On the Processes that Produce Hydrocarbon and Mineral Resources in Sedimentary Basins
Abstract
1. Introduction
2. Basin Processes
2.1. Mantle Dynamics
2.1.1. McKenzie’s Stretching Model for Basin Formation
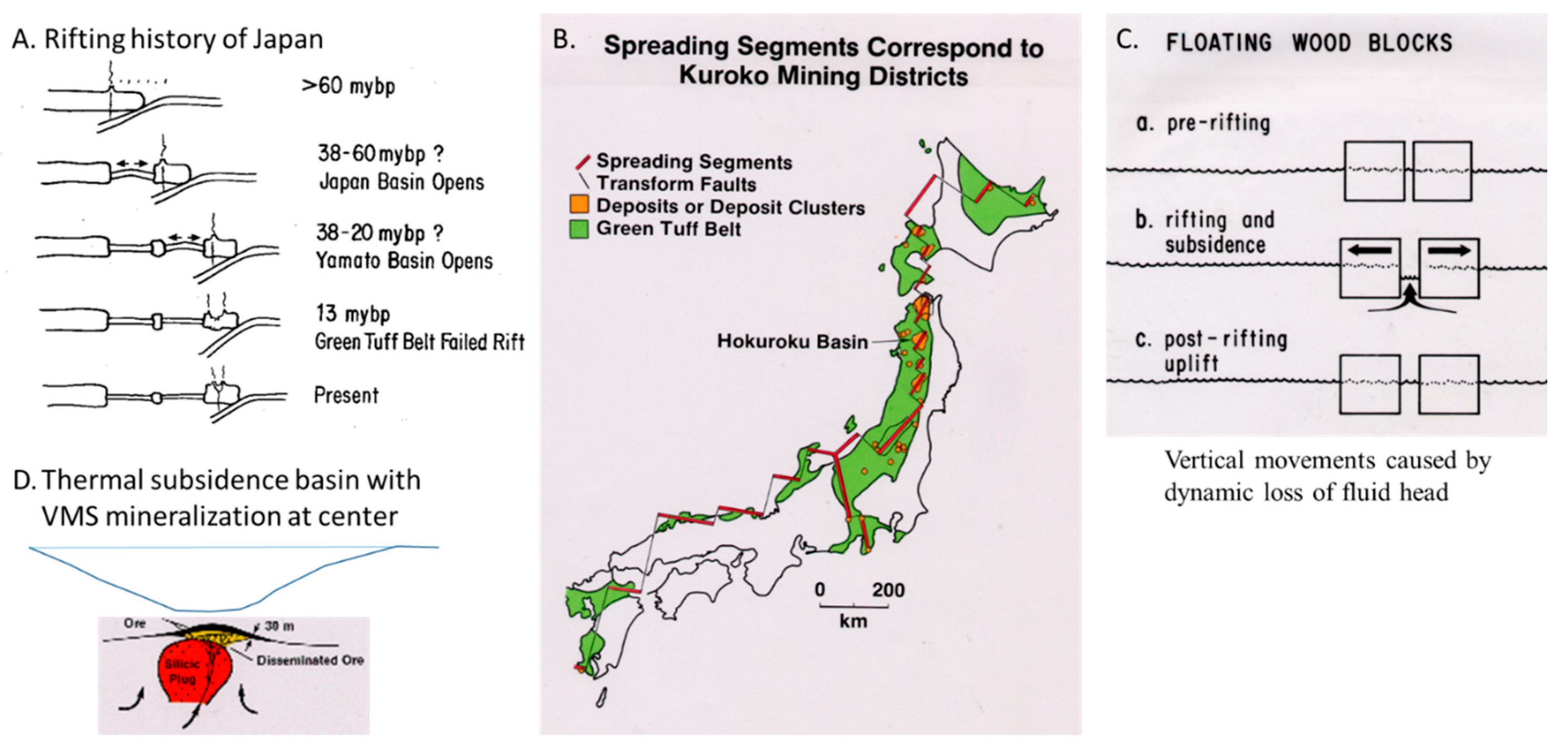
2.1.2. Rifting and Base Metal Deposition
2.1.3. Juxtaposition of Sediments of Contrasting Oxidation State
2.2. Fluid Dynamics
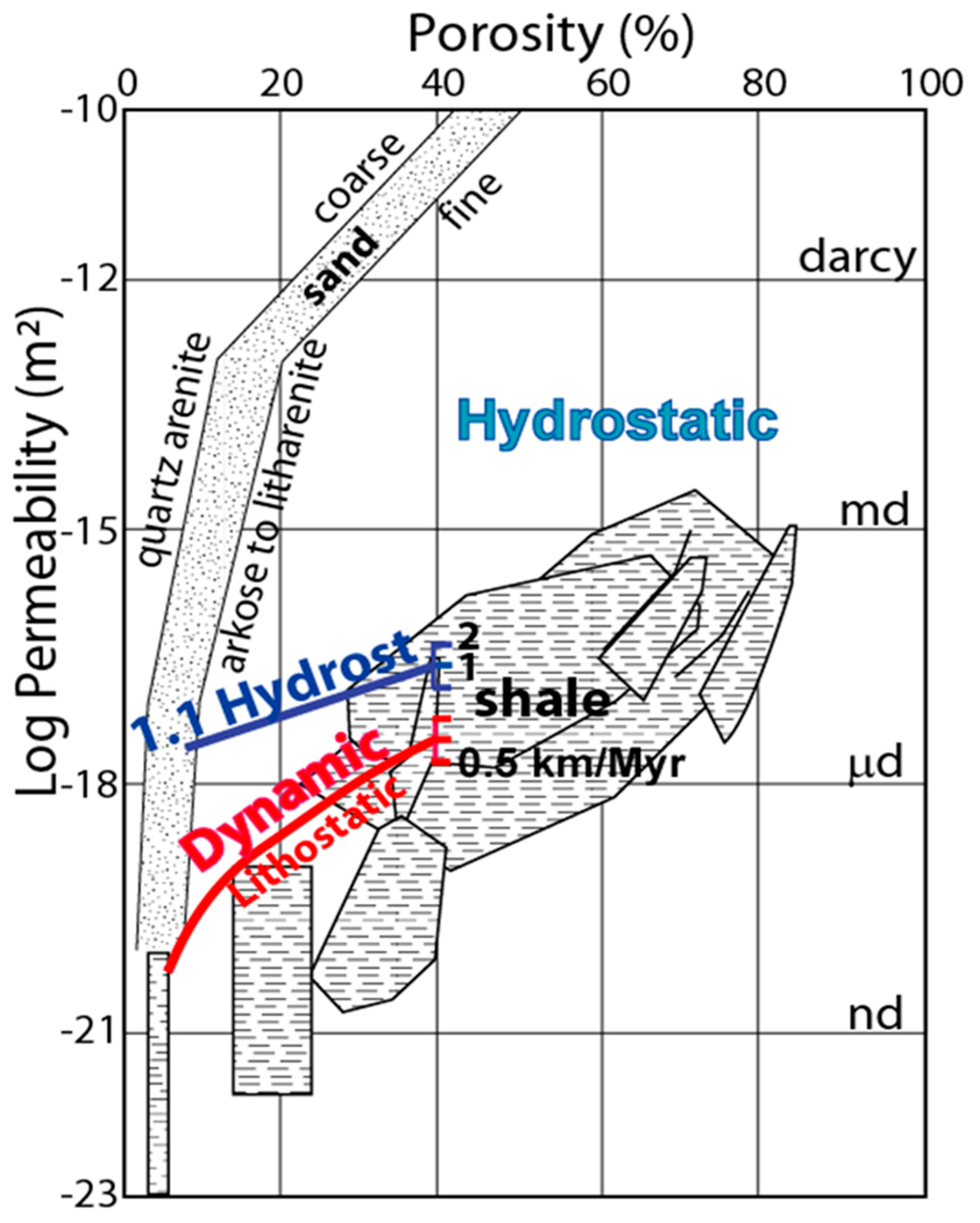
2.2.1. Dynamic Permeability
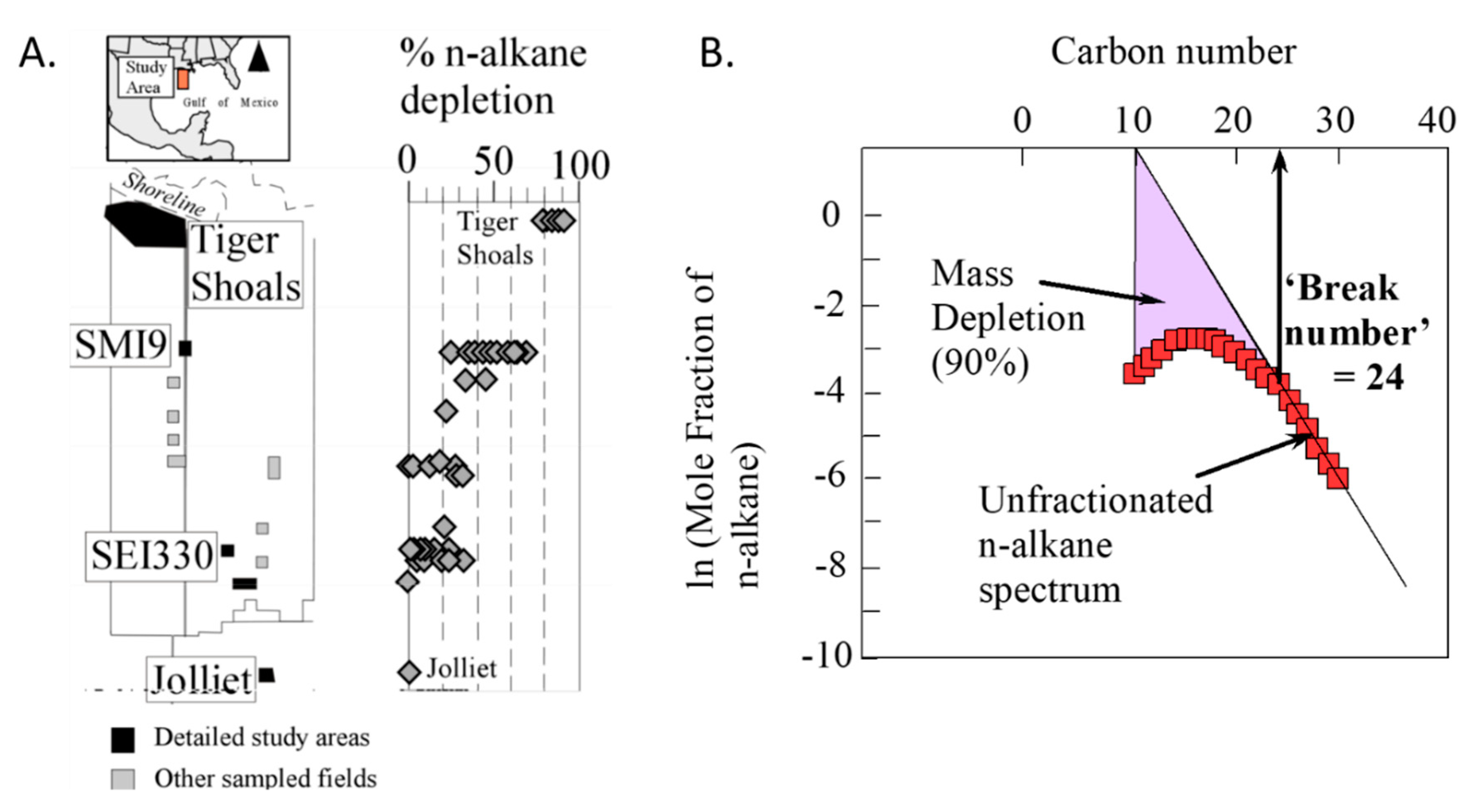
2.2.2. Hydrocarbon Migration
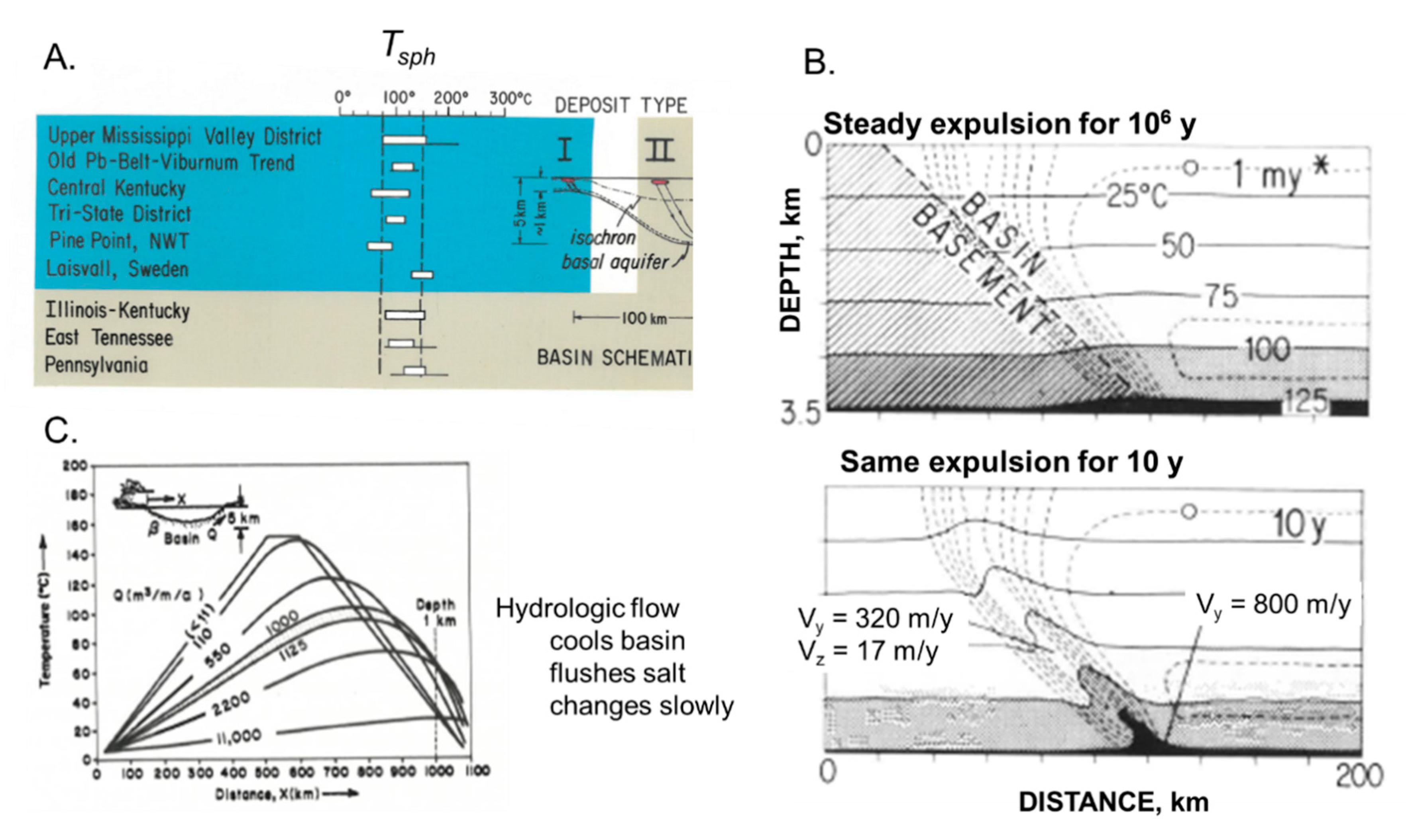
2.2.3. Nature of Fluid Flow in Basins
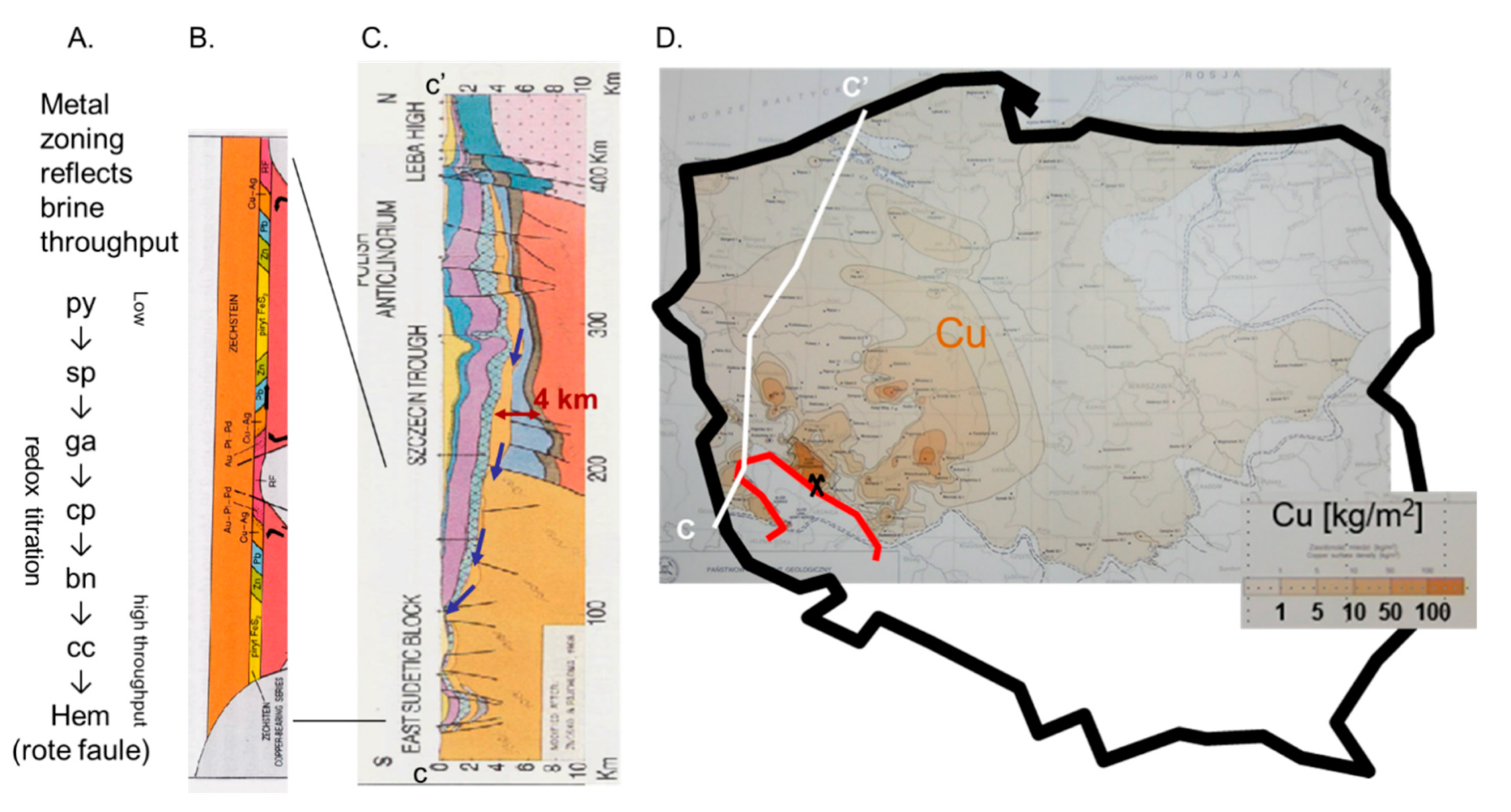
Steady Expulsion: The Kupferschiefer Deposits in Germany and Poland
Episodic Expulsion: Mississippi Valley Type Pb-Zn Deposits
2.3. Capillary Dynamics
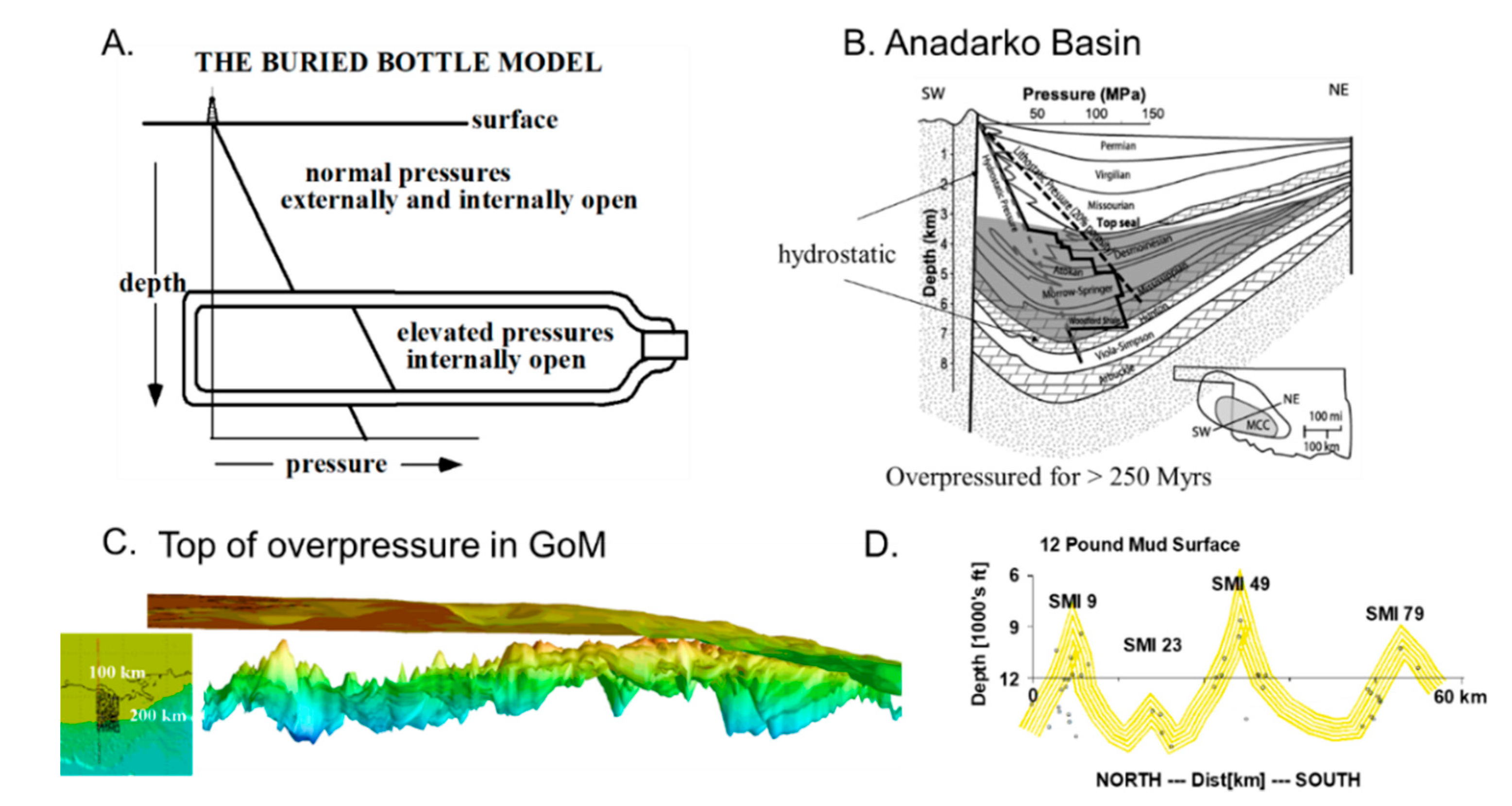
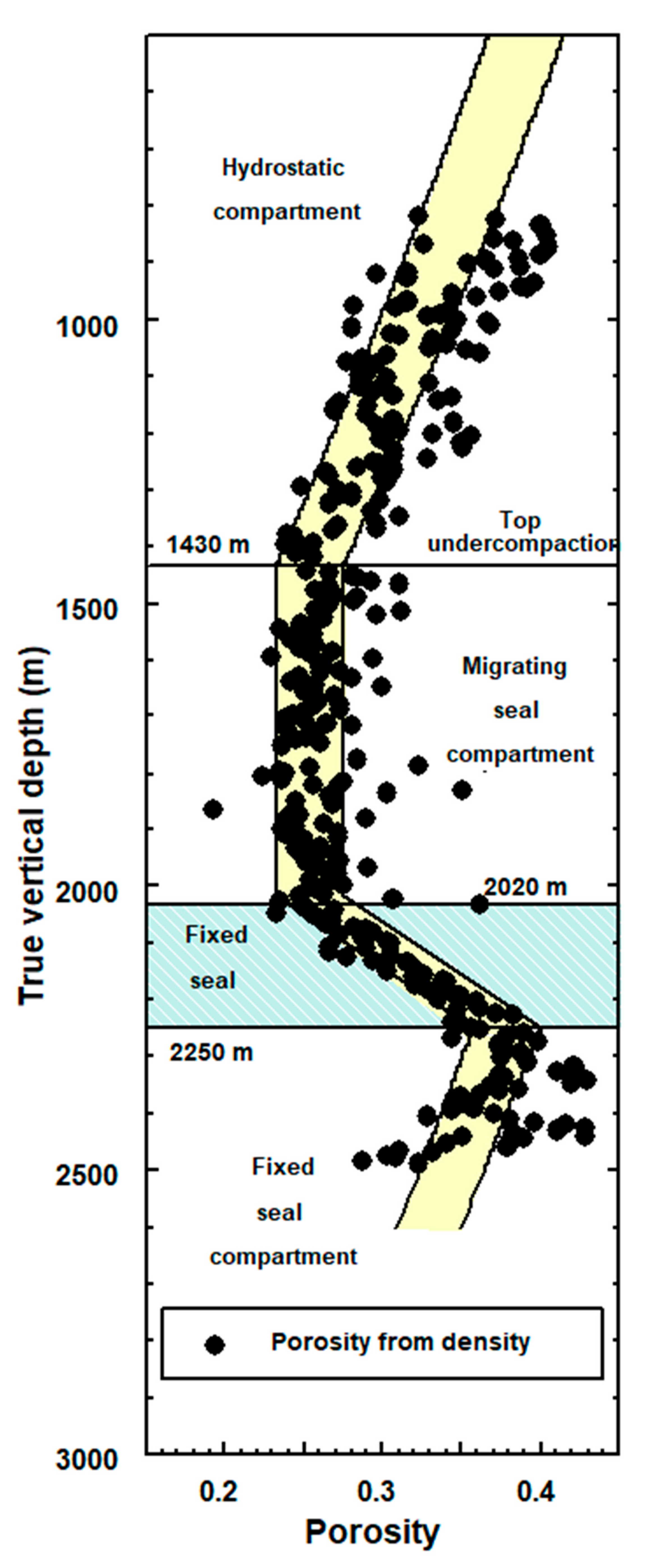
2.3.1. Basin Pressure Compartments
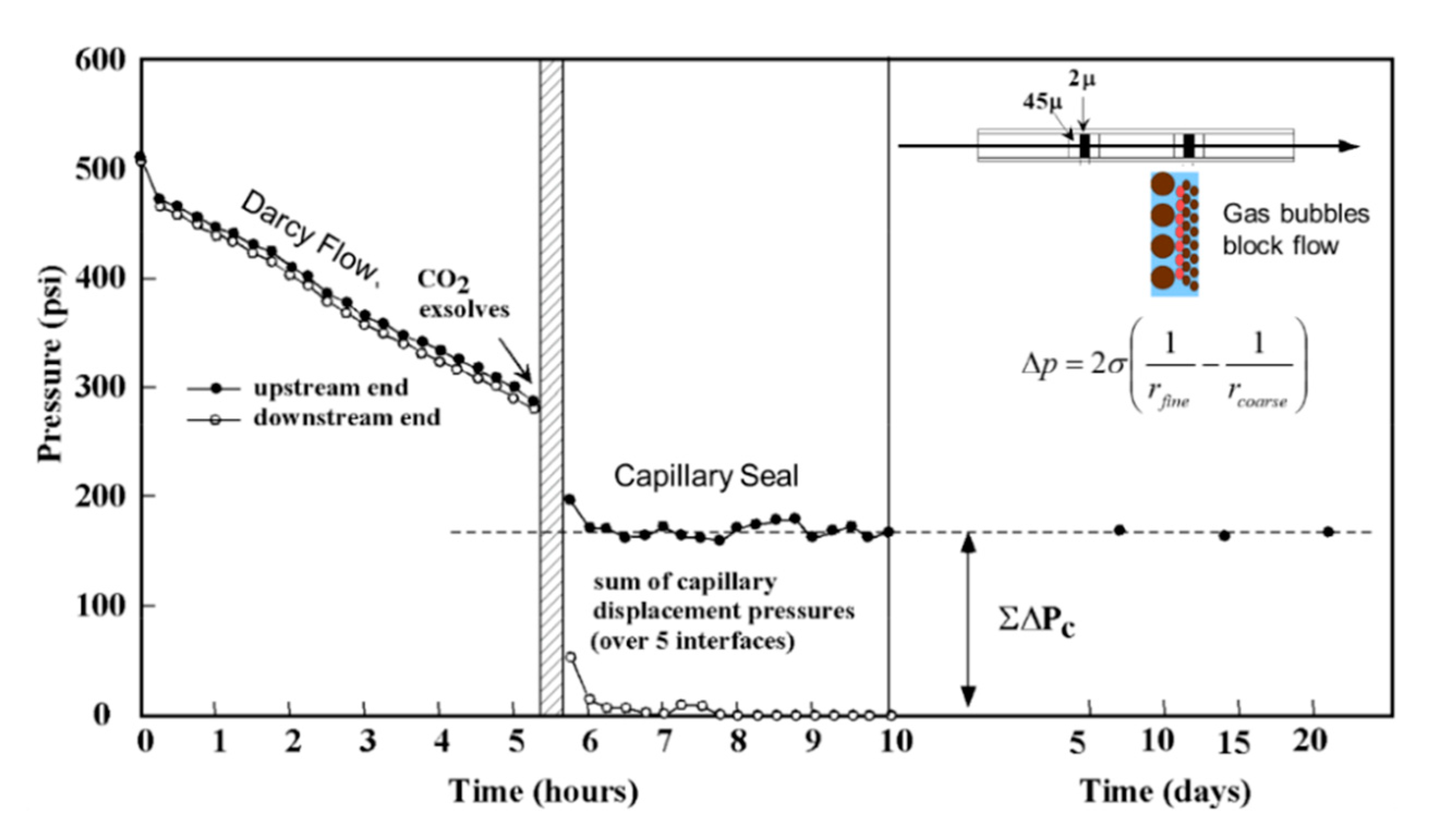
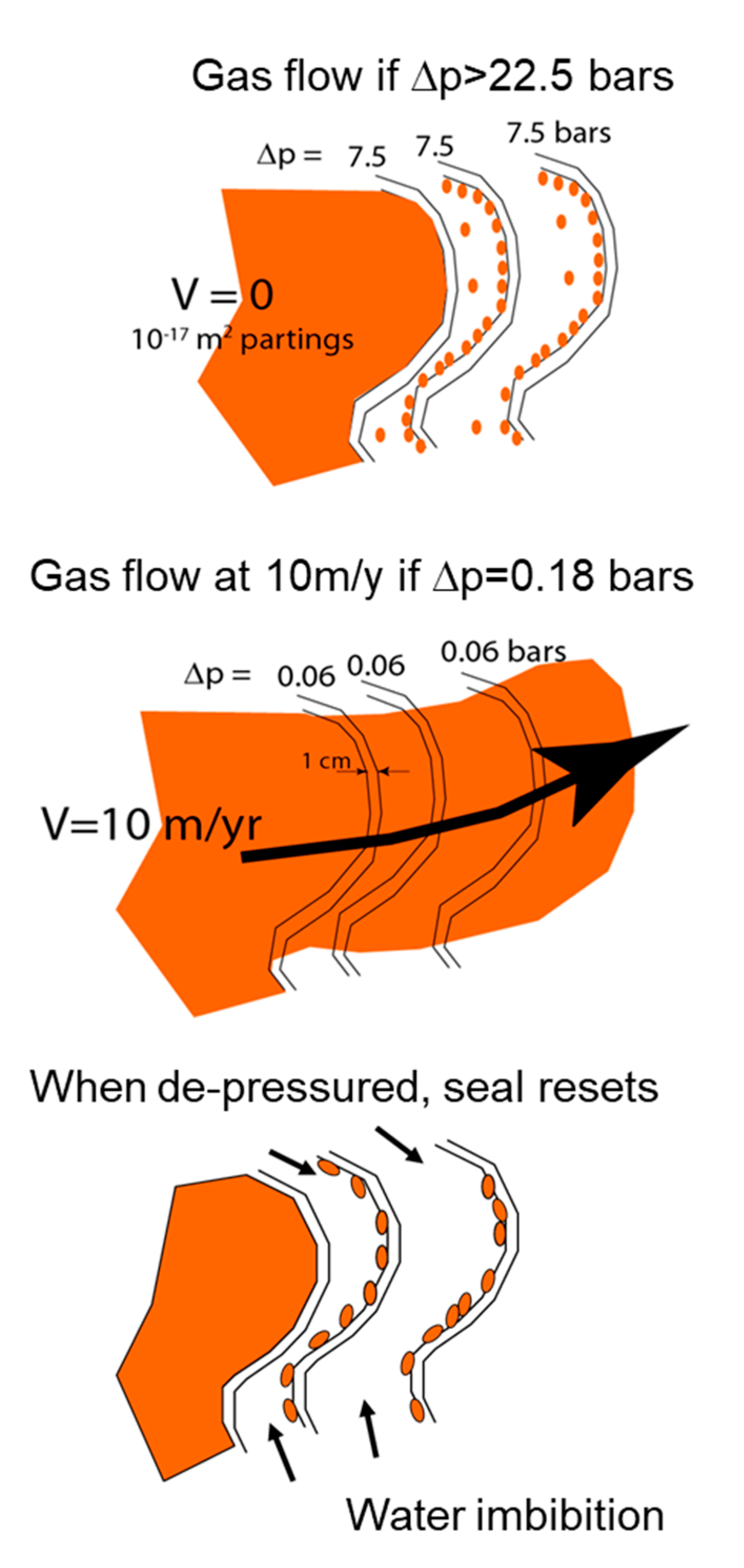
2.3.2. Capillary Seals
Pressure Compartmentation is Spontaneous and Natural
Incarcerated Gas
Seal Migration
Fluids Migrate Together
Fluid Release Valve
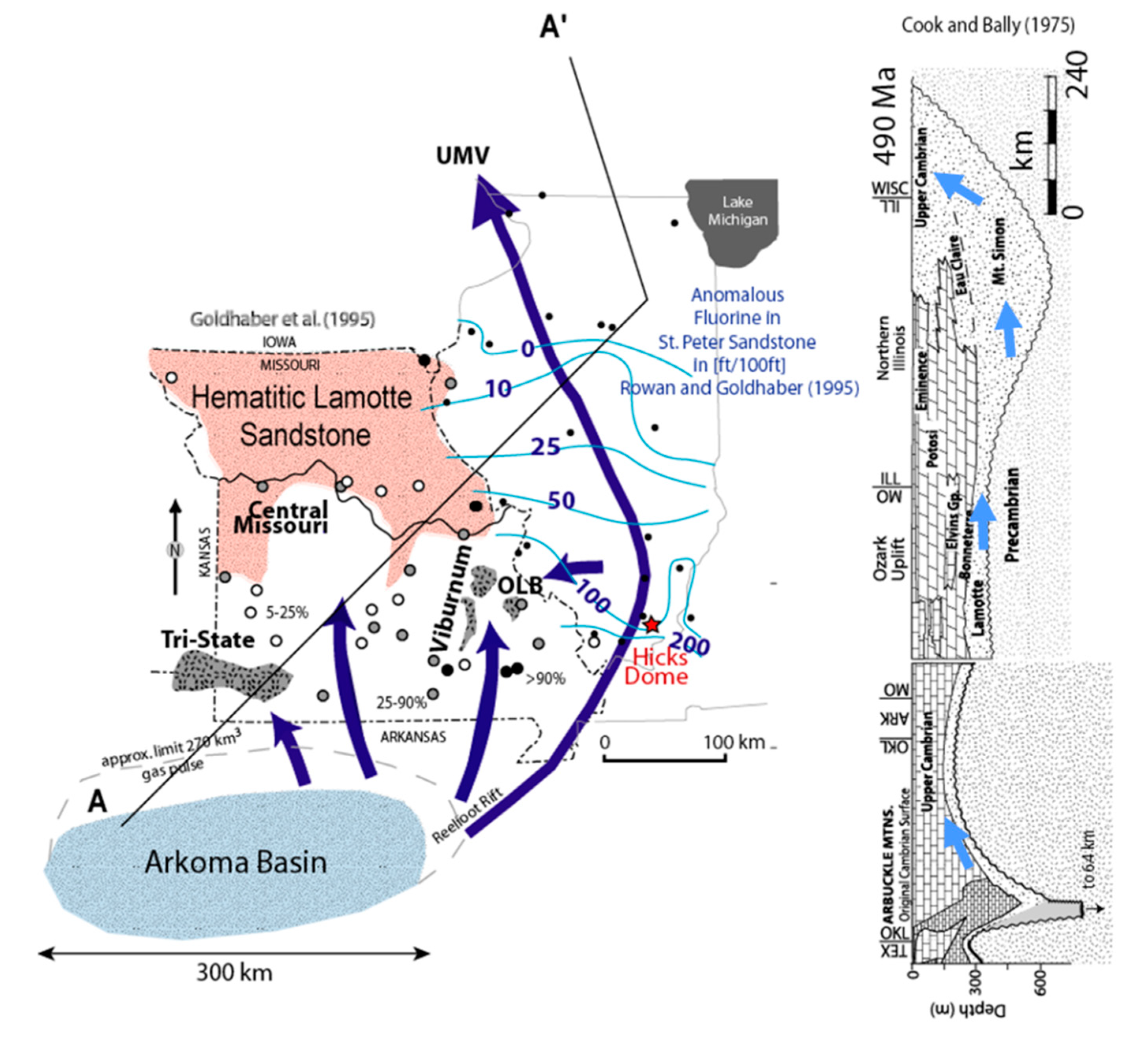
Gas Pulsar Formation of Mississippi Valley-Type (MVT) Deposits
Late Paleozoic Remagnetization of the North American Mid-Continent
Impact on Oil Production
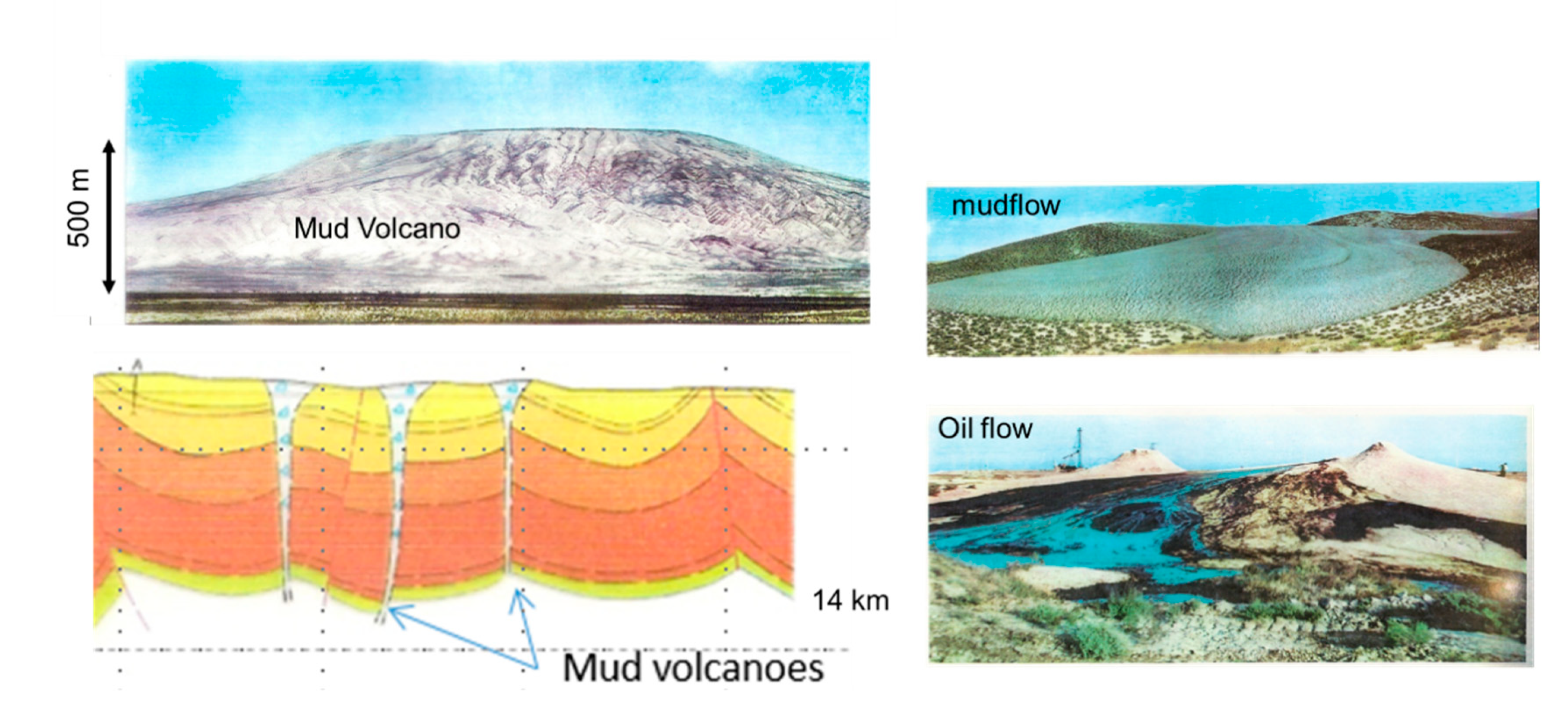
2.3.3. Mud Volcanoes
2.4. Non-Hydrocarbon Gas Dynamics
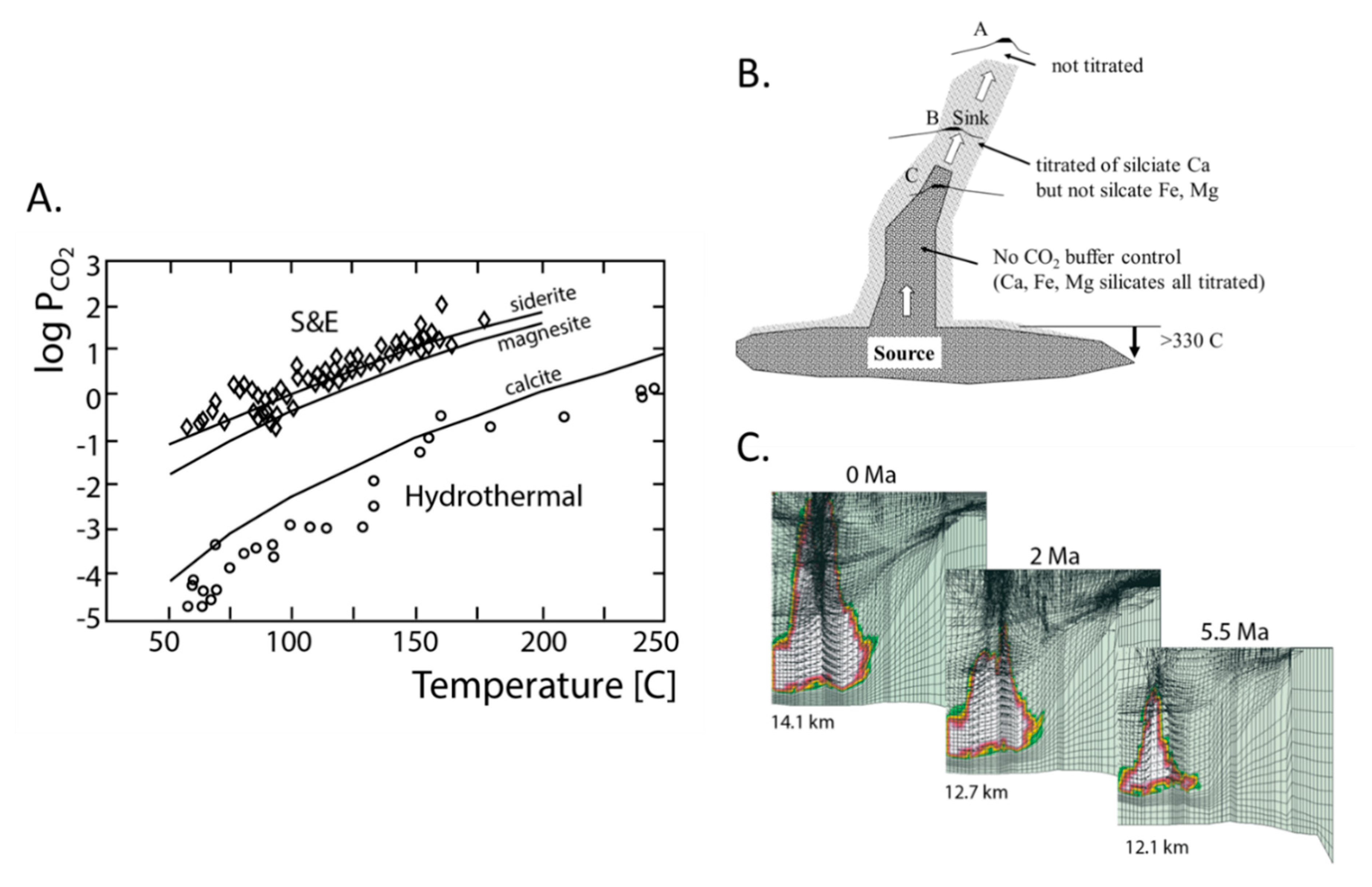
2.4.1. CO2 Generation and Titration
2.4.2. H2 Generation
3. Summary and Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- McKenzie, D. Some remarks on the development of sedimentary basins. Earth Planet. Sci. Lett. 1978, 40, 25–32. [Google Scholar] [CrossRef]
- Cathles, L.M.; Guber, A.L.; Lenagh, T.C.; Dudás, F.Ö. Kuroko-Type Massive Sulfide Deposits of Japan: Products of an Aborted Island-Arc Rift. In The Kuroko and Related Volcanogenic Massive Sulfide Deposits; Society of Economic Geologists: Littleton, CO, USA, 1983. [Google Scholar]
- German, C.R.; Petersen, S.; Hannington, M.D. Hydrothermal exploration of mid-ocean ridges: Where might the largest sulfide deposits be forming? Chem. Geol. 2016, 420, 114–126. [Google Scholar] [CrossRef]
