Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessment
2.2.1. Tower of London Test (TOLT)
2.2.2. Visual Span Test
2.3. Impulsivity Scores
2.4. EEG Data Acquisition and Preprocessing
2.5. DMN Seed Regions and FC Calculations
2.6. EEG Based Functional Connectivity Analysis in eLORETA
2.7. RF Classification Model and Parameters
3. Results
3.1. RF Classification
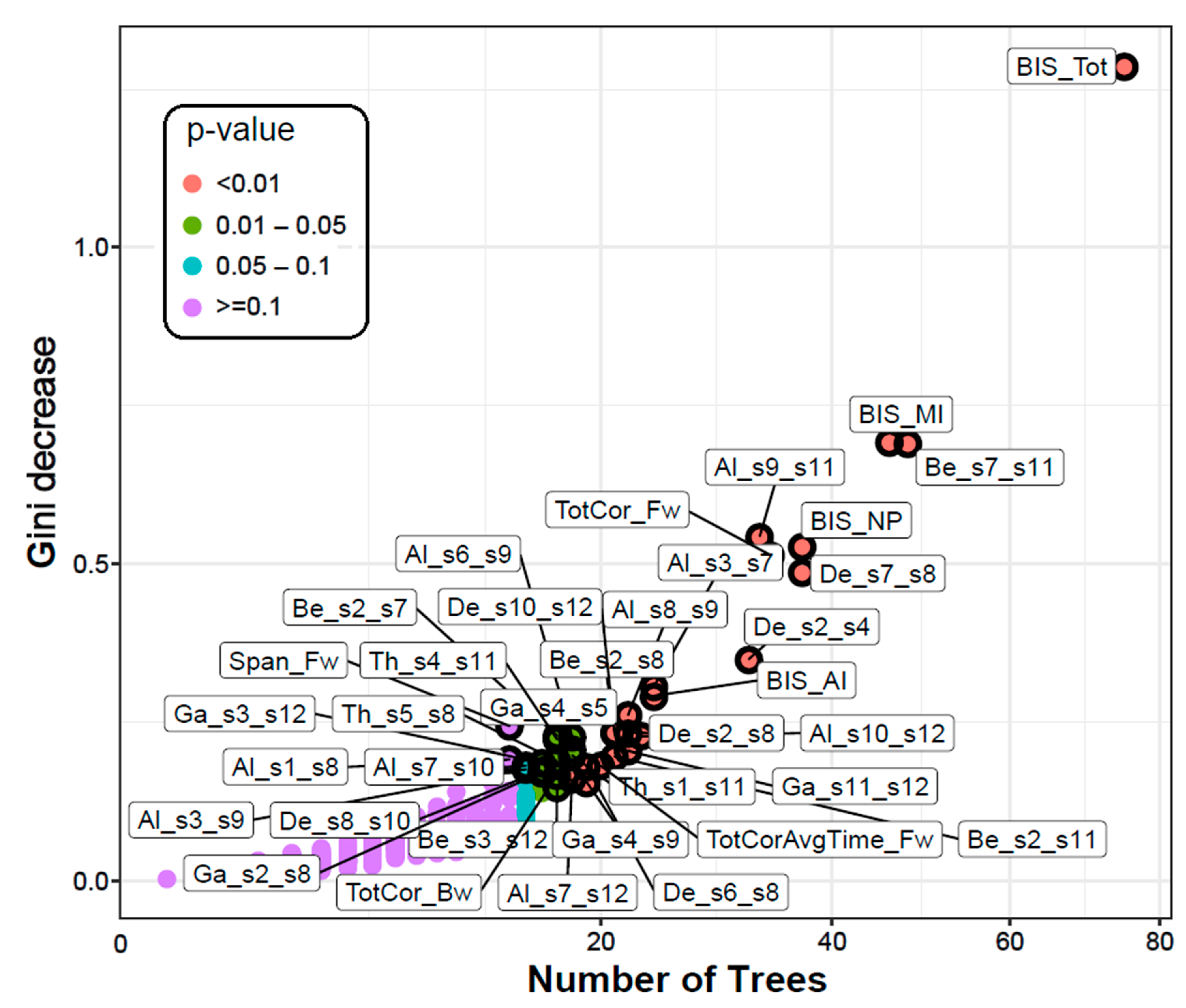
3.1.1. Classification Accuracy and Top Significant Variables
3.1.2. Multi-Way Importance
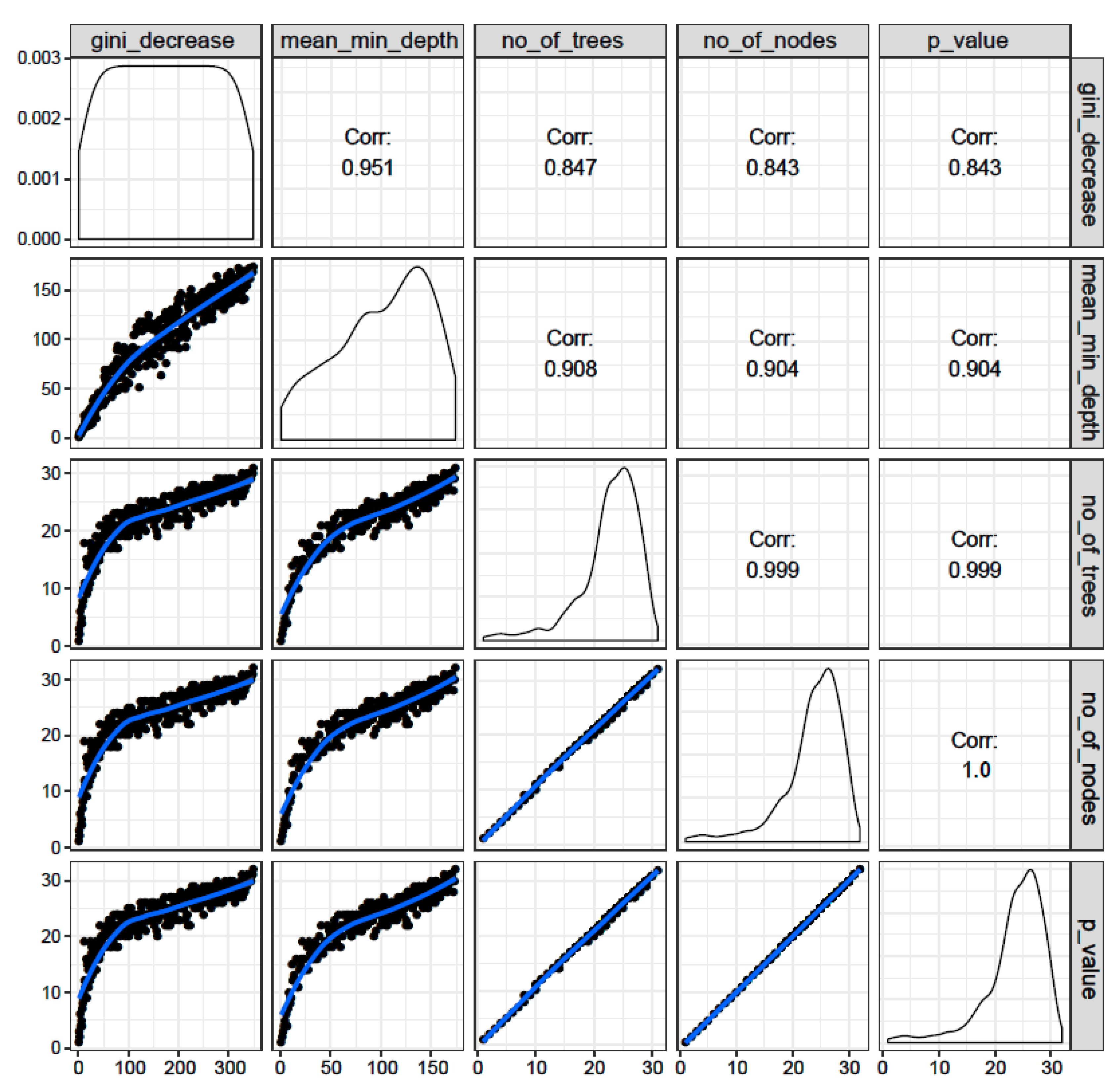
3.1.3. Distribution of Minimal Depth
3.1.4. Correlations among Rankings of Different RF Parameters
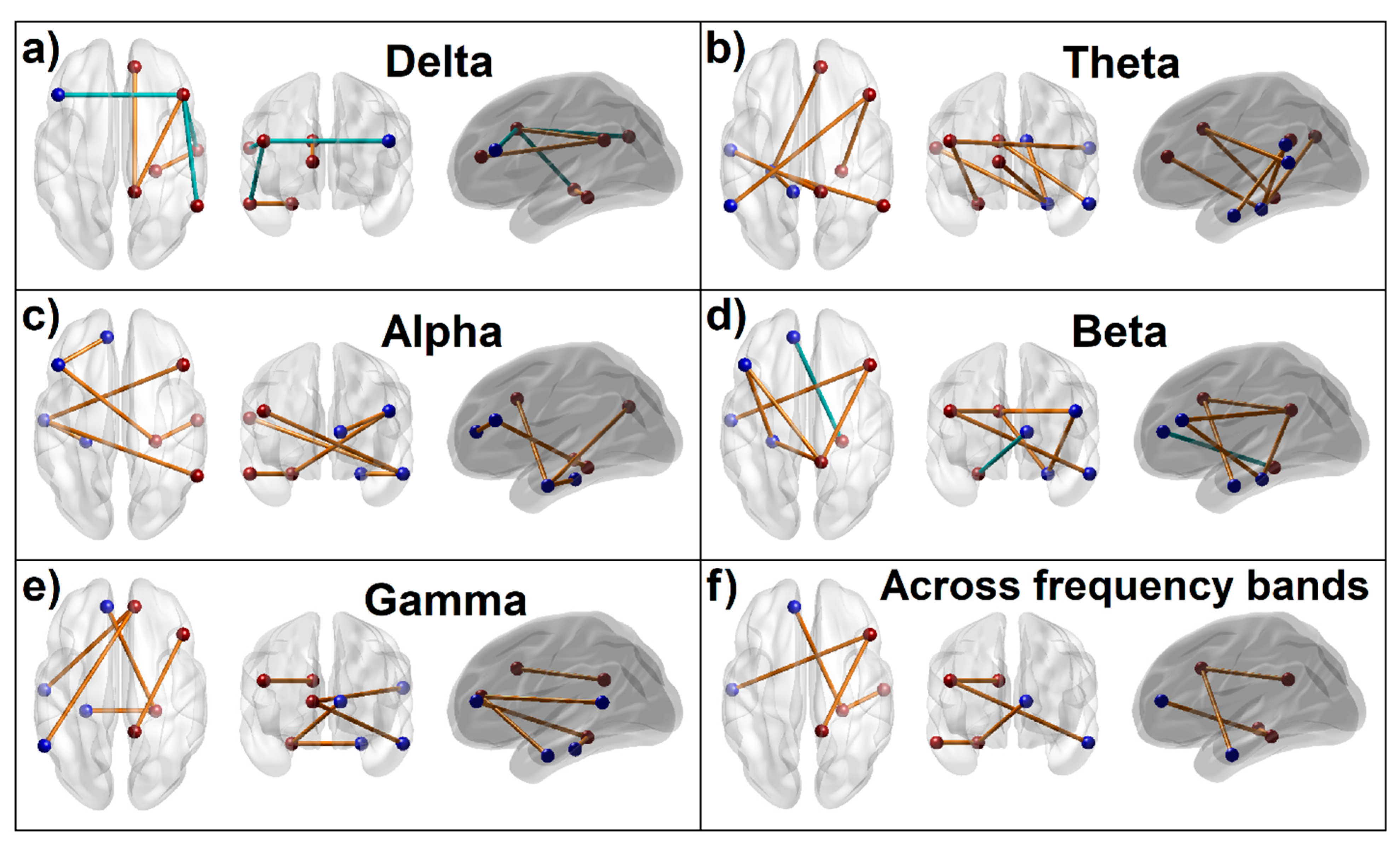
3.1.5. Connectivity Mapping of Significant FC Connections
3.2. Correlations between Significant Variables and Age
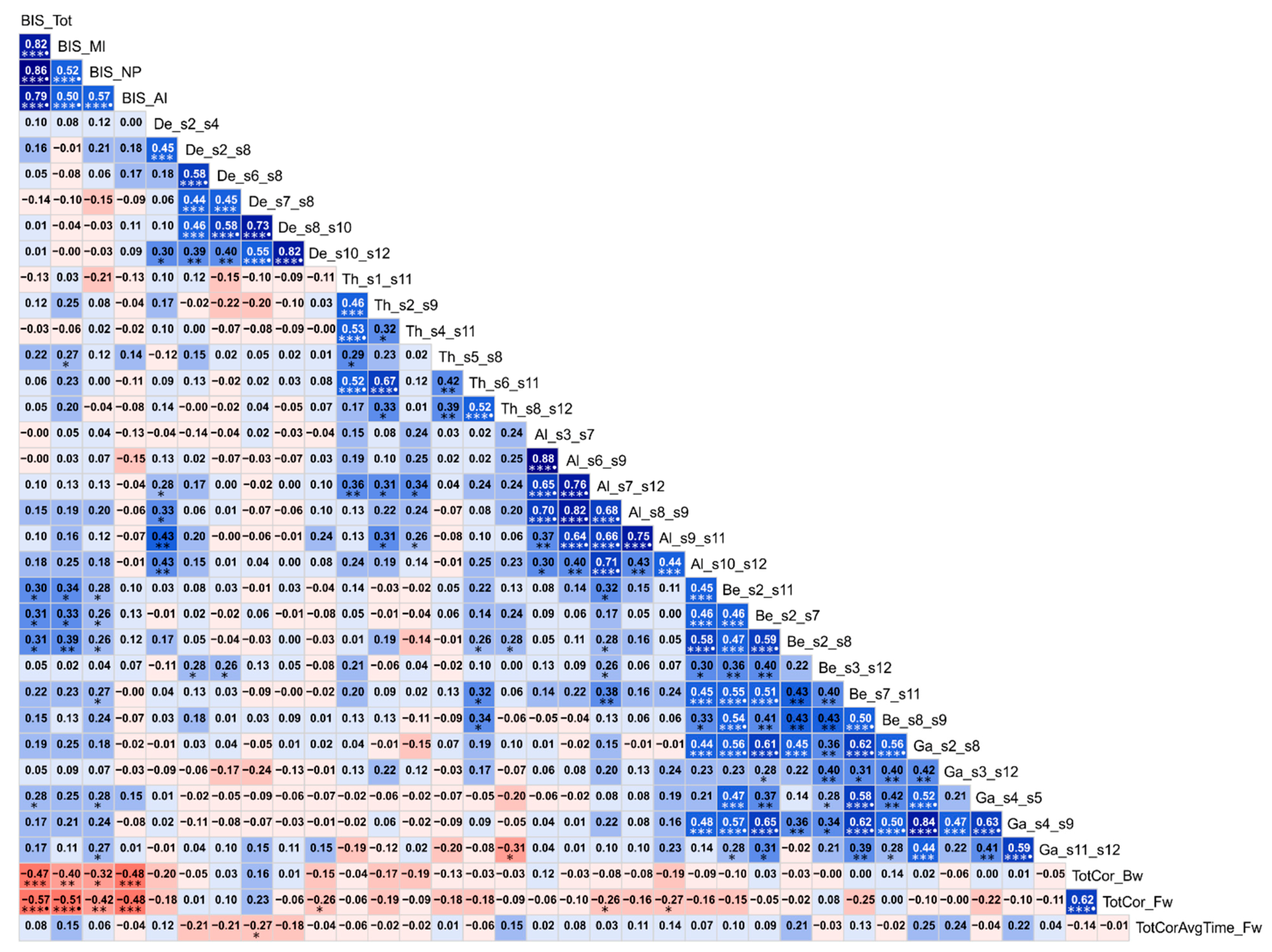
3.3. Correlations among the Top Significant Variables
4. Discussion
4.1. Aberrations in EEG Source DMN FC in AUD
4.1.1. Predominant Pattern of DMN Hyperconnectivity in AUD
4.1.2. Parahippocampal Hyperconnectivity in AUD
4.1.3. Hypoconnectivity of Prefrontal Nodes in AUD
4.2. Poor Neuropsychological Performance in AUD
4.3. Heightened Impulsivity in AUD
4.4. Associations among AUD, FC, Impulsivity, and Neurocognition
4.5. Potential Limitations and Suggestions for Future Research
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Trees: Decision trees whose results are aggregated into one final result for classifying the factors or outcomes. Each tree is constructed based on a random (bootstrapped) subsample of the observations.
- Node: A point in a tree, where a split occurs as a result of a ’test’ on an attribute leading to binary outcomes (e.g., whether a coin flip results in head or tail). A binary split at a node partitions the data from the parent node into two daughter nodes.
- Branch: The outcome of the test resulting in a split or two branches in a classification tree.
- Leaf: A terminal node that has no children or branches.
