Abstract
Background/Objectives. This manuscript presents an overview of advances in oncological radiotherapy as an effective treatment method for cancerous tumors, focusing on mechanisms of action within metabolite–antimetabolite systems. The urgency of this topic is underscored by the fact that cancer remains one of the leading causes of death worldwide: as of 2022, approximately 20 million new cases were diagnosed globally, accounting for about 0.25% of the total population. Given prognostic models predicting a steady increase in cancer incidence to 35 million cases by 2050, there is an urgent need for the latest developments in physics, chemistry, molecular biology, pharmacy, and strict adherence to oncological vigilance. The purpose of this work is to demonstrate the relationship between the nature and mechanisms of past diagnostic and therapeutic oncology approaches, their current improvements, and future prospects. Particular emphasis is placed on isotope technologies in the production of therapeutic nuclides, focusing on the mechanisms of formation of simple and complex theranostic compounds and their classification according to target specificity. Methods. The methodology involved searching, selecting, and analyzing information from PubMed, Scopus, and Web of Science databases, as well as from available official online sources over the past 20 years. The search was structured around the structure–mechanism–effect relationship of active pharmaceutical ingredients (APIs). The manuscript, including graphic materials, was prepared using a narrative synthesis method. Results. The results present a sequential analysis of materials related to isotope technology, particularly nucleus stability and instability. An explanation of theranostic principles enabled a detailed description of the action mechanisms of radiopharmaceuticals on various receptors within the metabolite–antimetabolite system using specific drug models. Attention is also given to radioactive nanotheranostics, exemplified by the mechanisms of action of radioactive nanoparticles such as Tc-99m, AuNPs, wwAgNPs, FeNPs, and others. Conclusions. Radiotheranostics, which combines the diagnostic properties of unstable nuclei with therapeutic effects, serves as an effective adjunctive and/or independent method for treating cancer patients. Despite the emergence of resistance to both chemotherapy and radiotherapy, existing nuclide resources provide protection against subsequent tumor metastasis. However, given the unfavorable cancer incidence prognosis over the next 25 years, the development of “preventive” drugs is recommended. Progress in this area will be facilitated by modern medical knowledge and a deeper understanding of ligand–receptor interactions to trigger apoptosis in rapidly proliferating cells.
1. Introduction
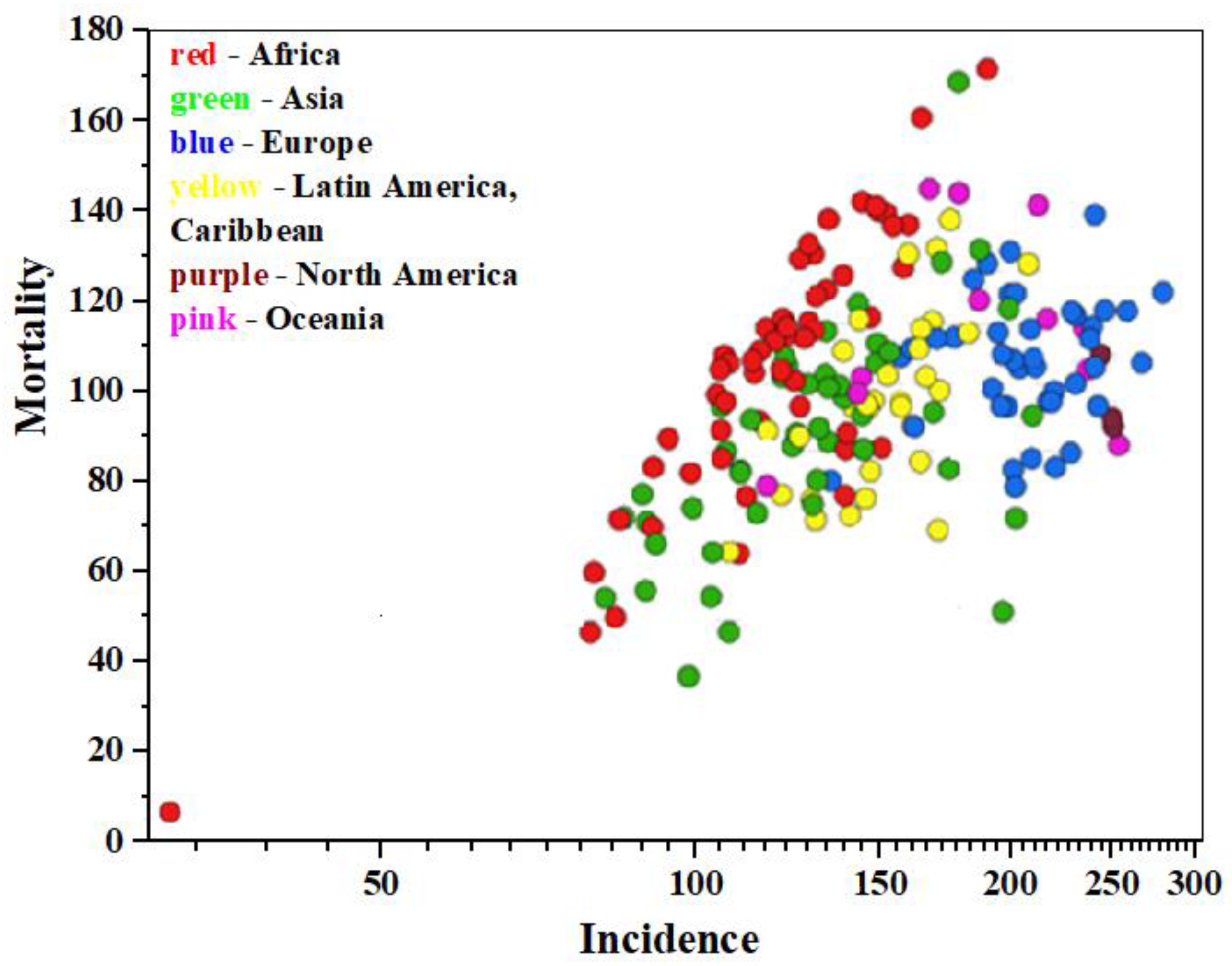
According to data from the International Agency for Research on Cancer (IARC https://gco.iarc.fr/today/en, accessed on 11 January 2025) as of 2022, approximately 20 million new cancer cases and 9.7 million cancer-related deaths were registered worldwide, including non-melanoma skin cancers (NMSCs) [1]. Data analysis shows [2] that cancer is diagnosed in about one in five individuals of both sexes—men and women—during their lifetime, while approximately one in nine men and one in twelve women die from it. Malignant neoplasms of the lung and breast (in women) are the most common types of cancer globally (12.4% and 11.6% of all new cases, respectively), colorectal cancer accounts for 9.6% in both sexes, prostate cancer for 7.3% in men, and stomach cancer for 4.9% in both sexes. Data visualization illustrating the incidence, mortality, and prevalence of 36 specific cancer types across 185 countries by sex and age group is presented in Figure 1 within the GLOBOCAN project (https://www.uicc.org/, accessed on 11 January 2025) [3].
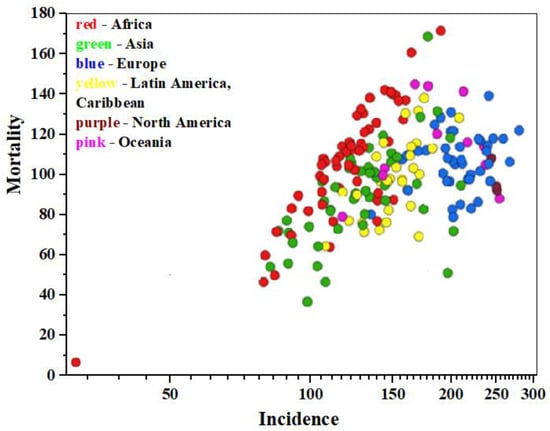
Figure 1.
The epidemiology diagram of «Mortality—Incidence» (age-standardized mortality rate per 100.000 population), based on 2022 IARC data, for both sexes, all cancers except NMSCs.
Prognostic models predict an increase in cancer cases to 35 million by 2050, particularly affecting countries with low and medium Human Development Index (HDI), emphasizing the need for access to cost-effective cancer treatment services and the importance of developing innovative solutions in medicine and rehabilitation [4].
The evolution of cancer therapy is not confined to the 20th century, despite notable breakthroughs such as the discovery of the radioactive elements radium and polonium by Marie and Pierre Curie, marking the beginning of radiotherapy [5]; combination immunotherapy for cancer by Paul Ehrlich’s, who coined the term “chemotherapy” [6]; and the discovery of new properties of cytotoxic agents exemplified by chemical warfare mustard agents—sulfur mustard (SM, 2,2′-dichloroethyl sulfide)—and the development of one of the first alkylating chemotherapeutic drugs, nitrogen mustard (NM, mechlorethamine), by Louis Goodman and Alfred Gilman [7,8,9]. Radiotherapy has been used since 1950, following the introduction of cobalt teletherapy, which enabled precise radiation delivery to tumors while minimizing damage to surrounding healthy tissue and facilitating combination with other treatment modalities [10].
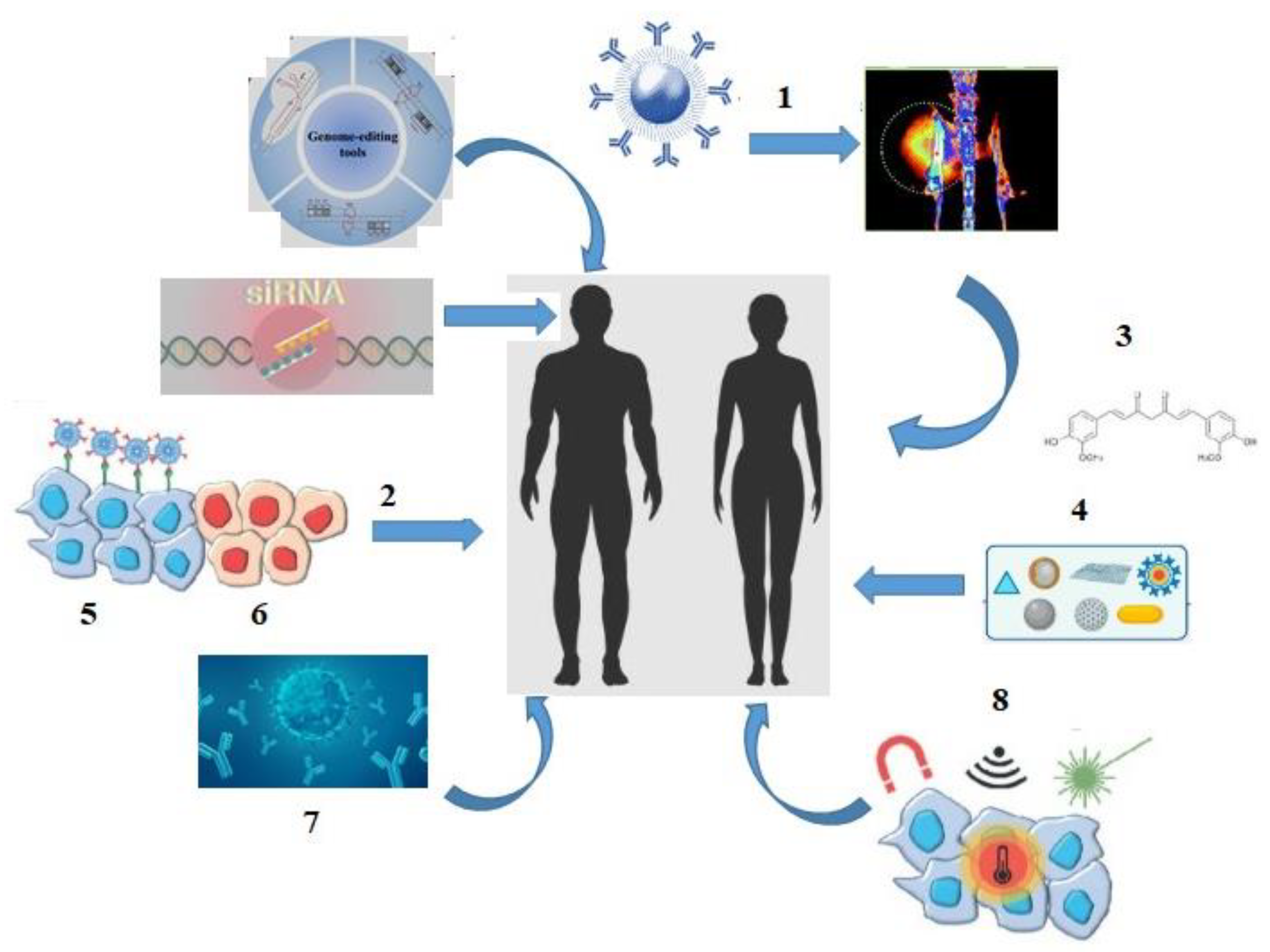
A significant improvement in patients’ quality of life and survival was driven by a dramatic shift in the cancer treatment landscape in the 21st century: theranostic methods involving conjugation of diagnostic radiopharmaceuticals with therapeutic agents; the implementation of gene therapy and nanomedicine combined with targeted therapy and efficient biodistribution of chemotherapeutic agents; delivery methods for small interfering RNA (siRNA); development of a monoclonal antibody («mABs-drug»), thermal ablation and magnetic hyperthermia techniques; use of natural antioxidants in cancer therapy; as well as innovative approaches such as radiomics (quantitative assessment of tumor characteristics) and pathomics (high-resolution tissue image analysis) (Figure 2) [11,12,13,14,15,16,17,18,19].

