Are Calculated Immune Markers with or Without Comorbidities Good Predictors of Colorectal Cancer Survival? The Results of a Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Clinicopathological and Laboratory Data Measurements
2.3. Statistical Analysis
3. Results
3.1. Longitudinal Data Analysis Results
3.2. Survival Analysis Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| CV | Cardiovascular |
| DSS | Disease-specific survival |
| OS | Overall survival |
| PFS | Progression-free survival |
| PIV | Pan-immune inflammation value |
| PNI | Prognostic nutritional index |
| SII | Systemic immune-inflammation index |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Herold, Z.; Herold, M.; Lohinszky, J.; Szasz, A.M.; Dank, M.; Somogyi, A. Longitudinal changes in personalized platelet count metrics are good indicators of initial 3-year outcome in colorectal cancer. World J. Clin. Cases 2022, 10, 6825–6844. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Hu, X.; Gao, T.; Zhou, H.; Li, B.; Zhou, C.; Yu, B.; Wang, G. Potential impact of platelet-to-lymphocyte ratio on prognosis in patients with colorectal cancer: A systematic review and meta-analysis. Front. Surg. 2023, 10, 1139503. [Google Scholar] [CrossRef] [PubMed]
- Naszai, M.; Kurjan, A.; Maughan, T.S. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 5983–5997. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Bartolotta, P.; Azzolina, D.; Gregori, D.; Fiscon, V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: Systematic review and meta-analysis. Langenbecks Arch. Surg. 2023, 408, 85. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef]
- Fuca, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: A systematic review and meta-analysis. Int. J. Color. Dis. 2019, 34, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, P.; Carkman, M.S. The relationship between the Prognostic Nutritional Index and lymphovascular and perineural invasion of the tumor in patients diagnosed with gastric cancer, and its effect on overall survival. Medicine 2024, 103, e40087. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Saliba, W.; Schwartz, J.B. Prevalence and proportion by age and sex of chronic health conditions in a large healthcare system. PLoS ONE 2024, 19, e0308031. [Google Scholar] [CrossRef]
- Pennisi, F.; Buzzoni, C.; Russo, A.G.; Gervasi, F.; Braga, M.; Renzi, C. Comorbidities, Socioeconomic Status, and Colorectal Cancer Diagnostic Route. JAMA Netw. Open 2025, 8, e258867. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, L.; Zhou, L.; Wang, X. Comorbidity Patterns in Patients Newly Diagnosed with Colorectal Cancer: Network-Based Study. JMIR Public Health Surveill. 2023, 9, e41999. [Google Scholar] [CrossRef]
- Rashmi, R.; Mohanty, S.K. Examining chronic disease onset across varying age groups of Indian adults using competing risk analysis. Sci. Rep. 2023, 13, 5848. [Google Scholar] [CrossRef]
- Cuthbert, C.A.; Hemmelgarn, B.R.; Xu, Y.; Cheung, W.Y. The effect of comorbidities on outcomes in colorectal cancer survivors: A population-based cohort study. J. Cancer Surviv. 2018, 12, 733–743. [Google Scholar] [CrossRef]