- Random Forest ensemble: Aggregation of individual decision trees in order to combine predictions (votes) from each tree. The class/group/outcome with most votes becomes the RF model’s prediction.
- Bagging: It’s the short form of ’bootstrap aggregating’, which is a method to improve classification by combining classifications of randomly generated training sets.
- Out of bag (OOB) estimate: The observations that are not part of the bootstrap subsample are referred to as out-of-bag (OOB) observations. The OOB error refers to the classification error based on this subsample and serves as a validation of Random Forest model accuracy.
- Gini (mean) decrease: It represents the importance of a specific feature/predictor/variable (Vi) for the classification or prediction. It’s the mean decrease in node impurity (classification error) of Vi. A higher Gini decrease indicates higher variable importance for Vi.
- Accuracy decrease: Mean decrease in prediction accuracy after Vi is not taken into account.
- Mean minimal depth: It refers to the number of nodes along the shortest path from the root node down to the nearest leaf node. Smaller depth for the Vi indicates its higher importance.
- Mtry: A preset number of features/variables/predictors randomly selected (from the entire list) for splitting at each node in the construction of each decision tree.
- ntree: A preset total number of trees to grow for a given model. Larger ‘ntree’ normally produce more stable models and more reliable predictions.
- Number of nodes: Total number of nodes that use Vi for splitting (it is usually equal to number of trees if trees are shallow).
- Times a root: Total number of trees in which Vi is used for splitting the root node (i.e., the whole sample is divided into two based on the value of Vi).
- P-value: Probability value of hypothesis testing based on a one-sided binomial test that indicates whether the observed number of successes (number of nodes in which Vi was used for splitting) exceeds the theoretical number of successes if they were random.