Figure 2.
The «landscape» of cancer treatment in the 21st century: 1—theranostics; 2—targeted therapy; 3—antioxidants; 4—nanoparticles; 5—tumor cells; 6—healthy cells; 7—monoclonal antibodies (mABs); 8—thermal ablation, magnetic hyperthermia.
Special attention from researchers and clinical practitioners is given to radiopharmacy, targeted radionuclide therapy, and the development and implementation of rapid diagnostic systems and compact measuring devices for determining isotope content in medical applications. This focus is driven by the advent of personalized medicine (https://www.genome.gov/genetics-glossary/Personalized-Medicine, accessed on 18 January 2025), whose core concept is the use of an individual’s genetic profile to inform decisions regarding disease prevention, diagnosis, and treatment [20,21].
2. Methods
The methodology for this review article involved developing our own search algorithm, which was based on the selection and analysis of information from the PubMed, Scopus, and Web of Science databases over the past 20 years. This approach was guided by the concept of the existing “structure–mechanism–effect” relationship of active pharmaceutical ingredients (API), encompassing various forms such as atoms, solid-phase (nano-) objects (d~1–100 nm), molecules, and complex particles.
In addition to review articles, practical research, and clinical cases, data from accessible official online sources were also considered, including the International Agency for Research on Cancer, the GLOBOCAN project, the National Human Genome Research Institute, the International Atomic Energy Agency, the Nuclear Research and Consultancy Group, Melanoma Unit, and NanoTherm.
Among the literary sources reviewed, articles from the last 5 years constituted approximately 60%, those from the last 20 years accounted for about 20%, and approximately 20% were older sources deemed valuable for the history of medicine.
The selection process involved screening abstracts and, more extensively, the full texts of articles. The final presentation of the material was conducted using a narrative synthesis approach, which included a systematic review and generalization of results from multiple studies, accompanied by our own conclusions. This approach was also applied to the presentation of graphic material.
3. Results
3.1. Isotope Technology—Nucleus Stability/Instability
Radiopharmacy utilizes two classes of isotopes for therapeutic purposes: stable isotopes, which do not undergo radioactive decay over time, and unstable isotopes (radioisotopes)—nuclei that undergo spontaneous radioactive decay, emitting ionizing radiation in order to transform into a stable form (https://www.iaea.org/ru/temy/radioizotopy, accessed on 25 January 2025). Nuclear stability is achieved through alpha, electron, or positron emission (in the form of gamma rays) [22,23]. There are three types of particulate radiation relevant to targeted radiopharmacy: alpha particles, beta particles, and Auger electrons, which can irradiate tissue volumes at multicellular, cellular, and subcellular scales (Table 1).

Table 1.
The types of radiation for targeted therapy.
The rupture of the DNA double helix (DSB) is considered the main and most destructive mechanism of damage to tumor cells. The foremost cause of DSB is replication across a nick, giving rise to chromatid breaks during S phase [27].
Stable isotopes are primarily used as tracers in pharmacokinetic studies, for example, to investigate human metabolism kinetics in vivo. The high sensitivity and specificity of tracers allow them to be tracked through complex processes of substance redistribution and transformation, including within living organisms. In human metabolism research, the most commonly used stable isotopes are those of hydrogen, oxygen, and nitrogen (13C, 15N, 2H и 18O), which can be incorporated into molecules and used as metabolic indicators [28]. It is believed that nuclear stability is maintained by boson pairing between protons and neutrons [29]. Disruption of boson pairing leads to instability. The so-called “proton stability boundary” demonstrates a narrow linear relationship for nuclei considered stable [30]. Such nuclei are characterized by the ratio of the number of neutrons «n» to protons «p» (Equation (1)):
where A = n + p is the mass number.
n/p = 0.98 + 0.015·A2/3,
The most stable nuclei are the so-called “magic nuclei”, where the number of protons or neutrons corresponds to one of the magic numbers: 2, 8, 20, 28, 50, 82, and 126 [31].
To date, more than 3500 unstable isotopes are known, of which around 80 occur naturally, about 200 have been artificially created, and fewer than 50 are regularly used in clinical practice. The production of radiopharmaceuticals requires a nuclear infrastructure encompassing the entire radionuclide manufacturing process (Nuclear Research and Consultancy Group (https://www.advancingnuclearmedicine.com/, accessed on 28 January 2025). Medical isotopes are produced either in nuclear reactors or in cyclotrons—cyclic accelerators of non-relativistic heavy charged particles. In reactors, nuclear fission chain reactions occur in a controlled and stable environment. Reactor fuel consists of low-enriched uranium U-235, which, upon fission, produces a neutron cloud. When neutrons collide with stable isotopes, radionuclides are generated, for example, the neutron-rich «parent» atom Mo-99. Reactor and cyclotron isotopes used in radiopharmacy are listed in Table 2.

Table 2.
Radiopharmaceuticals.
Despite years of experience in harnessing nuclear reactions for medical applications, both production routes—reactor and cyclotron—have limitations and drawbacks [34]. These include challenges in achieving high radionuclide purity and specific activity, the time-consuming and costly separation of therapeutic nuclides from radioactive impurities, limited availability due to reliance on historical uranium, actinium sources, or nuclear waste, which represent expensive investments, as well as the need for scale-up in the radiopharmaceutical market for small-batch GMP production [35].
Despite the advantages of targeted therapies, characterized by low toxicity profiles, they often exhibit low response rates to a single active pharmaceutical ingredient (API). Moreover, the emergence of resistance to targeted therapy is a significant clinical challenge, especially in patients with advanced tumors [36]. Therefore, there is a clear need for new translational strategies and targeted approaches to cancer treatment, particularly to overcome resistance.
3.2. Targeted Theranostics
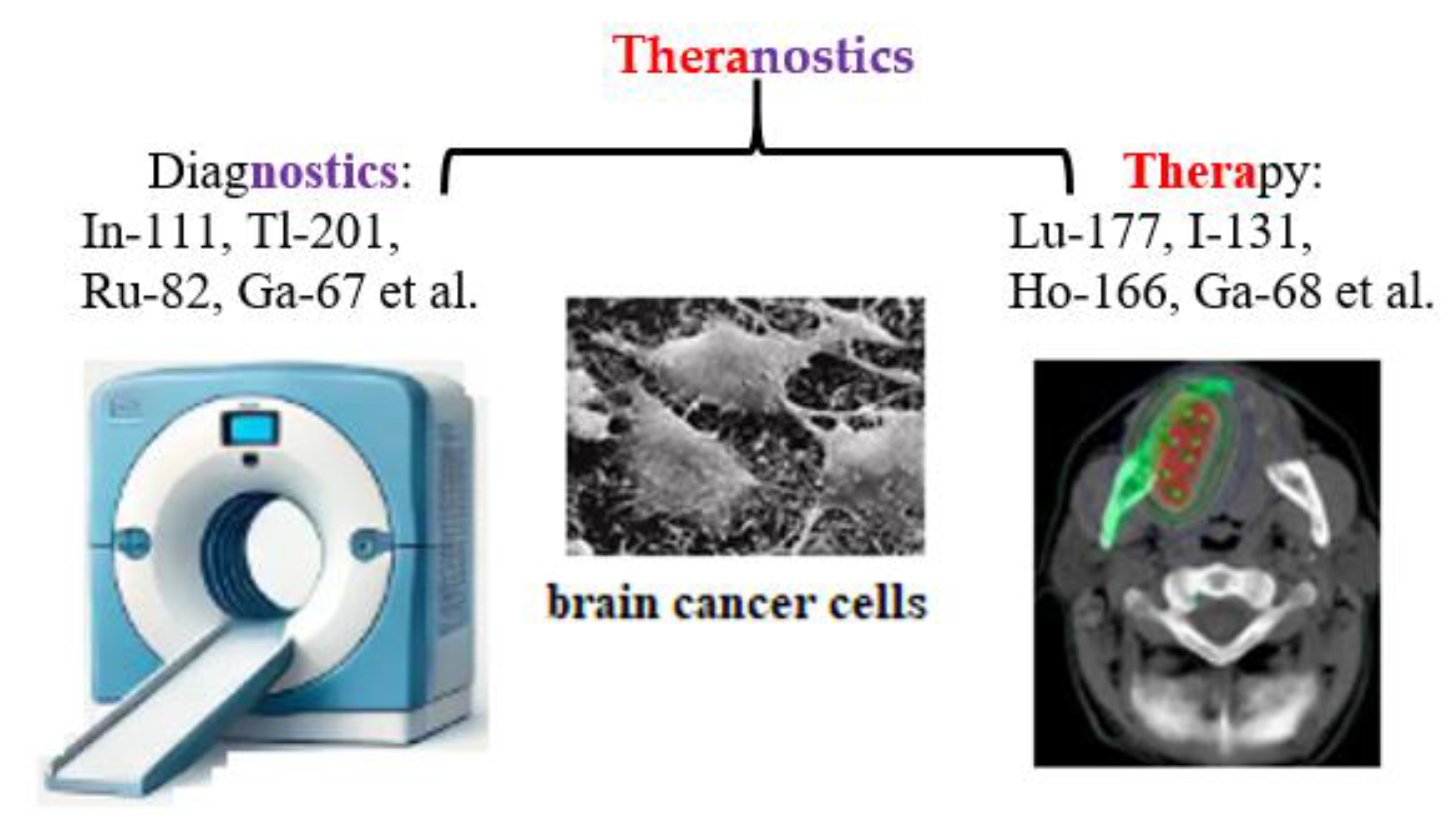
The term «theranostics», a combination of «therapeutic» and «diagnostic» was first introduced into nuclear medicine in 1998 at the intersection of precision and personalized medicine [37]. Theranostics combines diagnosis and treatment for continuous medical assessment of a patient’s condition using an appropriate combination of active pharmaceutical and radiopharmaceutical ingredients, as well as nuclear medical imaging methods: radiotracers, contrast agents, positron emission tomography (PET), and magnetic resonance imaging (MRI) (Figure 3).
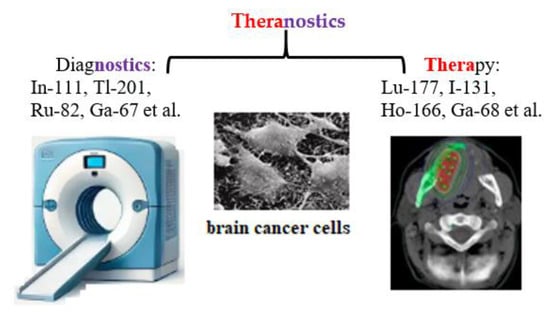
Figure 3.
Schematic representation of theranostics, integrating diagnostics and therapeutics in nuclear medicine.
A recent clinical study highlighted the ability of alpha-radiotherapy with high linear energy transfer (LET) (see Table 1) to overcome treatment resistance to beta-particle therapy [38]. This approach allows the selection of the sub-population of patients most likely to benefit from a targeted therapy in accordance with their “molecular profile” at a given time point, or, conversely, those patients for whom the risk of adverse effects is higher [39].