- Quintana, J.M.; Anton-Ladislao, A.; Lazaro, S.; Gonzalez, N.; Bare, M.; Fernandez-de-Larrea, N.; Redondo, M.; Escobar, A.; Sarasqueta, C.; Garcia-Gutierrez, S.; et al. Effect of comorbidities on long-term outcomes of colorectal cancer patients. Eur. J. Cancer Care 2022, 31, e13561. [Google Scholar] [CrossRef]
- Salika, T.; Lyratzopoulos, G.; Whitaker, K.L.; Waller, J.; Renzi, C. Do comorbidities influence help-seeking for cancer alarm symptoms? A population-based survey in England. J. Public Health 2018, 40, 340–349. [Google Scholar] [CrossRef]
- Jessup, J.; Goldberg, R.; Asare, E.; Benson, A.; Brierley, J.; Chang, G.; Chen, V.; Compton, C.; De Nardi, P.; Goodman, K.; et al. Colon and Rectum. In AJCC Cancer Staging Manual, 8th ed.; Amin, M., Edge, S., Greene, F., Byrd, D., Brookland, R., Washington, M., Gershenwald, J., Compton, C., Hess, K., Sullivan, D., et al., Eds.; Springer International Publishing: Chicago, IL, USA, 2018; pp. 251–274. [Google Scholar]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. 2018, 11, 264–273. [Google Scholar] [CrossRef]
- Pinato, D.J.; North, B.V.; Sharma, R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI). Br. J. Cancer 2012, 106, 1439–1445. [Google Scholar] [CrossRef]
- Tohme, S.; Chidi, A.P.; Sud, V.; Tsung, A. Prognostic Nutritional Index Is Associated with Survival in Patients with Unresectable Hepatocellular Carcinoma Treated with Radioembolization. J. Vasc. Interv. Radiol. 2017, 28, 470–472. [Google Scholar] [CrossRef]
- Burgos-Molina, A.M.; Tellez Santana, T.; Redondo, M.; Bravo Romero, M.J. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, L.; Gu, P.; Hua, Y.; Sun, Y.; Ni, S.; Zhou, X.; Tang, Z. Pan-immune-inflammation and its dynamics: Predictors of survival and immune-related adverse events in patients with advanced NSCLC receiving immunotherapy. BMC Cancer 2023, 23, 944. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, Q.; Li, Y. The association between pan-immune-inflammation value and chronic obstructive pulmonary disease: Data from NHANES 1999-2018. Front. Physiol. 2024, 15, 1440264. [Google Scholar] [CrossRef]
- Susok, L.; Said, S.; Reinert, D.; Mansour, R.; Scheel, C.H.; Becker, J.C.; Gambichler, T. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J. Cancer Res. Clin. Oncol. 2022, 148, 3103–3108. [Google Scholar] [CrossRef]
- Yang, X.C.; Liu, H.; Liu, D.C.; Tong, C.; Liang, X.W.; Chen, R.H. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1036890. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Zhang, Z.; Wang, K.; Sulayman, S.; Zeng, X.; Ababaike, S.; Guan, J.; Zhao, Z. Preoperative pan-immuno-inflammatory values and albumin-to-globulin ratio predict the prognosis of stage I-III colorectal cancer. Sci. Rep. 2025, 15, 11517. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Bae, S.U.; Kim, K.E.; Jeong, W.K.; Baek, S.K. Effects of the Strength, Assistance in walking, Rise from a chair, Climb stairs, and Falls score on postoperative clinical outcomes following colorectal cancer surgery: A retrospective study. Eur. J. Clin. Nutr. 2025, 79, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shimizu, T.; Ishizuka, M.; Suda, K.; Shibuya, N.; Hachiya, H.; Iso, Y.; Takagi, K.; Aoki, T.; Kubota, K. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I-III colorectal cancer patients undergoing surgery. Surg. Today 2022, 52, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, K.E.; Jeong, W.K.; Baek, S.K.; Bae, S.U. Effect of preoperative pan-immune-inflammation value on clinical and oncologic outcomes after colorectal cancer surgery: A retrospective study. Ann. Surg. Treat. Res. 2024, 106, 169–177. [Google Scholar] [CrossRef]
- Sato, R.; Oikawa, M.; Kakita, T.; Okada, T.; Abe, T.; Tsuchiya, H.; Akazawa, N.; Ohira, T.; Harada, Y.; Okano, H.; et al. A decreased preoperative platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value are associated with the poorer survival of patients with a stent inserted as a bridge to curative surgery for obstructive colorectal cancer. Surg. Today 2023, 53, 409–419. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Zhang, W.; Cheng, D.; Lu, Y.; Wang, C.; Li, J.; You, L.; Yu, J.; Guo, W.; et al. Pan-immune-inflammation value is associated with the clinical stage of colorectal cancer. Front. Surg. 2022, 9, 996844. [Google Scholar] [CrossRef]
- Liang, L.; Guo, X.; Ye, W.; Liu, Y. KRAS Gene Mutation Associated with Grade of Tumor Budding and Peripheral Immunoinflammatory Indices in Patients with Colorectal Cancer. Int. J. Gen. Med. 2024, 17, 4769–4780. [Google Scholar] [CrossRef]
- Perez-Martelo, M.; Gonzalez-Garcia, A.; Vidal-Insua, Y.; Blanco-Freire, C.; Brozos-Vazquez, E.M.; Abdulkader-Nallib, I.; Alvarez-Fernandez, J.; Lazare-Iglesias, H.; Garcia-Martinez, C.; Betancor, Y.Z.; et al. Clinical significance of baseline Pan-Immune-Inflammation Value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci. Rep. 2022, 12, 6893. [Google Scholar] [CrossRef]
- Corti, F.; Lonardi, S.; Intini, R.; Salati, M.; Fenocchio, E.; Belli, C.; Borelli, B.; Brambilla, M.; Prete, A.A.; Quara, V.; et al. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2021, 150, 155–167. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Menyhart, O.; Fekete, J.T.; Gyorffy, B. Inflammation and Colorectal Cancer: A Meta-Analysis of the Prognostic Significance of the Systemic Immune-Inflammation Index (SII) and the Systemic Inflammation Response Index (SIRI). Int. J. Mol. Sci. 2024, 25, 8441. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, B.; Li, Q.; Cao, W. Prognostic value and clinicopathological significance of pre-and post-treatment systemic immune-inflammation index in colorectal cancer patients: A meta-analysis. World J. Surg. Oncol. 2025, 23, 11. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Muntean, C.; Bolboaca, S.D.; Olariu, S. Exploratory Evaluation of Pre-Treatment Inflammation Profiles in Patients with Colorectal Cancer. Diseases 2024, 12, 61. [Google Scholar] [CrossRef]
- Sun, J.; Yang, R.; Wu, H.; Li, L.; Gu, Y. Prognostic value of preoperative combined with postoperative systemic immune-inflammation index for disease-free survival after radical rectal cancer surgery: A retrospective cohort study. Transl. Cancer Res. 2024, 13, 371–380. [Google Scholar] [CrossRef]
