, 119, 76–85. [Google Scholar] [CrossRef]
- Glasheen, C.; Pemberton, M.R.; Lipari, R.; Copello, E.A.; Mattson, M.E. Binge drinking and the risk of suicidal thoughts, plans, and attempts. Addict. Behav. 2015, 43, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hingson, R.W.; Zha, W. Binge drinking above and below twice the adolescent thresholds and health-risk behaviors. Alcohol. Clin. Exp. Res. 2018, 42, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.; Smith, V.C.; Levy, S.; Ammerman, S.D.; Gonzalez, P.K.; Ryan, S.A. Binge Drinking. Pediatrics 2015, 136, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-J.; Balan, S.; Price, R.K. Association of contextual factors with drug use and binge drinking among white, native American, and mixed-race adolescents in the general population. J. Youth Adolesc. 2012, 41, 1426–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, L.R.; Harness, S.D.; Swaim, R.C.; Beauvais, F. Rates of substance use of American Indian students in 8th, 10th, and 12th grades living on or near reservations: Update, 2009–2012. Public Health Rep. 2014, 129, 156–163. [Google Scholar] [CrossRef]
- Stanley, L.R.; Swaim, R.C. Latent classes of substance use among american indian and white students living on or near reservations, 2009-2013. Public Health Rep. 2018, 133, 432–441. [Google Scholar] [CrossRef]
- Lee, D.J.; Markides, K.S.; Ray, L.A. Epidemiology of self-reported past heavy drinking in Hispanic adults. Ethn. Health 1997, 2, 77–88. [Google Scholar] [CrossRef]
- Caetano, R.; Ramisetty-Mikler, S.; Rodriguez, L.A. The Hispanic Americans baseline alcohol survey (HABLAS): Rates and predictors of DUI across Hispanic national groups. Accid. Anal. Prev. 2008, 40, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Caetano, R.; Ramisetty-Mikler, S.; Rodriguez, L.A. The Hispanic Americans baseline alcohol survey (HABLAS): Rates and predictors of alcohol abuse and dependence across Hispanic national groups. J. Stud. Alcohol Drugs 2008, 69, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Curtin, S.C.; Warner, M.; Hedegaard, H. Increase in Suicide in the United States, 1999–2014. Available online: https://stacks.cdc.gov/view/cdc/39008 (accessed on 29 September 2020).
- Goldsmith, S.K.; Institute of Medicine (U.S.). Committee on pathophysiology & prevention of adolescent & adult suicide. In Reducing Suicide: A National Imperative; National Academies Press: Washington, DC, USA, 2002; p. 496. [Google Scholar]
- Department of Health and Human Services. Trends in Indian Health 2002–2003 Edition; Indian Health Service, U.S. Department of Health and Human Services: Rockville, MD, USA, 2009.
- Chartier, K.G.; Vaeth, P.A.; Caetano, R. Focus on: Ethnicity and the social and health harms from drinking. Alcohol Res. Curr. Rev. 2013, 35, 229–237. [Google Scholar]
- Herne, M.A.; Bartholomew, M.L.; Weahkee, R.L. Suicide mortality among American Indians and Alaska Natives, 1999–2009. Am. J. Public Health 2014, 104, S336–S342. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease, Control and Prevention. Alcohol and suicide among racial/ethnic populations—17 states, 2005–2006. MMWR. Morb. Mortal. Wkly. Rep. 2009, 58, 637–641. [Google Scholar]
- Oquendo, M.A.; Ellis, S.P.; Greenwald, S.; Malone, K.M.; Weissman, M.M.; Mann, J.J. Ethnic and sex differences in suicide rates relative to major depression in the United States. Am. J. Psychiatry 2001, 158, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services Office of the Surgeon General and National Action Alliance for Suicide Prevention. National Strategy for Suicide Prevention: Goals and Objectives for Action: A Report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention; Health and Human Services: Washington, DC, USA, 2012.
- The National Institute of Mental Health. Strategic Plan for Research. Available online: https://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml (accessed on 29 September 2020).