3.2.1. Radiopharmaceuticals—Antimetabolites
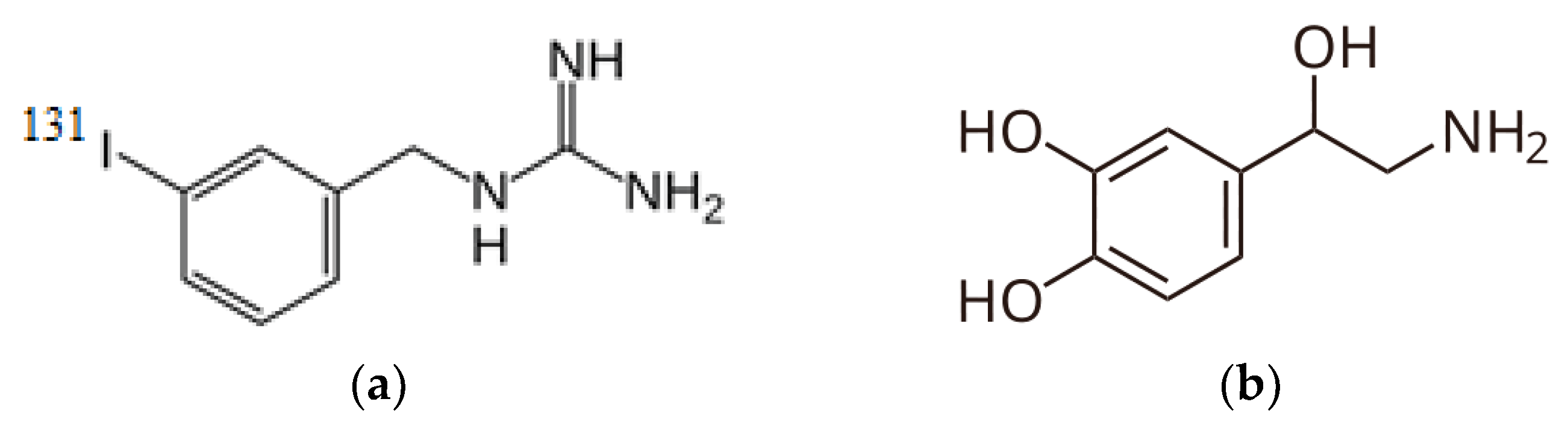
While «theranostics» entered nuclear medicine terminology relatively recently, the concept of nuclear theranostics was introduced in 1943 by Dr. S. M. Seidlin at Montefiore Hospital in New York City, who first used iodine-131 for diagnostic imaging, target expression confirmation, and radionuclide therapy of thyroid cancer in his patient [40]. In modern nuclear medicine, the combination of radionuclide I-131 and I-131-iodine-meta-iodobenzylguanidine (MIBG, Iobenguane, AzedraR). Iobenguane I-131—is a radiopharmaceutical ingredient representing a structural analog of the neurotransmitter noradrenaline, containing radioactive I-131 in the meta position relative to the alkylguanidine side chain (Figure 4). Iobenguane may be used to image or eradicate neuroendocrine tissues and tumor cells.

Figure 4.
Radiopharmaceutical agent’s structural formulas: (a) MIBG and (b) Noradrenaline.
The mechanism of action of MIBG theranostics is as follows: acting as a structural analog of the natural metabolite noradrenaline, meta-iodobenzylguanidine interacts with adrenergic receptors in adrenal, liver, heart, and spleen tissues, blocking them and thereby inhibiting signal transmission, thus exhibiting antimetabolite properties. MIBG is used for diagnosing primary and metastatic pheochromocytoma or neuroblastoma. The radiopharmaceutical was approved by the FDA on 19 September 2008.
The radioisotope I-131, as the therapeutic component in this theranostic, destroys tissues metabolizing noradrenaline, undergoing transformations (Equation (2)):
Combination of metabolic radiotherapy (MtRth) with other treatment methods is the most important direction for increasing the efficiency of cancer treatment and reducing the frequency of side effects in clinical practice. This goal can be achieved by combining MtRth with neoadjuvant (NCRT) and adjuvant chemoradiotherapy (ACRT) before surgery, for example, immunotherapy, proton beam therapy, hyperthermia, etc. [41].
3.2.2. Radionuclide Therapy with Peptide Receptors
Another example of a theranostic radionuclide of interest as a suitable combination of active radiopharmaceutical ingredients (ARPI) for diagnosis and therapy of neuroendocrine tumors (NETs), adenocarcinoma variants is lutetium-177.
- 177Lu–Dotatate radioligand therapy (RLT)
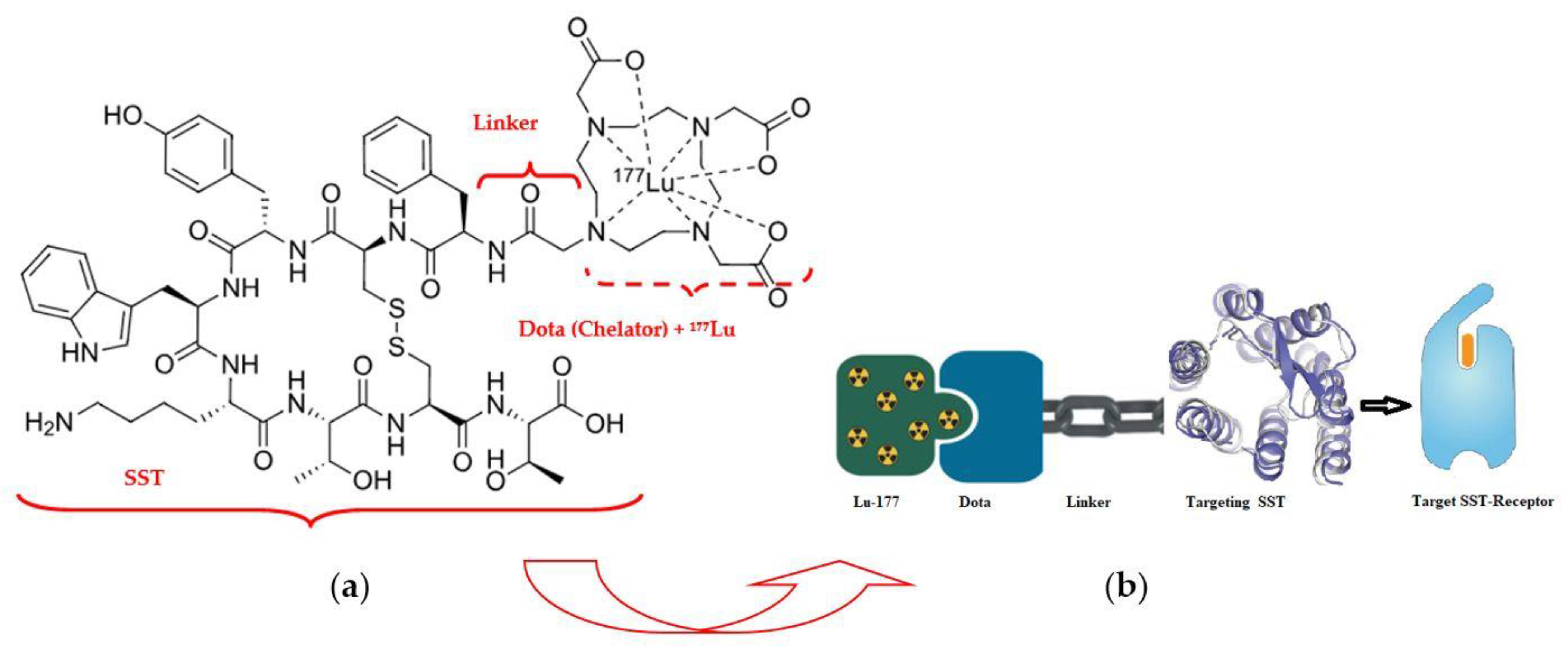
177Lu–Dotatate (LutatheraR) («Dota»—dodecanetetraacetic acid and «tate»—shortly somatostatin receptors) is a complex of a somatostatin-like peptide hormone (SST) and the octadentate ligand DOTA with a lutetium-177 central radionuclide atom (Figure 5) [42].
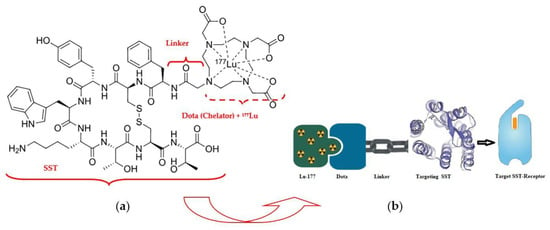
Figure 5.
Radiopharmaceutical Lu-177 dota-tate agent: (a) structural formula; (b) demonstration of the lutetium-177 (177Lu)–Dotatate theranostic action.
The pioneer of NET radiotherapy was Professor Eric Krenning at the Erasmus Medical Centre Rotterdam, who first presented clinical results in 2017 for peptide receptor radionuclide therapy (PRRT) of gastroenteropancreatic (GEP) NETs [43]. Neuroendocrine tumors of the midgut represent the most common type of malignant gastrointestinal neuroendocrine tumors and are associated with 5-year survival rates of less than 50% among persons with metastatic disease. 177Lu–Dotatate, as a radioactively labeled somatostatin analog, enables targeted delivery of radiation with a high therapeutic index to tumors expressing somatostatin receptors [44]. An alternative name for therapy with a similar mechanism is «radioligand therapy of somatostatin receptor» (RTSR) which has demonstrated prolonged median progression-free survival in heterogeneous NET patient populations [45].
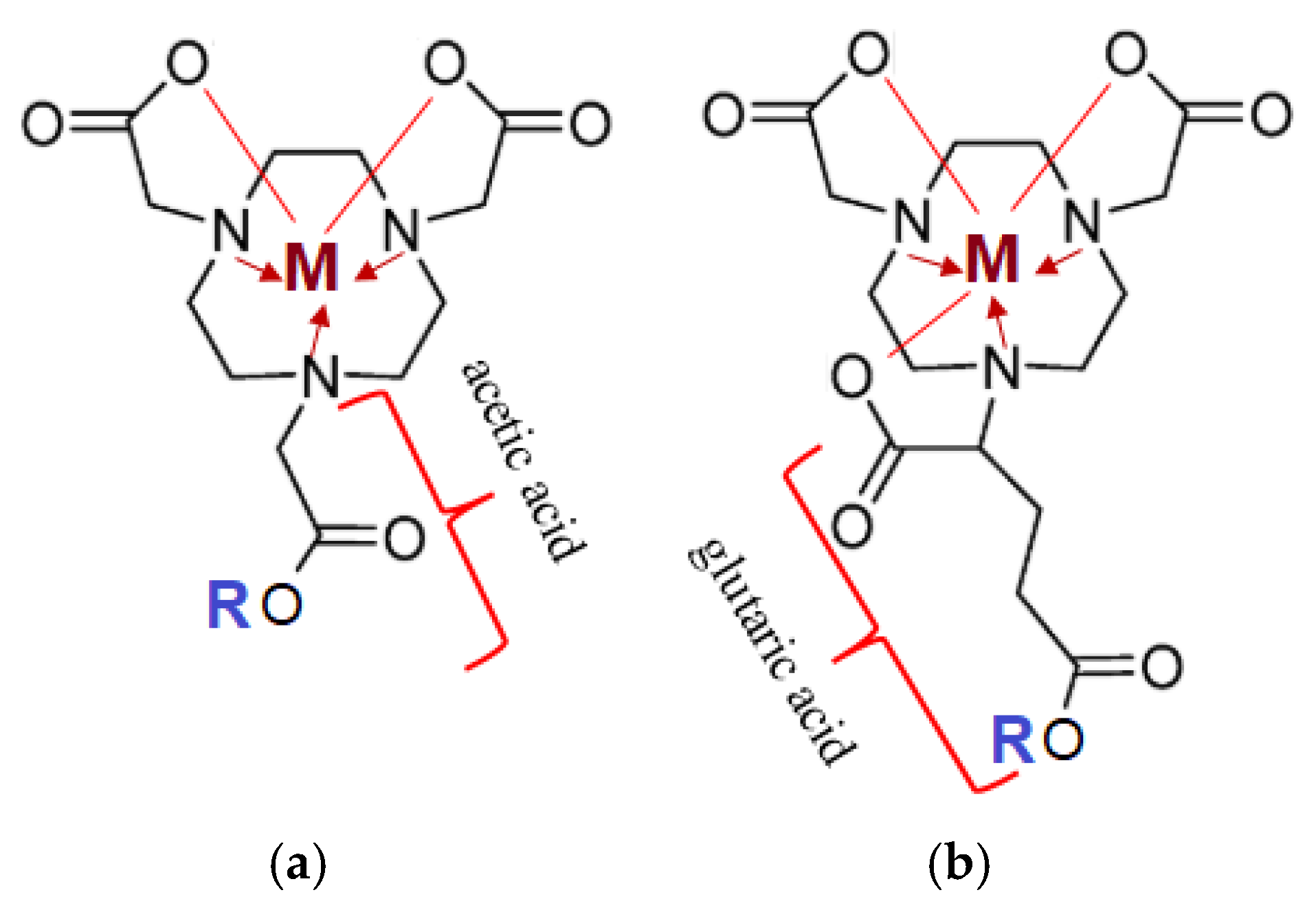
The most commonly used chelating agents for complex formation with radioactive metals, apart from DOTA, are NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) and NODAGA glutaric-derivatives (Figure 6) [46,47].
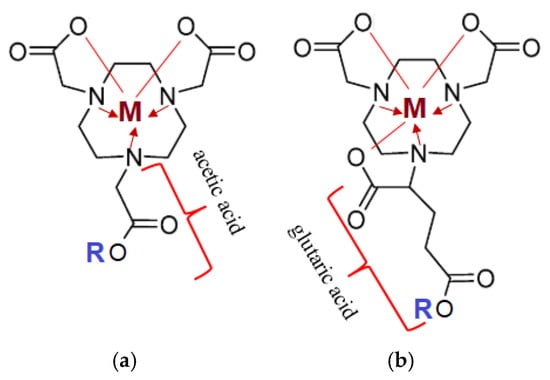
Figure 6.
