The Fossil Record of Darkling Beetles (Insecta: Coleoptera: Tenebrionidae)
Abstract
1. Introduction
2. Material and Methods
- Fossilworks: http://fossilworks.org/,
- International Commission on Stratigraphy: http://www.stratigraphy.org/,
- Catalogue of fossil Tenebrionidae: https://www.zin.ru/Animalia/Coleoptera/rus/teneb_ff.htm.
3. Brief History of the Study of Fossil Tenebrionidae
4. Catalogue of Fossil Tenebrionidae
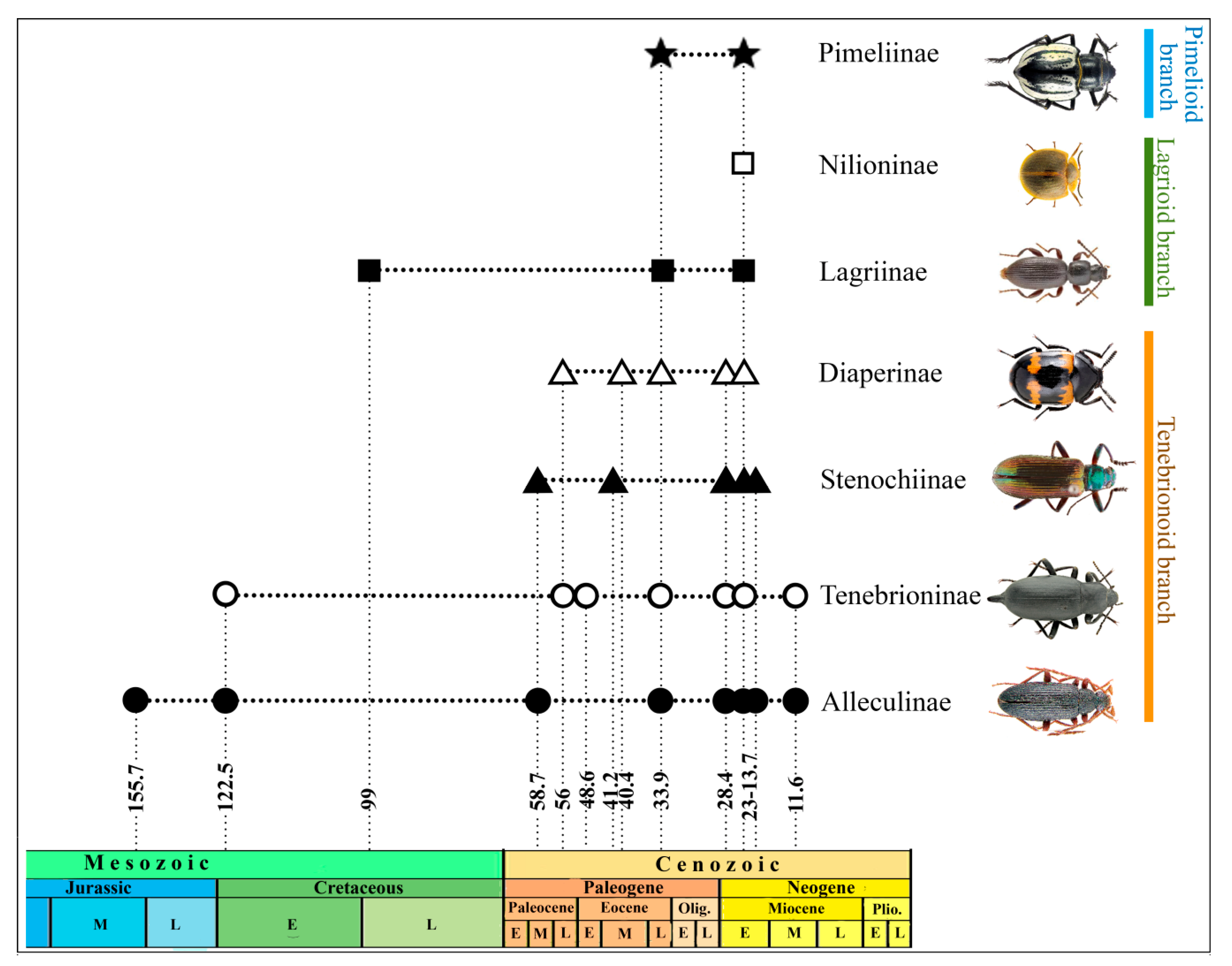
5. The Fossil Record of Major Tenebrionid Lineages
5.1. Tenebrionoid Branch.
5.1.1. Subfamily Alleculinae
5.1.2. Subfamily Tenebrioninae
5.1.3. Subfamily Stenochiinae
5.1.4. Subfamily Diaperinae
5.2. Pimelioid Branch
Subfamily Pimeliinae
5.3. Lagrioid Branch
5.3.1. Subfamily Lagriinae
5.3.2. Subfamily Nilioninae
6. The Fossil Record of Tenebrionidae in Evolutionary Reconstructions
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Matthews, E.G.; Lawrence, J.F.; Bouchard, P.; Steiner, W.E.; Ślipiński, S.A. 11.14. Tenebrionidae Latreille, 1802. In Handbook of Zoology. Arthropoda: Insecta. Part 38. Coleoptera, Beetles. Vol. 2. Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia Partim); Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; Walter de Gruyter: Berlin, Germany, 2010; pp. 574–659. [Google Scholar]
- Kergoat, G.J.; Soldati, L.; Clamens, A.L.; Jourdan, H.; Zahab, R.; Genson, G.; Bouchard, P.; Condamine, F.L. Higher-level molecular phylogeny of darkling beetles (Coleoptera, Tenebrionoidea, Tenebrionidae). Syst. Entomol. 2014, 39, 486–499. [Google Scholar] [CrossRef]
- Watt, J.C. A revised subfamily classification of Tenebrionidae (Coleoptera). New Zealand J. Zool. 1974, 1, 381–452. [Google Scholar] [CrossRef]
- Medvedev, G.S. Taxonomic significance of antennal sensilla of darkling beetles (Coleoptera, Tenebrionidae). In Trudy Vsesoyuznogo Entomologisheskogo Obshchestva. Vol. 58. Morphological Bases of the Systematics of Insects; Medvedev, G.S., Ed.; Nauka: Leningrad, Russia, 1977; pp. 61–86. (In Russian) [Google Scholar]
- Doyen, J.T. Familial and subfamilial classification of the Tenebrionoidea (Coleoptera) and a revised generic classification of the Coniontini (Tentyriidae). Quaest. Entomol. 1972, 8, 357–376. [Google Scholar]
- Doyen, J.T. Cladistic Relationships among Pimeliine Tenebrionidae (Coleoptera). J. N. Y. Entomol. Soc. 1993, 101, 443–514. [Google Scholar]
- Doyen, J.T.; Lawrence, J.F. Relationships and higher classification of some Tenebrionidae and Zopheridae (Coleoptera). Syst. Entomol. 1979, 4, 333–337. [Google Scholar] [CrossRef]
- Tschinkel, W.R.; Doyen, J.T. Comparative anatomy of the defensive glands, ovipositors and female tubes of tenebrionid beetles (Coleoptera). Int. J. Insect Morphol. Embryol. 1980, 9, 321–368. [Google Scholar] [CrossRef]
- Doyen, J.T.; Tschinkel, W.R. Phenetic and cladistic relationships among tenebrionid beetles (Coleoptera). Syst. Entomol. 1982, 7, 127–183. [Google Scholar] [CrossRef]