- Borges, G.; Breslau, J.; Su, M.; Miller, M.; Medina-Mora, M.E.; Aguilar-Gaxiola, S. Immigration and suicidal behavior among Mexicans and Mexican Americans. Am. J. Public Health 2009, 99, 728–733. [Google Scholar] [CrossRef]
- Sorenson, S.B.; Golding, J.M. Prevalence of suicide attempts in a Mexican-American population: Prevention implications of immigration and cultural issues. Suicide Life Threat. Behav. 1988, 18, 322–333. [Google Scholar] [CrossRef]
- Begleiter, H.; Porjesz, B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol. Clin. Exp. Res. 1999, 23, 1125–1135. [Google Scholar] [CrossRef]
- Frederick, J.A.; Iacono, W.G. Beyond the DSM: Defining endophenotypes for genetic studies of substance abuse. Curr. Psychiatry Rep. 2006, 8, 144–150. [Google Scholar] [CrossRef]
- Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Jones, K.A.; Padmanabhapillai, A.; Begleiter, H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neurophysiol. 2005, 116, 993–1018. [Google Scholar] [CrossRef]
- Ceballos, N.A.; Bauer, L.O.; Houston, R.J. Recent EEG and ERP findings in substance abusers. Clin. EEG Neurosci. 2009, 40, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Kamarajan, A.K.P.C.; Rangaswamy, M.; Pandey, A.K.; Porjesz, B. Event-related oscillations in alcoholism research: A review. J. Addict. Res. Ther. 2012, 7, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Campanella, S.; Pogarell, O.; Boutros, N. Event-related potentials in substance use disorders: A narrative review based on articles from 1984 to 2012. Clin. EEG Neurosci. 2014, 45, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, M.; Porjesz, B. Understanding alcohol use disorders with neuroelectrophysiology. Handb. Clin. Neurol. 2014, 125, 383–414. [Google Scholar] [CrossRef] [Green Version]
- Kamarajan, C.; Porjesz, B. Advances in electrophysiological research. Alcohol Res. Curr. Rev. 2015, 37, 53–87. [Google Scholar]
- Campanella, S.; Schroder, E.; Kajosch, H.; Noël, X.; Kornreich, C. Why cognitive event-related potentials (ERPs) should have a role in the management of alcohol disorders. Neurosci. Biobehav. Rev. 2019, 106, 234–244. [Google Scholar] [CrossRef]
- Begleiter, H.; Porjesz, B.; Bihari, B.; Kissin, B. Event-related brain potentials in boys at risk for alcoholism. Science 1984, 225, 1493–1496. [Google Scholar] [CrossRef]
- Berman, S.; Whipple, S.; Fitch, R.; Noble, E.P. P3 in young boys as a predictor of adolescent substance use. Alcohol 1993, 10, 69–76. [Google Scholar] [CrossRef]
- Elmasian, R.; Neville, H.; Woods, D.L.; Schuckit, M.; Bloom, F. Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proc. Natl. Acad. Sci. USA 1982, 79, 7900–7903. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, P.C.; Ehlers, C.L.; Garcia-Andrade, C.; Wall, T.L.; Sobel, D.F.; Phillips, E. Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology 1998, 18, 282–292. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Wall, T.L.; Garcia-Andrade, C.; Phillips, E. Visual P3 findings in Mission Indian youth: Relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001, 105, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.Y.; Steinhauer, S.R.; Zubin, J.; Baughman, T. Event-related potentials as markers for alcoholism risk in high density families. Alcohol. Clin. Exp. Res. 1988, 12, 545–554. [Google Scholar] [CrossRef]
- Hill, S.Y.; Steinhauer, S.R.; Park, J.; Zubin, J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol. Clin. Exp. Res. 1990, 14, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.Y.; Steinhauer, S.R.; Locke, J. Event-related potentials in alcoholic men, their high-risk male relatives, and low-risk male controls. Alcohol. Clin. Exp. Res. 1995, 19, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.Y.; Locke, J.; Steinhauer, S.R. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol. Psychiatry 1999, 46, 982–989. [Google Scholar] [CrossRef]
- Hill, S.Y.; Shen, S.; Locke, J.; Steinhauer, S.R.; Konicky, C.; Lowers, L.; Connolly, J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol. Psychiatry 1999, 46, 970–981. [Google Scholar] [CrossRef]
- O’Connor, S.; Hesselbrock, V.; Tasman, A.; DePalma, N. P3 amplitudes in two distinct tasks are decreased in young men with a history of paternal alcoholism. Alcohol 1987, 4, 323–330. [Google Scholar] [CrossRef]
