Abstract
Background/Objectives: Although numerous prognostic biomarkers have been proposed for colorectal cancer (CRC), their longitudinal evaluation remains limited. The aim of this study was to investigate longitudinal changes in biomarkers calculated from routinely used laboratory markers and their relationships to common chronic diseases (comorbidities). Methods: A retrospective longitudinal observational study was completed with the inclusion of 817 CRC patients and a total of 4542 measurement points. Pan-immune inflammation value (PIV), prognostic nutritional index (PNI), and systemic immune-inflammation index (SII) were calculated based on complete blood count and albumin measurement data. Results: Longitudinal data analyses confirmed the different values and slopes of the parameters tested at the different endpoints. Survivors had the lowest and most constant PIVs and SII values, and the highest and most slowly decreasing PNI values. Those patients with non-cancerous death had similar values to the previous cohort, but an increase/decrease occurred towards the death event. Patients with CRC-related death had significantly higher PIVs and SII values and significantly lower PNI values (p < 0.0001), and a significant increase/decrease was observed at the early observational periods. The presence of lymph node and/or distant metastases, adjuvant chemotherapy, and hypertension significantly affected PIVs and SII and/or PNI values. The changes in PIVs and SII and PNI values toward pathological values are poor prognostic signs (p < 0.0001). Conclusions: Each of the three calculated markers demonstrates suitability for longitudinal patient follow-up, and their pathological alterations over time serve as valuable prognostic indicators. They may also be useful to detect certain clinicopathological parameters early.
1. Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer type for females, males and for both sexes as well, with over 1.9 million new cases and over 900,000 deaths worldwide annually, making it the second leading cause of cancer deaths according to the GLOBOCAN 2022 data [1]. Despite the large number of known cases, to date, no perfect biomarker has been identified that can be used for diagnostic and/or prognostic purposes. In recent decades, a large variety of molecular and routinely used biomarkers have been proposed, including those derived, for example, from the ratios of already routinely used laboratory markers or personalized markers. The identification of such potential biomarkers is further complicated by the fact that the number of longitudinal studies in the literature with large enough sample sizes, long enough follow-up times, and with a sufficient number of biomarker measurements is scarce [2].
In the available literature, a large number of publications have previously addressed potentially predictive biomarkers derived from blood counts and/or other routine laboratory parameters. These biomarkers have the advantages of typically requiring low budgets, being used routinely, and not requiring the development/application of new/additional techniques. Furthermore, in many cases, these combinations can indirectly indicate complex systemic and/or physiological processes and/or interrelationships between these processes. Examinations of such markers can, for example, provide valuable information about, among other things, whether the patient’s condition is worsening because of the progression of the tumor or due to other conditions [3]. While in previous decades, markers such as platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio were more frequently examined [2,4,5,6,7], definitions of the pan-immune inflammation value (PIV) and the systemic immune-inflammation index (SII), which might give further data on the complex interactions within the tumor microenvironment, have recently appeared [8,9]. Moreover, the use of prognostic nutritional index (PNI) is also experiencing a renaissance [10,11,12]. It has to be mentioned though that essentially all previous studies investigated the effect of the latter three biomarkers of CRC in only single-timepoint analyses. Either a specific timepoint of interest (e.g., pre- or postoperatively, or after the first set of chemotherapy ± biological agents, etc.) or the difference between two timepoints (also known as “delta”) was investigated.
The incidence of CRC increases with age [13]. Similarly, the incidence of common chronic diseases, such as hypertension, type 2 diabetes mellitus, asthma, chronic obstructive pulmonary disease, obesity, and various cardiovascular diseases and/or events are also increasing with older age [14]. It is quite common for CRC patients to be diagnosed not only with CRC, but also with other, incidentally found comorbidities, or to have a known, pre-existing chronic disease [15,16,17]. Comorbidities are known to significantly affect CRC survival by contributing directly or indirectly to faster disease progression. Moreover, it has also been reported that comorbidities can prevent the use of the same oncological therapies, compared to CRC patients without any comorbidities [18,19]. However, the relationship between CRC and comorbidities is not totally negative. It should be emphasized that comorbidities can play a significant role in the early detection of CRC. Patients with various chronic diseases often report new symptoms to their doctor, which can significantly help with the earlier detection of the tumor [20].
The aim of this retrospective longitudinal observational study was to investigate the effects of the PVI, SII, PNI, and their changes throughout the disease on CRC survival. The secondary aim was to study the possible associations between these markers and the various clinicopathological features, the most common incidental and/or pre-existing comorbidities, and anamnestic data.
2. Materials and Methods
2.1. Patients and Study Design
A retrospective longitudinal observational study was conducted. Patient data was obtained from the medical database of Semmelweis University, Budapest. The list of possibly eligible patients was downloaded based on the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes C18 (malignant neoplasm of colon), C19 (malignant neoplasm of rectosigmoid junction), and C20 (malignant neoplasm of rectum). After the removal of ineligible cases, a total of 817 CRC patients’ anonymous and de-identified data was retrieved. All included CRC patients attended the outpatient clinics of the Department of Internal Medicine and Hematology, Semmelweis University, Budapest, and the Department of Internal Medicine and Oncology, Semmelweis University, Budapest, between 2006 and 2020. CRC was diagnosed using colonoscopy followed by the pathological confirmation of colorectal adenocarcinoma from biopsy specimens, and further imaging studies were performed to confirm metastases. Inclusion in the study required laboratory results performed at Semmelweis University at least at the time of CRC diagnosis. Exclusion criteria included age < 18 years; any previous malignancies; a histological CRC type other than adenocarcinoma; emergency cases such as perforation and/or obstruction due to CRC; known hematologic, inflammatory bowel, systemic autoimmune, mental, hepatic (other than Gilbert’s syndrome), acute infectious, and/or inadequately controlled thyroid diseases; known renal dysfunction; the usage of systemic corticosteroids 90 days prior to the baseline visit date; erythropoiesis-stimulating agents; and patients with an Eastern Cooperative Oncology Group (ECOG) performance status > 2 (Figure 1). This study was approved by the Regional and Institutional Committee of Science and Research Ethics, Semmelweis University (SE TUKEB 21-14/1994, approval date of latest modification: 23 February 2021). Handling of patient data was performed in accordance with the General Data Protection Regulation issued by the European Union, and in concordance with the WMA Declaration of Helsinki.