- Matthews, E.G.; Bouchard, P. (Eds.) Tenebrionid Beetles of Australia: Descriptions of Tribes, Keys to Genera, Catalogue of Species; Australian Biological Resources Study: Canberra, Australia, 2008; pp. 1–410. [Google Scholar]
- Aalbu, R.L.; Triplehorn, C.A.; Campbell, J.M.; Brown, K.W.; Somerby, R.E.; Thomas, D.B. Tenebrionidae Latreille 1802. In American Beetles, Vol. 2. Polyphaga: Scarabaeoidea through Curculionoidea; Arnett, R.H.J., Thomas, M.C., Skelley, P.E., Frank, J.H., Eds.; CRC Press: New York, NY, USA, 2002; pp. 463–509. [Google Scholar]
- Bouchard, P.; Lawrence, J.F.; Davies, A.E.; Newton, A.F. Synoptic classification of the world Tenebrionidae (Insecta: Coleoptera) with a review of family-group names. Ann. Zool. 2005, 55, 499–530. [Google Scholar]
- Nabozhenko, M.V.; Sadeghi, S. Foranotum perforatum gen. et sp. nov.—A new troglobitic darkling beetle (Coleoptera: Tenebrionidae: Kuhitangiinae: Foranotini trib. nov.) from a cave in Southern Zagros, Iran. Zootaxa 2017, 4338, 163–172. [Google Scholar] [CrossRef]
- Kergoat, G.L.; Bouchard, P.; Clamens, A.L.; Abbate, J.L.; Jourdan, H.; Jabbour-Zahab, R.; Genson, G.; Soldati, L.; Condamine, F.L. Cretaceous environmental changes led to high extinction rates in a hyperdiverse beetle family. BMC Evol. Biol. 2014, 14, 220. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Che, L.H.; Li, Y.; Liang, D.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Germar, E.F. (Ed.) Insectorum Protogaeae Specimen Sistens Insecta Carbonum Fossilium. Fauna Insectorum Europae. Fasc. 19; Kümmelii: Halae, CA, USA, 1837; pp. 1–27. [Google Scholar] [CrossRef]
- Heyden, C.V. Fossile Insekten aus der Rheinischen Braunkhohle. Palaeontographica. 1859, 8, 1–15. [Google Scholar]
- Heyden, C.V.; Heyden, L.V. Käfer und Polypen aus der Braunkohle des Siebengebirges. Palaeontographica 1866, 15, 131–156. [Google Scholar]
- Heer, O. Die Insektenfauna der Tertiargebilde von Oeningen und von Radoboj in Croatien. Erste Theil. Kaefer. Neue Denkschriften der Allgemeinen. Schweiz. Ges. Naturw. 1847, 8, 1–230. [Google Scholar]
- Heer, O. (Ed.) Die Urwelt der Schweiz; Schulthess: Zürich, Switzerland, 1865; pp. 1–622. [Google Scholar]
- Heer, O. Primitiae florae fossilis sachalinensis. Mém. Acad. Sci. St. Pétersb., VIIe Sér. 1878, 25, 1–61. [Google Scholar]
- Heer, O. (Ed.) Ueber die fossilen Insekten Grönlands. In Die Fossile Flora Grönlands 2; Wurster, J. & Co: Zurich, Switzerland, 1883; pp. 143–148. [Google Scholar]
- Scudder, S.H. Appendix A. The fossil insects collected in 1877, by Mr. G.M. Dawson, in the interior of British Columbia. In Report of Progress of the Geological Survey of Canada 1877–1878; Dawson Brothers: Ottawa, ON, Canada, 1879; pp. 175–185. [Google Scholar]
- Scudder, S.H. Adephagous and Clavicorn Coleoptera from the Tertiary Deposits at Florissant, Colorado with Descriptions of a Few Other Forms and a Systematic List of the Non-Rhynchophorus Tertiary Coleoptera of North America; Monogr. U. S. Geol. Surv.; US Government Printing Office: Washington, DC, USA, 1900; Volume 40, pp. 1–148.