Structural representation of chelating agents with an ion of metal (M+2,+3: Lu-175, Ga-69, Ga-71): (a) NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid); (b) NODAGA (1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid). The lines represent covalent polar bonds between the Me ion and the carboxyl group residue; the arrow represents covalent bonds via a donor-acceptor mechanism between a tertiary amine (donor) and the complexing agent (Me-acceptor).
Target complexes based onNOTA/NODAGA/DOTA/DODAGA also showed high affinity and selectivity for GRPR (Gastrin-Releasing Peptide Receptor), SSTR2 (Somatostatin Receptor Subtype 2) and MC1R (Melanocortin-subtype 1 receptor), receptors present on the surface of primary and secondary tumors of the prostate, mammary glands, pancreas, lungs, etc. [48].
Overall, PRRT with 177Lu-Dotatate is an effective treatment for patients with progressive, high-grade NETs, with an approximately 80% reduction in the risk of progression or death. Monitoring of renal and blood function during and after therapy is recommended to minimize this risk [49].
- 177Lu—vipivotide tetraxetan RLT
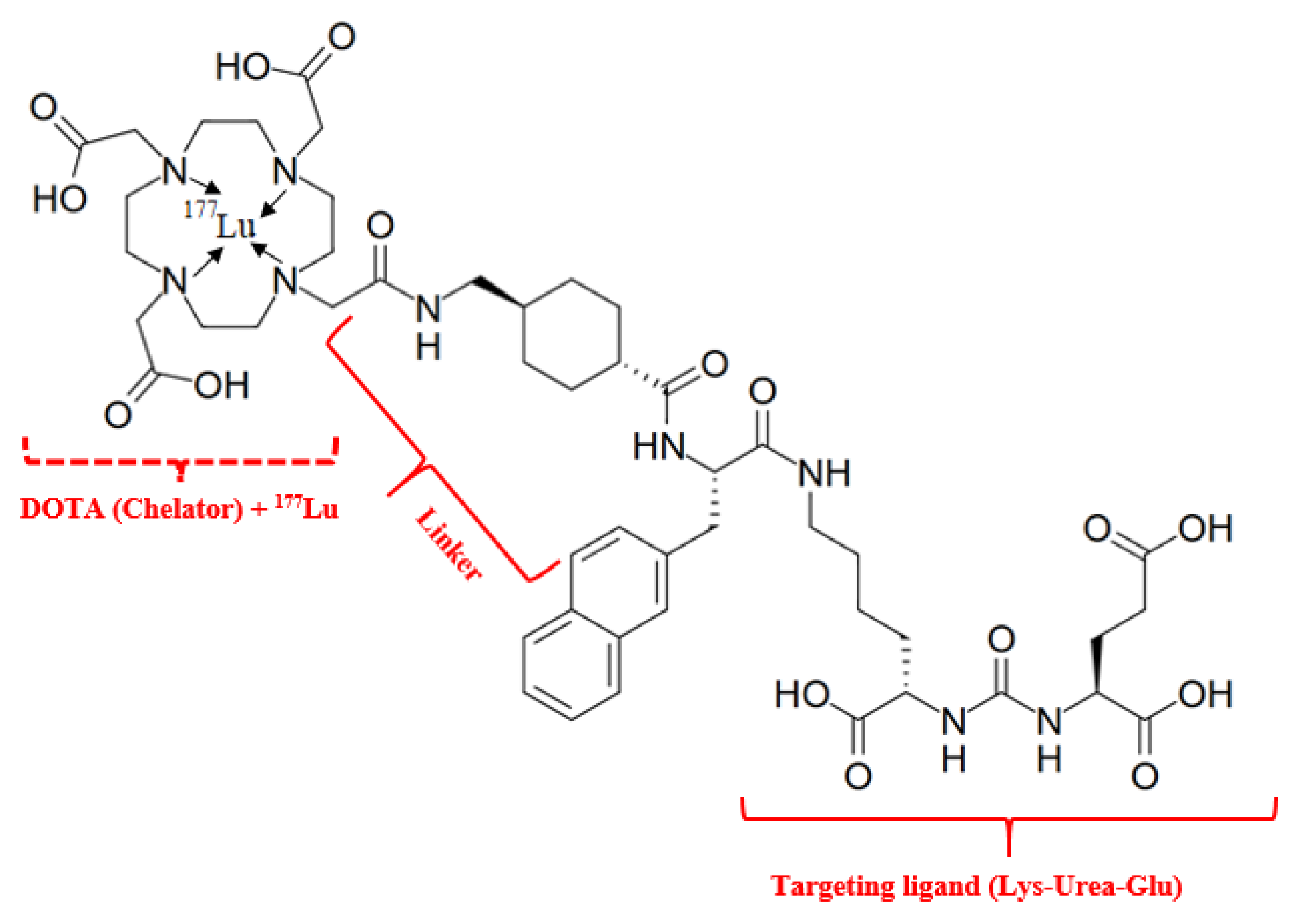
Lutetium-177 vipivotide tetraxetan (PluvictoR) (“vipivo”- targeting moiety Lys-Urea-Glu, the “tide” suffix—peptide nature of this moiety and “tetraxetan” is a DOTA derived from)—is a RLT drug first approved by the FDA on 23 March 2022, for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer (NIH, https://www.cancer.gov/publications/dictionaries/cancer-drug/def/lutetium-lu-177-vipivotide-tetraxetan, accessed on 16 April 2025). It is generally accepted that the mechanism of action of 177Lu-PSMA-617 is attributed to its radioligand activity [50]. The structure comprises the main fragments: radionuclide (Lu-177)—a source of β− radiation; targeting ligand (vipivotide)—a PSMA-binding peptide (Lys-Urea-Glu) that specifically targets prostate cancer cells, as PSMA is significantly overexpressed on their surface; chelator (tetraxetan)—a chemical moiety that securely binds the radionuclide to the targeting ligand; hydrophobic linker, composed of 2-naphthyl-L-Ala and cyclohexyl groups, it connects the targeting ligand to the chelator, influencing the compound’s pharmacological properties (Figure 7).

Figure 7.
Radiopharmaceutical Lu-177 vipivotide tetraxetan. IUPAC Name: 2-[4-[2-[[4-[[(2S)-1-[[(5S)-5-carboxy-5-[[(1S)-1,3-dicarboxypropyl]carbamoylamino]pentyl]amino]-3-naphthalen-2-yl-1-oxopropan-2-yl]carbamoyl]cyclohexyl]methylamino]-2-oxoethyl]-7,10-bis(carboxylatomethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetate;lutetium-177(3+).
The results of 177Lu-vipivotide tetraxetan therapy show a pronounced biochemical (decrease in the level of total PSA) response, as well as low toxicity (often in the form of the development of grade I xerostomia) [51].
- MC1R targeting. Radioactive theranostics of melanoma
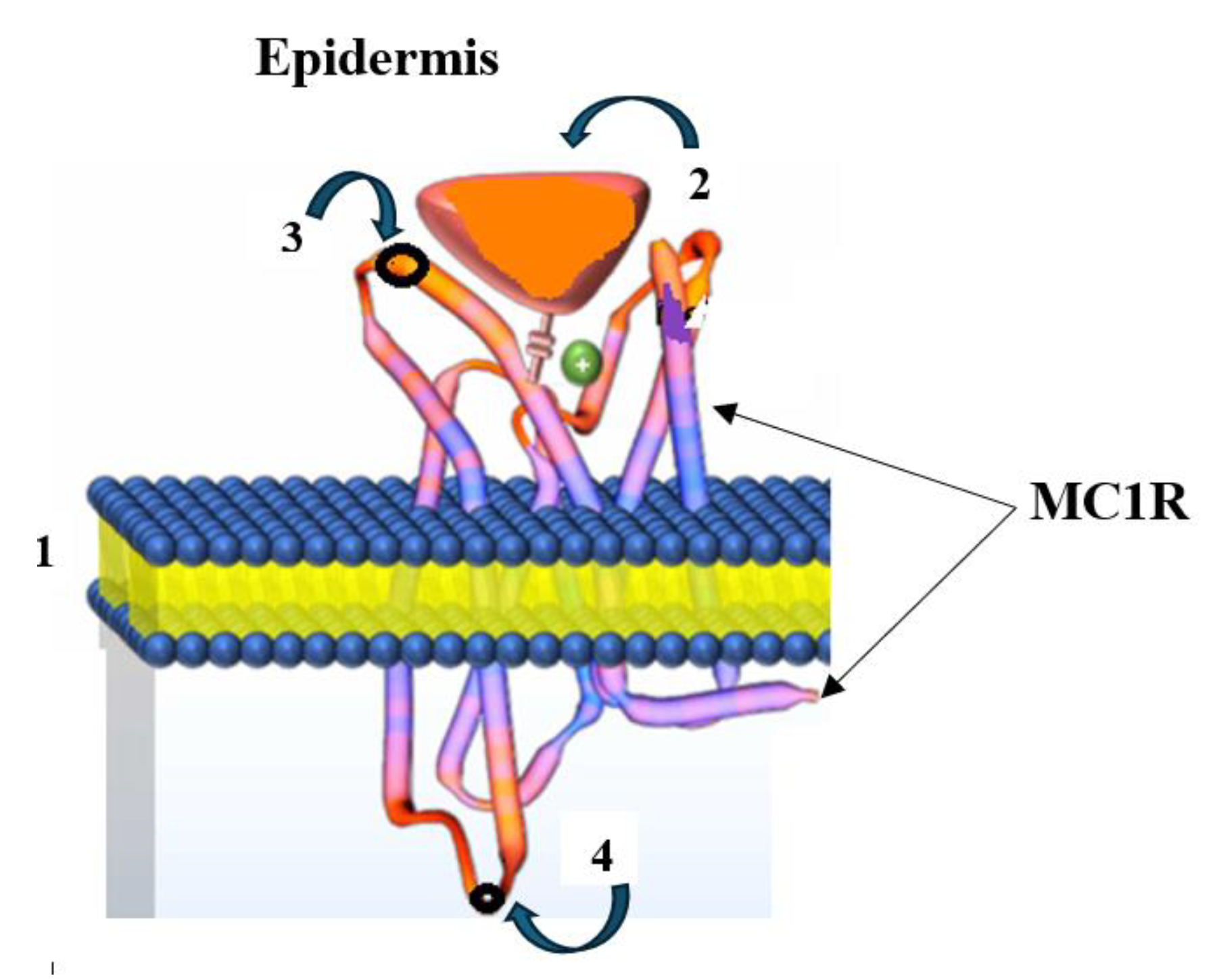
Skin cancer is the most common form of cancer, with melanoma being the most dangerous type. According to the World Health Organization, over 130,000 new cases of melanoma are diagnosed worldwide annually (https://melanomaunit.ru/vse-o-melanome/statistika/, accessed on 2 May 2025). Melanoma develops from pigment-producing cells—melanocytes—due to their malignant progression and early haematogenous and lymphogenous metastasis (metastatic melanoma, MtMn) [52]. A distinctive feature of MtMn is the elevated expression of the endocytic receptor Melanocortin 1 (MC1R) on the surface of human melanoma cells, making it a crucial tumor marker. The MC1R receptor is a melanocortin peptide that is mediated by G protein-coupled receptors (GPCRs) with an N-linked glycosylation site on its extracellular terminus and a palmitoylation site on the intracellular C-terminus. The extracellular N-terminal tail acts as a signaling anchor and plays a vital role in ligand affinity (Figure 8) [53].

Figure 8.
Structure of a melanocyte (from greek μέλας—“black” and κύτος—“cell”): 1—cell membrane; 2—α-melanocyte-stimulating hormone (α-MSH); 3—extracellular N-linked glycosylation site on the MC1R receptor’s extracellular terminus; 4—intracellular C-linked site.