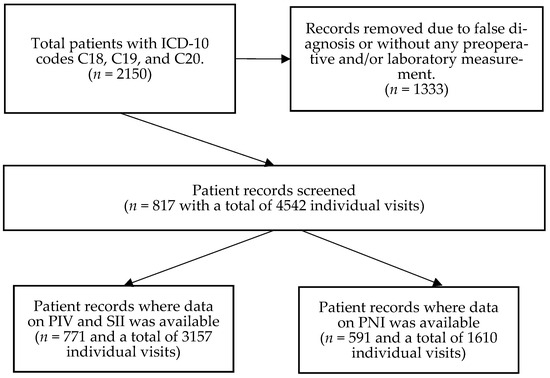
Figure 1.
After the removal of ineligible patients, a total of 817 colorectal cancer patients were identified, of whom 771 and 591 patients had at least one pan-immune inflammation (PIV) and systemic immune-inflammation index (SII) value, and prognostic nutritional index (PNI) value, respectively.
Patient visits to analyze were selected randomly. A total of 4542 visits (5.56 ± 3.93 visits/patient as average ± standard deviation) for the 817 patients were processed (Figure 1). PIVs and SII values could be calculated for 3157 of these 4542 visits (number of patients: 771; average visits: 4.09 ± 3.35), while PNIs were calculated for 1610 of the 4542 visits (number of patients: 591; average visits: 2.72 ± 2.46). Patients with a surgically resectable tumor had visits at the time of tumor diagnosis prior to any oncological procedures, at least 4–6 weeks after the primary tumor removal surgery and just before the first oncological treatment, and every 4–6 months thereafter. Patient visits with an irresectable tumor were recorded for every 4–6 months following the initial laboratory measurement performed at the time of CRC diagnosis. If a patient had no laboratory measurement(s) within the optimal window, the next measurement was recorded as close as possible. Laboratory measurements around recent blood transfusion(s) were omitted and another random visit was selected as close as possible.
2.2. Clinicopathological and Laboratory Data Measurements
Anamnestic data including disease history was collected at the time of the baseline visit. For appendicitis and appendectomy, and cholelithiasis and cholecystectomy, the surgery and condition events were combined, respectively. This had to be performed as a technical step because in several cases, the disease(s) could be managed conservatively, and in many cases, cholelithiasis was only detected during the preoperative imaging studies. Fasting blood measurement data, including complete blood count parameters, was recorded for every visit. All laboratory measurements were completed at the laboratories of Semmelweis University, Budapest. Staging was given by histopathological examination of surgical specimens and imaging studies; the American Joint Committee on Cancer (8th edition) grouping was used [21]. The location of CRC was described as rectal cancer, right-sided, left-sided, and at multiple sites, as described previously [22]. Chemotherapy was recorded as “adjuvant” if no distant metastasis by imaging was detected, and “palliative” if metastasis was present. Furthermore, the “palliative” group was further divided into “First-line”, “Second-line”, and “Third-line and above”. The usage of biological agents—such as bevacizumab or cetuximab—and oral regorafenib/trifluridine–tipiracil was recorded as a binary variable. Survival times were calculated from the time of CRC diagnosis until the patient’s death or until the termination of data collection (31 December 2024). Both overall survival (OS) and disease-specific survival (DSS) were calculated. In the case of the latter, tumor-related and -unrelated deaths were recorded as separate events. Patients alive at the end of the observational period were right-censored. The PIVs and SII and PNI values were calculated as described previously (Equations (1)–(3)) [8,9,23]. Moreover, PNI was categorized, based on its values, as normal, mild, moderate, and severe malnutrition if the values were ≥50, 50–45, 45–40, and <40, respectively, as determined previously [23,24]. Based on patients’ PNI values, the PNI grouping varied dynamically from visit to visit (if needed) and was not fixed per patient.
2.3. Statistical Analysis
Statistical analysis was performed within the R for Windows version 4.5.0 environment (R Foundation for Statistical Computing, 2025, Vienna, Austria). Natural-cubic-spline-adjusted random intercept linear mixed effect models were constructed to determine parameter changes with the course of the disease [R packages “nlme” (version 3.1-168) and “splines” (version 4.5.0)]. Two types of survival models were used. Simple competing risk models were used to investigate cumulative incidences, while for survival data associated with longitudinal data, competing risk survival models with a time-dependent covariate were applied (R package “survival”, version 3.8-3). p < 0.05 was considered statistically significant. Continuous data was reported as mean ± standard deviation, while the number of occurrences and their percentages in parentheses characterized the frequency data. Survival data was presented as the hazard ratio (HR) and its 95% confidence interval (95%CI). Figures were created using the R packages “survminer” (version 0.5.0) and “lattice” (version 0.22-7).
3. Results
As detailed in Methods, a total of 771 and 591 of the 817 patients with 3157 and 1610 individual visits had data available on their PIV/SII and PNI, respectively. The average age of the patients at the time of diagnosis was 65 ± 11 years, and there were no sex differences. There was a slight male dominance (56% vs. 44%). Since the study subjects’ comorbidities and relevant medical history were an important part of the study, recording the presence of the following comorbidities was of paramount importance: type 2 diabetes mellitus; thyroid diseases (e.g., Hashimoto’s hypothyroidism); minor and major cardiovascular (CV) diseases, such as hypertension, myocardial infarction, and stroke; and the presence/history of appendicitis and/or cholelithiasis with or without appendectomy and/or cholecystectomy. The complete list of anamnestic data of the whole, PIV/SII, and PNI cohorts can be seen in Table 1.

Table 1.
Complete anamnestic data of the study cohorts. Continuous, count, and survival data is presented as mean ± standard deviation, number of observations (percentage), and median survival time (95% confidence interval), respectively.