- Wickham, H.F. New Fossil Coleoptera from Florissant. Am. J. Sci. 1909, 28, 126–130. [Google Scholar] [CrossRef]
- Wickham, H.F. New fossil Coleoptera from Florissant, with notes on some already described. Am. J. Sci. 1910, 29, 47–51. [Google Scholar] [CrossRef]
- Wickham, H.F. Fossil Coleoptera from Florissant in the United States National Museum. Proc. U. S. Nat. Mus. 1913, 45, 283–303. [Google Scholar] [CrossRef]
- Wickham, H.F. New Miocene Coleoptera from Florissant. Bull. Mus. Comp. Zool. 1914, 53, 423–494. [Google Scholar]
- Klebs, R. Über Bernsteineinschlüsse in allgemeinen und die Coleopteren meiner Bernsteinsammlung. Schrift. Physikal. Ökonom. Gesell. Königsb. 1910, 51, 217–242. [Google Scholar]
- Haupt, H. Die Käfer (Coleoptera) aus der eozänen Braunkohle des Geiseltales. Geologica 1950, 6, 1–168. [Google Scholar]
- Medvedev, L.N. New Mesozoic beetles (Cucujoidea) of Asia. Paleontol. Zhurnal 1969, 1, 119–125. (In Russian) [Google Scholar]
- Larsson, S.G. (Ed.) Baltic Amber—A Palaeobiological Study: Entomonograph, Volume 1; Scand. Sci. Press Ltd.: Klampenborg, Denmark, 1978; pp. 1–192. [Google Scholar]
- Hieke, F.; Pietrzeniuk, E. Die BernsteinKäfer des Museums für Naturkunde, Berlin (Insecta, Coleoptera). Mitt. Zool. Mus. Berl. 1984, 60, 297–326. [Google Scholar]
- Spahr, U. (Ed.) Systematischer Katalog der Bersteinund Kopal-Käfer (Coleoptera); Staatliches Museum für Naturkunde: Karlsruhe, Germany, 1981; Volume 80, pp. 1–107. [Google Scholar]
- Hong, Y.C. (Ed.) Fossil Insects, Scorpionids and Araneids in the Diatoms of Shanwang; Geological Publishing House: Beijing, China, 1985; pp. 1–113. [Google Scholar]
- Zhang, J.F. (Ed.) Fossil Insects from Shanwang, Shandong, China; In Chinese with English Summary; Shandong Science & Technology Press: Jinan, China, 1989; pp. 1–459. [Google Scholar]
- Kaszab, Z.; Schawaller, W. Eine neue Schwarzkäfer-Gattung und -Art aus Dominikanischem Bernstein (Coleoptera, Tenebrionidae). Stutt. Beitr. Nat. Ser. B (Geol. Palaeontol.) 1984, 109, 1–6. [Google Scholar]
- Doyen, J.T.; Poinar, G.O. Tenebrionidae from Dominican amber (Coleoptera). Entomol. Scand. 1994, 25, 27–51. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G.; Merkl, O.; Kernegger, F. A new species of the genus Pentaphyllus Dejean, 1821 (Coleoptera, Tenebrionidae, Diaperinae) from the Baltic amber and checklist of the fossil Tenebrionidae. Zoosyst. Rossica 2008, 17, 131–137. [Google Scholar]
- Kirejtshuk, A.G.; Ponomarenko, A.G. Taxonomic List of Fossil Beetles of the Surder Scarabaeina (Part 3). Available online: https://www.zin.ru/Animalia/Coleoptera/rus/paleosy2.htm (accessed on 10 May 2018). (In Russia).
- Kirejtshuk, A.G.; Nabozhenko, M.V.; Nel, A. First Mesozoic representative of the subfamily Tenebrioninae (Coleoptera, Tenebrionidae) from the Lower Cretaceous of Yixian (China, Liaoning). Entomol. Obozr. 2011, 90, 548–552. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Chang, H.; Xu, L.; Pu, H.; Jia, S. A new species and a new genus of comb-clawed beetles (Coleoptera: Tenebrionidae: Alleculinae) from Lower Cretaceous of Yixian (China, Laoning). Paleontol. J. 2015, 49, 1420–1423. [Google Scholar] [CrossRef]
- Chang, H.L.; Nabozhenko, M.; Pu, H.Y.; Xu, L.; Jia, S.H.; Li, T.R. First record of fossil comb-clawed beetles of the tribe Cteniopodini (Insecta: Coleoptera: Tenebrionidae) from the Jehol Biota (Yixian formation of China), Lower Cretaceous. Cretac. Res. 2016, 57, 289–293. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Kirejtshuk, A.G. Cryptohelops menaticus—A new genus and species of the tribe Helopini (Coleoptera: Tenebrionidae) from the Paleocene of Menat (France). C. R. Palevol 2014, 13, 65–71. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Kirejtshuk, A.G. The oldest opatrine terrestrial darkling beetle (Coleoptera: Tenebrionidae: Tenebrioninae) from the Paleocene of Menat (France). Paläontol. Z. 2017, 91, 307–313. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G.; Nabozhenko, M.V.; Nel, A. New genus and species of the tribe Opatrini (Coleoptera, Tenebrionidae, Tenebrioninae) from the Lowermost Eocene amber of Paris Basin. Proc. Zool. Inst. RAS 2010, 314, 191–196. [Google Scholar]
- Alexeev, V.I.; Nabozhenko, M.V. A new fossil tenebrionid beetle of the tribe Palorini (Coleoptera: Tenebrionidae) from Eocene Baltic Amber. Coleopt. Bull. 2015, 69, 127–130. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Nabozhenko, M.V. Palorus platycotyloides sp.n., the second fossil representative of the tribe Palorini (Coleoptera: Tenebrionidae) from Baltic Amber. Acta Zool. Bulg. 2017, 69, 167–170. [Google Scholar]