- Eugster, H.P. Oil shales, evaporites and ore deposits. Geochim. Cosmochim. Acta 1985, 49, 619–635. [Google Scholar] [CrossRef]
- Malin, P.; Leary, P.; Cathles, L.M.; Barton, C.C. Observational and critical state physics descriptions of long-range flow structures. Geosciences 2019. submitted. [Google Scholar]
- Cathles, L.M.; Adams, J.J. Fluid Flow and Petroleum and Mineral Resources in the Upper (<20-km) Continental Crust. In One Hundredth Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 77–110. [Google Scholar]
- Nelson, P.H. Permeability-porosity Relationships in Sedimentary Rocks. Soc. Petrophys. Well-Log Anal. 1994, 35, 38–62. [Google Scholar]
- Nelson, P.H. Evolution of Permeability-Porosity Trends in Sandstones. In Proceedings of the SPWLA 41st Annual Logging Symposium, Dallas, TX, USA, 4–7 June 2000; p. 14. [Google Scholar]
- Neuzil, C.E. How permeable are clays and shales? Water Resour. Res. 1994, 30, 145–150. [Google Scholar] [CrossRef]
- England, W.A.; Mackenzie, A.S.; Mann, D.M.; Quigley, T.M. The movement and entrapment of petroleum fluids in the subsurface. J. Geol. Soc. 1987, 144, 327–347. [Google Scholar] [CrossRef]
- Løtveit, I.F.; Fjeldskaar, W.; Sydnes, M. Tilting and Flexural Stresses in Basins Due to Glaciations—An Example from the Barents Sea. Geosciences 2019, 9, 474. [Google Scholar] [CrossRef]
- Cathles, L.M.; Wizevich, M.; Losh, S. Volume II: Geology, geophysics, geochemistry, and GoCAD database. In Seal Control of Hydrocarbon Migration and Its Physical and Chemical Consequences; GRI-03/0065; Cathles, L.M., Ed.; Gas Research Institute: Chicago, IL, USA, 2002; p. 51. [Google Scholar]
- Whelan, J.K.; Eglinton, L. Volume III: Organic geochemical consequences in a North South transect in the northern Gulf of Mexico. In Seal Control of Hydrocarbon Migration and Its Physical and Chemical Consequences; GRI-03/0065; Cathles, L.M., Ed.; Gas Research Institute: Chicago, IL, USA, 2002; p. 85. [Google Scholar]
- Losh, S.; Cathles, L.M. Volume IV: Gas washing of oil and its implications. In Seal Control of Hydrocarbon Migration and Its Physical and Chemical Consequences; GRI-03/0065; Cathles, L.M., Ed.; Gas Research Institute: Chicago, IL, USA, 2002; p. 74. [Google Scholar]