3.1. Longitudinal Data Analysis Results
For the 771 and 591 patients with PIV/SII and PNI data available, an average of 4.09 ± 3.35 and 2.72 ± 2.46 visits per patient with a mean follow-up time of 75.25 ± 56.40 months and 75.81 ± 56.68 months were found, respectively. First, it was investigated whether the PIVs and SII, and PNI values differed between those patients who were (1.) alive, (2.) dead due to CRC-related reasons, or (3.) dead due to non-cancerous reasons at the end of the observation period. Both the PIVs and SII values were constant in the surviving patients, while the PNI values slowly decreased. CRC-related death was associated with constant, significantly higher PIVs (p < 0.0001) and SII (p < 0.0001) values, and significantly lower PNI (p < 0.0001) values. The PIVs (p = 0.5487) and SII (p = 0.5932) and PNI (p = 0.1306) values of those patients who also died, but not due to CRC, were more similar to those of the survivors, showing no significant changes. It should be highlighted, however, that a significant slope of change in the values of the indices was observed in both deceased cohorts. While this increase occurred more often in the first half of the study in patients who died of CRC (PIV: p = 0.0277; SII: p = 0.0179; PNI: p = 0.0228), it was more pronounced in the second half of the study in patients who died of other causes (PIV and SII: statistically not significant; PNI: p = 0.0068; Figure 2). To gather more information about this phenomenon, the cumulative incidence of the two endpoints was investigated using competing risk survival models. The results of the latter model were in line with the former observation: death due to CRC occurred more often in the earlier stages of the study, while other deaths occurred more frequently in the later stages (Figure 3).
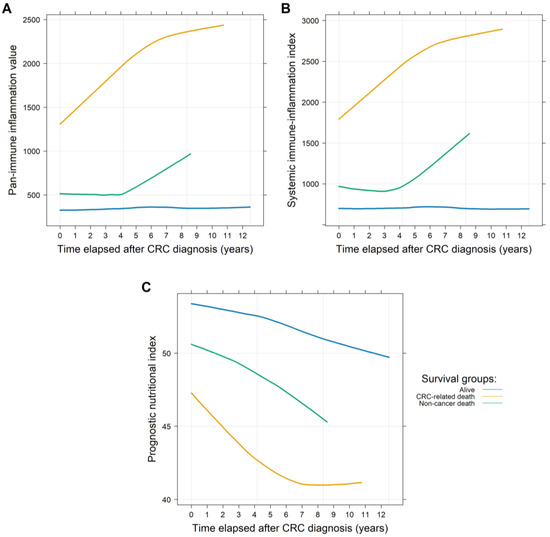
Figure 2.
Longitudinal changes in the pan-immune inflammation (PIV; (A)), the systemic immune-inflammation index (SII; (B)), and the prognostic nutritional index (PNI; (C)) values throughout the observational period of the study. Higher PIVs and SII values and lower PNI values are associated with worse clinical outcomes. Those patients who died due to colorectal cancer (CRC) had significantly higher PIVs and SII values and lower PNI values throughout the entire study. Those patients who died due to other reasons or were still alive at the end of the observational period had more constant and similar values, compared to those who died due to CRC.

Figure 3.
Cumulative incidences of two competing events throughout the observational period of this study. It was found that death due to colorectal cancer (CRC) occurred more frequently in the earlier part of the study, while death due to other causes was more frequent in the later period of the study.
The secondary question(s) of our study was whether the various comorbidities and/or clinicopathological parameters affected PIVs and SII and/or PNI values (Table 2). PIVs were significantly lower in females (p = 0.0286; Figure 4A). Stage IV CRC (at the time of diagnosis) caused a constant significant increase in PIVs (p < 0.0001) and SII values (p < 0.0001) and a significant decrease in PNI (p < 0.0001) values throughout the observation period, compared to those study participants with stage I-III disease. Both regional lymph node and synchronous distant metastases significantly affected the indices (p < 0.001). In the case of metachronous metastases, SII and PNI values were significantly higher (p = 0.0396) and lower (p = 0.0191), respectively, and only a marginal increase in PIVs (p = 0.0911) was observed. SII values were significantly higher in the case of rectal tumor (p = 0.0147). The various anti-cancer therapies had the following effects: Those patients receiving adjuvant chemotherapy had significantly better PIVs and SII and PNI values (p < 0.0001) compared to those receiving palliative chemotherapy or no chemotherapy at all. The use of biological agents was also associated with higher PIVs and SII values (Table 2).

Table 2.
p-values obtained from univariate longitudinal mixed effect models investigating whether any comorbidities and/or clinicopathological parameters related to colorectal cancer affected the pan-immune inflammation (PIV), prognostic nutritional index (PNI), or the systemic immune-inflammation index (SII) values.
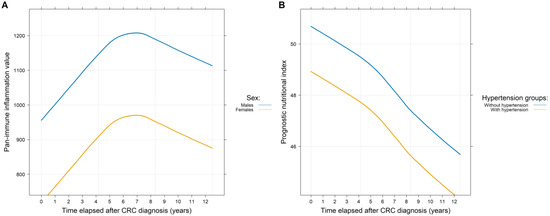
Figure 4.
Throughout the whole observation period of the study, there was a significant difference between the pan-immune inflammation (A) and the prognostic nutritional index (B) values of the two sexes and those patients with and without hypertension, respectively.
We examined the impact of comorbidities by analyzing each comorbidity separately and in combination as well. The following combinations were investigated: major CV events, all CV diseases excluding hypertension, and any comorbidities versus no comorbidities. For PIVs and SII values, no differences between the diseased and non-diseased groups could be demonstrated for either individual comorbidities or combinations. In contrast, the PNI was significantly worsened by the co-existence of hypertension (p = 0.0076; Figure 4B) and the occurrence of transient ischemic attack/lacunar stroke (p = 0.0229) and/or (pulmonary) embolism (p = 0.0292) in the patient’s medical history. A reduced PNI value was observed with older age (p < 0.0001) and when comparing the diseased and non-diseased groups (p = 0.0005; Table 2).