- Nabozhenko, M.V.; Perkovsky, E.E.; Chernei, L.S. A new species of the genus Nalassus Mulsant (Coleoptera: Tenebrionidae: Helopini) from the Baltic amber. Paleontol. J. 2016, 50, 947–952. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Kirejtshuk, A.G.; Merkl, O. Yantaroxenos colydioides gen. and sp.n. (Tenebrionidae: Lagriinae) from Baltic Amber. Ann. Zool. 2016, 66, 563–566. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Bukejs, A.; Telnov, D. Gonialaenini, a new tribe of Lagriinae (Coleoptera: Tenebrionidae) from Eocene Baltic Amber. Zootaxa 2019, 4565, 253–260. [Google Scholar] [CrossRef]
- Nabozhenko, M.; Chigray, I.; Bukejs, A. Taxonomic notes on the Eocene Helopini, and a review of the genus Isomira Mulsant, 1856 from Baltic amber (Coleoptera: Tenebrionidae). Insect Syst. Evol. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Soldati, F.; Nabozhenko, M. Asida groehni sp. nov., the first and the oldest fossil representative of the subfamily Pimeliinae from Eocene Baltic amber (Coleoptera: Tenebrionidae: Asidini). Ann. Zool. 2017, 67, 555–559. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Chigray, I.A. New and little known species of Alleculini (Coleoptera: Tenebrionidae: Alleculinae): extinсt from Eocene Baltic Amber and extant from Lebanon. Cauc. Entomol. Bull. 2018, 14, 171–176. [Google Scholar] [CrossRef]
- Telnov, D.; Bukejs, A.; Merkl, O. Description of a new fossil Statira Lepeletier et Audinet-Serville, 1828 (Coleoptera: Tenebrionidae: Lagriinae) from Baltic amber of the Sambian Peninsula. Zootaxa 2019, 4683, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F. A new species of Corticeus Piller & Mitterpacher, 1783 from Dominican amber (Coleoptera Tenebrionidae). Entomapeiron (P.S.) 2007, 2, 1–6. [Google Scholar]
- Vitali, F. A new species of Tyrtaeus Champion, 1913 from Dominican amber (Coleoptera Tenebrionidae). Entomapeiron (P.S.). 2008, 3, 11–16. [Google Scholar]
- Kirejtshuk, A.G.; Nel, A.; Kirejtshuk, P.A. Taxonomy of the reticulate beetles of the subfamily Cupedinae (Coleoptera: Archostemata), with a review of the historical development. Invertebr. Zool. 2016, 13, 61–190. [Google Scholar] [CrossRef]
- Batelka, J.; Engel, M.S.; Prokop, J. A remarkable diversity of parasitoid beetles (Ripiphoridae) in Cretaceous amber, with a summary of the Mesozoic record of Tenebrionoidea. Cretac. Res. 2018, 90, 296–310. [Google Scholar] [CrossRef]
- Arillo, A.; Ortuño, V. Catalogue of fossil insect species described from Dominican amber (Miocene). Stuttg. Beiträge Nat. B (Geol. Palaeontol.) 2005, 352, 1–68. [Google Scholar]
- Handlirsch, A. (Ed.) Die Fossilen Insekten und Die Phylogenie der Rezenten Formen. Ein Handbuch für Paläontologen und Zoologen Vol. 1, lfg. I-IV.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1906; pp. 1–640. [Google Scholar]
- Bachofen-Echt, A. (Ed.) Der Bernstein und Seine Einschlüsse; Springer: Wien, Austria, 1949; pp. 1–204. [Google Scholar]
- Abdullah, M. New heteromerous beetles (Coleoptera) from the Baltic amber of eastern Prussia and gum copal of Zanzibar. Trans. R. Entomol. Soc. Lond. 1964, 116, 329–349. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.H.; Shih, C.H.; Ren, D. Coleoptera—Beetles. In Rhythms of Insect Evolution: Evidence from the Jurassic and Cretaceous in Northern China, 1st ed.; Ren, D., Shih, C.H.K., Gao, T., Yao, Y.U., Wang, Y., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 337–428. [Google Scholar] [CrossRef]
- Seidlitz, G. Alleculidae. In Naturgeschichte der Insecten Deutschlands. Erste Abtheilung. Coleoptera. Fünfter Band. Zweite Hälfte. Lieferungen 1–3; Erichson, W.F., Ed.; Nicolaische Verlags-Buchhandlung: Berlin, Germany, 1896; pp. 1–305. [Google Scholar]
- Campbell, J.M. Fossil beetle of the genus Hymenorus (Coleoptera: Alleculidae) found in amber from Chiapas, Mexico. Univ. Calif. Publ. Entomol. 1963, 31, 41–42. [Google Scholar]
- Schaufuss, L.W. Einige Käfer aus dem baltischen Bernsteine. Berl. Entomol. Z. 1888, 32, 266–270. [Google Scholar] [CrossRef]
- Bousquet, Y.; Bouchard, P.; Campbell, J.M. Catalogue of the genus-group names in Alleculinae (Coleoptera: Tenebrionidae). Coleopt. Bull. 2015, 69, 131–151. [Google Scholar] [CrossRef]
- Berendt, G.C. (Ed.) Die im Bernstein befi Ndlichen Organischen Reste der Vorvelt; Nikolaische Buchhandlung: Danzig, Poland, 1845; pp. 1–38. [Google Scholar]
- Scudder, S.H. (Ed.) Index to the Known Fossil Insects of the World including Myriapods and Arachnids; US Government Printing Office: Washington, DC, USA, 1891; Volume 71, pp. 1–744. [CrossRef]
- Seidlitz, G.V. Nachträge und Berichtigungen zur Familie Tenebrionidae. In Naturgeschichte der Insecten Deutschlands. Erste Abteilung Coleoptera. Bd. 5. Hf. 8; Kiesenwetter, H.V., Seidlitz, G.V., Eds.; Nicolaische Verlags-Buchhandlung: Berlin, Germany, 1898; pp. 813–853. [Google Scholar]