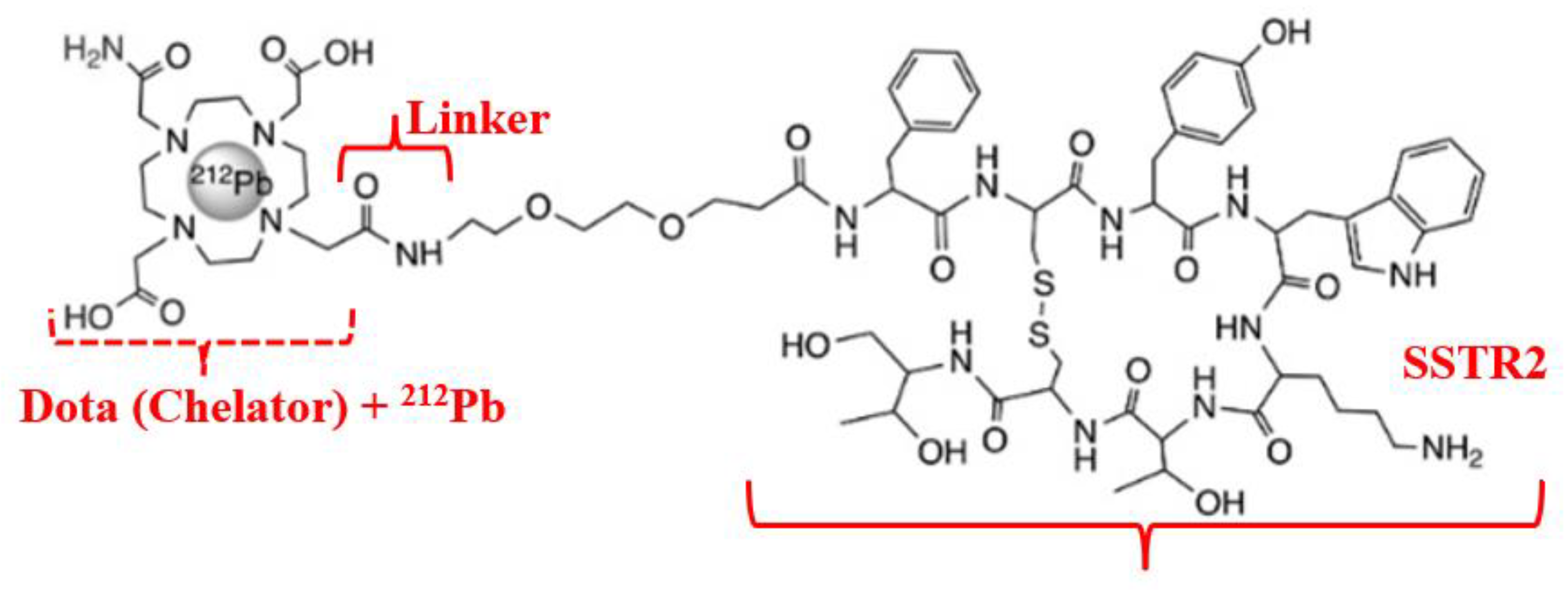
Therapy for MtMn generally aims to detect and subsequently exert cytostatic effects on secondary tumors (as a consequence of advanced melanoma). A theranostic approach is employed, based on targeted delivery of a radioactively labeled peptide (active pharmaceutical ingredient, antibody-based API) to the tumor, where the highly overexpressed MC1R is activated on the cell surface [54]. In this case, the selective peptide binding domain to the cell surface antigen is identified using a native related peptide (Figure 9).
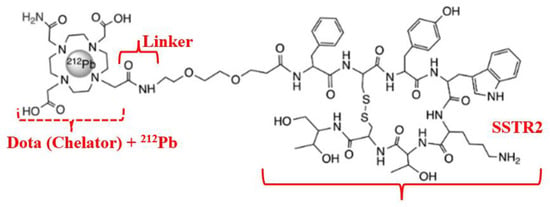
Figure 9.
Radiopharmaceutical Pb-212 dota-tate agent for MC1R targeting.
Deposition of high LET radiation over a short path length leads to increased frequency of DSB and specific destruction of MtMn tumor cells. Quantitative radiolabeling is typically achieved using elementally matched theranostic radioisotope pairs, such as 203Pb/212Pb (diagnostic/therapeutic) (see Table 2) [55,56,57]. The radioisotope Pb-212, as the therapeutic component in this theranostic, is obtained via decay transformations (Equation (3)) [58]:
3.2.3. Radionuclide Therapy with Hormone Receptors
- 18F-Fluoroestradiol
In May 2020, after decades of research, the United States Food and Drug Administration (FDA) approved the PET tracer radiopharmaceutical 6α-fluoro-17β-estradiol (18F-fluoroestradiol, FES) for clinical use in patients with estrogen receptor (ER)/progesterone receptor (PR)-positive recurrent or metastatic breast cancer as a complement to biopsy [59].
18F-fluoroestradiol binds to hormone receptors (HR) in the nuclei of ER-expressing cells, including those in the uterus or ovaries, enabling in vivo assessment of ER/PR expression throughout the body [60]. This distinguishes 18F-fluoroestradiol oт 18F-fluorodeoxyglucose (FDG, the glucose analog), which has limited sensitivity for detecting primary breast tumors [61].
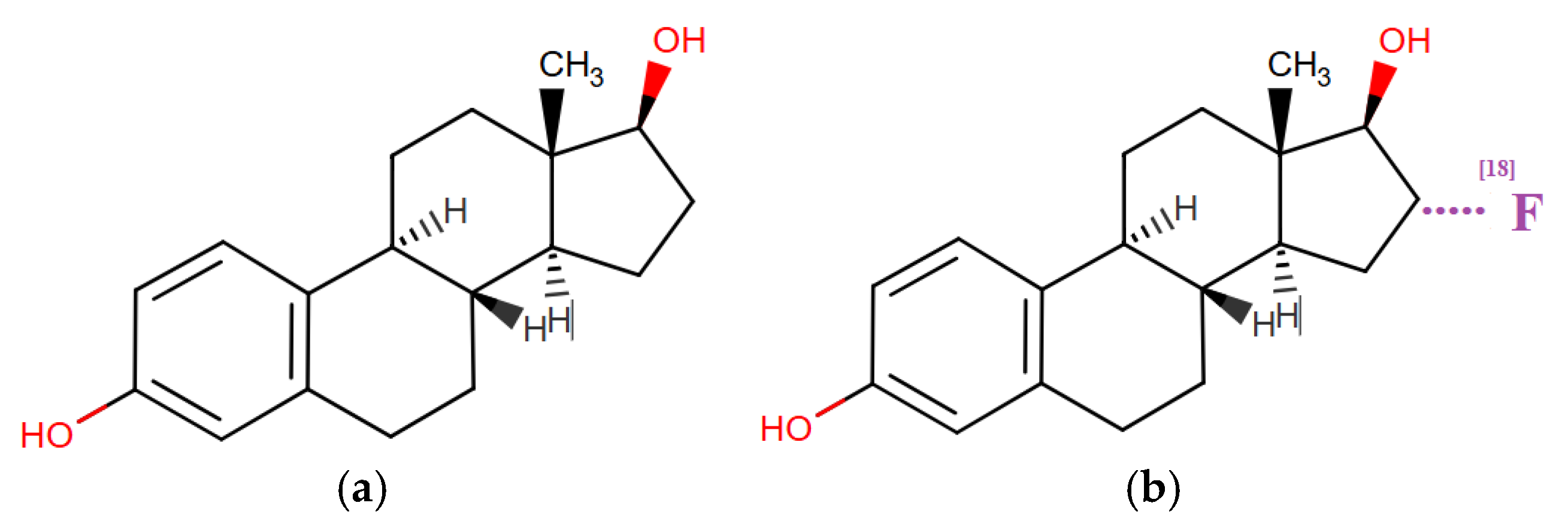
The molecular structures of estradiol and 18F-fluoroestradiol explain the mechanism of detecting breast cancer lesions or other estrogen receptor-positive organs (Figure 10).

Figure 10.
Molecular structures of APIs: (a) 17b-estradiol; (b) 6α-fluoro-17β-estradiol (18F-fluoroestradiol, FES). UPAC Name: (8R,9S,13S,14S,16R,17R)-16-(18F)fluoranyl-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a] phenanthrene-3,17-diol.
It can be seen that 18F-fluoroestradiol is a fluorinated derivative of the estrogenic steroid at the C18 position of hydrogenated cyclopentaphenanthrendiol, which explains the affinity and binding to estrogen receptors, allowing PET imaging of the lesions. 18F-fluoroestradiol has been used as a research agent since the 1980s and as a clinical agent since 2016 in France and 2020 in the United States, without any major adverse events reported to date [62].
3.2.4. Radiopharmaceuticals—Metabolites
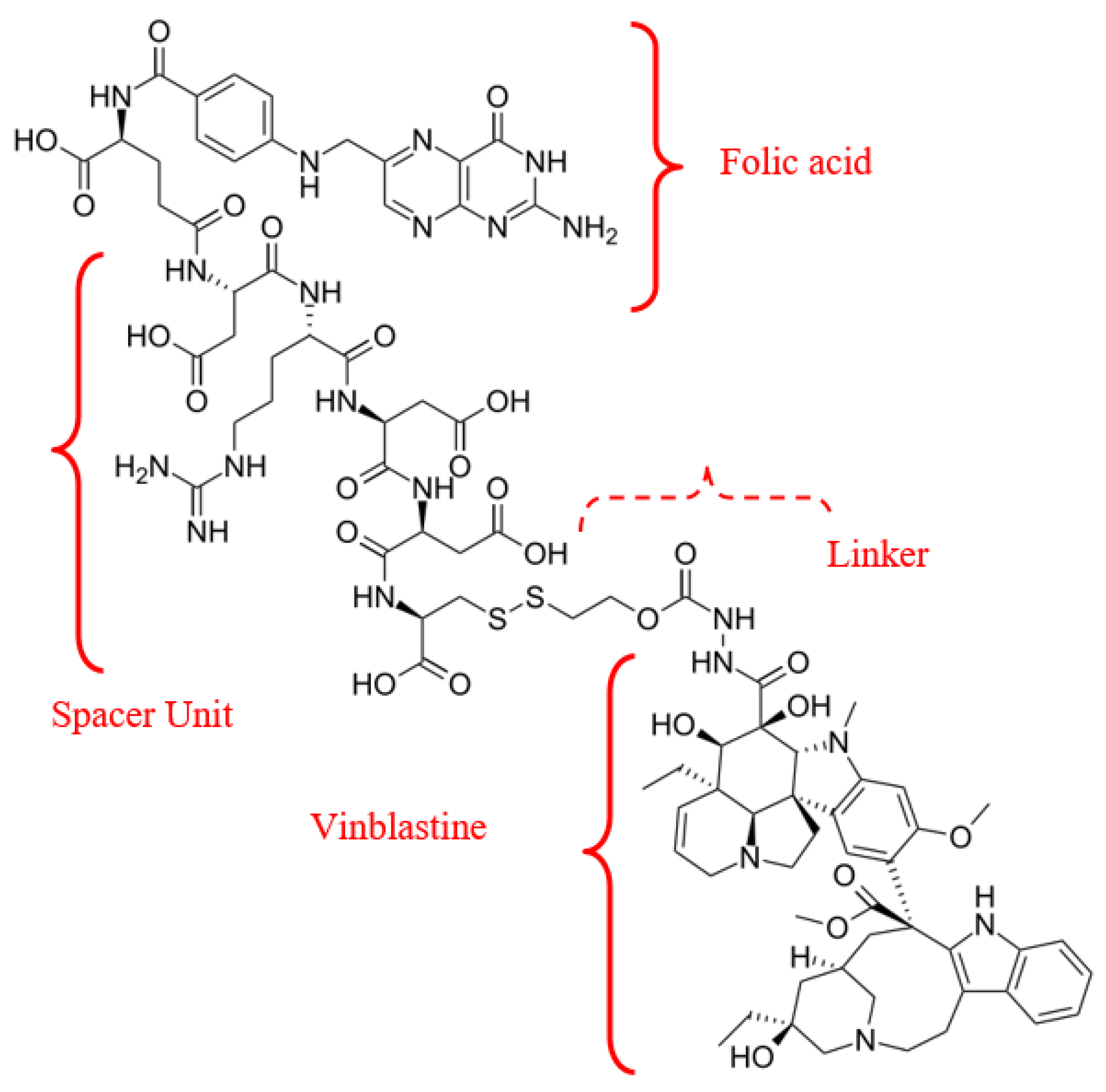
Delivery of receptor-targeted APIs acts as an appealing strategy for cancer treatment and diagnosis. A notable example of a combination of a natural metabolite (folate) and an antitumour agent (vinblastine) is the vintafolide/etarfolatide couple (Figure 11).

Figure 11.
Radiopharmaceutical Vintafolide agent’s structural formula.
Vinca alkaloids—a class of antitubulin agents acting at the G2-M metaphase of the cell cycle—are plant-derived alkaloids with cytostatic activity. Vinca alkaloids, derived from Vinca rosea or Catharanthus roseus, include first-generation compounds (vincristine and vinblastine), semisynthetic second-generation derivatives (vinorelbine and vindesine), and third-generation agents (vinflunine). Incorporating folic acid into the complex with the API and carrier ensures specific, targeted interaction with tumor cells, due to the expression of folate receptor beta (FR-α,-β), including on cancer cells [63]. Consequently, FRs may represent targets for specific delivery of therapeutic agents to activated. Moreover, studies indicate a positive correlation between FR-β expression on mesenchymal stem cells (MSC), cancer stage, and lymph node metastases [64].