- Malik, M.; Radecka, B.; Gelej, M.; Jackowska, A.; Filipczyk-Cisarz, E.; Zurowska, M.; Hetman, K.; Foszczynska-Kloda, M.; Kania-Zembaczynska, B.; Manka, D.; et al. Predictive and Prognostic Role of Systemic Immune-Inflammation Index (SII) in Metastatic Colorectal Cancer Patients Treated with Trifluridine/Tipiracil. Biomedicines 2024, 12, 2076. [Google Scholar] [CrossRef]
- Popovici, D.; Stanisav, C.; Sima, L.V.; Negru, A.; Murg, S.I.; Carabineanu, A. Influence of Biomarkers on Mortality among Patients with Hepatic Metastasis of Colorectal Cancer Treated with FOLFOX/CAPOX and FOLFIRI/CAPIRI, Including Anti-EGFR and Anti-VEGF Therapies. Medicina 2024, 60, 1003. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Azzali, I.; Bittoni, A.; Marisi, G.; Rebuzzi, F.; Molinari, C.; Bartolini, G.; Matteucci, L.; Sullo, F.G.; Debonis, S.A.; et al. Inflammatory indices as prognostic markers in metastatic colorectal cancer patients treated with chemotherapy plus Bevacizumab. Ther. Adv. Med. Oncol. 2023, 15, 17588359231212184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, H.; Guo, X.; Gu, K.; Wang, W.; Chen, X.; Ji, S.; Yang, H.; Zhu, J. The Change of Systemic Immune-Inflammation Index Independently Predicts Survival of Colorectal Cancer Patients after Curative Resection. Mediat. Inflamm. 2020, 2020, 4105809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Pang, S.; Yu, X.C.; Xue, Q.; Jiang, H.Y.; Liang, X.J.; Liu, L. Predictive Values of Postoperative and Dynamic Changes of Inflammation Indexes in Survival of Patients with Resected Colorectal Cancer. Curr. Med. Sci. 2018, 38, 798–808. [Google Scholar] [CrossRef]
- Shevchenko, I.; Serban, D.; Simion, L.; Motofei, I.; Cristea, B.M.; Dumitrescu, D.; Tudor, C.; Dascalu, A.M.; Serboiu, C.; Tribus, L.C.; et al. Clinical Significance of Blood Cell-Derived Inflammation Markers in Assessing Potential Early and Late Postoperative Complications in Patients with Colorectal Cancer: A Systematic Review. J. Clin. Med. 2025, 14, 2529. [Google Scholar] [CrossRef]
- Liu, C.Q.; Yu, Z.B.; Gan, J.X.; Mei, T.M. Preoperative blood markers and intra-abdominal infection after colorectal cancer resection. World J. Gastrointest. Surg. 2024, 16, 451–462. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, R.; Liu, H.; Zheng, L.; Zheng, X.; Li, Y.; Cui, H.; Qin, C.; Hu, J. New scoring system combining computed tomography body composition analysis and inflammatory-nutritional indicators to predict postoperative complications in stage II-III colon cancer. J. Gastroenterol. Hepatol. 2023, 38, 1520–1529. [Google Scholar] [CrossRef]
- Serban, R.E.; Popescu, D.M.; Boldeanu, M.V.; Florescu, D.N.; Serbanescu, M.S.; Sandru, V.; Panaitescu-Damian, A.; Fortofoiu, D.; Serban, R.C.; Gherghina, F.L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, K.; Yuan, Q.; Liu, L.; Miao, J.; Wang, H.; Ding, C.; Guan, W. The role of sarcopenia in pre- and postoperative inflammation: Implications of outcomes in patients with colorectal cancer. J. Gastrointest. Surg. 2024, 28, 1791–1798. [Google Scholar] [CrossRef]