- Deutschlands Petrefacten: Ein Systematisches Verzeichniss Aller in Deutschland und den Angrezenden Ländern Vorkommenden Petrefacten, Nebst Angabe der Synonymen und Fundorte; Giebel, C.G., Ed.; Verlag von Ambrosius Abel: Leipzig, Germany, 1852; pp. 1–706. [Google Scholar]
- Giebel, C.G. (Ed.) Fauna der Vorwelt Mit steter Berücksichtigung der Lebenden Thiere. Monographisch Dargestellt. B. 12; F.A. Brockhaus: Leipzig, Germany, 1856; pp. 1–511. [Google Scholar] [CrossRef]
- Scudder, S.H. (Ed.) Canadian Fossil Insects, Myriapods and Arachnids, 2. The Coleoptera Hitherto Found Fossil in Canada; Contributions to Canadian Palaeontology; Geolog. Surv.: Ottawa, ON, Canada, 1895; Volume 2, pp. 27–56. [Google Scholar]
- Scudder, S.H. (Ed.) Systematic Review of our Present Knowledge of Fossil Insects including Myriapods and Arachnids; Govt. Print. Off.: Washington, DC, USA, 1886; Volume 31, pp. 1–128. [CrossRef]
- Scudder, S.H. The tertiary insects of North America. Rep. United States Geol. Surv. territories. 1890, 13, 1–734. [Google Scholar] [CrossRef][Green Version]
- Heyden, C.V. Gliederthiere aus der Braunkohle des Niederrhein’s, der Wetterau und der Röhn. Palaeontographica 1862, 10, 62–82. [Google Scholar]
- Hörnschemeyer, T. Ein fossiler Tenebrionide Ceropria? messelense n. sp. (Coleoptera: Tenebrionidae: Diaperinae) aus dem Mitteleozän der Grube Messel bei Darmstadt. Cour. Forsch. Senckenberg 1994, 170, 75–83. [Google Scholar]
- Hope, F.W. Observations on succinic insects. Trans. R. Entomol. Soc. Lond. 1837, 2, 46–57. [Google Scholar] [CrossRef][Green Version]
- Meunier, F. Die Insektenreste aus dem Lutetien von Messel bei Darmstadt. Abh. Grösshesz. Hess. Geol. Landesanst. 1921, 7, 2–15. [Google Scholar]
- Pongrácz, A. Die eozäne Insektenfauna des Geiseltales. Nova Acta Leopold. 1935, 2, 485–572. [Google Scholar]
- Heyden, C.V.; Heyden, L.V. Fossile Insekten aus der Braunkohle von Salzhausen. Palaeontographica 1865, 14, 31–35. [Google Scholar]
- Cockerell, T.D.A. Fossil Arthropods in the British Museum, III. Ann. Mag. Nat. Hist. 1920, 6, 65–72. [Google Scholar] [CrossRef]
- Redtenbacher, L. Helops atticus. In Unger F. Die Fossile Flora Von Kumi Auf Der Insel Euboea. Denkschr. Österr. Akad. Wiss. Math. Naturwiss. Kl. 1867, 27, 33. [Google Scholar]
- Vitali, F. Systematic revision of the fossil cerambycids from Geiseltal (Coleoptera Cerambycidae). Entomapeiron (PS). 2008, 3, 1–10. [Google Scholar]
- Wang, B.; Zhang, H. The oldest Tenebrionoidea (Coleoptera) from the Middle Jurassic of China. J. Paleontol. 2011, 85, 266–270. [Google Scholar] [CrossRef]
- Shchegoleva-Barovskaya, T.I. Der erste Vertreter der Familie Mordellidae (Coleoptera) aus der Juraformation Turkestans. CR Acad. Sci. URSS 1929, 1, 25–29. [Google Scholar]
- Hsiao, Y.; Yu, Y.; Deng, C.; Pang, H. The first fossil wedge-shaped beetle (Coleoptera, Ripiphoridae) from the middle Jurassic of China. Eur. J. Taxon. 2017, 277, 1–13. [Google Scholar] [CrossRef]
- Krishtofovich, A.N. (Ed.) Paleobotany; Gostoptehizdat: Leningrad, Russia, 1957; pp. 1–650. (In Russian) [Google Scholar]
- Kirejtshuk, A.G. The Himalaya: Region of relicts and center of modern diversifications of biota by example of sap-beetles (Coleoptera, Nitidulidae). In Russian Himalayan Research: Past, Present, Future; Borkin, L.J., Ed.; Evropeisky Dom: St Petersburg, Russia, 2017; pp. 168–172. [Google Scholar]
- Striganova, B.R. Morphological peculiarities of and identification key to alleculid-larvae of the subfamily Alleculinae (Coleoptera). Zool. Zhurnal 1961, 40, 193–200. (in Russian). [Google Scholar]
- Dubrovin, N.N.; Kompantseva, T.V. Morpho-ecological review of larvae of Isomira (Coleoptera, Alleculidae) with the description of a new species from Central Asia. Zool. Zhurnal 1992, 71, 14–22. (In Russian) [Google Scholar]
- Nabozhenko, M.V. A revision of the genus Catomus Allard, 1876 and the allied genera (Coleoptera, Tenebrionidae) from the Caucasus, Middle Asia, and China. Entomol. Rev. 2006, 86, 1024–1072. [Google Scholar] [CrossRef]
- Nabozhenko, M.V. Darkling Beetles of the Tribe Helopini (Coleoptera: Tenebrionidae) of the World Fauna. Ph.D. Thesis, Zoological Institute of the Russian Academy of Sciences, St Petersburg, Russia, 2019; pp. 1–408. (In Russian). [Google Scholar]
- Kirejtshuk, A.G.; Nel, A. Current knowledge of Coleoptera (Insecta) from the Lowermost Eocene Oise amber. Insect Syst. Evol. 2013, 44, 175–201. [Google Scholar] [CrossRef]
- Kohlman-Adamska, A. A graphic reconstruction of an “amber” forest. In The Amber Treasure Trove, Pt I: The Tadeusz Giecewiczs Collection at the Museum of the Earth, Polish Academy of Sciences, Warsaw; Kosmowska-Ceranowicz, B., Ed.; Oficyna Wydawnicza Sadyba: Warsaw, Poland, 2001; pp. 15–17. [Google Scholar]
- Alekseev, V.; Alexeev, P. New Approaches for Reconstruction of the Ecosystem of an Eocene Amber Forest. Biol. Bull. 2016, 43, 75–86. [Google Scholar] [CrossRef]