- Porjesz, B.; Begleiter, H. Event-related potentials in individuals at risk for alcoholism. Alcohol 1990, 7, 465–469. [Google Scholar] [CrossRef]
- Porjesz, B.; Begleiter, H. Genetic basis of event-related potentials and their relationship to alcoholism and alcohol use. J. Clin. Neurophysiol. 1998, 15, 44–57. [Google Scholar] [CrossRef]
- Whipple, S.C.; Parker, E.S.; Noble, E.P. An atypical neurocognitive profile in alcoholic fathers and their sons. J. Stud. Alcohol 1988, 49, 240–244. [Google Scholar] [CrossRef]
- Andrew, C.; Fein, G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol. Clin. Exp. Res. 2010, 34, 669–680. [Google Scholar] [CrossRef]
- Criado, J.R.; Gizer, I.R.; Slutske, W.S.; Phillips, E.; Ehlers, C.L. Event-related oscillations to affective stimuli: Heritability, linkage and relationship to externalizing disorders. J. Psychiatr. Res. 2012, 46, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Phillips, E.; Wills, D.; Benedict, J.; Sanchez-Alavez, M. Phase locking of event-related oscillations is decreased in both young adult humans and rats with a history of adolescent alcohol exposure. Addict. Biol. 2019, 25, 12732. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Wills, D.N.; Phillips, E.; Havstad, J. Low voltage alpha EEG phenotype is associated with reduced amplitudes of alpha event-related oscillations, increased cortical phase synchrony, and a low level of response to alcohol. Int. J. Psychophysiol. 2015, 98, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Phillips, E.; Kim, C.; Wills, D.N.; Karriker-Jaffe, K.J.; Gilder, D.A. CR-19-0950: Event-related responses to alcohol-related stimuli in Mexican-American young adults: Relation to age, gender, comorbidity and “dark side” symptoms. Drug Alcohol Depend. 2019, 202, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Basar-Eroglu, C.; Rosen, B.; Schütt, A. A New Approach to Endogenous Event-Related Potentials in Man: Relation Between Eeg and P300-Wave. Int. J. Neurosci. 1984, 24, 1–21. [Google Scholar] [CrossRef]
- Başar, E.; Gönder, A.; Ungan, P. Comparative frequency analysis of single EEG—Evoked potential records. J. Biomed. Eng. 1980, 2, 9–14. [Google Scholar] [CrossRef]
- Başar, E.; Stampfer, H.G.; Baslar, E. Important associations among eeg-dynamics, event-related potentials, short-term memory and learning. Int. J. Neurosci. 1985, 26, 161–180. [Google Scholar] [CrossRef]
- Duncan-Johnson, C.C.; Donchin, E. The Time Constant in P300 Recording. Psychophysiol. 1979, 16, 53–55. [Google Scholar] [CrossRef]
- Jodo, E.; Kayama, Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 477–482. [Google Scholar] [CrossRef]
- Gilmore, C.S.; Fein, G. Induced Theta Activity as a Biomarker for a morbid effect of alcoholism on the brain in long-term abstinent alcoholics. Psychophysiology 2013, 27, 76–83. [Google Scholar] [CrossRef]
- Jones, K.A.; Porjesz, B.; Chorlian, D.; Rangaswamy, M.; Kamarajan, C.; Padmanabhapillai, A.; Stimus, A.; Begleiter, H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin. Neurophysiol. 2006, 117, 2128–2143. [Google Scholar] [CrossRef]
- Rangaswamy, M.; Jones, K.A.; Porjesz, B.; Chorlian, D.B.; Padmanabhapillai, A.; Kamarajan, C.; Kuperman, S.; Rohrbaugh, J.; O’Connor, S.J.; Bauer, L.O.; et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int. J. Psychophysiol. 2007, 63, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado, J.R.; Ehlers, C.L. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience 2009, 163, 506–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balconi, M.; Lucchiari, C. EEG correlates (event-related desynchronization) of emotional face elaboration: A temporal analysis. Neurosci. Lett. 2006, 392, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Schmiedt-Fehr, C.; Oniz, A.; Basar-Eroglu, C. Brain oscillations evoked by the face of a loved person. Brain Res. 2008, 1214, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Facial affect manifested by multiple oscillations. Int. J. Psychophysiol. 2009, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Review of evoked and event-related delta responses in the human brain. Int. J. Psychophysiol. 2016, 103, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Roach, B.J.; Mathalon, D.H. Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008, 34, 907–926. [Google Scholar] [CrossRef] [Green Version]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef]