References
- O’Brien, C.P.; McLellan, A.T. Myths about the treatment of addiction. Lancet 1996, 347, 237–240. [Google Scholar] [CrossRef]
- Koob, G.F. Neurocircuitry of alcohol addiction: Synthesis from animal models. Handb. Clin. Neurol. 2014, 125, 33–54. [Google Scholar] [PubMed]
- Zhu, X.; Cortes, C.R.; Mathur, K.; Tomasi, D.; Momenan, R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: A resting-state study. Addict. Biol. 2017, 22, 206–217. [Google Scholar] [CrossRef]
- Zahr, N.M.; Pfefferbaum, A. Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res 2017, 38, 183–206. [Google Scholar] [PubMed]
- Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Jones, K.A.; Padmanabhapillai, A.; Begleiter, H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neurophysiol. 2005, 116, 993–1018. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, M.; Porjesz, B. Understanding alcohol use disorders with neuroelectrophysiology. Handb. Clin. Neurol. 2014, 125, 383–414. [Google Scholar]
- Oscar-Berman, M.; Marinkovic, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Moselhy, H.F.; Georgiou, G.; Kahn, A. Frontal lobe changes in alcoholism: A review of the literature. Alcohol Alcohol. 2001, 36, 357–368. [Google Scholar] [CrossRef]
- Fritz, M.; Klawonn, A.M.; Zahr, N.M. Neuroimaging in alcohol use disorder: From mouse to man. J. Neurosci. Res. 2019. [Google Scholar] [CrossRef]
- Le Berre, A.P.; Fama, R.; Sullivan, E.V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C.; Porjesz, B. Advances in Electrophysiological Research. Alcohol Res 2015, 37, 53–87. [Google Scholar] [PubMed]
- Oscar-Berman, M.; Valmas, M.M.; Sawyer, K.S.; Ruiz, S.M.; Luhar, R.B.; Gravitz, Z.R. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb. Clin. Neurol. 2014, 125, 183–210. [Google Scholar]
- Crews, F.T.; Boettiger, C.A. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav. 2009, 93, 237–247. [Google Scholar] [CrossRef]
- Buhler, M.; Mann, K. Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcohol. Clin. Exp. Res. 2011, 35, 1771–1793. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ardekani, B.A.; Kamarajan, C.; Zhang, J.; Chorlian, D.B.; Byrne, K.N.; Pandey, G.; Meyers, J.L.; Kinreich, S.; Stimus, A.; et al. Lower Prefrontal and Hippocampal Volume and Diffusion Tensor Imaging Differences Reflect Structural and Functional Abnormalities in Abstinent Individuals with Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2018, 42, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Zahr, N.M. Structural and microstructral imaging of the brain in alcohol use disorders. In Alcohol and the Nervous System; Sullivan, E.V., Pfefferbaum, A., Eds.; Elsevier: New York, NY, USA, 2014; Volume 125, pp. 275–290. [Google Scholar]
- Crespi, C.; Galandra, C.; Canessa, N.; Manera, M.; Poggi, P.; Basso, G. Microstructural damage of white-matter tracts connecting large-scale networks is related to impaired executive profile in alcohol use disorder. Neuroimage Clin 2019, 25, 102141. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.; Galandra, C.; Manera, M.; Basso, G.; Poggi, P.; Canessa, N. Executive Impairment in Alcohol Use Disorder Reflects Structural Changes in Large-Scale Brain Networks: A Joint Independent Component Analysis on Gray-Matter and White-Matter Features. Front. Psychol. 2019, 10, 2479. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Pfefferbaum, A. Human Imaging Studies of Brain Circuitry Disrupted by Alcoholism. In Neurobiology of Alcohol Dependence; Academic Press: San Diego, CA, USA, 2014; pp. 131–151. [Google Scholar] [CrossRef]
- Chanraud, S.; Pitel, A.L.; Pfefferbaum, A.; Sullivan, E.V. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb. Cortex 2011, 21, 2272–2281. [Google Scholar] [CrossRef]
- Wetherill, R.R.; Bava, S.; Thompson, W.K.; Boucquey, V.; Pulido, C.; Yang, T.T.; Tapert, S.F. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012, 1432, 66–73. [Google Scholar] [CrossRef]
- Camchong, J.; Stenger, V.A.; Fein, G. Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2013, 37, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Chanraud, S.; Pitel, A.L.; Muller-Oehring, E.M.; Pfefferbaum, A.; Sullivan, E.V. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb. Cortex 2013, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Weiland, B.J.; Welsh, R.C.; Yau, W.Y.; Zucker, R.A.; Zubieta, J.K.; Heitzeg, M.M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013, 128, 130–139. [Google Scholar]
- Muller-Oehring, E.M.; Jung, Y.C.; Pfefferbaum, A.; Sullivan, E.V.; Schulte, T. The Resting Brain of Alcoholics. Cereb. Cortex 2015, 25, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, Y.; Dong, Y.; Ma, M.; Ma, Y.; Dong, Y.; Niu, Y.; Jiang, Y.; Wang, H.; Wang, Z.; et al. Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. Plos One 2016, 11, e0161956. [Google Scholar]
- Kim, S.; Im, S.; Lee, J.; Lee, S.G. Disrupted Control Network Connectivity in Abstinent Patients with Alcohol Dependence. Psychiatry Investig. 2017, 14, 325–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.; Du, X.; Kerich, M.; Lohoff, F.W.; Momenan, R. Random forest based classification of alcohol dependence patients and healthy controls using resting state MRI. Neurosci. Lett. 2018, 676, 27–33. [Google Scholar] [CrossRef]
- Fede, S.J.; Grodin, E.N.; Dean, S.F.; Diazgranados, N.; Momenan, R. Resting state connectivity best predicts alcohol use severity in moderate to heavy alcohol users. Neuroimage Clin 2019, 22, 101782. [Google Scholar] [CrossRef]
- Bzdok, D.; Meyer-Lindenberg, A. Machine Learning for Precision Psychiatry: Opportunities and Challenges. Biol Psychiatry Cogn Neurosci Neuroimaging 2018, 3, 223–230. [Google Scholar] [CrossRef]
- Janssen, R.J.; Mourao-Miranda, J.; Schnack, H.G. Making Individual Prognoses in Psychiatry Using Neuroimaging and Machine Learning. Biol Psychiatry Cogn Neurosci Neuroimaging 2018. [Google Scholar] [CrossRef]
- Cho, G.; Yim, J.; Choi, Y.; Ko, J.; Lee, S.H. Review of Machine Learning Algorithms for Diagnosing Mental Illness. Psychiatry Investig. 2019, 16, 262–269. [Google Scholar] [CrossRef]
- Mumtaz, W.; Vuong, P.L.; Xia, L.; Malik, A.S.; Rashid, R.B.A. An EEG-based machine learning method to screen alcohol use disorder. Cogn. Neurodyn. 2017, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kinreich, S.; Meyers, J.L.; Maron-Katz, A.; Kamarajan, C.; Pandey, A.K.; Chorlian, D.B.; Zhang, J.; Pandey, G.; Subbie-Saenz de Viteri, S.; Pitti, D.; et al. Predicting risk for Alcohol Use Disorder using longitudinal data with multimodal biomarkers and family history: A machine learning study. Mol. Psychiatry 2019. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sarica, A.; Cerasa, A.; Quattrone, A. Random Forest Algorithm for the Classification of Neuroimaging Data in Alzheimer’s Disease: A Systematic Review. Front. Aging Neurosci. 2017, 9, 329. [Google Scholar] [CrossRef]
- Kim, S.G.; Richter, W.; Ugurbil, K. Limitations of temporal resolution in functional MRI. Magn. Reson. Med. 1997, 37, 631–636. [Google Scholar] [CrossRef]
- St. Louis, E.K.; Frey, L.C. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Tzimourta, K.; Tsoulos, I.; Bilero, T.; Tzallas, A.; Tsipouras, M.; Giannakeas, N. Direct Assessment of Alcohol Consumption in Mental State Using Brain Computer Interfaces and Grammatical Evolution. Inventions 2018, 3, 51. [Google Scholar] [CrossRef]
- Anuragi, A.; Sisodia, D.S. Alcohol use disorder detection using EEG Signal features and flexible analytical wavelet transform. Biomed. Signal Process. Control 2019, 52, 384–393. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Aoki, Y.; Ishii, R.; Pascual-Marqui, R.D.; Canuet, L.; Ikeda, S.; Hata, M.; Imajo, K.; Matsuzaki, H.; Musha, T.; Asada, T.; et al. Detection of EEG-resting state independent networks by eLORETA-ICA method. Front. Hum. Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef]
- Olbrich, S.; Trankner, A.; Chittka, T.; Hegerl, U.; Schonknecht, P. Functional connectivity in major depression: Increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014, 222, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Kazui, H.; Tanaka, T.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Kanemoto, H.; Yoshiyama, K.; et al. Functional connectivity assessed by resting state EEG correlates with cognitive decline of Alzheimer’s disease - An eLORETA study. Clin. Neurophysiol. 2016, 127, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.B.; Mennes, M.; Zuo, X.N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Raichle, M.E. The restless brain: How intrinsic activity organizes brain function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140172. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Molnar-Szakacs, I.; Uddin, L.Q. Self-processing and the default mode network: Interactions with the mirror neuron system. Front. Hum. Neurosci. 2013, 7, 571. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef]
- Hampson, M.; Driesen, N.; Roth, J.K.; Gore, J.C.; Constable, R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging 2010, 28, 1051–1057. [Google Scholar] [CrossRef]