- Cathles, L.M.; Losh, S. Volume V: A modeling analysis of the hydrocarbon chemistry and gas washing, hydrocarbon fluxes, and reservoir filling. In Seal Control of Hydrocarbon Migration and Its Physical and Chemical Consequences; GRI-03/0065; Cathles, L.M., Ed.; Gas Research Institute: Chicago, IL, USA, 2002; p. 63. [Google Scholar]
- Cathles, L.M.; Shosa, J.D. Volume VI: A theoretical analysis of the inorganic alteration by flow of brines through seals. In Seal Control of Hydrocarbon Migration and Its Physical and Chemical Consequences; GRI-03/0065; Cathles, L.M., Ed.; Gas Research Institute: Chicago, IL, USA, 2002; p. 70. [Google Scholar]
- Cathles, L.M. Hydrocarbon generation, migration, and venting in a portion of the offshore Louisiana Gulf of Mexico basin. Lead. Edge 2004, 23, 760–770. [Google Scholar] [CrossRef]
- Losh, S.; Cathles, L.; Meulbroek, P. Gas washing of oil along a regional transect, offshore Louisiana. Org. Geochem. 2002, 33, 655–663. [Google Scholar] [CrossRef]
- Meulbroek, P. Equations of state in exploration. Org. Geochem. 2002, 33, 613–634. [Google Scholar] [CrossRef]
- Meulbroek, P.; Cathles, L.; Whelan, J. Phase fractionation at South Eugene Island Block 330. Org. Geochem. 1998, 29, 223–239. [Google Scholar] [CrossRef]
- Meulbroek, P.; Cathles, L.; Goddard, W.A. HCToolkit/EOS interface: An open source, multi-platform phase equilibria framework for exploring phase behaviour of complex mixtures. Geol. Soc. Lond. Spec. Publ. 2004, 237, 89–98. [Google Scholar] [CrossRef]
- Chen, D.F.; Cathles, L.M. A kinetic model for the pattern and amounts of hydrate precipitated from a gas steam: Application to the Bush Hill vent site, Green Canyon Block 185, Gulf of Mexico: A Kinetic Model for Hydrate Precipitation. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Van Wees, J.-D.; Stephenson, R.A.; Ziegler, P.A.; Bayer, U.; McCann, T.; Dadlez, R.; Gaupp, R.; Narkiewicz, M.; Bitzer, F.; Scheck, M. On the origin of the Southern Permian Basin, Central Europe. Mar. Pet. Geol. 2000, 17, 43–59. [Google Scholar] [CrossRef]
- Oszczepalski, S. Kupferschiefer in Southwestern Poland: Sedimentary environments, metal zoning, and ore controls. In Sediment-Hosted Stratiform Copper Deposits; Special Paper; Geological Association of Canada: St. John’s, NL, Canada, 1989; pp. 571–600. [Google Scholar]