3.2. Survival Analysis Results
Of the 771 patients for whom PIV/SII data was available, 282, 363, and 126 patients were alive at the end of the follow-up period, died from CRC, and died from other causes, respectively. Similar rates were found for the 591 patients with PNI data. Here 211, 285, and 95 subjects were alive, died due to CRC, and died due to other causes, respectively. First, the univariate effects of PIV, SII, and PNI changes over patients’ DSS were investigated. Increasing PIVs (HR: 1.00023; 95% CI: 1.00017–1.00029; p < 0.0001) and SII values (HR: 1.00019; 95% CI: 1.00015–1.00023; p < 0.0001; Figure 5B) and decreasing PNI (HR: 0.8797; 95% CI: 0.8543–0.9059; p < 0.0001) values are poor prognostic markers of shorter survival times. Moreover, the PNI data was analyzed by using the PNI category grouping. Compared to those patients who had normal PNI values, those with mild, moderate, and severe malnutrition had an increased hazard ratio of 4.7756 (95% CI: 2.4314–9.3800; p < 0.0001), 9.9865 (95% CI: 4.7315–21.0776; p < 0.0001), and 57.3284 (95% CI: 30.2639–108.5961; p < 0.0001), respectively (Figure 5A). The same observations were found for OS, with HR and 95% CI values changing only slightly.
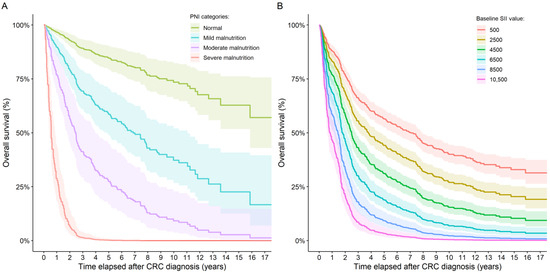
Figure 5.
Overall survival curves for (A) patients with different SII values and (B) patients in the four PNI categories. No group number could be given for the PNI categories because dynamic group switching between the four groups was allowed in the model. The SII values shown in the figure are hypothetical values only.
The DSS of patients was investigated in a multivariate setting as well. As the traditional factors for CRC significantly reduced any impact of comorbidities on survival, two sets of multivariate survival models were created. First, PIV, SII, and PNI were combined with sex and the various comorbidities. Each of the three study targets was shown to have a significant effect on survival. Of the parameters tested in the models, hypertension had a more significant effect, as did the gender of the patients in the SII model (Table 3). Second, a combination of CRC-specific parameters and the presence of comorbidities as a dummy variable were examined. As an expected result, higher disease stages and older age were significant predictors of shorter survival times. The same was true for higher PIVs and SII values and lower PVI values; all three were associated with worse patient survival. Moreover, the location of the primary tumor and the presence of any comorbidities were also poor prognostic signs in the SII and PNI models, respectively (Table 4).

Table 3.
p-value results of three multivariate survival models, where the three study targets were combined with sex and data on comorbidities and medical history.

Table 4.
p-value results of three multivariate survival models, where the three study targets were combined with the most commonly used colorectal-cancer-related clinicopathological parameters and a binarized version of data on comorbidities and medical history.
4. Discussion
Inflammation and immune response play crucial roles in the pathology, development, progression, and metastatic spread of CRC [25,26]. The applicability of several immune and/or inflammatory biomarkers has been investigated in recent years/decades. These biomarkers are often selected from among parameters already used in routine diagnostics, but their combination/ratios are used. By using such indices, one can generate specific ratios whose shift in one direction or the other can be an indirect pathological indicator of certain physiological processes., e.g., the lymphocyte-to-monocyte ratio can be used to gather information on whether the patient’s condition is worsening because of the progression of the tumor or due to other conditions [3]. Even though these markers can provide indirect information only about the tumorous disease(s) and their relationship between cancer and inflammation and/or immunity, they have been shown to be very good predictors over the past decades and have been used in a wide range of studies. But there are still blank spots in our knowledge. One of these is that well-detailed longitudinal analyses about these biomarkers are scarce. Most of the complete blood count index-based biomarkers have been shown to have a distinct prognostic value at diagnosis, before or after surgery, before or after chemo, before or after immunotherapy, etc. In fact, they often even show very strong associations with other clinicopathological markers. However, the accuracy of these predictions is somewhat biased, as the measurements and conclusions are usually taken from a single timepoint only or are based on “delta” values. To overcome this bias, longitudinal analyses are suggested. For example, in a previous publication [2], our group reported that some of the complete blood count index-based calculated biomarkers had different predictive powers at various stages of the disease. With the same in mind, in the current study, we investigated how PIVs and SII and PNI values change with the course of CRC.
The PIV is the youngest of the three biomarkers and was first described in CRC [8]. It was developed with the aim of providing indirect but sufficiently accurate information on both the inflammatory and immune processes that occur alongside tumorous diseases, and of integrating several already known pro-inflammatory, tumor microenvironment-associated, and/or immune-inflammatory markers [8,27]. Since then, its predictive capabilities have been demonstrated in a few other cancerous and non-cancerous diseases [28,29,30]. In CRC, a high vs. low PIV is associated with a two-times higher OS and progression-free survival (PFS) [31,32]. The PIV is a good predictor of pre-, peri-, and postoperative indicators [33,34,35], but some conflicting results have also been published [36]. In relation to the clinicopathological parameters of CRC, it was reported previously that the PIV was a good marker that aligned with clinical stage [37], and age ≥ 65 years, distant metastases, KRAS wild type, and deficiency in DNA mismatch repair were associated with higher PIVs [38]. The association with distant metastases and clinical staging was described in the current study as well. A novel observation from our results is that, to our knowledge, no previous study has investigated the longitudinal change in PIV in such detail. It was described that the PIVs of surviving patients did not change with the course of the disease, while the PIVs of deceased patients were significantly higher. Moreover, an early PIV increase was more characteristic for those patients who died due to CRC-related causes, and a later PIV increase was more frequent for those who died due to non-cancerous reasons. In the research of Pérez-Martelo et al. [39] and Corti et al. [40], the authors found that the introduction of chemotherapy or immunotherapy reduces, while disease progression increases the PIV [39,40], which was also observed in the current study. We identified significantly lower PIVs in female CRC patients throughout the whole study, which is in line with the study by Seo et al. [35], but is in contrast with most of the previous studies [8,28,29,37,39,40]. We hypothesize that the constant lower PIVs of females that we observed might have occurred due to the known sexual dimorphism associated with CRC, including but not limited to the protective effect of estrogen on systemic inflammation and the gut microbiome [41]. In addition to all of the above, we found that none of the investigated comorbidities or relevant medical history events had any effect on CRC patients’ PIVs.