- Poinar, G.; Brown, A.E. Descriptions of a broad-nosed weevil (Eudiagogini: Curculionidae) and false ladybird beetle (Nilionini: Nilionidae) in Dominican amber. Hist. Biol. 2011, 23, 231–235. [Google Scholar] [CrossRef]
- Aloquio, S.; Lopes-Andrade, C. Redescription of Immature Stages and Adults of Nilio (Nilio) brunneus (Coleoptera: Tenebrionidae: Nilioninae). Zoologia 2016, 33, e20150191. [Google Scholar] [CrossRef]
| Fossil Deposits | Era(period) Age, Ma |
|---|---|
| Early Jurassic, Rhaetian/Hettangian; Switzerland: Aargau, Schambelen | Mz(Ju) 201.6–196.5 |
| Middle/Late Jurassic, Callovian/Oxfordian; Kazakhstan: Karatau | Mz(Ju) 164.7–155.7 |
| Early Cretaceous, Aptian; China: Huangbanjigou, Chaomidian | Mz(Cr) 125.5–122.5 |
| Early Cretaceous, Santonian; Russia: Sakhalin, Mgachi | Mz(Cr) 85.8–84.9 |
| Early Paleocene, Danian; Argentina: El Sunchal | Pg(Pc) 66–55.8 |
| Middle Paleocene, Selandian; Denmark, Greenland: Aumarutigsat, Haseninsel (Hareøen), | Pg(Pc) 61.7–58.7 |
| Middle/Late Paleocene, Selandian/Thanetian; France: Menat | Pg(Pc) 61–56 |
| Earliest Eocene, Oise amber, Ypresian; France: Le Quesnoy | Pg(Eo) 55.8–48.6 |
| Early Eocene, Ypresian; Canada: Whipsaw Creek | Pg(Eo) 55.8–40.4 |
| Middle Eocene, Lutetian; Germany: Messel Pit | Pg(Eo-Mess) 48.6–40.4 |
| Middle Eocene, Lutetian; Germany: Geiseltal Halle | Pg(Eo-Geis) 48.6–40.4 |
| Middle Eocene, Lutetian; United Kingdom: Bournemouth | Pg(Eo-Bour) 48.6–40.4 |
| Late Eocene, Baltic Amber, Priabonian; coasts of the Baltic Sea | Pg(Eo-BA) 37.2–33.9 |
| Latest Eocene, Priabonian; USA, Colorado: Florissant, Twin Creek, Front Range near Pike’s Peak | Pg(Eo-Flor) 37.2–33.9 |
| Early Oligocene, Rupelian; France: Alsace, Haut-Rhine, 5 km SW Mulhouse, Brunstatt | Pg(Og) 33.9–28.4 |
| Late Oligocene/early Miocene, Chattian/Aquitanian; Germany: Rott | Ng(Mi) 28.4–23 |
| Early Miocene, Aquitanian; Switzerland: Lausanne, Molasse | Ng(Mi) 23–20.4 |
| Early Miocene, Mexican amber, Aquitanian/Burdigalian; Mexico: Simojovel area, Chiapas | Ng(Mi) 23–16 |
| Early Miocene, Burdigalian; China: Linqu County, Shanwang | Ng(Mi-Shan) 20.4–16 |
| Early Miocene, Burdigalian; Greece: Kumi, Euboea | Ng(Mi-Kumi) 20.4–16 |
| Early Miocene, Dominican amber, Burdigalian; Dominican Republic | Ng(Mi-Dom) 20.4–13.7 |
| Middle Miocene, Langhian/Serravallian; Germany: Lower Saxony, Salzhausen | Ng(Mi) 16.0–11.6 |
| Middle Miocene, Serravallian; Germany: Baden-Württemberg, Oeningen | Ng(Mi)12.7–11.6 |
| No. | Taxon | Age and Site of Finding | Sources |
|---|---|---|---|
| Subfamily Lagriinae | |||
| Tribe Lagriini Latreille, 1825 | |||
| 1 | Lagria sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 2 | Statira sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 3 | Statira baltica Telnov, Bukejs et Merkl, 2019 | Pg(Eo-BA) 37.2–33.9 | [55] |
| 4 | Statira dermoidea Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tribe Laenini | |||
| 5 | Laena sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,62,63] |
| Tribe Gonialaenini Nabozhenko, Telnov et Bukejs, 2019 | |||
| 6 | Gonialaena† groehni Nabozhenko, Telnov et Bukejs, 2019 | Pg(Eo-BA) 37.2–33.9 | [51] |
| Tribe Lupropini Lesne, 1926 | |||
| 7 | Luprops sp. | Pg(Eo-BA) 37.2–33.9 | [29] |
| 8 | Lorelus angulatus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 9 | Lorelus foraminosus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 10 | Lorelus minutulus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 11 | Lorelus wolcotti Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tribe Belopini Reitter, 1917 | |||
| 12 | Yantaroxenos† colydioides Nabozhenko, Kirejtshuk et Merkl, 2016 | Pg(Eo-BA) 37.2–33.9 | [50] |
| Subfamily Pimeliinae | |||
| Tribe Asidini Fleming, 1821 | |||
| 13 | Asida (Planasida) groehni F. Soldati et Nabozhenko (2017 | Pg(Eo-BA) 37.2–33.9 | [53] |
| 14 | Pelecyphorus (Stenosides) primus (Wickham, 1910) (Ologlyptus), transferred to Pelecyphorus by F. Soldati & Nabozhenko (2017) | Pg(Eo-Flor) 37.2–33.9 | [26,39,53] |
| Tribe Stenosini Schaum, 1859 | |||
| 15 | Miostenosis† lacordairei Wickham, 1913 | Pg(Eo-Flor) 37.2–33.9 | [27,39] |
| Tribe Edrotini Lacordaire, 1859 | |||
| 16 | Trientoma hascens Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Subfamily Alleculinae | |||
| Tribe Alleculini Laporte, 1840 | |||
| Subtribe Alleculina Laporte, 1840 | |||
| 17 | Jurallecula† grossa L. Medvedev, 1969 | Mz(Ju) 164.7–155.7 | [31,39,59,64] |
| 18 | Allecula austriaca Zhang, 1989 | Ng(Mi-Shan) 20.4–16 | [36,39] |
| 19 | Allecula dominula (Heer, 1847) (Cistela) | Ng(Mi) 12.7–11.6 | [19,39,65] |
| 20 | Allecula sp. | Pg(Eo-BA) 37.2–33.9 | [29,32] |
| 21 | Hymenorus haydeni Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39] |