- Cathles, L.M.; Oszczepalski, S.; Jowett, E.C. Mass balance evaluation of the late diagenetic hypothesis for Kupferschiefer Cu mineralization in the Lubin Basin of southwestern Poland. Econ. Geol. 1993, 88, 948–956. [Google Scholar] [CrossRef]
- Oszczepalski, S.; Speczik, S.; Zieliński, K.; Chmielewski, A. The Kupferschiefer Deposits and Prospects in SW Poland: Past, Present and Future. Minerals 2019, 9, 592. [Google Scholar] [CrossRef]
- Jowett, E.C.; Rydzewsk, A.; Jowett, R.J. The Kupferschiefer Cu–Ag ore deposits in Poland: A re-appraisal of the evidence of their origin and presentation of a new genetic model. Can. J. Earth Sci. 1987, 24, 2016–2037. [Google Scholar] [CrossRef]
- Ziegler, P.A. Evolution of the Arctic-North Atlantic and the Western Tethys/Book and Map; American Association of Petroleum Geologists: Tulsa, OK, USA, 1988. [Google Scholar]
- Schito, A.; Corrado, S.; Trolese, M.; Aldega, L.; Caricchi, C.; Cirilli, S.; Grigo, D.; Guedes, A.; Romano, C.; Spina, A.; et al. Assessment of thermal evolution of Paleozoic successions of the Holy Cross Mountains (Poland). Mar. Pet. Geol. 2017, 80, 112–132. [Google Scholar] [CrossRef]
- Oszsczepalski, S.; Rydzewski, A.; Speczik, S. Rote Fäule-related Au–Pt–Pd mineralization in SW Poland: New data. In Proceeding of the Fifth Biennial SGA Meeting, London, UK, 22–25 August 1999. [Google Scholar]
- White, W.S. A paleohydrologic model for mineralization of the White Pine copper deposit, northern Michigan. Econ. Geol. 1971, 66, 1–13. [Google Scholar] [CrossRef]
- Brown, A.C. Sediment-hosted Stratiform Copper Deposits. Geosci. Canada 1992, 19, 125–141. [Google Scholar]
- Mauk, J.L.; Kelly, W.C.; van der Pluijm, B.A.; Seasor, R.W. Relations between deformation and sediment-hosted copper mineralization: Evidence from the White Pine part of the Midcontinent rift system. Geology 1992, 20, 427–430. [Google Scholar] [CrossRef]
- Oszczepalski, S.; Rydzewski, A.; Geologiczny, P.I. Metallogenic Atlas of the Zechstein Copper-Bearing Series in Poland; Państwowy Instytut Geologiczny: Warsaw, Poland, 1997. [Google Scholar]
- Downorowicz, S. Geothermics of the copper ore deposit of the Fore-Sudetic monocline: Prace Inst. Ge-. ol. (Poland) 1983, 106, 88. [Google Scholar]