Similarly to PIV, SII is also an indirect immune-inflammatory biomarker, which is also found to be highly associated with OS, PFS, recurrence-free survival, and other survival indicators [42,43,44,45], independent of the anti-cancer treatment the patients receive [46,47,48]. An increase in SII following tumor removal surgery is associated with worse survival prognosis [49,50] and with more frequent postoperative complications [51,52,53]. Higher SII levels are associated with higher TNM stages, higher tumor grades, sarcopenia, KRAS mutations, lymphovascular invasion, older age, and right-sided CRC [38,44,54,55,56,57,58,59]. It is also suggested that the SII can be predictive in the early detection of CRCs or can even distinguish between CRC and adenomatous polyps [58,60,61]. In the current study, the same situation could be described for SII as for PIV, detailed above. It showed the same changes in the survivor, tumor, and other cause of death cohorts as the PIV, and higher SII values were associated with more advanced tumor stages and with rectal cancer. The dynamic change undergoing/following chemotherapy was in line with a previous study’s results [62]. No relationship with any of the comorbidities and/or medical history events investigated were identified.
PNI has been investigated in various cancerous diseases, including CRC, since the 1980s [63]. It was described early in the literature that the chronic systemic inflammation caused by cancer negatively affects the nutritional status of the patients [64]; therefore, PNI can also be used as a biomarker that can give indirect information about both the inflammatory and the nutritional status of cancer patients. Similarly to the previous two immune-inflammatory indices, a pathological and/or lower PNI value is known to be associated with worse survival outcomes [11,65,66,67,68], with significantly more postoperative complications [69], and with older age and worst clinicopathological parameters [67,68,70]. These observations could be repeated in the current study as well. Moreover, we found that CRC survivors had a very slow decrease in PNI value, similar to what is known to occur in healthy people [71]. In contrast, the PNI values decreased significantly in those patients who died throughout our study. The decrease was more intense in the first part of our observational period when the death was due to CRC, and a much smaller and later decrease was found in those who died due to non-cancerous causes. We also found that those CRC patients who had co-existing hypertension had constantly lower PNI levels throughout the study. PNI and hypertension have a known relationship [72,73], and we hypothesize that the observation we described might occur due to the following process: It is known that CRC can often be associated with hypoalbuminemia [74] and lymphopenia [75]. Both CRC and hypertension are known to cause chronic systemic inflammation, which can result in an inadequate malnutritional status [76]. This state is often undiagnosed, making it a silent but serious health risk, especially in people with chronic diseases [77]. And when the two diseases co-exist, these self-exciting processes can create a vicious cycle that eventually leads to even further decline, which can also be influenced by the further negative effects of chemotherapy.
Limitations of This Study
This study has some limitations, starting with the retrospective design and the fact that the data was obtained from a single center only, which limits generalizability and might have introduced biases regarding the selection of cases, incomplete data, and limited control over confounders. The retrospective design has influenced our ability to further analyze the effects of other clinical parameters and their relationship with the PIV, SII, and PNI. Additional limiting factors were as follows: (1.) Not every laboratory measurement from the patients could be downloaded from the medical system, only randomly selected ones. (2.) Due to certain infrastructure and funding changes, not all laboratory measurements were available for all visits. To reduce biases connected to the latter two factors, we chose statistical methods that could robustly address the problem of missing values. (3.) Data on surgical details (type of operation, Clavien Dindo Classification categories, etc.) could not be collected, as a large number of patients were operated on at another institute. (4.) Although the literature data suggests that poor differentiation is significantly associated with systemic inflammation and high SII values [43,78], no data on histologic grading was collected.
5. Conclusions
In summary, our findings revealed that the PIV, SII, and PNI are potentially good indicators of patient survival in CRC at any given point throughout the course of the disease. We believe that the future use of the PIV, SII, and PNI would fit into the current treatment structure in a way that would complement rather than replace any other traditional routine markers, such as CEA, CA19-9, etc. It has to be highlighted, however, that due to the limitations of our study, such as the single-center retrospective design, the results presented are not fully generalizable; therefore, their use in everyday oncological practice should be further studied, possibly in a prospective multi-center study. There is a complex relationship between the various complete blood count indices, immune-inflammatory biomarkers, and CRC, and the tendencies indicated by the PIV, SII, and PNI suggest that further parameters and/or their combination, such as the measurement of macrophages and myeloid-derived suppressor cells, should be investigated as well. Our finding that PNI, CRC, and hypertension have a strong association warrants that cardiometabolic and/or nutritional adjustments are necessary prior any oncological interventions, possibly as a new oncological algorithm. Overall, we recommend the development of complex oncological treatment programs involving other specialists, which will ultimately help to detect changes in the disease state as early as possible.
Author Contributions
Conceptualization, Z.H., M.H. and A.M.S.; methodology, M.H. and A.M.S.; formal analysis, Z.H.; investigation, M.H., G.S., R.S. and Z.H.; resources, G.S., J.L., A.S., A.M.S. and M.D.; data curation, Z.H. and M.H.; writing—original draft preparation, Z.H. and M.H.; writing—review and editing, G.S., R.S., J.L., A.S., A.M.S. and M.D.; visualization, Z.H.; supervision, A.M.S. and M.D.; project administration, Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
A.M.S. was supported by the NKKP ADVANCED 150998 grant from the National Research, Excellence and Innovation Office of Hungary.
Institutional Review Board Statement
This study was approved by the Regional and Institutional Committee of Science and Research Ethics, Semmelweis University (SE TUKEB 21-14/1994, approval date of latest modification: 23 February 2021).