- Singer, W. Neuronal Synchrony: A versatile code for the definition of relations? Neuron 1999, 24, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Varela, F.; Lachaux, J.-P.; Rodriguez, E.; Martinerie, J. The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001, 2, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Wills, D.N.; Desikan, A.; Phillips, E.; Havstad, J. Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Dev. Neurosci. 2014, 36, 175–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Wall, T.L.; Betancourt, M.; Gilder, D.A. The clinical course of alcoholism in 243 Mission Indians. Am. J. Psychiatry 2004, 161, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Spence, J.C.H.; Wall, T.L.; Gilder, D.A.; Carr, L.G. Association of ALDH1 promoter polymorphisms with alcohol-related phenotypes in southwest California Indians. Alcohol. Clin. Exp. Res. 2004, 28, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Slutske, W.S.; Gilder, D.A.; Lau, P.; Wilhelmsen, K.C. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol. Clin. Exp. Res. 2006, 30, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, C.L.; Gilder, D.A.; Slutske, W.S.; Lind, P.A.; Wilhelmsen, K.C. Externalizing disorders in American Indians: Comorbidity and a genome wide linkage analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Gilder, D.A.; Criado, J.R.; Caetano, R. Acculturation stress, anxiety disorders, and alcohol dependence in a select population of young adult Mexican Americans. J. Addict. Med. 2009, 3, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Gizer, I.R.; Phillips, E.; Wilhelmsen, K. EEG alpha phenotypes: Linkage analyses and relation to alcohol dependence in an American Indian community study. BMC Med. Genet. 2010, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Gilder, D.A.; Criado, J.R.; Caetano, R. Sleep quality and alcohol-use disorders in a select population of young-adult Mexican Americans. J. Stud. Alcohol Drugs 2010, 71, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Phillips, E.; Criado, J.R.; Gilder, D.A. N4 component responses to pre-pulse startle stimuli in young adults: Relationship to alcohol dependence. Psychiatry Res. 2011, 188, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Gizer, I.R.; Gilder, D.A.; Yehuda, R. Lifetime history of traumatic events in an American Indian community sample: Heritability and relation to substance dependence, affective disorder, conduct disorder and PTSD. J. Psychiatr. Res. 2013, 47, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Gizer, I.R.; Gilder, D.A.; Ellingson, J.M.; Yehuda, R. Measuring historical trauma in an American Indian community sample: Contributions of substance dependence, affective disorder, conduct disorder and PTSD. Drug Alcohol Depend. 2013, 133, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Stouffer, G.M.; Gilder, D.A. Associations between a history of binge drinking during adolescence and self-reported responses to alcohol in young adult Native and Mexican Americans. Alcohol. Clin. Exp. Res. 2014, 38, 2039–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, C.L.; Stouffer, G.M.; Corey, L.; Gilder, D.A. The clinical course of DSM-5 alcohol use disorders in young adult native and Mexican Americans. Am. J. Addict. 2015, 24, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Kim, C.; Gilder, D.A.; Stouffer, G.M.; Caetano, R.; Yehuda, R. Lifetime history of traumatic events in a young adult Mexican American sample: Relation to substance dependence, affective disorder, acculturation stress, and PTSD. J. Psychiatr. Res. 2016, 83, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Wills, D.; Gilder, D.A. A history of binge drinking during adolescence is associated with poorer sleep quality in young adult Mexican Americans and American Indians. Psychopharmacology 2018, 235, 1775–1782. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Gilder, D.A.; Gizer, I.R.; Wilhelmsen, K. Indexing the ‘dark side of addiction’: Substance-induced affective symptoms and alcohol use disorders. Addiction 2018, 114, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Erwin, R.J.; Gur, R.C.; Gur, R.E.; Skolnick, B.; Mawhinney-Hee, M.; Smailis, J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992, 42, 231–240. [Google Scholar] [CrossRef]