- Hallager, W.S.; Carpenter, A.B.; Campbell, W.L. Smectite clays in red beds as a source of base metals. In Geological Society America Abstracts Programs; Geological Society America: Boulder, CO, USA, 1991; pp. 31–32. [Google Scholar]
- Tooms, J.S. Review of Knowledge of Metalliferous Brines and Related Deposits; Institution of Mining & Metallurgy: London, UK, 1970. [Google Scholar]
- Piestrzyński, A.; Pieczonka, J.; Głuszek, A. Redbed-type gold mineralisation, Kupferschiefer, south-west Poland. Min. Dep. 2002, 37, 512–528. [Google Scholar] [CrossRef]
- Eldridge, C.S.; Barton, P.; Ohmoto, H. Mineral textures and their bearing on formation of Kuroko orebodies. In The Kuroko and Related Volcanogenic Massive Sulfide Deposits; Economic Geology Monograph; Economic Geology Publishing Co.: Littleton, CO, USA, 1983; pp. 241–281. [Google Scholar]
- Cox, P.; Lindsey, D.A.; Singer, D.A.; Diggles, M.F. Sediment-Hosted Copper Deposits of the World: Deposit Models and Database. US Geol. Surv. Open-File Rep. 2003, 3, 50. [Google Scholar]
- Cathles, L.M. Changes in sub-water table fluid flow at the end of the Proterozoic and its implications for gas pulsars and MVT lead–zinc deposits. Geofluids 2007, 7, 209–226. [Google Scholar] [CrossRef]
- Sverjensky, D.A. The origin of a mississippi valley-type deposit in the Viburnum Trend, Southeast Missouri. Econ. Geol. 1981, 76, 1848–1872. [Google Scholar] [CrossRef]
- Hagni, R.D. Tri-State Ore Deposits: The Character of Their Host Rocks and Theis Genesis. In Cu, Zn, Pb and Ag Deposits; en Wolf, K.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 6. [Google Scholar]
- Cathles, L.M. A Discussion of Flow Mechanisms Responsible for Alteration and Mineralization in the Cambrian Aquifers of the Ouachita-Arkoma Basin-Ozark System: Chapter 8: Diagenesis and Basin Hydrodynamics; American Association of Petroleum Geologists: Tulsa, OK, USA, 1993. [Google Scholar]
- Cathles, L.M.; Smith, A.T. Thermal constraints on the formation of mississippi valley-type lead-zinc deposits and their implications for episodic basin dewatering and deposit genesis. Econ. Geol. 1983, 78, 983–1002. [Google Scholar] [CrossRef]