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012, 16, 584–592. [Google Scholar] [CrossRef]
- Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Kinreich, S.; Pandey, G.; Chorlian, D.B.; Meyers, J.L.; Zhang, J.; Bermudez, E.; Stimus, A.T.; et al. Random Forest Classification of Alcohol Use Disorder Using fMRI Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Brain Sci 2020, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M. Cellular substrates of brain rhythms. In Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 31–83. [Google Scholar]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Zinn, M.L.; Zinn, M.A.; Jason, L.A. Intrinsic Functional Hypoconnectivity in Core Neurocognitive Networks Suggests Central Nervous System Pathology in Patients with Myalgic Encephalomyelitis: A Pilot Study. Appl. Psychophysiol. Biofeedback 2016, 41, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Della Marca, G.; Brunetti, R.; Carbone, G.A.; Massullo, C.; Valenti, E.M.; Amoroso, N.; Maestoso, G.; Contardi, A.; Farina, B. Default Mode Network alterations in alexithymia: An EEG power spectra and connectivity study. Sci. Rep. 2016, 6, 36653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Mohan, A.; De Ridder, D.; Sunaert, S.; Vanneste, S. The neural correlates of the unified percept of alcohol-related craving: A fMRI and EEG study. Sci. Rep. 2018, 8, 923. [Google Scholar] [CrossRef]
- He, B.; Yang, L.; Wilke, C.; Yuan, H. Electrophysiological imaging of brain activity and connectivity-challenges and opportunities. Ieee Trans. Biomed. Eng. 2011, 58, 1918–1931. [Google Scholar]
- David, O.; Garnero, L.; Cosmelli, D.; Varela, F.J. Estimation of neural dynamics from MEG/EEG cortical current density maps: Application to the reconstruction of large-scale cortical synchrony. Ieee Trans. Biomed. Eng. 2002, 49, 975–987. [Google Scholar] [CrossRef]
- Basar, E. Brain Function and Oscillations. Vol. I: Principles and Approaches; Springer Verlag: Berlin, Germany, 1999. [Google Scholar]
- Parsons, O.A. Neuropsychological deficits in alcoholics: Facts and fancies. Alcohol. Clin. Exp. Res. 1977, 1, 51–56. [Google Scholar] [CrossRef]
- Oscar-Berman, M. Neuropsychological vulnerabilities in chronic alcoholism. In Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism (NIAAA) Research Monograph No. 34; Noronha, A., Eckardt, M.J., Warren, K., Eds.; NIAAA: Bethesda, MD, USA, 2000; pp. 437–471. [Google Scholar]
- Kamarajan, C.; Rangaswamy, M.; Manz, N.; Chorlian, D.B.; Pandey, A.K.; Roopesh, B.N.; Porjesz, B. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum. Brain Mapp. 2012, 33, 1019–1039. [Google Scholar] [CrossRef]
- Bucholz, K.K.; Cadoret, R.; Cloninger, C.R.; Dinwiddie, S.H.; Hesselbrock, V.M.; Nurnberger, J.I., Jr.; Reich, T.; Schmidt, I.; Schuckit, M.A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J Stud Alcohol 1994, 55, 149–158. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Shallice, T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 199–209. [Google Scholar] [PubMed]
- Davis, H.P.; Keller, F. Colorado Assessment Tests (CATs), Version 1.2; Colorado Springs: Colorado, CO, USA, 2002. [Google Scholar]
- Berch, D.B.; Krikorian, R.; Huha, E.M. The Corsi block-tapping task: Methodological and theoretical considerations. Brain Cogn. 1998, 38, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Milner, B. Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Stanford, M.S.; Mathias, C.W.; Dougherty, D.M.; Lake, S.L.; Anderson, N.E.; Patton, J.H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers. Individ. Dif. 2009, 47, 385–395. [Google Scholar] [CrossRef]
- Jasper, H.H. Report of the committee on methods of clinical examination in electroencephalography: 1957. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. Am. J. Eeg Technol. 1985, 25, 83–92. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Modified nomenclature for the “10%” electrode system. J. Clin. Neurophysiol. 1988, 5, 183–186. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Thatcher, R.W.; North, D.M.; Biver, C.J. LORETA EEG phase reset of the default mode network. Front. Hum. Neurosci. 2014, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Dansereau, C.; Benhajali, Y.; Risterucci, C.; Pich, E.M.; Orban, P.; Arnold, D.; Bellec, P. Statistical power and prediction accuracy in multisite resting-state fMRI connectivity. Neuroimage 2017, 149, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Canuet, L.; Ishii, R.; Pascual-Marqui, R.D.; Iwase, M.; Kurimoto, R.; Aoki, Y.; Ikeda, S.; Takahashi, H.; Nakahachi, T.; Takeda, M. Resting-state EEG source localization and functional connectivity in schizophrenia-like psychosis of epilepsy. Plos One 2011, 6, e27863. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. Neuroimage 2007, 34, 1600–1611. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: Frequency decomposition. arXiv 2007, 0711.1455. Available online: https://arxiv.org/abs/0711.1455 (accessed on 1 January 2020).
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, 0710.3341. Available online: https://arxiv.org/abs/0710.3341 (accessed on 1 January 2020).
- Babiloni, C.; Triggiani, A.I.; Lizio, R.; Cordone, S.; Tattoli, G.; Bevilacqua, V.; Soricelli, A.; Ferri, R.; Nobili, F.; Gesualdo, L.; et al. Classification of Single Normal and Alzheimer’s Disease Individuals from Cortical Sources of Resting State EEG Rhythms. Front. Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Talwar, S.; Jaiswal, A.K.; Banerjee, A. Quantitative Evaluation in Estimating Sources Underlying Brain Oscillations Using Current Source Density Methods and Beamformer Approaches. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Mulert, C.; Jager, L.; Schmitt, R.; Bussfeld, P.; Pogarell, O.; Moller, H.J.; Juckel, G.; Hegerl, U. Integration of fMRI and simultaneous EEG: Towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage 2004, 22, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Vitacco, D.; Brandeis, D.; Pascual-Marqui, R.; Martin, E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum. Brain Mapp. 2002, 17, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Worrell, G.A.; Lagerlund, T.D.; Sharbrough, F.W.; Brinkmann, B.H.; Busacker, N.E.; Cicora, K.M.; O’Brien, T.J. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000, 12, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.; Jelic, V.; Pascual-Marqui, R.D.; Wahlund, L.; Julin, P.; Linden, D.E.; Maurer, K.; Winblad, B.; Nordberg, A. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer’s disease. Clin. Neurophysiol. 2000, 111, 1817–1824. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Oakes, T.R.; Fox, A.S.; Chung, M.K.; Larson, C.L.; Abercrombie, H.C.; Schaefer, S.M.; Benca, R.M.; Davidson, R.J. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol. Psychiatry 2004, 9, 393–405. [Google Scholar] [CrossRef]
- Zumsteg, D.; Lozano, A.M.; Wennberg, R.A. Depth electrode recorded cerebral responses with deep brain stimulation of the anterior thalamus for epilepsy. Clin. Neurophysiol. 2006, 117, 1602–1609. [Google Scholar] [CrossRef]
- Lopez-Caneda, E.; Cadaveira, F.; Crego, A.; Gomez-Suarez, A.; Corral, M.; Parada, M.; Caamano-Isorna, F.; Rodriguez Holguin, S. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: A follow-up study. Addiction 2012, 107, 1796–1808. [Google Scholar] [CrossRef]
- Olbrich, S.; Olbrich, H.; Adamaszek, M.; Jahn, I.; Hegerl, U.; Stengler, K. Altered EEG lagged coherence during rest in obsessive-compulsive disorder. Clin. Neurophysiol. 2013, 124, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Caneda, E.; Cadaveira, F.; Correas, A.; Crego, A.; Maestu, F.; Rodriguez Holguin, S. The Brain of Binge Drinkers at Rest: Alterations in Theta and Beta Oscillations in First-Year College Students with a Binge Drinking Pattern. Front. Behav. Neurosci. 2017, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Wiener, M. Breiman and Cutler’s Random Forests for Classification and Regression, R Package Version 4.6-14; 2018. Available online: https://cran.r-project.org/web/packages/randomForest/ (accessed on 2 January 2019).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Benesty, M.; et al. Classification and Regression Training, R Package Version 6.0-84; 2019. Available online: https://cran.r-project.org/web/packages/caret (accessed on 4 September 2019).
- Paluszynska, A.; Biecek, P.; Jiang, Y. randomForestExplainer: Explaining and Visualizing Random Forests in Terms of Variable Importance, R Package Version 0.10.0; 2019. Available online: https://cran.r-project.org/web/packages/randomForestExplainer (accessed on 3 April 2019).
- Qi, Y. Random Forest for Bioinformatics. In Ensemble Machine Learning; Zhang, C., Ma, Y., Eds.; Springer US: Boston, MA, USA, 2012; pp. 307–323. [Google Scholar]
- Couronne, R.; Probst, P.; Boulesteix, A.L. Random forest versus logistic regression: A large-scale benchmark experiment. Bmc Bioinform. 2018, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Cutler, A. Random Forest. Available online: https://www.stat.berkeley.edu/~breiman/RandomForests/cc_home.htm#ooberr (accessed on 1 June 2019).