- Criado, J.R.; Ehlers, C.L. Electrophysiological responses to affective stimuli in Southwest California Indians: Relationship to alcohol dependence. J. Stud. Alcohol Drugs 2007, 68, 813–823. [Google Scholar] [CrossRef]
- Criado, J.R.; Ehlers, C.L. Electrophysiological responses to affective stimuli in Mexican Americans: Relationship to alcohol dependence and personality traits. Pharmacol. Biochem. Behav. 2007, 88, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Phillips, E.; Finnerman, G.; Gilder, D.; Lau, P.; Criado, J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol. Teratol. 2007, 29, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.; Ehlers, C.L. Gender differences in electrophysiological responses to facial stimuli. Biol. Psychiatry 1998, 44, 281–289. [Google Scholar] [CrossRef]
- Orozco, S.; Wall, T.L.; Ehlers, C.L. Influence of alcohol on electrophysiological responses to facial stimuli. Alcohol 1999, 18, 11–16. [Google Scholar] [CrossRef]
- Bucholz, K.K.; Cadoret, R.; Cloninger, C.R.; Dinwiddie, S.H.; Hesselbrock, V.M.; Nurnberger, J.I.; Reich, T.; Schmidt, I.; Schuckit, M. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J. Stud. Alcohol. 1994, 55, 149–158. [Google Scholar] [CrossRef]
- Bauer, L. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol. Depend. 1997, 44, 1–10. [Google Scholar] [CrossRef]
- Hill, S.Y.; Steinhauer, S.R.; Locke-Wellman, J.; Ulrich, R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: A prospective study. Biol. Psychiatry 2009, 66, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Gabor, D. Theory of communication. Part 1: The analysis of information. J. Inst. Electr. Eng. Part 3 Radio Commun. Eng. 1946, 93, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, R.; Mansinha, L.; Lowe, R. Localization of the complex spectrum: The S transform. IEEE Trans. Signal Process. 1996, 44, 998–1001. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Wills, D.N.; Havstad, J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012, 1450, 67–79. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.H. G–test of goodness-of-fit. In Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2014; pp. 53–58. [Google Scholar]
- SPSS Statistical Software. SPSS, v26; IBM Corporation: Armonk, NY, USA, 2018. [Google Scholar]
- Johnston, L.D.; O’Malley, P.M.; Miech, R.A.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use; Institute for Social Research, The University of Michigan: Ann Arbor, MI, USA, 2014. [Google Scholar]
- Cheadle, J.E.; Whitbeck, L.B. Alcohol use trajectories and problem drinking over the course of adolescence: A study of North American indigenous youth and their caretakers. J. Health Soc. Behav. 2011, 52, 228–245. [Google Scholar] [CrossRef]
- Cruz, R.A.; King, K.M.; Mechammil, M.; Bámaca-Colbert, M.; Robins, R.W. Mexican-origin youth substance use trajectories: Associations with cultural and family factors. Dev. Psychol. 2018, 54, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Cwik, M.F.; Rosenstock, S.; Tingey, L.; Redmond, C.; Goklish, N.; Larzelere-Hinton, F.; Barlow, A. Exploration of pathways to binge drinking among American Indian adolescents. Prev. Sci. 2017, 18, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Swaim, R.C.; Stanley, L.R. Substance use among american indian youths on reservations compared with a national sample of US adolescents. JAMA Netw. Open 2018, 1, e180382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2013 National Survey on Drug Use and Health: Detailed Tables. 2013. Available online: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013/NSDUH-DetTabs2013.htm#tab1.20b (accessed on 5 October 2020).
- Tortolero, S.R.; Roberts, R.E. Differences in nonfatal suicide behaviors among Mexican and European American middle school children. Suicide Life Threat. Behav. 2001, 31, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System. Available online: https://www.cdc.gov/injury/wisqars/ (accessed on 29 September 2020).