- Cathles, L.M. A simple analytical method for calculating temperature perturbations in a basin caused by the flow of water through thin, shallow-dipping aquifers. Appl. Geochem. 1987, 2, 649–655. [Google Scholar] [CrossRef]
- Powley, D.E. Subsurface Fluid Compartments. In Gas Research Institute Deep Gas Sands Workshop; Gas Research Institute: Chicago, IL, USA, 1987. [Google Scholar]
- Ortoleva, P.J. Basin Compartments and Seals; Memoir 61; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; p. 477. [Google Scholar]
- Al-Shaieb, Z.; Puckette, J.O.; Abdalla, A.A.; Ely, P.B. Megacompartment Complex in the Anadarko Basin: A Completely Sealed Overpressured Phenomenon. In Basin Compartments and Seals; Orteleva, P.J., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; pp. 55–68. [Google Scholar]
- Schowalter, T.T. Mechanics of Secondary Hydrocarbon Migration and Entrapment. AAPG Bull. 1979, 63, 723–760. [Google Scholar]
- Bradley, J.S.; Powley, D.E. Pressure Compartments in Sedimentary Basins: A Review. In Basin Compartments and Seals; Orteleva, P.J., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; pp. 3–26. [Google Scholar]
- Shosa, J.D.; Cathles, L.M. Experimental investigation of capillary blockage of two phase flow in layered porous media. Pet. Syst. Deep-Water Basins Glob. Gulf Mexico Exp. Houston Texas GCSSEPM GCS 2001, 21, 725–739. [Google Scholar]
- Law, B.E. Basin-Centered Gas Systems. AAPG Bull. 2002, 86, 1891–1919. [Google Scholar]
- Ryder, R.T.; Zagorski, W.A. Nature, origin, and production characteristics of the Lower Silurian regional oil and gas accumulation, central Appalachian basin, United States. AAPG Bull. 2003, 87, 847–872. [Google Scholar] [CrossRef]
- Masters, J.A. Lower Cretaceous Oil and Gas in Western Canada. Elmworth: Case Study of a Deep Basin Gas Field; AAPG Memoir 38; American Association of Petroleum Geologists: Tulsa, OK, USA, 1984; pp. 1–33. [Google Scholar]
- Engelder, T.; Cathles, M.L.; Bryndzia, T.L. The fate of residual treatment water in gas shale. J. Unconv. Oil Gas Resour. 2014, 7, 33–48. [Google Scholar] [CrossRef]
- Revil, A.; Cathles, L.M. The porosity-depth pattern defined by 40 wells in Eugene Island South Addition, Block 330 Area, and its relation to pore pressure, fluid leakage, and seal migration. Pet. Syst. Deep-Water Basins Glob. Gulf Mexico Exp. Houston Texas GCSSEPM GCS 2001, 21, 687–712. [Google Scholar]