- Begleiter, H.; Porjesz, B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol. Clin. Exp. Res. 1999, 23, 1125–1135. [Google Scholar] [CrossRef]
- Justus, A.N.; Finn, P.R.; Steinmetz, J.E. P300, disinhibited personality, and early-onset alcohol problems. Alcohol. Clin. Exp. Res. 2001, 25, 1457–1466. [Google Scholar] [CrossRef]
- Iacono, W.G.; Carlson, S.R.; Malone, S.M.; McGue, M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch. Gen. Psychiatry 2002, 59, 750–757. [Google Scholar] [CrossRef]
- Kamarajan, C.; Porjesz, B.; Jones, K.A.; Choi, K.; Chorlian, D.B.; Padmanabhapillai, A.; Rangaswamy, M.; Stimus, A.T.; Begleiter, H. Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a Go/No-Go task. Biol. Psychol. 2005, 69, 353–373. [Google Scholar] [CrossRef]
- Jones, A.; Christiansen, P.; Nederkoorn, C.; Houben, K.; Field, M. Fluctuating disinhibition: Implications for the understanding and treatment of alcohol and other substance use disorders. Front Psychiatry 2013, 4, 140. [Google Scholar] [CrossRef]
- Proctor, W.R.; Diao, L.; Freund, R.K.; Browning, M.D.; Wu, P.H. Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J. Physiol. 2006, 575, 145–159. [Google Scholar] [CrossRef]
- Kumar, S.; Porcu, P.; Werner, D.F.; Matthews, D.B.; Diaz-Granados, J.L.; Helfand, R.S.; Morrow, A.L. The role of GABA(A) receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacol. (Berl.) 2009, 205, 529–564. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Yorgason, J.T.; Nelson, A.C.; Lewis, N.; Nufer, T.M.; Edwards, J.G.; Steffensen, S.C. Glutamate Transmission to Ventral Tegmental Area GABA Neurons Is Altered by Acute and Chronic Ethanol. Alcohol. Clin. Exp. Res. 2018, 42, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Porjesz, B.; Almasy, L.; Edenberg, H.J.; Wang, K.; Chorlian, D.B.; Foroud, T.; Goate, A.; Rice, J.P.; O’Connor, S.J.; Rohrbaugh, J.; et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc. Natl. Acad. Sci. USA 2002, 99, 3729–3733. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; Dick, D.M.; Xuei, X.; Tian, H.; Almasy, L.; Bauer, L.O.; Crowe, R.R.; Goate, A.; Hesselbrock, V.; Jones, K.; et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 2004, 74, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.F.; Hicks, B.M.; Patrick, C.J.; Carlson, S.R.; Iacono, W.G.; McGue, M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J. Abnorm. Psychol. 2002, 111, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Iacono, W.G.; Malone, S.M.; McGue, M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int. J. Psychophysiol. 2003, 48, 147–178. [Google Scholar] [CrossRef]
- Chen, A.C.; Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Tang, Y.; Jones, K.A.; Chorlian, D.B.; Stimus, A.T.; Begleiter, H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol. Clin. Exp. Res. 2007, 31, 156–165. [Google Scholar] [CrossRef]
- Imperatori, C.; Fabbricatore, M.; Innamorati, M.; Farina, B.; Quintiliani, M.I.; Lamis, D.A.; Mazzucchi, E.; Contardi, A.; Vollono, C.; Della Marca, G. Modification of EEG functional connectivity and EEG power spectra in overweight and obese patients with food addiction: An eLORETA study. Brain Imaging Behav 2015, 9, 703–716. [Google Scholar] [CrossRef]
- Ward, A.M.; Schultz, A.P.; Huijbers, W.; Van Dijk, K.R.; Hedden, T.; Sperling, R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2013. [Google Scholar] [CrossRef]
- White, A.M. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health 2003, 27, 186–196. [Google Scholar]
- Lee, H.; Roh, S.; Kim, D.J. Alcohol-induced blackout. Int. J. Environ. Res. Public Health 2009, 6, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.E.; Grant, J.E. Alcohol-induced blackout. Phenomenology, biological basis, and gender differences. J. Addict. Med. 2010, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, R.R.; Fromme, K. Alcohol-Induced Blackouts: A Review of Recent Clinical Research with Practical Implications and Recommendations for Future Studies. Alcohol. Clin. Exp. Res. 2016, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.; Hensel, A.; Wolf, H.; Weiss, T.; Gertz, H.J. Does the hippocampal atrophy correlate with the cortical theta power in elderly subjects with a range of cognitive impairment? J. Clin. Neurophysiol. 2007, 24, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zang, Y.; He, Y.; Liang, M.; Zhang, X.; Tian, L.; Wu, T.; Jiang, T.; Li, K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage 2006, 31, 496–504. [Google Scholar] [CrossRef]
- James, G.A.; Tripathi, S.P.; Ojemann, J.G.; Gross, R.E.; Drane, D.L. Diminished default mode network recruitment of the hippocampus and parahippocampus in temporal lobe epilepsy. J. Neurosurg. 2013, 119, 288–300. [Google Scholar] [CrossRef]
- Haneef, Z.; Lenartowicz, A.; Yeh, H.J.; Levin, H.S.; Engel, J., Jr.; Stern, J.M. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014, 55, 137–145. [Google Scholar] [CrossRef]
- Salami, A.; Pudas, S.; Nyberg, L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc. Natl. Acad. Sci. USA 2014, 111, 17654–17659. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Y.; Fu, X.M.; Li, N.; Wang, C.X.; Zhang, H.; Qian, R.B.; Xu, H.S.; Hu, X.; Zhang, D.R. Abnormal brain default-mode network functional connectivity in drug addicts. Plos One 2011, 6, e16560. [Google Scholar] [CrossRef]
- Bubb, E.J.; Kinnavane, L.; Aggleton, J.P. Hippocampal - diencephalic - cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci Adv 2017, 1, 1–20. [Google Scholar] [CrossRef]