Informed Consent Statement
Patient data was retrieved anonymously from the medical database of Semmelweis University in a retrospective manner. A general consent form for academic assessment studies of all admitted patients was signed upon their treatment in the relevant years. Signed patient informed consent for further research was not required at that time given the anonymized, de-identified data.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AJCC | American Joint Committee on Cancer |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| CV | Cardiovascular |
| DSS | Disease-specific survival |
| OS | Overall survival |
| PFS | Progression-free survival |
| PIV | Pan-immune inflammation value |
| PNI | Prognostic nutritional index |
| SII | Systemic immune-inflammation index |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Herold, Z.; Herold, M.; Lohinszky, J.; Szasz, A.M.; Dank, M.; Somogyi, A. Longitudinal changes in personalized platelet count metrics are good indicators of initial 3-year outcome in colorectal cancer. World J. Clin. Cases 2022, 10, 6825–6844. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Hu, X.; Gao, T.; Zhou, H.; Li, B.; Zhou, C.; Yu, B.; Wang, G. Potential impact of platelet-to-lymphocyte ratio on prognosis in patients with colorectal cancer: A systematic review and meta-analysis. Front. Surg. 2023, 10, 1139503. [Google Scholar] [CrossRef] [PubMed]
- Naszai, M.; Kurjan, A.; Maughan, T.S. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 5983–5997. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Bartolotta, P.; Azzolina, D.; Gregori, D.; Fiscon, V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: Systematic review and meta-analysis. Langenbecks Arch. Surg. 2023, 408, 85. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef]
- Fuca, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: A systematic review and meta-analysis. Int. J. Color. Dis. 2019, 34, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, P.; Carkman, M.S. The relationship between the Prognostic Nutritional Index and lymphovascular and perineural invasion of the tumor in patients diagnosed with gastric cancer, and its effect on overall survival. Medicine 2024, 103, e40087. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Saliba, W.; Schwartz, J.B. Prevalence and proportion by age and sex of chronic health conditions in a large healthcare system. PLoS ONE 2024, 19, e0308031. [Google Scholar] [CrossRef]
- Pennisi, F.; Buzzoni, C.; Russo, A.G.; Gervasi, F.; Braga, M.; Renzi, C. Comorbidities, Socioeconomic Status, and Colorectal Cancer Diagnostic Route. JAMA Netw. Open 2025, 8, e258867. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, L.; Zhou, L.; Wang, X. Comorbidity Patterns in Patients Newly Diagnosed with Colorectal Cancer: Network-Based Study. JMIR Public Health Surveill. 2023, 9, e41999. [Google Scholar] [CrossRef]
- Rashmi, R.; Mohanty, S.K. Examining chronic disease onset across varying age groups of Indian adults using competing risk analysis. Sci. Rep. 2023, 13, 5848. [Google Scholar] [CrossRef]
- Cuthbert, C.A.; Hemmelgarn, B.R.; Xu, Y.; Cheung, W.Y. The effect of comorbidities on outcomes in colorectal cancer survivors: A population-based cohort study. J. Cancer Surviv. 2018, 12, 733–743. [Google Scholar] [CrossRef]
- Quintana, J.M.; Anton-Ladislao, A.; Lazaro, S.; Gonzalez, N.; Bare, M.; Fernandez-de-Larrea, N.; Redondo, M.; Escobar, A.; Sarasqueta, C.; Garcia-Gutierrez, S.; et al. Effect of comorbidities on long-term outcomes of colorectal cancer patients. Eur. J. Cancer Care 2022, 31, e13561. [Google Scholar] [CrossRef]
- Salika, T.; Lyratzopoulos, G.; Whitaker, K.L.; Waller, J.; Renzi, C. Do comorbidities influence help-seeking for cancer alarm symptoms? A population-based survey in England. J. Public Health 2018, 40, 340–349. [Google Scholar] [CrossRef]
- Jessup, J.; Goldberg, R.; Asare, E.; Benson, A.; Brierley, J.; Chang, G.; Chen, V.; Compton, C.; De Nardi, P.; Goodman, K.; et al. Colon and Rectum. In AJCC Cancer Staging Manual, 8th ed.; Amin, M., Edge, S., Greene, F., Byrd, D., Brookland, R., Washington, M., Gershenwald, J., Compton, C., Hess, K., Sullivan, D., et al., Eds.; Springer International Publishing: Chicago, IL, USA, 2018; pp. 251–274. [Google Scholar]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. 2018, 11, 264–273. [Google Scholar] [CrossRef]
- Pinato, D.J.; North, B.V.; Sharma, R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI). Br. J. Cancer 2012, 106, 1439–1445. [Google Scholar] [CrossRef]
- Tohme, S.; Chidi, A.P.; Sud, V.; Tsung, A. Prognostic Nutritional Index Is Associated with Survival in Patients with Unresectable Hepatocellular Carcinoma Treated with Radioembolization. J. Vasc. Interv. Radiol. 2017, 28, 470–472. [Google Scholar] [CrossRef]
- Burgos-Molina, A.M.; Tellez Santana, T.; Redondo, M.; Bravo Romero, M.J. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, L.; Gu, P.; Hua, Y.; Sun, Y.; Ni, S.; Zhou, X.; Tang, Z. Pan-immune-inflammation and its dynamics: Predictors of survival and immune-related adverse events in patients with advanced NSCLC receiving immunotherapy. BMC Cancer 2023, 23, 944. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, Q.; Li, Y. The association between pan-immune-inflammation value and chronic obstructive pulmonary disease: Data from NHANES 1999-2018. Front. Physiol. 2024, 15, 1440264. [Google Scholar] [CrossRef]
- Susok, L.; Said, S.; Reinert, D.; Mansour, R.; Scheel, C.H.; Becker, J.C.; Gambichler, T. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J. Cancer Res. Clin. Oncol. 2022, 148, 3103–3108. [Google Scholar] [CrossRef]