| 22 | Hymenorus chiapasensis Campbell, 1963 | Ng(Mi) 23–16 | [39,66] |
| 23 | Hymenorus oculatus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 24 | Hymenalia sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 25 | Pseudocistela gracilis Förster, 1891 | Pg(Og) 33.9–28.4 | [27,39,65] |
| 26 | Parahymenorus sp. | Ng(Mi-Dom) 20.4–13.7 | [38,39] |
| 27 | Lobopoda annosa Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 28 | Lobopoda sp. | Ng(Mi-Dom) 20.4–13.7 | [38,39] |
| Subtribe Mycetocharina Gistel, 1848 | |||
| 29 | Mycetochara sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 30 | Mycetocharoides† baumeisteri Schaufuss, 1888 | Pg(Eo-BA) 37.2–33.9 | [39,65,67,68] |
| Subtribe Gonoderina Seidlitz, 1896 | |||
| 31 | Gonodera antiqua (Wickham, 1913) (Cistela) | Pg(Eo-Flor) 37.2–33.9 | [27,39] |
| 32 | Gonodera vulcanica (Wickham, 1914) (Cistela) | Pg(Eo-Flor) 37.2–33.9 | [27,39] |
| 33 | Gonodera baygushevae Nabozhenko et I. Chigray, 2018 | Pg(Eo-BA) 37.2–33.9 | [54] |
| 34 | Gonodera sp. (Cistela); transferred to Gonodera by Spahr (1981) | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,69] |
| 35 | Isomira (Mucheimira) avula Seidlitz, 1896 (originally in the subgenus Asiomira; transferred by Nabozhenko et al. 2019) | Pg(Eo-BA) 37.2–33.9 | [39,52,65] |
| 36 | Isomira (Isomira) hoffeinsorum Nabozhenko in Nabozhenko, I. Chigray et Bukejs, 2019 | Pg(Eo-BA) 37.2–33.9 | [52] |
| 37 | Isomira sp. aff. hoffeinsorum | Pg(Eo-BA) 37.2–33.9 | [52] |
| 38 | Isomira (subgenus incertus) florissantensis Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39] |
| 39 | Isomira (subgenus incertus) sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 40 | Capnochroa senilis Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39] |
| Tribe Cteniopodini Solier, 1835 | |||
| 41 | Platycteniopus† diversoculatus Chang, Nabozhenko, Pu, Xu, Jia et Li, 2016 | Mz(Cr) 125.5–122.5 | [43,59,64] |
| 42 | Calcarocistela† kirejtshuki Nabozhenko in Nabozhenko, Chang, Xu, Pu et Jia, 2015 | Mz(Cr) 125.5–122.5 | [42,59] |
| 43 | Cteniopus sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,39] |
| 44 | Cteniopinus sp. | Pg(Eo-BA) 37.2–33.9 | [33,39] |
| 45 | Sinocistela† gymnelytra Zhang, 1989 | Ng(Mi-Shan) 20.4–16 | [36,39] |
| 46 | Sinocistela† silpha Zhang, 1989 | Ng(Mi-Shan) 20.4–16 | [36,39] |
| Subfamily Tenebrioninae | |||
| Tribe Palorini | |||
| 47 | Palorus sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,47,48,62,63] |
| 48 | Palorus platycotyloides Alekseev et Nabozhenko, 2017 | Pg(Eo-BA) 37.2–33.9 | [48] |
| 49 | Vabole† triplehorni Alekseev et Nabozhenko, 2015 | Pg(Eo-BA) 37.2–33.9 | [47,48] |
| Tribe Toxicini | |||
| Subtribe Eudysantina | |||
| 50 | Wattius reflexus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tribe Bolitophagini | |||
| 51 | “Bolitophagus” vetustus Heyden et Heyden, 1866 | Ng(Mi) 28.4–23 | [18,39,70,71] |
| 52 | Bolitophagus sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,61,69,70,71,72,73,74,75] |
| 53 | Rhipidandrus quadripapillatus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 54 | Proteleates† centralis Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39] (as Protelerates in the tribe Blaptini)] |
| Tribe Tenebrionini | |||
| 55 | Tenebrio primigenius Scudder, 1879 | Pg(Eo) 55.8–40.4 | [23,70,71,76] |
| 56 | Tenebrio effossus Germar, 1837 | Ng(Mi) 28.4–23 | [16,39,70,71,72] |
| 57 | Tenebrio senex Heyden, 1859 | Ng(Mi) 28.4–23 | [17,39,70,71] |
| Tribe Alphitobiini | |||
| 58 | Alphitopsis† initialis Kirejtshuk, Nabozhenko et Nel, 2011 | [41,59,64] | |
| Tribe Amarygmini | |||
| 59 | Meracantha lacustris Wickham, 1909 | Pg(Eo-Flor) 37.2–33.9 | [28,39] |
| 60 | Cymatothes dominicus Doyen et Poinar, 1994 | Pg(Eo-Flor) 37.2–33.9 | [38,39,57] |
| Tribe Helopini | |||
| 61 | Cryptohelops† menaticus Nabozhenko et Kirejtshuk, 2014 | Pg(Pc) 61–56 | [44,49,52] |
| 62 | Stenohelops (Stenolassus) klebsi (Nabozhenko, Perkovsky et Chernei, 2016) (originally as Nalassus, was transferred to Stenohelops by Nabozhenko et al. (2019)) | Pg(Eo-BA) 37.2–33.9 | [49,52] |
| 63 | Helops meissneri Heer, 1847 | (Ng(Mi) 12.7–11.6 | [19,39,72,73] |
| 64 | “Helops” sp. (genus incerta sedis) | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,49,62,63] |
| Tribe Triboliini | |||
| 65 | Tribolium sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,62,63] |
| 66 | Hypodena marginalis Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tribe Ulomini | |||
| 67 | Uloma sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,62,63] |
| 68 | Uloma avia C. Heyden, 1862 | Ng(Mi) 28.4–23 | [70,71,77] |
| Tribe Pedinini | |||
| Subtribe Leichenina | |||
| 69 | Leichenum sp. | Pg(Eo-BA) 37.2–33.9 | [29,32,34,39,62,63] |
| Tribe Opatrini | |||