- Buzsaki, G.; Freeman, W. Editorial overview: Brain rhythms and dynamic coordination. Curr. Opin. Neurobiol. 2015, 31, v–ix. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Chapter 2 - Anatomy of the Prefrontal Cortex. In The Prefrontal Cortex (Fourth Edition); Fuster, J.M., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. 7–58. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Chapter 5 - Human Neuropsychology. In The Prefrontal Cortex (Fourth Edition); Fuster, J.M., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. 171–219. [Google Scholar]
- Sullivan, E.V.; Pfefferbaum, A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacol. (Berl.) 2005, 180, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.T.; Bo, P.; Giardini, A.; Soragna, D. Chronic alcoholism and the frontal lobe: Which executive functions are imparied? Acta Neurol. Scand. 2002, 105, 276–281. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Sullivan, E.V.; Mathalon, D.H.; Lim, K.O. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol. Clin. Exp. Res. 1997, 21, 521–529. [Google Scholar] [CrossRef]
- Harper, C.; Kril, J.; Daly, J. Are we drinking our neurones away? Br. Med. J. (Clin. Res. Ed) 1987, 294, 534–536. [Google Scholar] [CrossRef]
- Noel, X.; Paternot, J.; Van der Linden, M.; Sferrazza, R.; Verhas, M.; Hanak, C.; Kornreich, C.; Martin, P.; De Mol, J.; Pelc, I.; et al. Correlation between inhibition, working memory and delimited frontal area blood flow measure by 99mTc-Bicisate SPECT in alcohol-dependent patients. Alcohol Alcohol. 2001, 36, 556–563. [Google Scholar] [CrossRef]
- Noel, X.; Sferrazza, R.; Van Der Linden, M.; Paternot, J.; Verhas, M.; Hanak, C.; Pelc, I.; Verbanck, P. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002, 37, 347–354. [Google Scholar] [CrossRef]
- Golkowski, D.; Ranft, A.; Kiel, T.; Riedl, V.; Kohl, P.; Rohrer, G.; Pientka, J.; Berger, S.; Preibisch, C.; Zimmer, C.; et al. Coherence of BOLD signal and electrical activity in the human brain during deep sevoflurane anesthesia. Brain Behav. 2017, 7, e00679. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Muller-Oehring, E.M.; Le Berre, A.P.; Serventi, M.; Kalon, E.; Haas, A.L.; Padula, C.B.; Schulte, T. Brain activation to cannabis- and alcohol-related words in alcohol use disorder. Psychiatry Res Neuroimaging 2019, 294, 111005. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Sinha, R. Prefrontal Limbic-Striatal Circuits and Alcohol Addiction in Humans. In Neurobiology of Alcohol Dependence; Academic Press: San Diego, CA, USA, 2014; pp. 49–63. [Google Scholar] [CrossRef]
- Rosen, V.M.; Engle, R.W. Forward and backward serial recall. Intelligence 1997, 25, 37–47. [Google Scholar] [CrossRef]
- Tuulio-Henriksson, A.; Arajarvi, R.; Partonen, T.; Haukka, J.; Varilo, T.; Schreck, M.; Cannon, T.; Lonnqvist, J. Familial loading associates with impairment in visual span among healthy siblings of schizophrenia patients. Biol. Psychiatry 2003, 54, 623–628. [Google Scholar] [CrossRef]
- Parsons, O.A.; Butters, N.; Nathan, P.E. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment; The Guilford Press: New York, NY, USA, 1987; p. 414. [Google Scholar]
- Tarter, R.E.; Ryan, C.M. Neuropsychology of alcoholism. Etiology, phenomenology, process, and outcome. Recent Dev. Alcohol. 1983, 1, 449–469. [Google Scholar] [PubMed]
- Oscar-Berman, M. Neuropsychological consequences of long-term chronic alcoholism. Am. Sci. 1980, 68, 410–419. [Google Scholar] [PubMed]
- Day, A.M.; Kahler, C.W.; Ahern, D.C.; Clark, U.S. Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Curr. Drug Abus. Rev. 2015, 8, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.J.; Luty, J.; Bogdan, N.A.; Sahakian, B.J.; Clark, L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacol. (Berl.) 2009, 207, 163–172. [Google Scholar] [CrossRef]
- Rangaswamy, M.; Porjesz, B.; Chorlian, D.B.; Wang, K.; Jones, K.A.; Bauer, L.O.; Rohrbaugh, J.; O’Connor, S.J.; Kuperman, S.; Reich, T.; et al. Beta power in the EEG of alcoholics. Biol. Psychiatry 2002, 52, 831–842. [Google Scholar] [CrossRef]
- Meier, N.M.; Perrig, W.; Koenig, T. Is excessive electroencephalography beta activity associated with delinquent behavior in men with attention-deficit hyperactivity disorder symptomatology? Neuropsychobiology 2014, 70, 210–219. [Google Scholar] [CrossRef]
- Moeller, F.G.; Barratt, E.S.; Fischer, C.J.; Dougherty, D.M.; Reilly, E.L.; Mathias, C.W.; Swann, A.C. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology 2004, 50, 167–173. [Google Scholar] [CrossRef]
- Carlson, S.R.; Thai, S.; McLarnon, M.E. Visual P3 amplitude and self-reported psychopathic personality traits: Frontal reduction is associated with self-centered impulsivity. Psychophysiology 2009, 46, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Barratt, E.S.; Wigg, C. Impulsiveness, aggression, reading, and the P300 of the event-related potential. Pers. Individ. Dif. 1997, 22, 439–445. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.M.; Kim, Y.J.; Kim, D.J.; Choi, S.W.; Kwon, J.S.; Choi, J.S. Resting-state EEG activity related to impulsivity in gambling disorder. J Behav Addict 2017, 6, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Diaz, A.; Mendoza-Quinones, R.; Melie-Garcia, L.; Martinez-Montes, E.; Sanabria-Diaz, G.; Romero-Quintana, Y.; Salazar-Guerra, I.; Carballoso-Acosta, M.; Caballero-Moreno, A. Functional Connectivity and Quantitative EEG in Women with Alcohol Use Disorders: A Resting-State Study. Brain Topogr. 2016, 29, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Tanaka, T.; Kazui, H.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Sato, S.; Suzuki, Y.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease Correlate With Electroencephalography Parameters Assessed by Exact Low-Resolution Electromagnetic Tomography (eLORETA). Clin. Eeg Neurosci. 2017, 48, 338–347. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Gender differences in brain functional connectivity density. Hum. Brain Mapp. 2012, 33, 849–860. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Laterality patterns of brain functional connectivity: Gender effects. Cereb. Cortex 2012, 22, 1455–1462. [Google Scholar] [CrossRef]