- O’Keefe, V.M.; The Celebrating Life Team; Haroz, E.E.; Goklish, N.; Ivanich, J.; Cwik, M.F.; Barlow, A. Employing a sequential multiple assignment randomized trial (SMART) to evaluate the impact of brief risk and protective factor prevention interventions for American Indian Youth Suicide. BMC Public Health 2019, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Caetano, R.; Kaplan, M.S.; Kerr, W.C.; McFarland, B.H.; Giesbrecht, N.; Kaplan, Z.B. Suicide, alcohol intoxication, and age among Whites and American Indians/Alaskan Natives. Alcohol. Clin. Exp. Res. 2019, 44, 492–500. [Google Scholar] [CrossRef]
- Başar, E.; Güntekin, B.; Oniz, A. Principles of oscillatory brain dynamics and a treatise of recognition of faces and facial expressions. Prog. Brain Res. 2006, 159, 43–62. [Google Scholar]
- Basar, E.; Ozgoren, M.; Oniz, A.; Schmiedt, C.; Basar-Oroglu, C. Brain oscillations differentiate the picture of one’s own grandmother. Int. J. Psychophysiol. 2007, 64, 81–90. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kamarajan, C.; Manz, N.; Chorlian, D.B.; Stimus, A.; Porjesz, B. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2015, 65, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Fein, G.; Key, K.; Szymanski, M.D. ERP and RT delays in long-term abstinent alcoholics in processing of emotional facial expressions during gender and emotion categorization tasks. Alcohol. Clin. Exp. Res. 2010, 34, 1127–1139. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Y.; Chan, J.S.Y.; Yang, F.-C.; Cui, F. Dysfunctional early processing of facial expressions in hazardous drinkers: Evidence from an ERP study. Sci. Rep. 2017, 7, 13360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlet, K.; Schlagenhauf, F.; Richter, A.; Naundorf, K.; Dornhof, L.; Weinfurtner, C.E.J.; König, F.; Walaszek, B.; Schubert, F.; Müller, C.A.; et al. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict. Biol. 2013, 19, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Clark, U.S.; Oscar-Berman, M.; Shagrin, B.; Pencina, M. Alcoholism and judgments of affective stimuli. Neuropsychology 2007, 21, 346–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Hondt, F.; De Timary, P.; Bruneau, Y.; Maurage, P. Categorical perception of emotional facial expressions in alcohol-dependence. Drug Alcohol Depend. 2015, 156, 267–274. [Google Scholar] [CrossRef]
- Freeman, C.R.; Wiers, C.E.; Sloan, M.E.; Zehra, A.; Ramirez, V.; Wang, G.; Volkow, N.D. Emotion recognition biases in alcohol use disorder. Alcohol. Clin. Exp. Res. 2018, 42, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Gowin, J.L.; Vatsalya, V.; Westman, J.G.; Schwandt, M.L.; Bartlett, S.; Heilig, M.; Momenan, R.; Ramchandani, V.A. The effect of varenicline on the neural processing of fearful faces and the subjective effects of alcohol in heavy drinkers. Alcohol. Clin. Exp. Res. 2016, 40, 979–987. [Google Scholar] [CrossRef]
- Hoffman, L.A.; Lewis, B.; Nixon, S.J. Neurophysiological and interpersonal correlates of emotional face processing in alcohol use disorder. Alcohol. Clin. Exp. Res. 2019, 43, 1928–1936. [Google Scholar] [CrossRef]
- Lewis, B.; Price, J.L.; Garcia, C.C.; Nixon, S.J. Emotional face processing among treatment-seeking individuals with alcohol use disorders: Investigating sex differences and relationships with interpersonal functioning. Alcohol Alcohol. 2019, 54, 361–369. [Google Scholar] [CrossRef]
- Maurage, P.; Campanella, S.; Philippot, P.; Martin, S.; De Timary, P. Face processing in chronic alcoholism: A specific deficit for emotional features. Alcohol. Clin. Exp. Res. 2008, 32, 600–606. [Google Scholar] [CrossRef]
- Osório, F.L.; Donadon, M.F. Recognition of facial expressions by alcoholic patients: A systematic literature review. Neuropsychiatr. Dis. Treat. 2014, 10, 1655. [Google Scholar] [CrossRef] [Green Version]