- Yang, X.C.; Liu, H.; Liu, D.C.; Tong, C.; Liang, X.W.; Chen, R.H. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1036890. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Zhang, Z.; Wang, K.; Sulayman, S.; Zeng, X.; Ababaike, S.; Guan, J.; Zhao, Z. Preoperative pan-immuno-inflammatory values and albumin-to-globulin ratio predict the prognosis of stage I-III colorectal cancer. Sci. Rep. 2025, 15, 11517. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Bae, S.U.; Kim, K.E.; Jeong, W.K.; Baek, S.K. Effects of the Strength, Assistance in walking, Rise from a chair, Climb stairs, and Falls score on postoperative clinical outcomes following colorectal cancer surgery: A retrospective study. Eur. J. Clin. Nutr. 2025, 79, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shimizu, T.; Ishizuka, M.; Suda, K.; Shibuya, N.; Hachiya, H.; Iso, Y.; Takagi, K.; Aoki, T.; Kubota, K. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I-III colorectal cancer patients undergoing surgery. Surg. Today 2022, 52, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, K.E.; Jeong, W.K.; Baek, S.K.; Bae, S.U. Effect of preoperative pan-immune-inflammation value on clinical and oncologic outcomes after colorectal cancer surgery: A retrospective study. Ann. Surg. Treat. Res. 2024, 106, 169–177. [Google Scholar] [CrossRef]
- Sato, R.; Oikawa, M.; Kakita, T.; Okada, T.; Abe, T.; Tsuchiya, H.; Akazawa, N.; Ohira, T.; Harada, Y.; Okano, H.; et al. A decreased preoperative platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value are associated with the poorer survival of patients with a stent inserted as a bridge to curative surgery for obstructive colorectal cancer. Surg. Today 2023, 53, 409–419. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Zhang, W.; Cheng, D.; Lu, Y.; Wang, C.; Li, J.; You, L.; Yu, J.; Guo, W.; et al. Pan-immune-inflammation value is associated with the clinical stage of colorectal cancer. Front. Surg. 2022, 9, 996844. [Google Scholar] [CrossRef]
- Liang, L.; Guo, X.; Ye, W.; Liu, Y. KRAS Gene Mutation Associated with Grade of Tumor Budding and Peripheral Immunoinflammatory Indices in Patients with Colorectal Cancer. Int. J. Gen. Med. 2024, 17, 4769–4780. [Google Scholar] [CrossRef]
- Perez-Martelo, M.; Gonzalez-Garcia, A.; Vidal-Insua, Y.; Blanco-Freire, C.; Brozos-Vazquez, E.M.; Abdulkader-Nallib, I.; Alvarez-Fernandez, J.; Lazare-Iglesias, H.; Garcia-Martinez, C.; Betancor, Y.Z.; et al. Clinical significance of baseline Pan-Immune-Inflammation Value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci. Rep. 2022, 12, 6893. [Google Scholar] [CrossRef]
- Corti, F.; Lonardi, S.; Intini, R.; Salati, M.; Fenocchio, E.; Belli, C.; Borelli, B.; Brambilla, M.; Prete, A.A.; Quara, V.; et al. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2021, 150, 155–167. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Menyhart, O.; Fekete, J.T.; Gyorffy, B. Inflammation and Colorectal Cancer: A Meta-Analysis of the Prognostic Significance of the Systemic Immune-Inflammation Index (SII) and the Systemic Inflammation Response Index (SIRI). Int. J. Mol. Sci. 2024, 25, 8441. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, B.; Li, Q.; Cao, W. Prognostic value and clinicopathological significance of pre-and post-treatment systemic immune-inflammation index in colorectal cancer patients: A meta-analysis. World J. Surg. Oncol. 2025, 23, 11. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Muntean, C.; Bolboaca, S.D.; Olariu, S. Exploratory Evaluation of Pre-Treatment Inflammation Profiles in Patients with Colorectal Cancer. Diseases 2024, 12, 61. [Google Scholar] [CrossRef]
- Sun, J.; Yang, R.; Wu, H.; Li, L.; Gu, Y. Prognostic value of preoperative combined with postoperative systemic immune-inflammation index for disease-free survival after radical rectal cancer surgery: A retrospective cohort study. Transl. Cancer Res. 2024, 13, 371–380. [Google Scholar] [CrossRef]
- Malik, M.; Radecka, B.; Gelej, M.; Jackowska, A.; Filipczyk-Cisarz, E.; Zurowska, M.; Hetman, K.; Foszczynska-Kloda, M.; Kania-Zembaczynska, B.; Manka, D.; et al. Predictive and Prognostic Role of Systemic Immune-Inflammation Index (SII) in Metastatic Colorectal Cancer Patients Treated with Trifluridine/Tipiracil. Biomedicines 2024, 12, 2076. [Google Scholar] [CrossRef]
- Popovici, D.; Stanisav, C.; Sima, L.V.; Negru, A.; Murg, S.I.; Carabineanu, A. Influence of Biomarkers on Mortality among Patients with Hepatic Metastasis of Colorectal Cancer Treated with FOLFOX/CAPOX and FOLFIRI/CAPIRI, Including Anti-EGFR and Anti-VEGF Therapies. Medicina 2024, 60, 1003. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Azzali, I.; Bittoni, A.; Marisi, G.; Rebuzzi, F.; Molinari, C.; Bartolini, G.; Matteucci, L.; Sullo, F.G.; Debonis, S.A.; et al. Inflammatory indices as prognostic markers in metastatic colorectal cancer patients treated with chemotherapy plus Bevacizumab. Ther. Adv. Med. Oncol. 2023, 15, 17588359231212184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, H.; Guo, X.; Gu, K.; Wang, W.; Chen, X.; Ji, S.; Yang, H.; Zhu, J. The Change of Systemic Immune-Inflammation Index Independently Predicts Survival of Colorectal Cancer Patients after Curative Resection. Mediat. Inflamm. 2020, 2020, 4105809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Pang, S.; Yu, X.C.; Xue, Q.; Jiang, H.Y.; Liang, X.J.; Liu, L. Predictive Values of Postoperative and Dynamic Changes of Inflammation Indexes in Survival of Patients with Resected Colorectal Cancer. Curr. Med. Sci. 2018, 38, 798–808. [Google Scholar] [CrossRef]
- Shevchenko, I.; Serban, D.; Simion, L.; Motofei, I.; Cristea, B.M.; Dumitrescu, D.; Tudor, C.; Dascalu, A.M.; Serboiu, C.; Tribus, L.C.; et al. Clinical Significance of Blood Cell-Derived Inflammation Markers in Assessing Potential Early and Late Postoperative Complications in Patients with Colorectal Cancer: A Systematic Review. J. Clin. Med. 2025, 14, 2529. [Google Scholar] [CrossRef]