- Goldstein, R.B.; Dawson, D.A.; Chou, S.P.; Grant, B.F. Sex differences in prevalence and comorbidity of alcohol and drug use disorders: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Stud. Alcohol Drugs 2012, 73, 938–950. [Google Scholar] [CrossRef]
- Wagner, M.; Fuchs, M.; Kastner, J. sLORETA, eLORETA, and SWARM in the presence of noise and multiple sources. In Biomagnetism: Interdisciplinary Research and Exploration; Kakigi, R., Yokosawa, K., Kuri, S., Eds.; Hokkaido University Press: Tokyo, Japan, 2008; pp. 74–76. [Google Scholar]
- Thompson, P.M.; Ge, T.; Glahn, D.C.; Jahanshad, N.; Nichols, T.E. Genetics of the connectome. Neuroimage 2013, 80, 475–488. [Google Scholar] [CrossRef]
- Pluta, D.; Yu, Z.; Shen, T.; Chen, C.; Xue, G.; Ombao, H. Statistical methods and challenges in connectome genetics. Stat. Probab. Lett. 2018. [Google Scholar] [CrossRef]
- Jahanshad, N.; Hibar, D.P.; Ryles, A.; Toga, A.W.; McMahon, K.L.; de Zubicaray, G.I.; Hansell, N.K.; Montgomery, G.W.; Martin, N.G.; Wright, M.J.; et al. Discovery of Genes That Affect Human Brain Connectivity: A Genome-Wide Analysis of the Connectome. Proc Ieee Int Symp Biomed Imaging 2012, 542–545. [Google Scholar]
- Fox, M.D.; Buckner, R.L.; White, M.P.; Greicius, M.D.; Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 2012, 72, 595–603. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Manning, P.; Glue, P.; Cape, G.; Langguth, B.; Vanneste, S. Anterior Cingulate Implant for Alcohol Dependence: Case Report. Neurosurgery 2016, 78, E883–E893. [Google Scholar] [CrossRef] [PubMed]
- Gruzelier, J. A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cogn Process 2009, 10 (Suppl. 1), S101–S109. [Google Scholar] [CrossRef]
- Khemiri, L.; Brynte, C.; Stunkel, A.; Klingberg, T.; Jayaram-Lindstrom, N. Working Memory Training in Alcohol Use Disorder: A Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2019, 43, 135–146. [Google Scholar] [CrossRef]

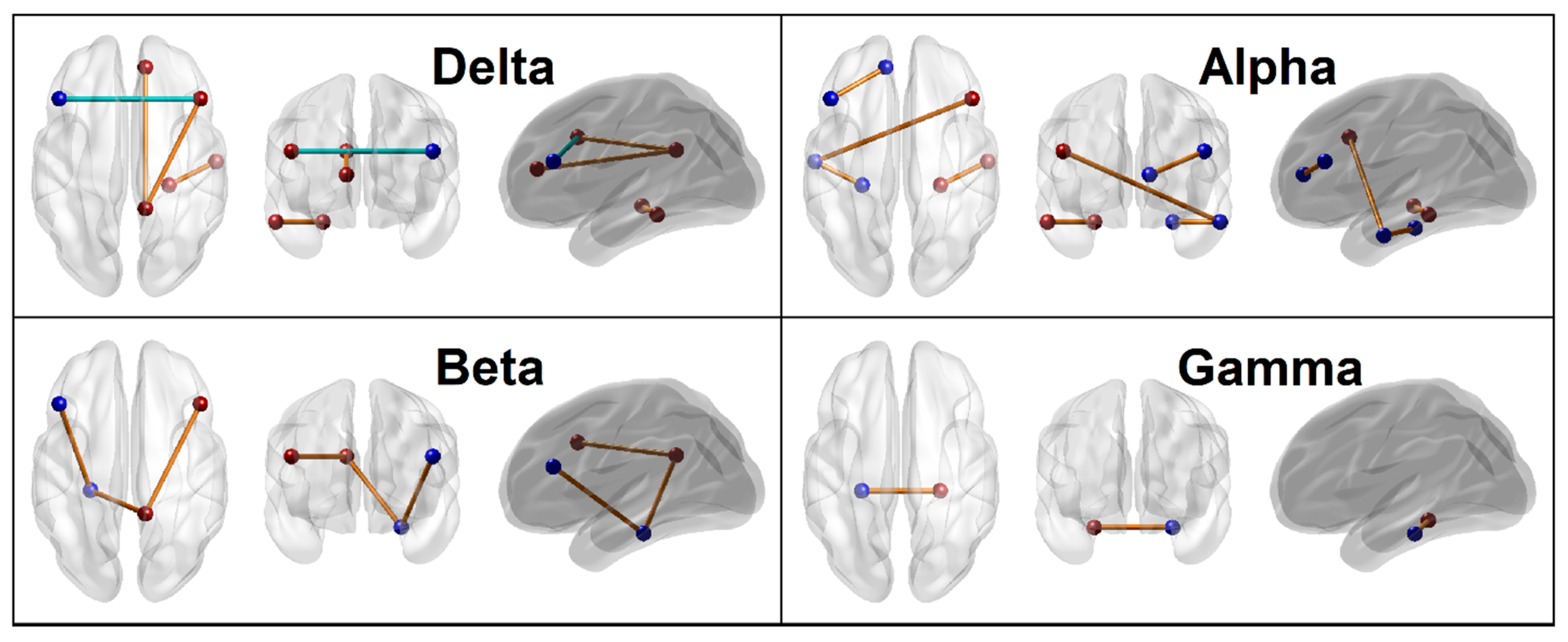
| Variable | AUD | CTL | ||||
|---|---|---|---|---|---|---|
| N* | Mean | SD | N* | Mean | SD | |
| Age (in years) | 30 | 41.42 | 7.31 | 30 | 27.44 | 4.74 |
| Education (in Years) | 30 | 11.93 | 2.35 | 30 | 15.77 | 1.87 |
| Age of onset (regular alcohol use) | 30 | 15.77 | 2.58 | 12 | 20.50 | 3.80 |
| Alcohol: Drinks/day (heaviest period of alcohol use) | 30 | 12.08 | 10.02 | 12 | 2.88 | 1.93 |
| Alcohol: Days/month (heaviest period of alcohol use) | 30 | 20.30 | 9.01 | 12 | 3.35 | 3.64 |
| Alcohol: Drinks (last 6 months) | 30 | 2.68 | 6.61 | 18 | 2.61 | 1.98 |
| Alcohol: Days (last 6 months) | 30 | 3.97 | 8.02 | 18 | 2.94 | 3.62 |
| Length of abstinence from drinking (in months) | 30 | 22.43 | 28.16 | 18 | 1.9 | 4.99 |
| Tobacco: Times/day (last 6 months) | 20 | 9.90 | 5.80 | 6 | 2.33 | 1.63 |
| Tobacco: Days/month (last 6 months) | 20 | 28.35 | 4.83 | 6 | 14.17 | 13.82 |
| Marijuana: Times in last 6 months | 10 | 98.80 | 91.38 | 4 | 18.75 | 27.61 |
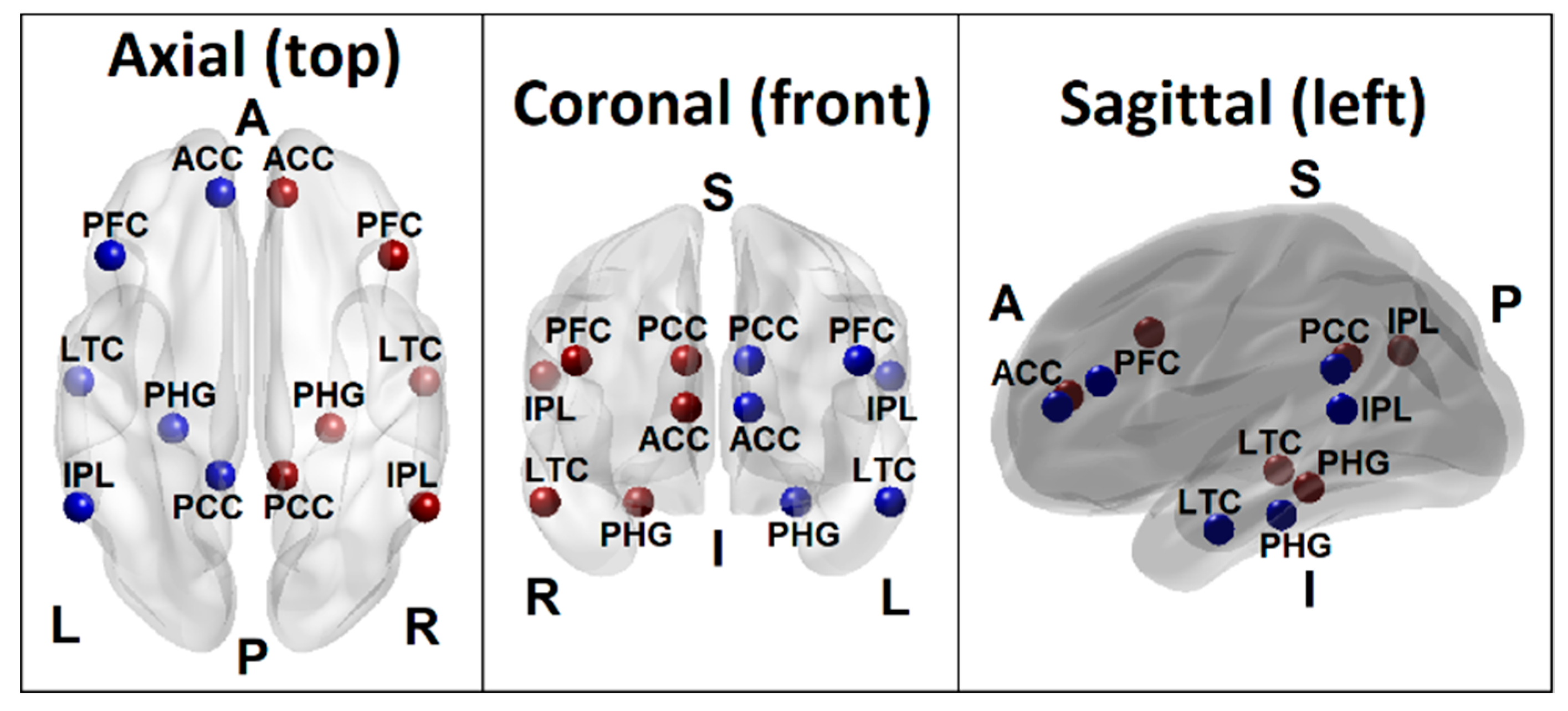
| Seed | Region Name | Region Code | BA | MNI (X) | MNI (Y) | MNI (Z) |
|---|---|---|---|---|---|---|
| s1 | Left posterior cingulate cortex | L.PCC | 23 | −10 | −45 | 25 |
| s2 | Right posterior cingulate cortex | R.PCC | 23 | 10 | −45 | 25 |
| s3 | Left anterior cingulate cortex | L.ACC | 32 | −10 | 45 | 10 |
| s4 | Right anterior cingulate cortex | R.ACC | 32 | 10 | 45 | 10 |
| s5 | Left inferior parietal lobule | L.IPL | 40 | −55 | −55 | 20 |
| s6 | Right inferior parietal lobule | R.IPL | 40 | 55 | −55 | 20 |
| s7 | Left prefrontal cortex | L.PFC | 46 | −45 | 25 | 25 |
| s8 | Right prefrontal cortex | R.PFC | 46 | 45 | 25 | 25 |
| s9 | Left lateral temporal cortex | L.LTC | 21 | −55 | −15 | −20 |
| s10 | Right lateral temporal cortex | R.LTC | 21 | 55 | −15 | −20 |
| s11 | Left parahippocampal gyrus | L.PHG | 36 | −25 | −30 | −20 |
| s12 | Right parahippocampal gyrus | R.PHG | 36 | 25 | −30 | −20 |
| Variable | Mean Minimal Depth | No. of Trees | No. of Nodes | Times a Root | Accuracy Decrease | Gini Decrease | p-Value | Direction |
|---|---|---|---|---|---|---|---|---|
| BIS_Tot | 1.0667 | 75 | 75 | 27 | 0.0129 | 1.2864 | <0.0000 | Alc > Ctl |
| Be_s7_s11 (L.PFC–L.PHG) | 2.1804 | 48 | 49 | 20 | 0.0052 | 0.6907 | <0.0000 | Alc > Ctl |
| BIS_MI | 2.3889 | 46 | 47 | 18 | 0.0059 | 0.6928 | <0.0000 | Alc > Ctl |
| BIS_NP | 2.7201 | 37 | 37 | 13 | 0.0016 | 0.5271 | <0.0000 | Alc > Ctl |
| De_s7_s8 (L.PFC–R.PFC) | 2.8268 | 37 | 37 | 10 | 0.0021 | 0.4859 | <0.0000 | Ctl > Alc |
| TotCor_Fw | 2.7327 | 34 | 35 | 17 | 0.0058 | 0.5137 | <0.0000 | Ctl > Alc |
| Al_s9_s11 (L.LTC–L.PHG) | 2.7503 | 33 | 33 | 18 | 0.0035 | 0.5437 | <0.0000 | Alc > Ctl |
| De_s2_s4 (R.PCC–R.ACC) | 3.1145 | 32 | 32 | 7 | 0.0034 | 0.3497 | <0.0000 | Alc > Ctl |
| Al_s3_s7 (L.ACC–L.PFC) | 3.3615 | 24 | 25 | 5 | 0.0007 | 0.3068 | <0.0000 | Alc > Ctl |
| Al_s10_s12 (R.LTC–R.PHG) | 3.3791 | 23 | 24 | 6 | 0.0023 | 0.2278 | 0.0001 | Alc > Ctl |
| BIS_AI | 3.3615 | 24 | 24 | 6 | 0.0022 | 0.2915 | 0.0001 | Alc > Ctl |
| Al_s8_s9 (R.PFC–L.LTC) | 3.3300 | 22 | 23 | 11 | 0.0015 | 0.2610 | 0.0002 | Alc > Ctl |
| De_s2_s8 (R.PCC–R.PFC) | 3.5566 | 22 | 22 | 2 | −0.0006 | 0.2333 | 0.0004 | Alc > Ctl |
| Ga_s11_s12 (L.PHG–R.PHG) | 3.5700 | 22 | 22 | 3 | 0.0011 | 0.2048 | 0.0004 | Alc > Ctl |
| Be_s2_s11 (R.PCC–L.PHG) | 3.5209 | 21 | 21 | 5 | 0.0004 | 0.1963 | 0.0010 | Alc > Ctl |
| Be_s2_s8 (R.PCC–R.PFC) | 3.5209 | 21 | 21 | 7 | 0.0007 | 0.2348 | 0.0010 | Alc > Ctl |
| De_s10_s12 (R.LTC–R.PHG) | 3.5475 | 21 | 21 | 4 | 0.0004 | 0.2319 | 0.0010 | Alc > Ctl |