Vintafolide is a folate-targeted (FR-α) chemotherapeutic conjugate (folate-vitamin B9 + vinca alkaloid) in clinical stage development as a treatment for folate receptor-positive cancers [65]. The mechanism of action of this theranostic is as follows: vintafolide minimizes the off-target toxicity by delivering the vinca molecule directly and specifically to cancer cells that over-express the folate-receptor [66]. Once delivered to the cancer cell surface, Vintafolide is internalized into the cancer cell via endocytosis, a natural cellular process. Once inside the cell, Endocyte’s proprietary linker technology releases the chemotherapy to eliminate the cancer cell. In preclinical models, vintafolide demonstrated potent antitumour activity (IC50 1–10 nM), exhibiting sparing effects on healthy tissues.
3.3. Nanotheranostics—Prerequisites for Developments
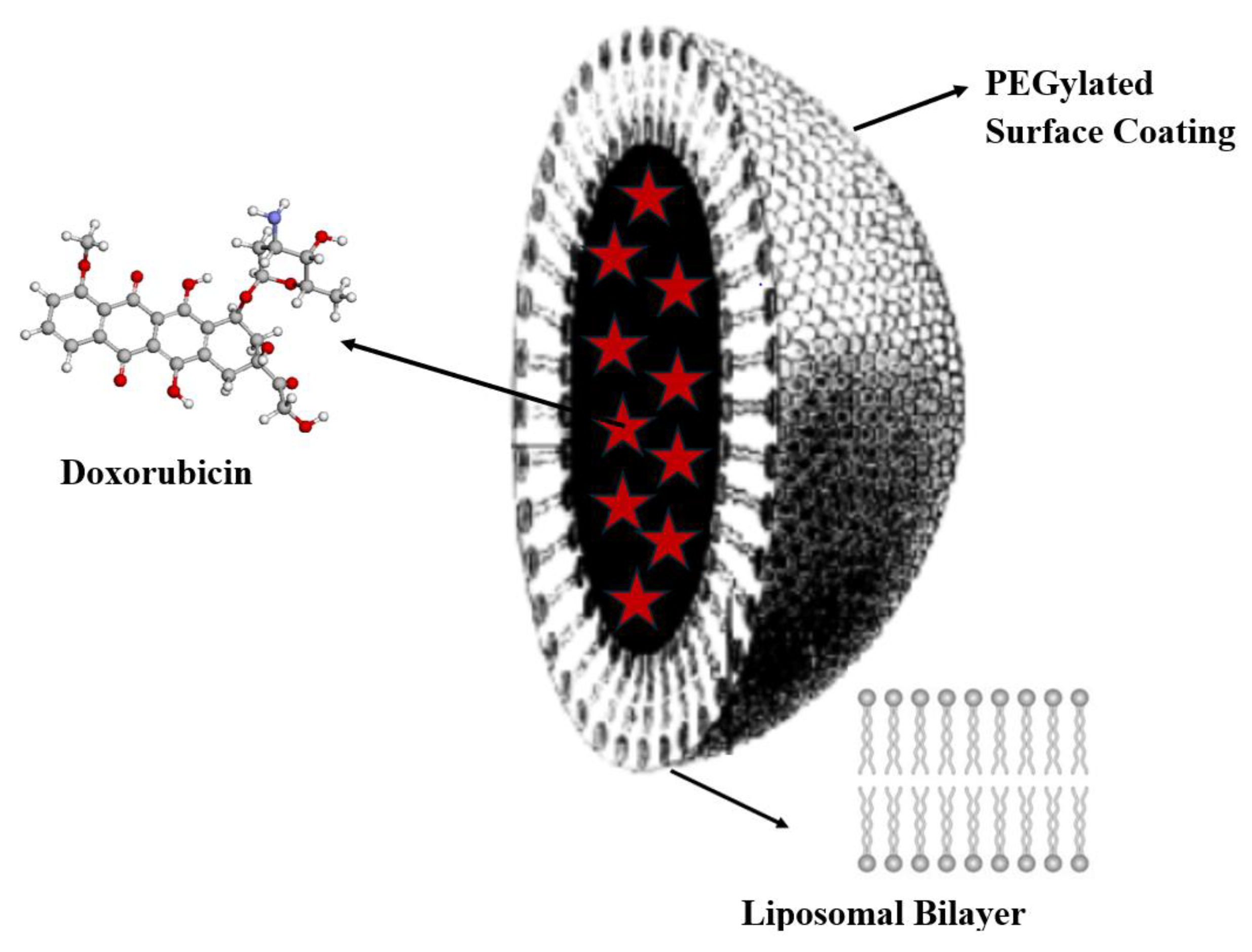
The impetus for developing antitumour drugs and treatment methods, which have become standard protocols still used today, was given in 1971 with the introduction of the National Cancer Act and the expansion of the National Cancer Institute’s (NCI) powers [67]. Many revolutionary discoveries in cancer molecular biology followed: restriction enzymes passage of national cancer act (1971); hybridomas and monoclonal antibodies 50% tracking of cancer statistics by seer program (1975); cellular origin of retroviral oncogenes (1979); epidermal growth factor and receptor 1981 suppression of tumor growth by p53 (1984); G proteins and cell (1984), etc. [68]. However, it took another 25 years of work before cancer treatment using nanoparticles as targeted delivery vehicles for diagnosis and therapy was realized: the first FDA-approved nanoparticle-based cancer drug was liposomal Doxil® in 1995 (Figure 12).

Figure 12.
The structure of the Doxil® liposomal dosage form, containing doxorubicin nanoencapsulated in a liquid vesicle core (red star symbols), stabilized with methoxypolyethylene glycol (d~80–100 nm).
The goal of developing liposomal Doxil® was to reduce the side effects of doxorubicin therapy, such as cardiomyopathy, by targeted delivery to the organ of interest [69,70]. Components of the liposomal dosage form (see Figure 9) collectively facilitate physiological endocytosis, endosomal escape, and release of the API cargo into the cytosol for translation [71].
Extensive experiments were conducted to isolate, stabilize, and study the physicochemical properties of nanoparticles whose sizes are comparable to the de Broglie wavelength of their charge carriers (i.e., electrons and holes) [72]. When this condition is met, a quantum effect occurs, whereby particles behave like zero-dimensional quantum dots that obey the rules of quantum mechanics [73]. In nanocrystals, electron wave functions are confined due to the increasing discreteness (lack of continuity) of energy levels, unlike bulk material of the same substance, resulting in higher energy and wider band gaps (Equation (4)):
where ΔE is the energy shift between discrete and continuous electrons in nano- and bulk-sized materials; n2 is the principal quantum number; h is Planck’s constant; m is the effective mass, and a is the quantum dot radius.
Special optical properties, caused by surface plasmon excitation in metallic nanoparticles and their ability to self-organize, can be exploited in medicine.
Key postulates and discoveries for future nanomedicine include the paradigm of assembling atoms into particles with the possibility of directed manipulation (“plenty of room at the bottom”), proposed by Richard Feynman in 1959 [74,75]; the discovery of liposomes by Alec Bangham in mid-1960s [76,77]; the introduction of the term “nanotechnology” by Norio Taniguchi in 1974 [78]. According to The National Nanotechnology Initiative (NNI), nanotechnology («nano» from the Greek word means «dwarf») is “a science, engineering, and technology conducted at the nanoscale (1 to 100 nm), where unique phenomena enable novel applications in a wide range of fields, from chemistry, physics and biology, to medicine, engineering and electronics” [79].
The dawn of the era of nanocytostatics (NCTCs) dictates the following characteristics for drugs to be effective in treatment: nanocarriers must reach the tumor with adequate API content; the drug pharmacokinetics (PK), biodistribution (BD), should be controlled by the nanocarriers and demonstrate a highly prolonged plasma circulation time; NCTCs should be available to tumor cells either by drug release at the tumor site or by the nanocarriers internalizing with the drug into tumor cells [80].
3.3.1. Radioactive Nanotheranostics
Attachment of therapeutic radionuclides to liposomes has shown significant promise in cancer treatment [78]. Based on the needs of modern oncological medicine, which include developing effective methods of noninvasively tracking and quantifying the distribution of liposomes, as targeted delivery vehicles for APIs in the body, methods for labeling liposomes with theranostic radionuclides have been proposed [79]. Nuclear methods, including radionuclide techniques, encompass highly sensitive modalities such as positron emission tomography (PET), gamma-emitting techniques such as single-photon emission tomography (SPECT), and planar scintigraphy. Scintigraphy (oт лaт. “scintilla”—spark or flicker) involves visualizing target liposomes in vivo using externally placed nuclear cameras.
The most commonly used nuclide for radiolabeling liposomes is Technetium-99m (99mTc). Its advantages include availability, relatively low cost, imaging capability, and an optimal half-life (see Table 2), and allowing imaging over 24 h [80].
Technetium-99m is a decay product of molybdenum-99 and undergoes gamma decay to form the ground state of technetium-99. Technetium-99 in the ground state can further decay to ruthenium (element 44). The overall synthesis and decay scheme is shown in (Equation (5)):
The second most widely used radionuclide for liposome radiolabeling is Indium-111 (111In), followed by iodine radioisotopes (see Table 2).
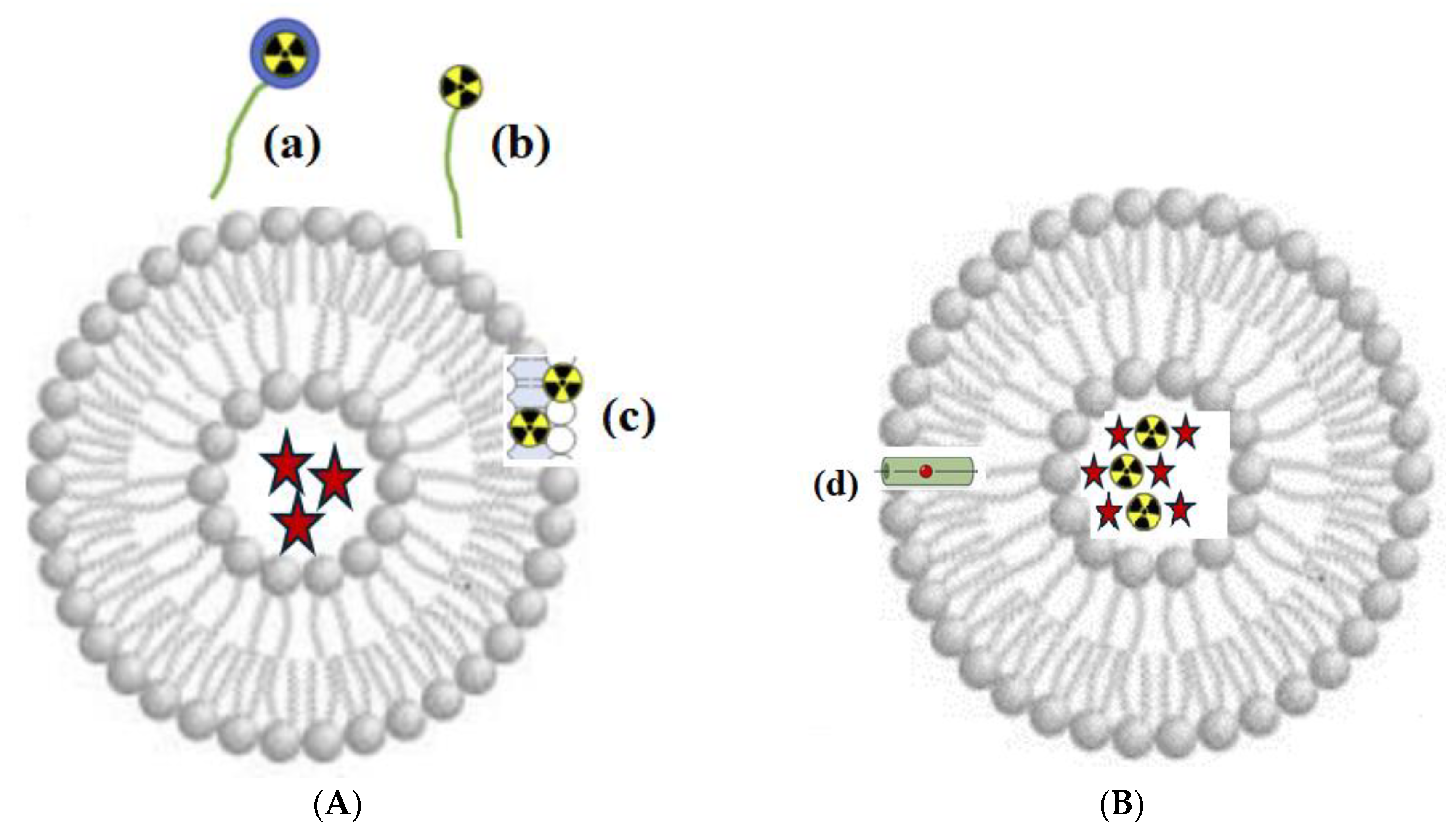
Depending on the method of radionuclide attachment, surface and internal nanoscale liposomal targeted systems are distinguished (Figure 13).

Figure 13.