- Chang, J.S.; Cheng, H.H.; Huang, S.C.; Lin, H.H.; Chang, S.C.; Lin, C.C. The impact of inflammatory markers on prognosis of stage II colon cancers depends on tumour sidedness. ANZ J. Surg. 2023, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, H.; Gul, M.O.; Kina, U.; Gurleyik, M.G. Use of the Systemic Immune Inflammation Index, TNM classification, and CEA in pre- and post- prognostic evaluation of sporadic colorectal cancer. Ann. Ital. Chir. 2022, 92, 319–324. [Google Scholar] [PubMed]
- Hernandez-Ainsa, M.; Velamazan, R.; Lanas, A.; Carrera-Lasfuentes, P.; Piazuelo, E. Blood-Cell-Based Inflammatory Markers as a Useful Tool for Early Diagnosis in Colorectal Cancer. Front. Med. 2022, 9, 843074. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Akiyama, T.; Kato, R.; Sawayama, H.; Ogawa, K.; Yoshida, N.; Baba, H. Prognostic Significance of Systemic Inflammation Indices by K-ras Status in Patients With Metastatic Colorectal Cancer. Dis. Colon Rectum 2023, 66, e809–e817. [Google Scholar] [CrossRef]
- Feng, Z.; Lin, H.; Yang, X.; Cao, S.; Gu, X.; Zhang, Z.; Deng, W. Diagnostic Value of Inflammation-Related Indicators in Distinguishing Early Colon Cancer and Adenomatous Polyps. Cancer Control 2023, 30, 10732748231180745. [Google Scholar] [CrossRef]
- Hasirci, I.; Sahin, A. Importance of the neutrophil-lymphocyte ratio and systemic immune-inflammation index in predicting colorectal pathologies in fecal occult blood-positive patients. J. Clin. Lab. Anal. 2023, 37, e24878. [Google Scholar] [CrossRef]
- Manojlovic, N.; Savic, G.; Nikolic, B.; Rancic, N. Dynamic monitoring of carcinoembryonic antigen, CA19-9 and inflammation-based indices in patients with advanced colorectal cancer undergoing chemotherapy. World J. Clin. Cases 2022, 10, 899–918. [Google Scholar] [CrossRef]
- Goseki, N.; Okamoto, A.; Onodera, T. Postoperative nutritional assessment in gastric and colorectal cancer. Nihon Geka Gakkai Zasshi 1986, 87, 853–858. [Google Scholar] [PubMed]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Sakamoto, Y.; Nakagawa, S.; Izumi, D.; Kosumi, K.; Taki, K.; Higashi, T.; Miyata, T.; Miyamoto, Y.; Yoshida, N.; et al. Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int. J. Clin. Oncol. 2017, 22, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Mohri, Y.; Inoue, Y.; Tanaka, K.; Hiro, J.; Uchida, K.; Kusunoki, M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J. Surg. 2013, 37, 2688–2692. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, P.; Chen, X.; Song, Y.; Shi, J.; Zhao, J.; Sun, J.; Xu, Y.; Wang, Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: Results from a retrospective cohort study and a meta-analysis. Oncotarget 2016, 7, 58543–58552. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, E.; Hilsden, R.; Hawel, J.D.; Elnahas, A.I.; Schlachta, C.M.; Alkhamesi, N.A. Preoperative malnutrition in patients with colorectal cancer. Can. J. Surg. 2021, 64, E621–E629. [Google Scholar] [CrossRef]