| TotCorAvgTime_Fw | 3.5251 | 20 | 20 | 6 | 0.0005 | 0.1813 | 0.0023 | Alc > Ctl |
| Al_s7_s12 (L.PFC–R.PHG) | 3.6802 | 18 | 19 | 4 | 0.0011 | 0.1598 | 0.0049 | Alc > Ctl |
| Ga_s4_s5 (R.ACC–L.IPL) | 3.6268 | 18 | 19 | 6 | 0.0001 | 0.2065 | 0.0049 | Alc > Ctl |
| De_s6_s8 (R.IPL–R.PFC) | 3.6226 | 19 | 19 | 5 | 0.0009 | 0.1804 | 0.0049 | Ctl > Alc |
| Ga_s4_s9 (R.ACC–L.LTC) | 3.5826 | 19 | 19 | 3 | −0.0004 | 0.1558 | 0.0049 | Alc > Ctl |
| Al_s6_s9 (R.IPL–L.LTC) | 3.5068 | 18 | 18 | 9 | 0.0007 | 0.2273 | 0.0101 | Alc > Ctl |
| Th_s1_s11 (L.PCC–L.PHG) | 3.5868 | 18 | 18 | 4 | 0.0011 | 0.1981 | 0.0101 | Alc > Ctl |
| TotCor_Bw | 3.6177 | 17 | 17 | 5 | 0.0007 | 0.1670 | 0.0199 | Ctl > Alc |
| Be_s2_s7 (R.PCC–L.PFC) | 3.6044 | 17 | 17 | 6 | 0.0008 | 0.2282 | 0.0199 | Alc > Ctl |
| Be_s3_s12 (L.ACC–R.PHG) | 3.7644 | 17 | 17 | 3 | 0.0004 | 0.1473 | 0.0199 | Ctl > Alc |
| Be_s8_s9 (R.PFC–L.LTC) | 3.7377 | 17 | 17 | 0 | −0.0005 | 0.1421 | 0.0199 | Alc > Ctl |
| Th_s4_s11 (R.ACC–L.PHG) | 3.5510 | 17 | 17 | 7 | 0.0006 | 0.2227 | 0.0199 | Alc > Ctl |
| Th_s5_s8 (L.IPL–R.PFC) | 3.5910 | 17 | 17 | 6 | 0.0001 | 0.1889 | 0.0199 | Alc > Ctl |
| Th_s6_s11 (R.IPL–L.PHG) | 3.7244 | 17 | 17 | 4 | 0.0002 | 0.1411 | 0.0199 | Alc > Ctl |
| De_s8_s10 (R.PFC–L.PCC) | 3.7953 | 16 | 16 | 2 | −0.0009 | 0.1738 | 0.0371 | Ctl > Alc |
| Ga_s2_s8 (R.PCC–R.PFC) | 3.7153 | 16 | 16 | 3 | 0.0003 | 0.1712 | 0.0371 | Alc > Ctl |
| Ga_s3_s12 (L.ACC–R.PHG) | 3.7153 | 16 | 16 | 4 | 0.0005 | 0.1858 | 0.0371 | Alc > Ctl |
| Th_s2_s9 (R.PCC–L.LTC) | 3.8219 | 16 | 16 | 2 | 0.0006 | 0.1398 | 0.0371 | Alc > Ctl |
| Th_s8_s12 (R.PFC–R.PHG) | 3.7019 | 16 | 16 | 1 | 0.0005 | 0.1510 | 0.0371 | Alc > Ctl |
| Variable | AUD | CTL | ALL§ | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| BIS_Tot | 0.1602 | 0.3977 | 0.0317 | 0.8681 | 0.1177 | 0.3746 |
| BIS_MI | 0.2666 | 0.1543 | 0.0642 | 0.7361 | 0.2084 | 0.1132 |
| BIS_NP | 0.0331 | 0.8620 | 0.1458 | 0.4422 | 0.0724 | 0.5856 |
| BIS_AI | 0.1106 | 0.5608 | −0.1913 | 0.3113 | 0.0107 | 0.9360 |
| TotCor_Bw | −0.2701 | 0.1489 | −0.1263 | 0.5061 | −0.2290 | 0.0810 |
| TotCor_Fw | −0.4236 | 0.0197*○ | −0.0432 | 0.8206 | −0.2521 | 0.0541 |
| TotCorAvgTime_Fw | −0.0204 | 0.9147 | 0.1663 | 0.3799 | 0.0360 | 0.7866 |
| Al_s10_s12 (R.LTC–R.PHG) | 0.0608 | 0.7495 | 0.1184 | 0.5332 | 0.0691 | 0.6032 |
| De_s10_s12 (R.LTC–R.PHG) | 0.1868 | 0.3230 | −0.1175 | 0.5362 | 0.0172 | 0.8970 |
| De_s2_s4 (R.PCC–R.ACC) | −0.2006 | 0.2879 | −0.0383 | 0.8406 | −0.1578 | 0.2328 |
| De_s2_s8 (R.PCC–R.PFC) | 0.0516 | 0.7865 | −0.0467 | 0.8063 | 0.0030 | 0.9818 |
| De_s6_s8 (R.IPL–R.PFC) | 0.0688 | 0.7178 | −0.3452 | 0.0617 | −0.1581 | 0.2316 |
| De_s7_s8 (L.PFC–R.PFC) | 0.1907 | 0.3128 | −0.1755 | 0.3535 | −0.0493 | 0.7109 |
| De_s8_s10 (R.PFC–L.PCC) | 0.2100 | 0.2654 | −0.1116 | 0.5572 | −0.0111 | 0.9334 |
| Th_s1_s11 (L.PCC–L.PHG) | 0.1724 | 0.3624 | 0.1572 | 0.4067 | 0.1570 | 0.2350 |
| Th_s2_s9 (R.PCC–L.LTC) | 0.2825 | 0.1303 | 0.1024 | 0.5904 | 0.2351 | 0.0731 |
| Th_s4_s11 (R.ACC–L.PHG) | 0.0936 | 0.6226 | 0.0431 | 0.8212 | 0.0657 | 0.6213 |
| Th_s5_s8 (L.IPL–R.PFC) | 0.3338 | 0.0714 | −0.0117 | 0.9511 | 0.2095 | 0.1113 |
| Th_s6_s11 (R.IPL–L.PHG) | 0.3025 | 0.1042 | 0.0510 | 0.7891 | 0.2465 | 0.0599 |
| Th_s8_s12 (R.PFC–R.PHG) | 0.1858 | 0.3255 | −0.0576 | 0.7623 | 0.1254 | 0.3438 |
| Al_s3_s7 (L.ACC–L.PFC) | 0.0406 | 0.8312 | −0.1670 | 0.3778 | 0.0257 | 0.8466 |
| Al_s6_s9 (R.IPL–L.LTC) | 0.0335 | 0.8604 | 0.4156 | 0.0224*○ | 0.0672 | 0.6129 |
| Al_s7_s12 (L.PFC–R.PHG) | 0.3021 | 0.1047 | 0.0661 | 0.7285 | 0.2500 | 0.0562 |
| Al_s8_s9 (R.PFC–L.LTC) | −0.0817 | 0.6677 | 0.0147 | 0.9385 | −0.0666 | 0.6165 |
| Al_s9_s11 (L.LTC–L.PHG) | 0.1162 | 0.5409 | 0.1822 | 0.3353 | 0.1168 | 0.3782 |
| Be_s2_s11 (R.PCC–L.PHG) | 0.3870 | 0.0346*○ | 0.3622 | 0.0492*○ | 0.3802 | 0.0030**○ |
| Be_s2_s7 (R.PCC–L.PFC) | −0.1554 | 0.4122 | −0.0574 | 0.7630 | −0.1330 | 0.3151 |
| Be_s2_s8 (R.PCC–R.PFC) | −0.1615 | 0.3938 | 0.1613 | 0.3946 | −0.0907 | 0.4947 |
| Be_s3_s12 (L.ACC–R.PHG) | 0.3241 | 0.0806 | 0.0168 | 0.9298 | 0.1630 | 0.2174 |
| Be_s7_s11 (L.PFC–L.PHG) | 0.3038 | 0.1026 | −0.0195 | 0.9185 | 0.2373 | 0.0703 |
| Be_s8_s9 (R.PFC–L.LTC) | 0.0397 | 0.8348 | 0.1475 | 0.4365 | 0.0708 | 0.5941 |
| Ga_s11_s12 (L.PHG–R.PHG) | 0.1716 | 0.3644 | −0.3246 | 0.0801 | 0.0623 | 0.6392 |
| Ga_s2_s8 (R.PCC–R.PFC) | 0.1697 | 0.3700 | −0.3420 | 0.0643 | 0.1024 | 0.4401 |
| Ga_s3_s12 (L.ACC–R.PHG) | 0.3124 | 0.0928 | 0.2271 | 0.2275 | 0.2673 | 0.0407*○ |
| Ga_s4_s5 (R.ACC–L.IPL) | 0.0611 | 0.7485 | −0.2805 | 0.1333 | −0.0137 | 0.9182 |
| Ga_s4_s9 (R.ACC–L.LTC) | 0.2004 | 0.2884 | −0.2603 | 0.1647 | 0.1467 | 0.2676 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Chorlian, D.B.; Kinreich, S.; Pandey, G.; Meyers, J.L.; Zhang, J.; Kuang, W.; Stimus, A.T.; et al. Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Behav. Sci. 2020, 10, 62. https://doi.org/10.3390/bs10030062
Kamarajan C, Ardekani BA, Pandey AK, Chorlian DB, Kinreich S, Pandey G, Meyers JL, Zhang J, Kuang W, Stimus AT, et al. Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Behavioral Sciences. 2020; 10(3):62. https://doi.org/10.3390/bs10030062
Chicago/Turabian StyleKamarajan, Chella, Babak A. Ardekani, Ashwini K. Pandey, David B. Chorlian, Sivan Kinreich, Gayathri Pandey, Jacquelyn L. Meyers, Jian Zhang, Weipeng Kuang, Arthur T. Stimus, and et al. 2020. "Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures" Behavioral Sciences 10, no. 3: 62. https://doi.org/10.3390/bs10030062
APA StyleKamarajan, C., Ardekani, B. A., Pandey, A. K., Chorlian, D. B., Kinreich, S., Pandey, G., Meyers, J. L., Zhang, J., Kuang, W., Stimus, A. T., & Porjesz, B. (2020). Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Behavioral Sciences, 10(3), 62. https://doi.org/10.3390/bs10030062