- Castellano, F.; Bartoli, F.; Crocamo, C.; Gamba, G.; Tremolada, M.; Santambrogio, J.; Clerici, M.; Carrà, G. Facial emotion recognition in alcohol and substance use disorders: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 59, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Luckenbaugh, D.A.; Moolchan, E.T.; Temple, V.A.; Jenness, J.; Korelitz, K.E.; London, E.D.; Kimes, A.S. Decision-making and facial emotion recognition as predictors of substance-use initiation among adolescents. Addict. Behav. 2010, 35, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Lannoy, S.; Benzerouk, F.; Maurage, P.; Barrière, S.; Billieux, J.; Naassila, M.; Kaladjian, A.; Gierski, F. Disrupted Fear and Sadness Recognition in Binge Drinking: A Combined Group and Individual Analysis. Alcohol. Clin. Exp. Res. 2019, 43, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Lannoy, S.; Dormal, V.; Brion, M.; Gaudelus, B.; Billieux, J.; Maurage, P. Affective impairments in binge drinking: Investigation through emotional facial expression decoding. Compr. Psychiatry 2018, 83, 59–63. [Google Scholar] [CrossRef]
- Leganes-Fonteneau, M.; Pi-Ruano, M.; Tejero, P. Early Signs of Emotional Recognition Deficits in Adolescent High-Binge Drinkers. Subst. Use Misuse 2019, 55, 218–229. [Google Scholar] [CrossRef]
- Maniglio, R.; Gusciglio, F.; Lofrese, V.; Murri, M.B.; Tamburello, A.; Innamorati, M. Biased processing of neutral facial expressions is associated with depressive symptoms and suicide ideation in individuals at risk for major depression due to affective temperaments. Compr. Psychiatry 2014, 55, 518–525. [Google Scholar] [CrossRef]
- Richard-Devantoy, S.; Guillaume, S.; Olié, E.; Courtet, P.; Jollant, F. Altered explicit recognition of facial disgust associated with predisposition to suicidal behavior but not depression. J. Affect. Disord. 2013, 150, 590–593. [Google Scholar] [CrossRef]
- Seymour, K.E.; Jones, R.N.; Cushman, G.K.; Galvan, T.; Puzia, M.E.; Kim, K.L.; Spirito, A.; Dickstein, D.P. Emotional face recognition in adolescent suicide attempters and adolescents engaging in non-suicidal self-injury. Eur. Child Adolesc. Psychiatry 2015, 25, 247–259. [Google Scholar] [CrossRef]
- Tsypes, A.; Burkhouse, K.L.; Gibb, B.E. Classification of facial expressions of emotion and risk for suicidal ideation in children of depressed mothers: Evidence from cross-sectional and prospective analyses. J. Affect. Disord. 2016, 197, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Albanese, B.J.; Macatee, R.J.; Gallyer, A.J.; Stanley, I.H.; Joiner, T.E.; Schmidt, N.B. Impaired Conflict Detection Differentiates Suicide Attempters from Ideating Nonattempters: Evidence from Event-Related Potentials. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2019, 4, 902–912. [Google Scholar] [CrossRef]
- Tavakoli, P.; Boafo, A.; Dale, A.; Robillard, R.; Greenham, S.L.; Campbell, K. Event-Related Potential Measures of Attention Capture in Adolescent Inpatients with Acute Suicidal Behavior. Front. Psychiatry 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; McLaughlin, K.A.; Busso, D.S.; Brueck, S.; Peverill, M.; Sheridan, M.A. Neural Correlates of Emotion Regulation and Adolescent Suicidal Ideation. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2017, 3, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Crane, N.A.; Gorka, S.M.; Phan, K.L.; Childs, E. Amygdala-orbitofrontal functional connectivity mediates the relationship between sensation seeking and alcohol use among binge-drinking adults. Drug Alcohol Depend. 2018, 192, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, M.A.; Lee, S.-H.; Yu, Y.; Zhu, H.; Crews, F.T.; Robinson, D.L.; Shih, Y.-Y.I. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict. Biol. 2017, 23, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.M.; Jones, S.A.; Harman, G.; Patching-Bunch, J.; Nagel, B.J. Associations between nucleus accumbens structural connectivity, brain function, and initiation of binge drinking. Addict. Biol. 2019, 25, e12767. [Google Scholar] [CrossRef]