- Li, J.; Zhu, N.; Wang, C.; You, L.; Guo, W.; Yuan, Z.; Qi, S.; Zhao, H.; Yu, J.; Huang, Y. Preoperative albumin-to-globulin ratio and prognostic nutritional index predict the prognosis of colorectal cancer: A retrospective study. Sci. Rep. 2023, 13, 17272. [Google Scholar] [CrossRef]
- Yang, G.; Wang, D.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Li, T.; Lin, Y.; Luo, H. Normal reference intervals of prognostic nutritional index in healthy adults: A large multi-center observational study from Western China. J. Clin. Lab. Anal. 2021, 35, e23830. [Google Scholar] [CrossRef]
- Tang, J.; Yang, L.; Yang, G.Y.; Li, Y.H.; Zhu, Y.S.; Li, H.; Gao, X.M. Prognostic nutritional index as a predictor of cardiovascular and all-cause mortality in American adults with hypertension: Results from the NHANES database. Front. Cardiovasc. Med. 2024, 11, 1465379. [Google Scholar] [CrossRef]
- Yilmaz, F.; Keles, M.; Bora, F. Relationship between the prognostic nutritional index and resistant hypertension in patients with essential hypertension. Clin. Exp. Hypertens. 2022, 44, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Christina, N.M.; Tjahyanto, T.; Lie, J.G.; Santoso, T.A.; Albertus, H.; Octavianus, D.; Putri, D.; Andrew, J.; Jatinugroho, Y.D.; Shiady, C.; et al. Hypoalbuminemia and colorectal cancer patients: Any correlation?: A systematic review and meta-analysis. Medicine 2023, 102, e32938. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, J.; Jia, H.; Huang, L.; Li, D.; Li, Q.; Li, X. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget 2016, 7, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Blay Cortes, G.; Botella Romero, F.; Fernandez Garcia, J.M.; Pita Gutierrez, F.; Ramirez Arroyo, V.; Breton Lesmes, I.; Seen Semg Semergen, S. Continuity of care in disease-related malnutrition and nutritional medical treatment. Endocrinol. Diabetes Nutr. 2022, 69, 897–909. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Makinen, M.J.; Vayrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
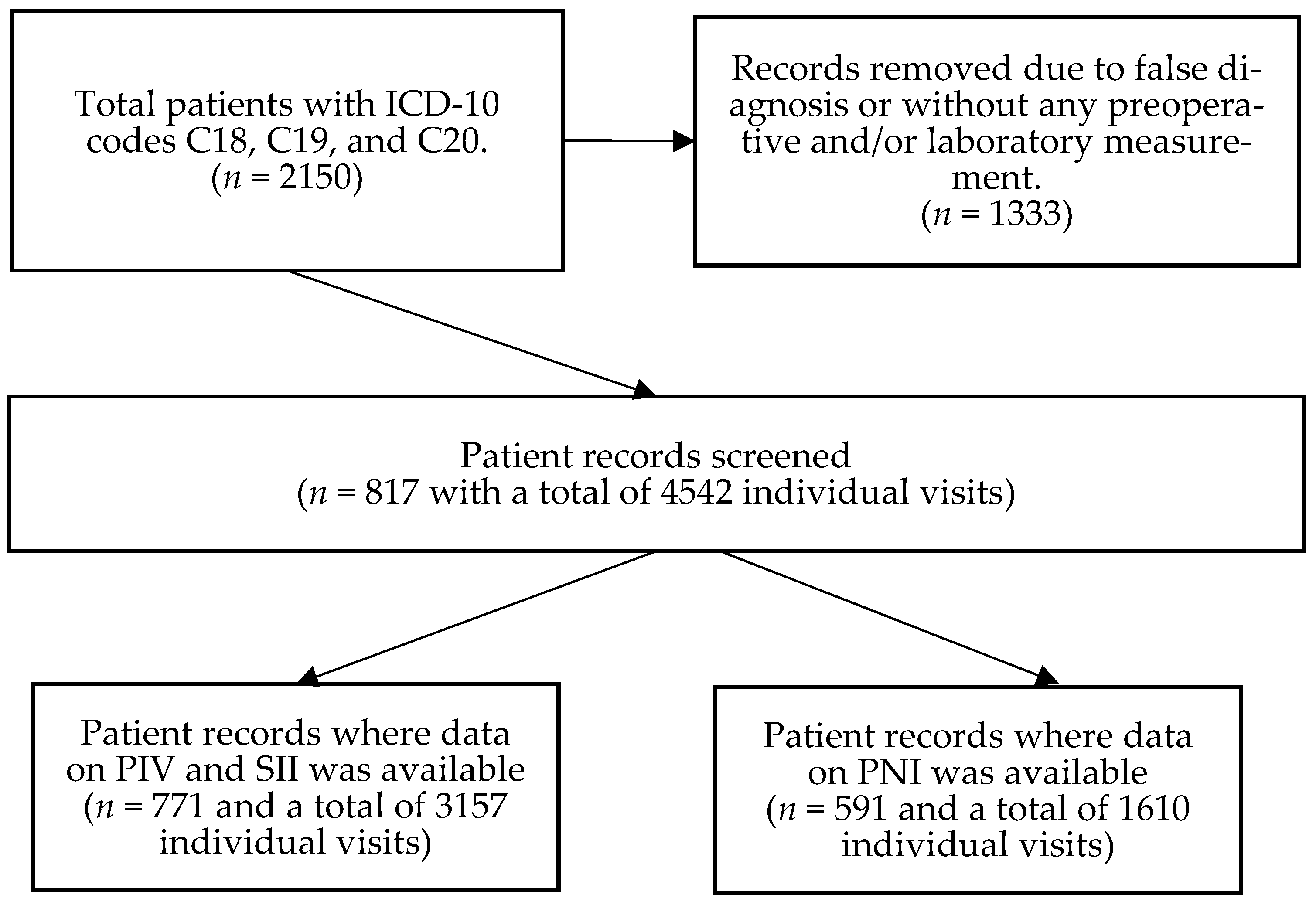
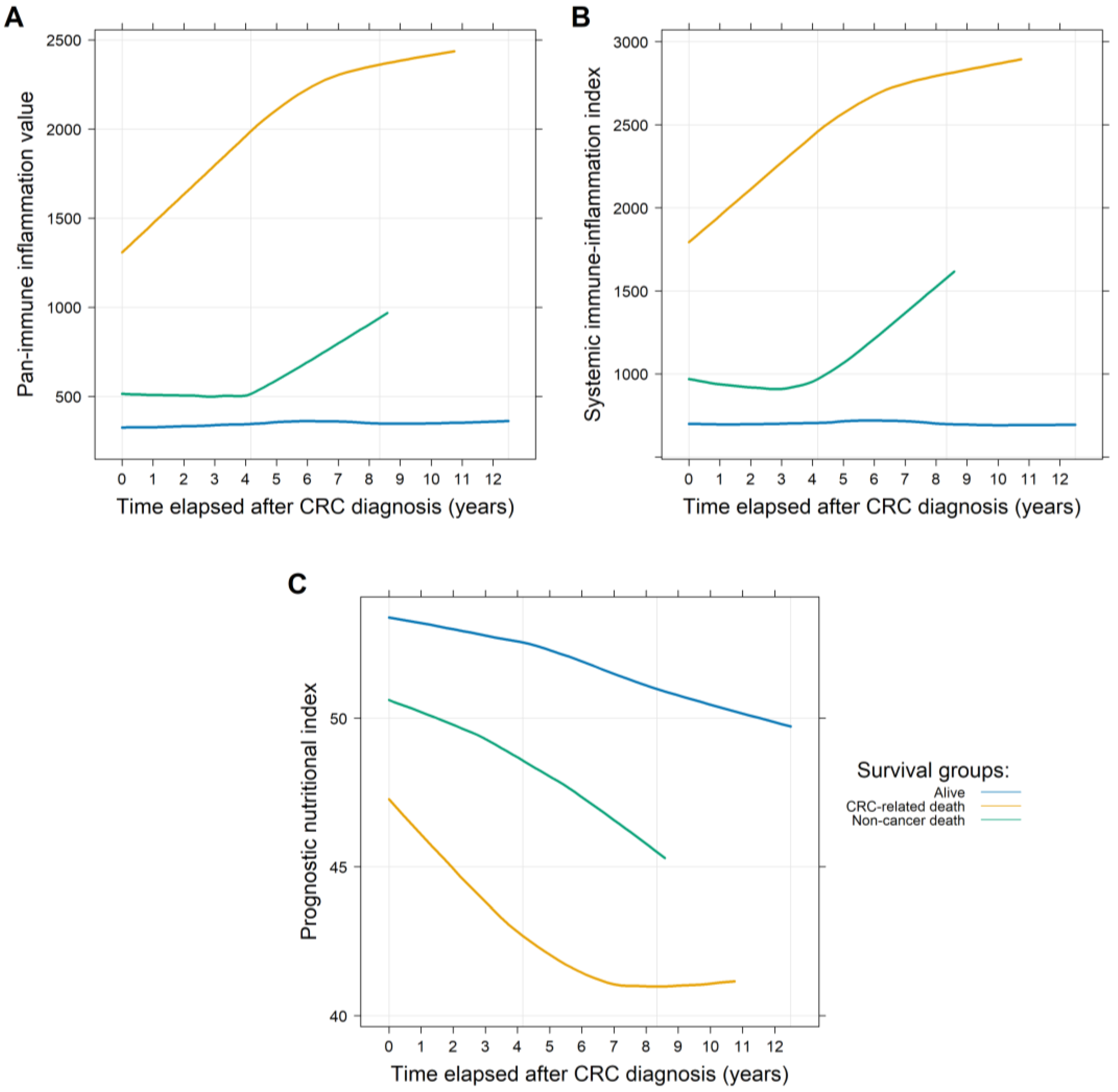
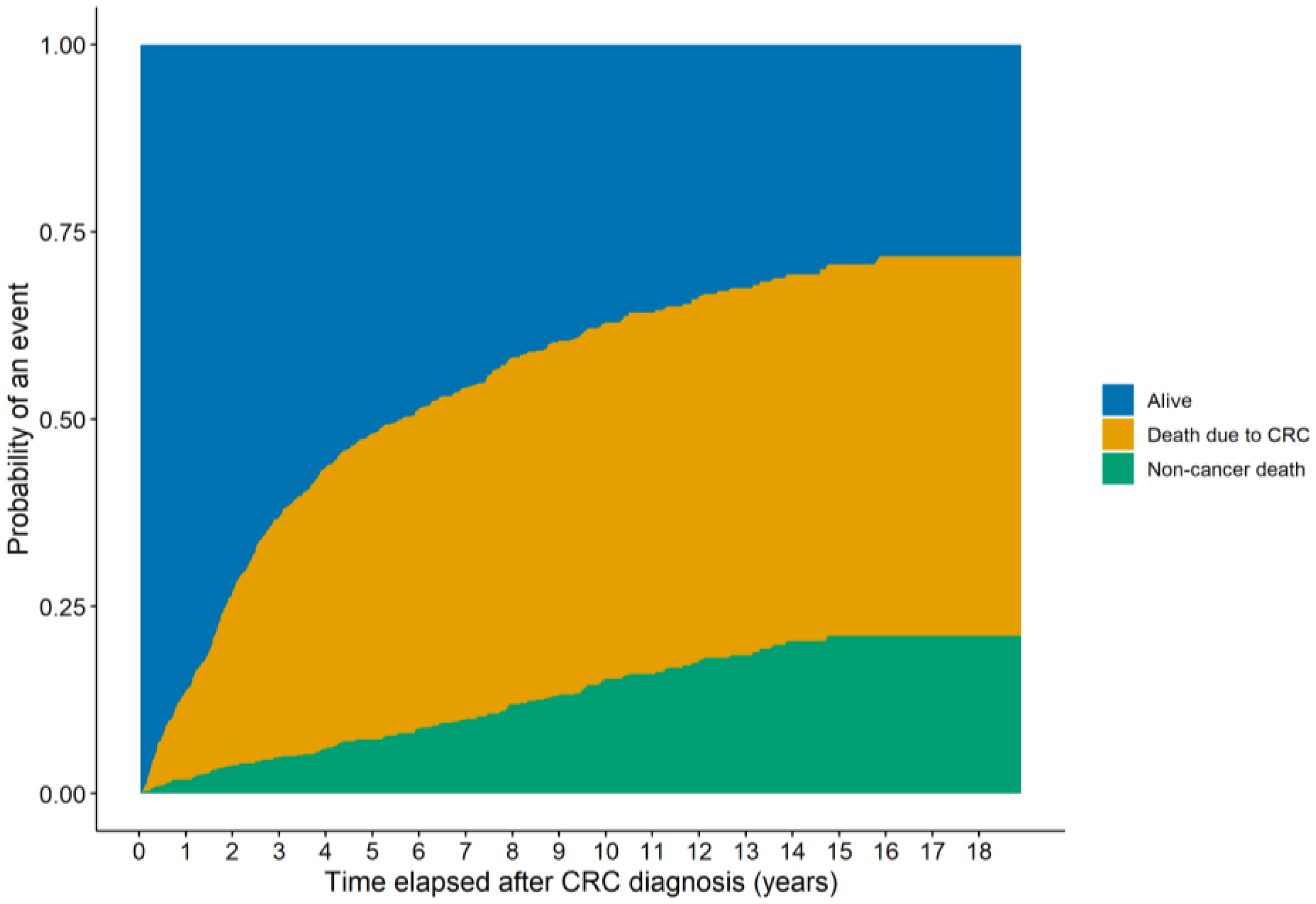


| Parameter | Complete Cohort (n = 817) | Patients with PIV/SII Data (n = 771) | Patients with PNI Data (n = 591) |
|---|---|---|---|
| Age (years) | 65.27 ± 10.97 | 65.29 ± 10.96 | 65.26 ± 11.08 |
| Sex (male/female) | 456:361 (55.81%:44.19%) | 426:345 (55.25%:44.75%) | 316:275 (53.47%:46.53%) |
| Primary tumor location | |||
| - Left-sided | 266 (32.56%) | 250 (32.43%) | 185 (31.30%) |
| - Right-sided | 239 (29.25%) | 232 (30.09%) | 183 (30.96%) |
| - Rectal | 293 (35.86%) | 272 (35.28%) | 209 (35.36%) |
| - Multiple sites | 19 (2.33%) | 17 (2.20%) | 14 (2.37%) |
| AJCC stage (I/II/III/IV) | 102:209:182:324 (12.48%:25.58%:22.28%:39.66%) | 96:204:176:295 (12.45%:26.46%:22.83%:38.26%) | 66:159:133:233 (11.17%:26.90%:22.50%:39.42%) |
| Regional lymph node metastasis | 438 (53.61%) | 408 (52.92%) | 315 (53.50%) |
| Distant metastasis | |||
| - Synchronous | 323 (39.53%) | 294 (38.13%) | 232 (39.26%) |
| - Metachronous | 98 (12.00%) | 97 (12.58%) | 75 (12.69%) |
| Inoperable cases | 67 (8.20%) | 47 (6.10%) | 35 (5.92%) |