- Goldhaber, M.B.; Church, S.E.; Doe, B.R.; Aleinikoff, J.N.; Podosek, F.A.; Brannon, J.C.; Mosier, E.L.; Taylor, C.D.; Gent, C.A. Lead and sulfur isotope investigation of Paleozoic sedimentary rocks from the southern Midcontinent of the United States; implications for paleohydrology and ore genesis of the Southeast Missouri lead belts. Econ. Geol. 1995, 90, 1875–1910. [Google Scholar] [CrossRef]
- Rowan, E.L.; Goldhaber, M.B. Duration of mineralization and fluid-flow history of the Upper Mississippi Valley zinc-lead district. Geology 1995, 23, 609–612. [Google Scholar] [CrossRef]
- Cook, T.D.; Bally, A.W. (Eds.) Stratigraphic Atlas of North and Central America [cartographic Material]; Princeton University Press: Princeton, NJ, USA, 1975; p. 271. [Google Scholar]
- Lu, G.; Marshak, S.; Kent, D.V. Characteristics of magnetic carriers responsible for Late Paleozoic remagnetization in carbonate strata of the mid-continent, U.S.A. Earth Planet. Sci. Lett. 1990, 99, 351–361. [Google Scholar]
- Voo, R.V.D.; Torsvik, T.H. The history of remagnetization of sedimentary rocks: Deceptions, developments and discoveries. Geol. Soc. Lond. Spec. Publ. 2012, 371, 23–53. [Google Scholar]
- Oliver, J. Fluids expelled tectonically from orogenic belts: Their role in hydrocarbon migration and other geologic phenomena. Geology 1986, 14, 99–102. [Google Scholar] [CrossRef]
- McCabe, C.; Elmore, R.D. The occurrence and origin of Late Paleozoic remagnetization in the sedimentary rocks of North America. Rev. Geophys. 1989, 27, 471–494. [Google Scholar] [CrossRef]
- Erendi, A.; Cathles, L.M. Gas capillary inhibition to oil production. Pet. Syst. Deep-Water Basins Glob. Gulf Mexico Exp. Houston Texas GCSSEPM GCS 2001, 21, 607–618. [Google Scholar]
- Jakubov, A.A.; Ali-Zade, A.A.; Zeinalov, M.M. Mud Volcanoes of the Azerbaijan SSR. Atlas; The Academy of Sciences of the Azerbaijan SSR: Baku, Azerbaijan, 1971. [Google Scholar]
- Smith, J.T.; Ehrenberg, S.N. Correlation of carbon dioxide abundance with temperature in clastic hydrocarbon reservoirs: Relationship to inorganic chemical equilibrium. Mar. Pet. Geol. 1989, 6, 129–135. [Google Scholar] [CrossRef]