- Liu, C.Q.; Yu, Z.B.; Gan, J.X.; Mei, T.M. Preoperative blood markers and intra-abdominal infection after colorectal cancer resection. World J. Gastrointest. Surg. 2024, 16, 451–462. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, R.; Liu, H.; Zheng, L.; Zheng, X.; Li, Y.; Cui, H.; Qin, C.; Hu, J. New scoring system combining computed tomography body composition analysis and inflammatory-nutritional indicators to predict postoperative complications in stage II-III colon cancer. J. Gastroenterol. Hepatol. 2023, 38, 1520–1529. [Google Scholar] [CrossRef]
- Serban, R.E.; Popescu, D.M.; Boldeanu, M.V.; Florescu, D.N.; Serbanescu, M.S.; Sandru, V.; Panaitescu-Damian, A.; Fortofoiu, D.; Serban, R.C.; Gherghina, F.L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, K.; Yuan, Q.; Liu, L.; Miao, J.; Wang, H.; Ding, C.; Guan, W. The role of sarcopenia in pre- and postoperative inflammation: Implications of outcomes in patients with colorectal cancer. J. Gastrointest. Surg. 2024, 28, 1791–1798. [Google Scholar] [CrossRef]
- Chang, J.S.; Cheng, H.H.; Huang, S.C.; Lin, H.H.; Chang, S.C.; Lin, C.C. The impact of inflammatory markers on prognosis of stage II colon cancers depends on tumour sidedness. ANZ J. Surg. 2023, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, H.; Gul, M.O.; Kina, U.; Gurleyik, M.G. Use of the Systemic Immune Inflammation Index, TNM classification, and CEA in pre- and post- prognostic evaluation of sporadic colorectal cancer. Ann. Ital. Chir. 2022, 92, 319–324. [Google Scholar] [PubMed]
- Hernandez-Ainsa, M.; Velamazan, R.; Lanas, A.; Carrera-Lasfuentes, P.; Piazuelo, E. Blood-Cell-Based Inflammatory Markers as a Useful Tool for Early Diagnosis in Colorectal Cancer. Front. Med. 2022, 9, 843074. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Akiyama, T.; Kato, R.; Sawayama, H.; Ogawa, K.; Yoshida, N.; Baba, H. Prognostic Significance of Systemic Inflammation Indices by K-ras Status in Patients With Metastatic Colorectal Cancer. Dis. Colon Rectum 2023, 66, e809–e817. [Google Scholar] [CrossRef]
- Feng, Z.; Lin, H.; Yang, X.; Cao, S.; Gu, X.; Zhang, Z.; Deng, W. Diagnostic Value of Inflammation-Related Indicators in Distinguishing Early Colon Cancer and Adenomatous Polyps. Cancer Control 2023, 30, 10732748231180745. [Google Scholar] [CrossRef]
- Hasirci, I.; Sahin, A. Importance of the neutrophil-lymphocyte ratio and systemic immune-inflammation index in predicting colorectal pathologies in fecal occult blood-positive patients. J. Clin. Lab. Anal. 2023, 37, e24878. [Google Scholar] [CrossRef]
- Manojlovic, N.; Savic, G.; Nikolic, B.; Rancic, N. Dynamic monitoring of carcinoembryonic antigen, CA19-9 and inflammation-based indices in patients with advanced colorectal cancer undergoing chemotherapy. World J. Clin. Cases 2022, 10, 899–918. [Google Scholar] [CrossRef]
- Goseki, N.; Okamoto, A.; Onodera, T. Postoperative nutritional assessment in gastric and colorectal cancer. Nihon Geka Gakkai Zasshi 1986, 87, 853–858. [Google Scholar] [PubMed]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Sakamoto, Y.; Nakagawa, S.; Izumi, D.; Kosumi, K.; Taki, K.; Higashi, T.; Miyata, T.; Miyamoto, Y.; Yoshida, N.; et al. Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int. J. Clin. Oncol. 2017, 22, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Mohri, Y.; Inoue, Y.; Tanaka, K.; Hiro, J.; Uchida, K.; Kusunoki, M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J. Surg. 2013, 37, 2688–2692. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Chen, X.; Song, Y.; Shi, J.; Zhao, J.; Sun, J.; Xu, Y.; Wang, Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: Results from a retrospective cohort study and a meta-analysis. Oncotarget 2016, 7, 58543–58552. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, E.; Hilsden, R.; Hawel, J.D.; Elnahas, A.I.; Schlachta, C.M.; Alkhamesi, N.A. Preoperative malnutrition in patients with colorectal cancer. Can. J. Surg. 2021, 64, E621–E629. [Google Scholar] [CrossRef]
- Li, J.; Zhu, N.; Wang, C.; You, L.; Guo, W.; Yuan, Z.; Qi, S.; Zhao, H.; Yu, J.; Huang, Y. Preoperative albumin-to-globulin ratio and prognostic nutritional index predict the prognosis of colorectal cancer: A retrospective study. Sci. Rep. 2023, 13, 17272. [Google Scholar] [CrossRef]
- Yang, G.; Wang, D.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Li, T.; Lin, Y.; Luo, H. Normal reference intervals of prognostic nutritional index in healthy adults: A large multi-center observational study from Western China. J. Clin. Lab. Anal. 2021, 35, e23830. [Google Scholar] [CrossRef]
- Tang, J.; Yang, L.; Yang, G.Y.; Li, Y.H.; Zhu, Y.S.; Li, H.; Gao, X.M. Prognostic nutritional index as a predictor of cardiovascular and all-cause mortality in American adults with hypertension: Results from the NHANES database. Front. Cardiovasc. Med. 2024, 11, 1465379. [Google Scholar] [CrossRef]
- Yilmaz, F.; Keles, M.; Bora, F. Relationship between the prognostic nutritional index and resistant hypertension in patients with essential hypertension. Clin. Exp. Hypertens. 2022, 44, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Christina, N.M.; Tjahyanto, T.; Lie, J.G.; Santoso, T.A.; Albertus, H.; Octavianus, D.; Putri, D.; Andrew, J.; Jatinugroho, Y.D.; Shiady, C.; et al. Hypoalbuminemia and colorectal cancer patients: Any correlation?: A systematic review and meta-analysis. Medicine 2023, 102, e32938. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, J.; Jia, H.; Huang, L.; Li, D.; Li, Q.; Li, X. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget 2016, 7, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Blay Cortes, G.; Botella Romero, F.; Fernandez Garcia, J.M.; Pita Gutierrez, F.; Ramirez Arroyo, V.; Breton Lesmes, I.; Seen Semg Semergen, S. Continuity of care in disease-related malnutrition and nutritional medical treatment. Endocrinol. Diabetes Nutr. 2022, 69, 897–909. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Makinen, M.J.; Vayrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).