Principle of radioactive labeling of liposomes. (A) Surface radioactive labeling: (a) radionuclide with chelator (Me); (b) radionuclide without chelator, linked to the liposome membrane via a PEG chain; and (c) radionuclide embedded in the lipid bilayer. (B) Intraliposomal radioactive labeling: radionuclide (black and yellow) and API (red star symbols), encapsulated in the aqueous core; (d) ionophore channel for transporting radionuclides across the bilayer.
3.3.2. Nanoparticles
Radioactive nanoparticles have found a unique application as an inhalable nanoaerosol containing radionuclides. The advantage of using radioactive nanoparticles is the ability to increase the effectiveness of lung imaging, using computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) as a standard method in lung disease diagnosis [81]. Examples of nanoparticles used for this purpose in medicine are presented in Table 3 [82,83,84].

Table 3.
Examples of radioactive nanoparticles used in imaging and therapy, with their diagnostic modality and associated advantages/disadvantages.
The examples of metallic nanoparticles of gold, silver, or iron (III) oxide presented in Table 3 have also attracted attention due to their potential for drug delivery (Au, Ag) and imaging (Fe) [85,86,87,88,89]. Metallic and oxide nanoparticles possess unique advantages described above, as well as a high surface area-to-volume ratio and the ability to penetrate biological barriers. Several formulations based on metallic nanoparticles have undergone clinical trials, and some have already been approved by the FDA, such as NanoTherm® (superparamagnetic iron oxide nanoparticles—SPION https://www.nanothermtx.com/, accessed on 12 June 2025) for the treatment of glioblastoma using magnetic hyperthermia (induction) therapy. Equipment for magnetic induction hyperthermia (MIH) converts magnetic energy into heat. This approach is considered an effective “green” cancer therapy due to its high safety and efficacy, attributable to the greater sensitivity of cancer cells to temperature compared to normal cells: cancer cells can be destroyed when the temperature reaches the target range of 42–46 °C [90].
Gold nanoparticles (AuNPs) are regarded as active components in cancer therapy and photothermal treatment (thermal ablation, TrAbl): AuNPs absorb electromagnetic radiation (near-infrared light, λ = 650–950 nm), acting as a heat source [91,92]. The heat flux density from the nano-source depends on the local electromagnetic field (Equation (6)):
where Ex (electromagnetic field) is the solution of the Helmholtz equation with a radiation boundary condition; ω is the incident angular frequency; e0 is the permittivity of a vacuum; and er is the complex relative permittivity of materials.
The efficiency of ablation is directly related to the size, shape, and agglomeration of nanoparticles (within the de Broglie wavelength) and their therapeutic concentration. However, as the size increases, so does the likelihood of damage to healthy tissues surrounding the tumor.
Regarding silver nanoparticles (AgNPs), according to research findings [93], AgNPs act as inducers of reactive oxygen species (ROS) within cells, causing oxidative stress through lipid peroxidation. Intracellular ROS production induced by AgNPs should be considered a key indicator of toxicity and may be viewed as the initial step in toxicity cascades: due to depletion of antioxidant capability (DAC), cells undergo programmed cell death (apoptosis) at the initiation stage of the toxicity cascade [94].
Radiotheranostics, which combines the diagnostic action of unstable nuclei with therapeutic effects, serves as an effective adjunct and/or standalone method in the treatment of oncology patients. Despite emerging resistance to both chemo- and radiotherapy, existing radionuclide resources provide protection against subsequent tumor metastasis. However, given the unfavorable prognosis for cancer incidence over the next 25 years, the development of “ahead-of-the-curve” pharmaceuticals is welcomed. This will be facilitated by current medical knowledge and understanding of ligand–receptor interaction mechanisms to trigger apoptosis in rapidly proliferating cells, as detailed in this research review.
4. Future Directions
The development of antitumour drugs for targeted delivery to organs expressing higher levels of endocytic receptors represents a complex set of challenges related to resistance, limitations, and opportunities that affect their efficacy. One promising avenue for cancer therapy, beyond the identification and study of new nuclear isotopes [95,96], is the development and application of genetically modified and unmodified oncolytic viruses capable of also targeting the tumor microenvironment [97,98].
All new methods introduced into cancer therapy promise to be as effective as possible in eradicating all cancer types [99]. However, experience shows that a universal «cure for cancer» is unlikely ever to be found [100]. Yet, in the hands of scientists, the development of adjunct therapies to existing treatments can gradually reduce oncogenicity and contribute to improved survival rates.
Author Contributions
Conceptualization, E.V.U.; methodology, E.V.U. and I.A.V.; software, A.S.; validation, D.V.A.; formal analysis, E.V.U.; resources, A.A.T.; data curation, I.V.K.; writing—original draft preparation, E.V.U.; writing—review and editing, A.S., D.V.A., I.A.V., I.V.K. and R.A.Z.; supervision, R.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RUDN University Scientific Projects Grant System, grant number 033322-2-000.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IARC | International Agency for Research on Cancer |
| NCI | National Cancer Institute |
| NMSCs | Nonmelanoma skin cancers |
| SM | Sulfur mustard |
| NM | Nitrogen mustard |
| HDI | Human Development Index |
| siRNA | Small interfering RNA |
| mABs-drug | monoclonal AntiBody |
| MtRth | Metabolic RadioTherapy |
| DSB | DNA double-strand break |
| IT | Isomeric Transition |
| ISOLDE | Isotope Separator On-Line Detector |
| API | Active Pharmaceutical Ingredient |
| PET | Positron emission tomography |
| MRI | Magnetic resonance imaging |
| LET | Linear energy transfer |
| MIBG | Meta-iodobenzylguanidine |
| ARPI | Active radiopharmaceutical ingredients |
| RLT | Radioligand therapy |
| NCRT | Neoadjuvant chemoradiotherapy |
| ACRT | Adjuvant chemoradiotherapy |
| NETs | Neuroendocrine tumors |
| SST | Somatostatin |
| PRRT | Peptide receptor radionuclide therapy |
| PSMA | Prostate-specific membrane antigen |
| GEP | Gastroenteropancreatic |
| RTSR | Radioligand therapy of somatostatin receptor |
| MtMn | Metastatic melanoma |
| MC1R | Melanocortin- subtype 1 receptor |
| GPCRs | G protein-coupled receptors |
| α-MSH | α-Melanocyte-stimulating hormone |
| FES | 18F-fluoroestradiol |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| HR | Hormone Receptor |
| FDG | 8F-fluorodeoxyglucose |
| FR-α,β | Folate receptor alpha,beta |
| MSC | Mesenchymal stem cells |
| NNI | The National Nanotechnology Initiative |
| NCTCs | Nanocytostatic Therapeutic Complexes |
| PK | Pharmacokinetics |
| BD | Biodistribution |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-Triacetic Acid |
| NODAG | Glutaric Acid Derivative of NOTA |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetraacetic Acid |
| DODAGA | Derivative of DOTA |
| NCTCs | Nanocytostatics |
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanoparticles |
| TrAbl | Thermal ablation |
| SPIONs | SuperParamagnetic Iron Oxide Nanoparticles |
| MIH | Magnetic induction hyperthermia |
| ROS | Reactive oxygen species |
| DAC | Depletion of antioxidant capability |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Abergel, R.; Aris, J.; Bolch, W.E.; Dewji, S.A.; Golden, A.; Hooper, D.A.; Margot, D.; Menker, C.G.; Paunesku, T.; Schaue, D.; et al. The enduring legacy of Marie Curie: Impacts of radium in 21st century radiological and medical sciences. Int. J. Radiat. Biol. 2022, 98, 267–275. [Google Scholar] [CrossRef]
- Elliott, R.L. Combination cancer immunotherapy “Expanding Paul Ehrlich’s Magic Bullet Concept”. Surg. Oncol. 2012, 21, 53–55. [Google Scholar] [CrossRef]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar] [CrossRef]
- Liner, K.; Brown, C.; McGirt, L.Y. Clinical potential of mechlorethamine gel for the topical treatment of mycosis fungoides-type cutaneous T-cell lymphoma: A review on current efficacy and safety data. Drug Des. Devel. Ther. 2018, 12, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Nitrogen mustard therapy. Use of methyl-bis(beta-chloroethyl) amine hydrochloride and tris(beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 1984, 251, 2255–2261. [Google Scholar] [CrossRef]
- Ravichandran, R. Radioactive Cobalt-60 Teletherapy Machine—Estimates of Personnel Dose in Mock Emergency in Patient Release during “Source Stuck Situation”. J. Med. Phys. 2017, 42, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kudchodkar, S.B.; Chung, C.N.; Park, Y.K.; Xu, Z.; Pardi, N.; Abdel-Mohsen, M.; Muthumani, K. Expanding the Reach of Monoclonal Antibodies: A Review of Synthetic Nucleic Acid Delivery in Immunotherapy. Antibodies 2023, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; Leporatti, S. Nanomedicine in Cancer Targeting and Therapy. J. Pers. Med. 2022, 12, 1312. [Google Scholar] [CrossRef]
- Henderson, M.L.; Zieba, J.K.; Li, X.; Campbell, D.B.; Williams, M.R.; Vogt, D.L.; Bupp, C.P.; Edgerly, Y.M.; Rajasekaran, S.; Hartog, N.L. Gene Therapy for Genetic Syndromes: Understanding the Current State to Guide Future Care. BioTech 2024, 13, 1. [Google Scholar] [CrossRef]
- Song, Y.; Zou, J.; Castellanos, E.A.; Matsuura, N.; Ronald, J.A.; Shuhendler, A.; Weber, W.A.; Gilad, A.A.; Müller, C.; Witney, T.H.; et al. Theranostics—A sure cure for cancer after 100 years? Theranostics 2024, 14, 2464–2488. [Google Scholar] [CrossRef]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The landscape of nanoparticle-based siRNA delivery and therapeutic development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef]
- Bonnet, B.; Tournier, L.; Deschamps, F.; Yevich, S.; Marabelle, A.; Robert, C.; Albiges, L.; Besse, B.; Bonnet, V.; De Baère, T. Thermal Ablation Combined with Immune Checkpoint Blockers: A 10-Year Monocentric Experience. Cancers 2024, 16, 855. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.S.; Salari, E.; Chen, X.; Switchenko, J.; Eaton, B.R.; Zhong, J.; Yang, X.; Shu, H.-K.G.; Sudmeier, L.J. Radiomic Analysis of Treatment Effect for Patients with Radiation Necrosis Treated with Pentoxifylline and Vitamin E. Tomography 2024, 10, 1501–1512. [Google Scholar] [CrossRef]
- Scott, E.C.; Baines, A.C.; Gong, Y. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US); Institute of Medicine (US); Committee on State of the Science of Nuclear Medicine. Advancing Nuclear Medicine Through Innovation; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Otto, T. Personal dose-equivalent conversion coefficients for 1252 radionuclides. Radiat. Prot. Dosim. 2016, 168, 1–10. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef] [PubMed]
- Alpha Emitter Radiation Therapy National Cancer Institute (NCI). Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/alpha-emitter-radiation-therapy (accessed on 17 May 2025).
- Selected Beta Particle Emitting Radionuclides for Therapeutic Nuclear Medicine by Open Medscience. Available online: https://openmedscience.com (accessed on 7 January 2025).