- Pan, L.A.; Hassel, S.; Segreti, A.M.; Nau, S.A.; Brent, D.A.; Phillips, M.L. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med. 2013, 43, 2129–2142. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.A.Y.; Wang, F.; Liu, J.; Blond, B.N.; Wallace, A.; Liu, J.; Spencer, L.; Lippard, E.T.C.; Purves, K.L.; Landeros-Weisenberger, A.; et al. Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. Am. J. Psychiatry 2017, 174, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Coleman, L.G., Jr.; He, J.; Lee, J.; Styner, M.; Crews, F.T. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, C.L.; Criado, J.R.; Wills, D.N.; Liu, W.; Crews, F.T. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience 2011, 199, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Kyzar, E.J.; Bohnsack, J.P.; Kokare, D.M.; Teppen, T.; Pandey, S.C. Adolescent alcohol exposure epigenetically regulates CREB signaling in the adult amygdala. Sci. Rep. 2018, 8, 10376. [Google Scholar] [CrossRef]
- Sanchez-Alavez, M.; Ehlers, C.L. Event-related oscillations (ERO) during an active discrimination task: Effects of lesions of the nucleus basalis magnocellularis. Int. J. Psychophysiol. 2016, 103, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Demographic Characteristic | No Suicidal Activity | Suicidal Thoughts | Suicidal Acts | No Adolescent Binge | Adolescent Binge | Extreme Adolescent Binge | Overall (n = 1184 1) |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Race | * | * | * | ||||
| American Indian | 330 (27.9) | 86 (7.3) | 63 (5.3) | 152 (13.1) | 136 (11.7) | 180 (15.5) | 479 (40.5) |
| Mexican American | 469 (39.6) | 177 (14.9) | 59 (5.0) | 440 (37.8) | 200 (17.2) | 55 (4.7) | 705 (59.5) |
| Gender | * | * | |||||
| Male | 353 (29.8) | 116 (9.8) | 38 (3.2) | 238 (20.5) | 136 (11.7) | 131 (11.3) | 507 (42.8) |
| Female | 446 (37.7) | 147 (12.4) | 84 (7.1) | 354 (30.4) | 200 (17.2) | 104 (8.9) | 677 (57.2) |
| Married | |||||||
| Yes | 96 (8.1) | 26 (2.2) | 10 (0.8) | 79 (6.8) | 35 (3.0) | 17 (1.5) | 132 (11.1) |
| No | 703 (59.4) | 237 (20.0) | 112 (9.5) | 513 (44.1) | 301 (25.9) | 218 (18.7) | 1052 (88.9) |
| Employed | * | ||||||
| Yes | 392 (33.7) | 131 (11.3) | 59 (5.1) | 331 (28.9) | 163 (14.2) | 77 (6.7) | 582 (50.0) |
| No | 393 (33.8) | 130 (11.2) | 59 (5.1) | 256 (22.4) | 165 (14.4) | 152 (13.3) | 582 (50.0) |
| Income ≥ $20,000/year | * | ||||||
| Yes | 521 (48.6) | 178 (16.6) | 72 (6.7) | 408 (38.6) | 237 (22.4) | 119 (11.3) | 771 (71.9) |
| No | 199 (18.5) | 64 (6.0) | 39 (3.6) | 136 (12.9) | 69 (6.5) | 87 (8.2) | 302 (28.1) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 22.86 ± 4.0 | 22.89 ± 3. 9 | 22.63 ± 3.6 | 23.14 ± 3.9 | 22.78 ± 4.0 | 22.2 ± 3.9 * | 22.84 ± 3.8 |
| Education (years) | 12.71 ± 2.0 | 12.91 ± 2.0 | 12.11 ± 1.8 * | 13.14 ± 1.9 | 12.74 ± 1.7 * | 11.55 ± 1.5 * | 12.69 ± 1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlers, C.L.; Wills, D.N.; Karriker-Jaffe, K.J.; Gilder, D.A.; Phillips, E.; Bernert, R.A. Delta Event-Related Oscillations Are Related to a History of Extreme Binge Drinking in Adolescence and Lifetime Suicide Risk. Behav. Sci. 2020, 10, 154. https://doi.org/10.3390/bs10100154
Ehlers CL, Wills DN, Karriker-Jaffe KJ, Gilder DA, Phillips E, Bernert RA. Delta Event-Related Oscillations Are Related to a History of Extreme Binge Drinking in Adolescence and Lifetime Suicide Risk. Behavioral Sciences. 2020; 10(10):154. https://doi.org/10.3390/bs10100154
Chicago/Turabian StyleEhlers, Cindy L., Derek N. Wills, Katherine J. Karriker-Jaffe, David A. Gilder, Evelyn Phillips, and Rebecca A. Bernert. 2020. "Delta Event-Related Oscillations Are Related to a History of Extreme Binge Drinking in Adolescence and Lifetime Suicide Risk" Behavioral Sciences 10, no. 10: 154. https://doi.org/10.3390/bs10100154