| Chemotherapy | |||
| - None | 248 (30.35%) | 233 (30.22%) | 172 (29.10%) |
| - Adjuvant | 244 (29.87%) | 238 (30.87%) | 178 (30.12%) |
| - Palliative | |||
| ○ First-line | 124 (15.18%) | 110 (14.27%) | 90 (15.23%) |
| ○ Second-line | 96 (11.75%) | 87 (11.28%) | 70 (11.84%) |
| ○ Third-line or above | 105 (12.85%) | 103 (13.36%) | 81 (13.71%) |
| Use of biological agents | 224 (27.42%) | 212 (27.50%) | 173 (29.27%) |
| Use of regorafenib/trifluridine–tipiracil | 53 (6.49%) | 53 (6.87%) | 45 (7.61%) |
| Patient with any relevant medical history and/or comorbidity | 666 (81.52%) | 627 (81.32%) | 490 (82.91%) |
| Medical history | |||
| - Type 2 diabetes mellitus | 204 (24.97%) | 193 (25.03%) | 144 (24.37%) |
| - Thyroid disease 1 | 82 (10.04%) | 77 (9.99%) | 61 (10.32%) |
| - Hypertension (untreated/treated) | 58:519 (7.10%:63.53%) | 52:490 (6.74%:63.55%) | 37:394 (6.26%:66.67%) |
| - Major CV events 2 | 87 (10.65%) | 83 (10.77%) | 65 (11.00%) |
| - Other CV diseases 3 | 64 (7.83%) | 59 (7.65%) | 52 (8.80%) |
| - Appendicitis with or without appendectomy | 142 (17.38%) | 136 (17.64%) | 107 (18.10%) |
| - Cholelithiasis with or without cholecystectomy | 191 (23.38%) | 185 (23.99%) | 143 (24.20%) |
| Median survival time (months) | 68.04 (55.95–82.50) | 58.78 (47.04–75.17) | 59.76 (44.15–82.50) |
| Parameter | PIV | SII | PNI |
|---|---|---|---|
| Age (years) | 0.6798 | 0.2921 | <0.0001 |
| Sex | 0.0286 | 0.2294 | 0.3724 |
| Primary tumor location | 0.1430 | 0.0268 | 0.6604 |
| AJCC stage (I–III vs. IV) | <0.0001 | <0.0001 | <0.0001 |
| Regional lymph node metastasis | <0.0001 | <0.0001 | 0.0021 |
| Synchronous distant metastasis | <0.0001 | <0.0001 | <0.0001 |
| Metachronous distant metastasis | 0.0911 | 0.0396 | 0.0911 |
| Chemotherapy (none vs. adjuvant/palliative) | <0.0001 | <0.0001 | <0.0001 |
| Use of biological agents | 0.0021 | 0.0383 | 0.1340 |
| Use of regorafenib/trifluridine–tipiracil | 0.3777 | 0.8311 | 0.7411 |
| Patient with any relevant medical history and/or comorbidity | 0.4740 | 0.5667 | 0.0005 |
| Medical history | |||
| - Type 2 diabetes mellitus | 0.4600 | 0.4553 | 0.8570 |
| - Thyroid disease 1 | 0.1218 | 0.2272 | 0.8027 |
| - Hypertension | 0.4442 | 0.6826 | 0.0076 |
| - Major CV events 2 | 0.8685 | 0.4778 | 0.5689 |
| - CV diseases 3 incl. major CV events but excluding hypertension | 0.4587 | 0.8093 | 0.1523 |
| - Appendicitis with or without appendectomy | 0.4970 | 0.7622 | 0.7221 |
| - Cholelithiasis with or without cholecystectomy | 0.5974 | 0.3869 | 0.5124 |
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Pan-immune inflammation value (increase per unit) | <0.0001 | – | – |
| Systemic immune-inflammation index (increase per unit) | – | <0.0001 | – |
| Prognostic nutritional index (increase per unit) | – | – | <0.0001 |
| Sex [male (ref.) vs. female] | 0.1296 | 0.0057 | 0.1624 |
| Medical history | |||
| - Type 2 diabetes mellitus [no (ref.) vs. yes] | 0.3277 | 0.2530 | 0.2001 |
| - Thyroid disease 1 [no (ref.) vs. yes] | 0.4635 | 0.3910 | 0.1672 |
| - Hypertension [no (ref.) vs. yes] | |||