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, A.; Undavalli, S.B.; Kumar, A.J. DNA mutilation: A telltale sign of cancer inception. J. Oral Maxillofac. Pathol. 2023, 27, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Syroeshkin, A.V.; Uspenskaya, E.V.; Levitskaya, O.V.; Kuzmina, E.S.; Kazimova, I.V.; Quynh, H.T.N.; Pleteneva, T.V. New Approaches to Determining the D/H Ratio in Aqueous Media Based on Diffuse Laser Light Scattering for Promising Application in Deuterium-Depleted Water Analysis in Antitumor Therapy. Sci. Pharm. 2024, 92, 63. [Google Scholar] [CrossRef]
- Yang, S.N. Two-Boson Exchange Physics: A Brief Review. Few-Body Syst. 2013, 54, 45–51. [Google Scholar] [CrossRef]
- Adamian, G.G.; Antonenko, N.V.; Diaz-Torres, A. How to extend the chart of nuclides? Eur. Phys. J. 2020, 56, 47. [Google Scholar] [CrossRef]
- Arsenyev, N.N.; Severyukhin, A.P. Electric Dipole Polarizability of Magic Nuclei. Mosc. Univ. Phys. 2024, 79, 200–207. [Google Scholar] [CrossRef]
- Burkhardt, C.; Bühler, L.; Viertl, D.; Stora, T. New Isotopes for the Treatment of Pancreatic Cancer in Collaboration with CERN: A Mini Review. Front. Med. 2021, 8, 674656. [Google Scholar] [CrossRef]
- Bara, S.; Jajčišinová, E.; Cocolios, T.E.; Andel, B.; Antalic, S.; Camaiani, A.; Costache, C.; Dockx, K.; Farooq-Smith, G.J.; Kellerbauer, A.; et al. Half-life determination of 215At and 221Ra with high-purity radioactive ion beams. Appl. Radiat. Isot. 2024, 208, 111289. [Google Scholar] [CrossRef]
- El-Azony, K.M.; Mohamed, N.M.A.; Aloraini, D.A. Advantages and disadvantages of nuclear reactions used in reactors or cyclotrons, in addition to a theoretical study based on photodisintegration on natural indium for 111Ag production. Nucl. Sci. Tech. 2022, 33, 14. [Google Scholar] [CrossRef]
- Moya, E.; Cerrato, C.; Bedoya, L.M. Radiopharmaceutical small-scale preparation in Europe: Will we be able to harmonize the situation? EJNMMI Radiopharm. Chem. 2024, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Foy, K.C.; Kaumaya, P.T. Cancer immunotherapy: Present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 2013, 15, 166–176. [Google Scholar]
- Idée, J.M.; Louguet, S.; Ballet, S.; Corot, C. Theranostics and contrast-agents for medical imaging: A pharmaceutical company viewpoint. Quant. Imaging Med. Surg. 2013, 3, 292–297. [Google Scholar] [PubMed]
- Okamoto, S.; Shiga, T.; Tamaki, N. Clinical Perspectives of Theranostics. Molecules 2021, 26, 2232. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Siegel, E. The beginnings of radioiodine therapy of metastatic thyroid carcinoma: A memoir of Samuel M. Seidlin, M. D. (1895–1955) and his celebrated patient. Cancer Biother. Radiopharm. 1999, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lișcu, H.D.; Verga, N.; Atasiei, D.I.; Ilie, A.-T.; Vrabie, M.; Roșu, L.; Poștaru, A.; Glăvan, S.; Lucaș, A.; Dinulescu, M.; et al. Therapeutic Management of Locally Advanced Rectal Cancer: Existing and Prospective Approaches. J. Clin. Med. 2025, 14, 912. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu] Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Burki, T.K. 177Lu-Dotatate for midgut neuroendocrine tumours. Lancet Oncol. 2017, 18, e74. [Google Scholar] [CrossRef]
- Imhof, A.; Brunner, P.; Marincek, N. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.M.B. The radiopharmaceutical science of monoclonal antibodies and peptides for imaging and targeted in situ radiotherapy of malignancies. In Handbook of Pharmaceutical Biotechnology; Gad, S.C., Ed.; John Wiley & Sons: Toronto, ON, Canada, 2007; pp. 987–1053. [Google Scholar]
- Chambers, C.G.; Wang, J.; Sakr, T.M.; Miao, Y.; Smith, C.J. NOTA and NODAGA Radionuclide Complexing Agents: Versatile Approaches for Advancements in Radiochemistry. Molecules 2025, 30, 2095. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Engelbrecht, S.; Läppchen, T.; Rominger, A.; Gourni, E. GRPR-targeting radiotheranostics for breast cancer management. Front. Med. 2023, 10, 1250799. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Li, A.A.; Geliashvili, T.M.; Rumyantsev, A.A.; Pronin, A.I.; Krylov, A.S.; Baranova, O.D.; Parnas, A.V.; Ilyakov, V.S.; Vorobyeva, D.A. Impressive response to 177Lu-PSMA-617 therapy in a patient with metastatic castration-resistant prostate cancer refractory to apalutamide, docetaxel and metastasis-directed therapy. Onkourologiya Cancer Urol. 2024, 20, 98–103. (In Russian) [Google Scholar] [CrossRef]
- Heyder, N.A.; Kleinau, G.; Speck, D. Structures of active melanocortin-4 receptor–Gs-protein complexes with NDP-α-MSH and setmelanotide. Cell Res. 2021, 31, 1176–1189. [Google Scholar] [CrossRef]
- Suominen, A.; Suni, A.; Ruohonen, S.; Szabó, Z.; Pohjolainen, L.; Cai, M.; Savontaus, E.; Talman, V.; Kerkelä, R.; Petteri, R. Melanocortin 1 Receptor Regulates Pathological and Physiological Cardiac Remodeling. J. Am. Heart Assoc. 2025, 14, 037961. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.; Kim, W.; Shin, D. Melanocortin 1 Receptor (MC1R): Pharmacological and Therapeutic Aspects. Int. J. Mol. Sci. 2023, 24, 12152. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for labeling proteins and peptides with lead-212. Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.; Said, S.A.; Al-abyad, M. Evaluation of different production routes for the radio medical isotope 203Pb using TALYS 1.4 and EMPIRE 3.1 code calculations. Appl. Radiate Isot. 2014, 91, 109–113. [Google Scholar] [CrossRef]
- McNeil, B.L.; Robertson, A.K.H.; Fu, W. Production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair. EJNMMI Radiopharm. Chem. 2021, 6, 6. [Google Scholar] [CrossRef]
- Kosenko, V.V.; Trapkova, A.A.; Kalmykov, S.N. Regulation of radiopharmaceutical products. Bulletin of the Scientific Centre for Expert Evaluation of Medicinal Products. Regul. Res. Med. Eval. 2022, 12, 379–388. [Google Scholar]
- Venema, C.M.; Apollonio, G.; Hospers, G.A.; Schröder, C.P.; Dierckx, R.A.; Vries, E.F.; Glaudemans, A.W. Recommendations and Technical Aspects of 16α-[18F]Fluoro-17β-Estradiol PET to Image the Estrogen Receptor In Vivo: The Groningen Experience. Clin. Nucl. Med. 2016, 41, 844–851. [Google Scholar] [CrossRef]
- Parnas, A.V.; Pronin, A.I.; Ilyakov, V.S.; Meshcheryakova, N.A.; Kamolova, Z.K.; Mikhaylov, A.I. [18F]-Fluoroestradiol PET/CT: A modern look at nuclear medicine applications. Tumors Female Reprod. Syst. 2021, 17, 20–26. [Google Scholar] [CrossRef]
- Talbot, J.N.; Gligorov, J.; Nataf, V.; Montravers, F.; Huchet, V.; Michaud, L.; Ohnona, J.; Balogova, S.; Cussenot, O.; Daraï, E.; et al. Current applications of PET imaging of sex hormone receptors with a fluorinated analogue of estradiol or of testosterone. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 4–17. [Google Scholar]
- O’Brien, S.R.; Edmonds, C.E.; Lanzo, S.M.; Weeks, J.K.; Mankoff, D.A.; Pantel, A.R. 18F-Fluoroestradiol: Current Applications and Future Directions. Radiographics 2023, 43, e220143. [Google Scholar] [CrossRef] [PubMed]
- Banyal, A.; Tiwari, S.; Sharma, A.; Chanana, I.; Patel, S.K.S.; Kulshrestha, S.; Kumar, P. Vinca alkaloids as a potential cancer therapeutics: Recent update and future challenges. 3 Biotech 2023, 13, 211. [Google Scholar] [CrossRef]
- Zhou, Y.; Unno, K.; Hyjek, E.; Liu, H.; Zimmerman, T.; Karmakar, S.; Putt, K.S.; Shen, J.; Low, P.S.; Wickrema, A. Expression of functional folate receptors in multiple myeloma. Leuk. Lymphoma 2018, 59, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Y.; Wang, C.; Xia, Y.; Yu, T.; Tang, M.; Meng, K.; Yin, L.; Yang, Y.; Shen, L.; et al. The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics. MedComm 2024, 5, e663. [Google Scholar] [CrossRef]
- Vergote, I.; Leamon, C.P. Vintafolide: A novel targeted therapy for the treatment of folate receptor expressing tumors. Ther. Adv. Med. Oncol. 2015, 7, 206–218. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 13342, Vinblastine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vinblastine (accessed on 23 May 2025).
- Surh, Y.J. The 50-Year War on Cancer Revisited: Should We Continue to Fight the Enemy Within? J. Cancer Prev. 2021, 26, 219–223. [Google Scholar] [CrossRef]
- DeVita, V.T.; Rosenberg, S.A., Jr. Two hundred years of cancer research. N. Engl. J. Med. 2012, 366, 2207–2214. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and humanstudies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Vysikaylo, P.I.; Samsonenko, N.V.; Semin, M.V. De Broglie wave in vacuum, matter and nanostructures. J. Phys. Conf. Ser. 2020, 1560, 012006. [Google Scholar] [CrossRef]
- Daniel, M.C.; Didier, A. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Yeo, J.; Jung, G.S.; Martín-Martínez, F.J.; Ling, S.; Gu, G.X.; Qin, Z.; Buehler, M.J. Materials-by-Design: Computation, Synthesis, and Characterization from Atoms to Structures. Phys. Scr. 2018, 93, 053003. [Google Scholar] [CrossRef]
- Ghaffar, K.A.; Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- National Nanotechnology Initiative (NNI). Available online: www.nano.gov (accessed on 1 June 2025).
- Satterlee, A.B.; Yuan, H.; Huang, L. A radio-theranostic nanoparticle with high specific drug loading for cancer therapy and imaging. J. Control. Release 2015, 217, 170–182. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.A.; Bao, A. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. Radioact. Liposomes 2008, 1, 69–83. [Google Scholar]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Kane, S.M.; Padda, I.S.; Patel, P.; Davis, D.D. Technetium-99m. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Munir, M.; Setiawan, H.; Awaludin, R.; Kett, V.L. Aerosolised micro and nanoparticle: Formulation and delivery method for lung imaging. Clin. Transl. Imaging 2023, 11, 33–50. [Google Scholar] [CrossRef]
- Silva, M.C.; Silva, A.S.; Fernandez-Lodeiro, J. Supercritical CO2-assisted spray drying of strawberry-like gold-coated magnetite nanocomposites in chitosan powders for inhalation. Materials 2017, 10, 74. [Google Scholar] [CrossRef]
- Huynh, M.; Kempson, I.; Bezak, E.; Phillips, W. Predictive modeling of hypoxic head and neck cancers during fractionated radiotherapy with gold nanoparticle radiosensitization. Med. Phys. 2021, 48, 3120–3133. [Google Scholar] [CrossRef]
- Patchin, E.S.; Anderson, D.S.; Silva, R.M. Size-dependent deposition, translocation, and microglial activation of inhaled silver nanoparticles in the rodent nose and brain. Environ. Health Perspect. 2016, 124, 1870–1875. [Google Scholar] [CrossRef]
- Gomes, M.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Advancing Brain Targeting: Cost-Effective Surface-Modified Nanoparticles for Faster Market Entry. Pharmaceutics 2025, 17, 661. [Google Scholar] [CrossRef]
- Kiwumulo, H.; Muwonge, H.; Lubwama, M. Iron oxide nanoparticles in leukemia: Design, diagnostic applications, and therapeutic strategies. J. Egypt. Natl. Canc. Inst. 2025, 37, 44. [Google Scholar]
- Lian, Y.; Wang, L.; Cao, J. Recent advances on the magnetic nanoparticle–based nanocomposites for magnetic induction hyperthermia of tumor: A short review. Adv. Compos. Hybrid Mater. 2021, 4, 925–937. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Grosges, T.; Barchiesi, D. Gold Nanoparticles as Photothermal Agent in Cancer Therapy: Theoretical Study of Concentration and Agglomeration Effects on Temperature. Appl. Sci. 2022, 12, 3315. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Lee, K.W.; Park, T.G. Optical Imaging of Intracellular Reactive Oxygen Species for the Assessment of the Cytotoxicity of Nanoparticles. Biomaterials 2011, 32, 2556–2565. [Google Scholar] [CrossRef]
- Niwase, T.; Watanabe, Y.X.; Hirayama, Y.; Mukai, M.; Schury, P.; Andreyev, A.N.; Hashimoto, T.; Iimura, S.; Ishiyama, H.; Ito, Y.; et al. Discovery of New Isotope 241U and Systematic High-Precision Atomic Mass Measurements of Neutron-Rich Pa-Pu Nuclei Produced via Multinucleon Transfer Reactions. Phys. Rev. Lett. 2023, 130, 132502. [Google Scholar] [PubMed]
- Tarasov, O.B.; Gade, A.A.; Fukushima, K.; Hausmann, M.; Kwan, E.; Portillo, M.; Smith, M.; Ahn, D.S.; Bazin, D.; Chyzh, R.; et al. Observation of New Isotopes in the Fragmentation of Pt198 at FRIB. Phys. Rev. Lett. 2024, 132, 072501. [Google Scholar]
- Liu, X. Development and application of oncolytic viruses as the nemesis of tumor cells. Front. Microbiol. 2023, 14, 1188526. [Google Scholar] [CrossRef]
- Science-Based Medicine. Tag: Oncolytic Viruses. Available online: https://sciencebasedmedicine.org/tag/oncolytic-viruses/ (accessed on 30 May 2025).
- Milestones in Cancer Research and Discovery. National Cancer Institute (NIH). Available online: https://www.cancer.gov/research/progress/250-years-milestones (accessed on 18 June 2025).
- Downar, J. Cancer: It’s time to change the sign. CMAJ 2010, 182, 1588. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).