- Cathles, L.M.; Schoell, M. Modeling CO2 generation, migration, and titration in sedimentary basins. Geofluids 2007, 7, 441–450. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Cathles, L.M. Explaining the pulsating emission of H2 from a sedimentary basin in Brazil. Geosciences. (in preparation).
- Sicking, C.; Malin, P. Permeability structure mapping using fracture seismics. Geosciences 2019, 9, 34. [Google Scholar]
- Leach, D.L.; Bradley, D.; Lewchuk, M.T.; Symons, D.T.; Marsily, G.; Brannon, J. Mississippi Valley-type lead–zinc deposits through geological time: implications from recent age-dating research. Miner. Depos. 2001, 36, 711–740. [Google Scholar] [CrossRef]
| Surface Density Interval | Log Average Metal Added | ACu | APb | Azn | MCu | MPb | MZn |
|---|---|---|---|---|---|---|---|
| (kg m−2) | 1000 km2 | 106 tons | |||||
| 1 to 5 | 2.24 | 60.0 | 70.0 | 162.0 | 134 | 157 | 363 |
| 5 to 10 | 4.83 | 32.0 | 42.9 | 82.4 | 155 | 207 | 398 |
| 10 to 50 | 13.05 | 20.0 | 27.0 | 58.0 | 261 | 352 | 757 |
| 50 to 100 | 39.04 | 3.3 | 5.4 | 0.1 | 129 | 211 | 5 |
| 100 to 500 | 121.23 | 1.2 | 145 | 0 | 0 | ||
| Sum | 116.5 | 145.3 | 302.5 | 824 | 927 | 1523 | |
| Brine (km3) | 200,000 | ppm | 4.1 | 4.6 | 7.6 | ||
| Rotliegende [35], ppm | 1 | 50 | 50 | ||||
| Akkrum field [36], ppm | <0.5 | 50 | 60 | ||||
| Chelekin, 50–80 °C, [37], ppm | 0.9 to 15 | ||||||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cathles, L. On the Processes that Produce Hydrocarbon and Mineral Resources in Sedimentary Basins. Geosciences 2019, 9, 520. https://doi.org/10.3390/geosciences9120520
Cathles L. On the Processes that Produce Hydrocarbon and Mineral Resources in Sedimentary Basins. Geosciences. 2019; 9(12):520. https://doi.org/10.3390/geosciences9120520
Chicago/Turabian StyleCathles, Lawrence. 2019. "On the Processes that Produce Hydrocarbon and Mineral Resources in Sedimentary Basins" Geosciences 9, no. 12: 520. https://doi.org/10.3390/geosciences9120520
APA StyleCathles, L. (2019). On the Processes that Produce Hydrocarbon and Mineral Resources in Sedimentary Basins. Geosciences, 9(12), 520. https://doi.org/10.3390/geosciences9120520