| ○ None (ref.) vs. untreated hypertension | 0.3049 | 0.5455 | 0.5521 |
| ○ None (ref.) vs. treated hypertension | 0.0092 | 0.0754 | 0.0860 |
| - CV diseases 2 incl. major CV events 3 but excluding hypertension [no (ref.) vs. yes] | 0.9813 | 0.2624 | 0.3729 |
| - Appendicitis with or without appendectomy [no (ref.) vs. yes] | 0.6518 | 0.2197 | 0.1576 |
| - Cholelithiasis with or without cholecystectomy [no (ref.) vs. yes] | 0.7488 | 0.6521 | 0.1201 |
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Pan-immune inflammation value (increase per unit) | <0.0001 | – | – |
| Systemic immune-inflammation index (increase per unit) | – | <0.0001 | – |
| Prognostic nutritional index (increase per unit) | – | – | <0.0001 |
| Age (years) | <0.0001 | 0.0002 | 0.8240 |
| Sex [male (ref.) vs. female] | 0.8328 | 0.3808 | 0.1842 |
| Patient with any relevant medical history and/or comorbidities [no (ref.) vs. yes] | 0.7084 | 0.9684 | 0.0488 |
| Primary tumor location | |||
| - Left-sided (ref.) vs. right-sided | 0.9618 | 0.9426 | 0.0572 |
| - Left-sided (ref.) vs. rectum | 0.5043 | 0.1921 | 0.0096 |
| - Left-sided (ref.) vs. multiplex | 0.0901 | 0.0697 | 0.7061 |
| - Right-sided (ref.) vs. rectum | 0.5031 | 0.2367 | 0.9482 |
| - Right-sided (ref.) vs. multiplex | 0.0888 | 0.0703 | 0.2147 |
| - Rectum (ref.) vs. multiplex | 0.1193 | 0.1260 | 0.1709 |
| AJCC stage | |||
| - Stage I (ref.) vs. stage II | 0.2199 | 0.2001 | 0.1467 |
| - Stage I (ref.) vs. stage III | 0.0048 | 0.0039 | 0.0239 |
| - Stage I (ref.) vs. stage IV | <0.0001 | <0.0001 | 0.0002 |
| - Stage II (ref.) vs. stage III | 0.0214 | 0.1882 | 0.0327 |
| - Stage II (ref.) vs. stage IV | <0.0001 | <0.0001 | <0.0001 |
| - Stage III (ref.) vs. stage IV | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herold, Z.; Herold, M.; Szentmartoni, G.; Szalasy, R.; Lohinszky, J.; Somogyi, A.; Szasz, A.M.; Dank, M. Are Calculated Immune Markers with or Without Comorbidities Good Predictors of Colorectal Cancer Survival? The Results of a Longitudinal Study. Med. Sci. 2025, 13, 108. https://doi.org/10.3390/medsci13030108
Herold Z, Herold M, Szentmartoni G, Szalasy R, Lohinszky J, Somogyi A, Szasz AM, Dank M. Are Calculated Immune Markers with or Without Comorbidities Good Predictors of Colorectal Cancer Survival? The Results of a Longitudinal Study. Medical Sciences. 2025; 13(3):108. https://doi.org/10.3390/medsci13030108
Chicago/Turabian StyleHerold, Zoltan, Magdolna Herold, Gyongyver Szentmartoni, Reka Szalasy, Julia Lohinszky, Aniko Somogyi, Attila Marcell Szasz, and Magdolna Dank. 2025. "Are Calculated Immune Markers with or Without Comorbidities Good Predictors of Colorectal Cancer Survival? The Results of a Longitudinal Study" Medical Sciences 13, no. 3: 108. https://doi.org/10.3390/medsci13030108
APA StyleHerold, Z., Herold, M., Szentmartoni, G., Szalasy, R., Lohinszky, J., Somogyi, A., Szasz, A. M., & Dank, M. (2025). Are Calculated Immune Markers with or Without Comorbidities Good Predictors of Colorectal Cancer Survival? The Results of a Longitudinal Study. Medical Sciences, 13(3), 108. https://doi.org/10.3390/medsci13030108