| Subtribe Opatrina | |||
| 70 | Ephalus adumbratus Scudder, 1900 | Pg(Eo-Flor) 37.2–33.9 | [24,39,45] |
| 71 | Paleosclerum† pohli Nabozhenko et Kirejtshuk, 2017 | Pg(Pc) 61–56 | [45] |
| 72 | Ulus minutus Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39,45] |
| 73 | Gonocephalum pristinum (Heyden et Heyden, 1866) | Ng(Mi) 28.4–23 | [18,39], [70] (as Opatrum), [71] (as Opatrum) |
| Subtribe Neopachypterina | |||
| 74 | Eupachypterus† eocenicus | Pg(Eo) 55.8–48.6 | [45,46] |
| Subfamily Diaperinae | |||
| Tribe Diaperini | |||
| 75 | Ceropria (?) messelense Hornschemeyer, 1994 | Pg(Eo-Mess) 48.6–40.4 | [39,78] |
| 76 | Platydema bethunei Wickham, 1913 | Pg(Eo-Flor) 37.2–33.9 | [27,39] |
| 77 | Platydema antiquorum Wickham, 1913 | Pg(Eo-Flor) 37.2–33.9 | [27,39] |
| 78 | Platydema geinitzi Heyden et Heyden, 1866 | Ng(Mi) 28.4–23 | [18,39,70,71] |
| 79 | Pentaphyllus cioides Kirejtshuk, Merkl et Kernegger, 2008 | Pg(Eo-BA) 37.2–33.9 | [39] |
| 80 | Liodema phalacroides Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 81 | Neomida senicula Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tribe Scaphidemini | |||
| 82 | New fossil genus† and a new species | Pg(Pc) 61–56 | (Nabozhenko, Kirejtshuk, in litt) |
| Tribe Hypophlaeini | |||
| 83 | Corticeus tertarius Vitali, 2007 | Ng(Mi-Dom) 20.4–13.7 | [56] |
| 84 | “Hypophloeus” sp. (Corticeus) | Pg(Eo-BA) 37.2–33.9 | [34,79] |
| Tribe Gnathidiini | |||
| Subtribe Anopidiina | |||
| 85 | Tyrtaeus azureus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 86 | Tyrtaeus elongatus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 87 | Tyrtaeus flavoantennatus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 88 | Tyrtaeus thoracicus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 89 | Tyrtaeus cupreorutilans Vitali, 2008 | Ng(Mi-Dom) 20.4–13.7 | [57] |
| Subfamily Stenochiinae | |||
| 90 | Pseudohelops† groenlandicus Haupt, 1950 (nom nov. by Haupt (1950) = Helops molassicus Heer, 1883 nec Helops molassicus Heyden, 1865) | Pg(Pc) 61.7–58.7 | [22,30,39,70,71] |
| 91 | Pyrochalcaspis† geiseltalensis Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 92 | Eodromus† agilis (Meunier, 1915) (= aeneocupreus Pongrácz, 1935) | Pg(Eo-Geis) 48.6–40.4 | [30,39,80] |
| 93 | Eodromus† helopoides Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 94 | Eodromus† parvus Haupt, 1956 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 95 | Eodromus† punctatostriatus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 96 | Eodromus† punctatosulcatus Pongrácz, 1935 | Pg(Eo-Geis) 48.6–40.4 | [30,39,81] |
| 97 | Caryosoma† rugosum Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 98 | Parakeleusticus† postumus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 99 | Mimohelops† venosus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 100 | Anthracohelops† gigas Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 101 | Anthracohelops† minutus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 102 | Anthcarohelops† molassicus (Heyden, 1865) Transferred from Helops to Anthracohelops Haupt, 1950 by Haupt [30] | Ng(Mi) 23–20.4 | [22,30,39,70,71] |
| 103 | Anthcarohelops† wetteravicus (Heyden et Heyden, 1865) Transferred from Helops to Anthracohelops by Haupt [30] | Ng(Mi) 16.0–11.6 | [22,30,39,70,71,75,82] |
| 104 | Nesocyrtosoma antiquus (Kaszab et Schawaller, 1984) (originally described is Hesiodobates; transferred to Nesocyrtosoma by Doyen and Poinar [38]) | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 105 | Nesocyrtosoma antiquus Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 106 | Nesocyrtosoma celadonum Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 107 | Nesocyrtosoma hadratum Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 108 | Nesocyrtosoma impensum Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| 109 | Nesocyrtosoma phthanatum Doyen et Poinar, 1994 | Ng(Mi-Dom) 20.4–13.7 | [38,39,57] |
| Tenebrionidae, family incertae sedis | |||
| 110 | Parapiophorus† nitidus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 111 | Eohelaeus† perpunctatus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 112 | Eohelaeus† sublaevis Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 113 | Eoallognosis† limbellus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 114 | Rhinohelaeites† longipes Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 115 | Rhinohelaeites† punctatulus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 116 | Rhinohelaeites† undulatus Haupt, 1950 | Pg(Eo-Geis) 48.6–40.4 | [30,39] |
| 117 | Tenebrionites† alatus Cockerell, 1920 | Pg(Eo-Flor) 37.2–33.9 | [39,83] |
| 118 | Tenebrionites† anglicus Cockerell, 1920 | Pg(Eo-Bour) 48.6–40.4 | [39,83] |
| 119 | Tenebrionites† inclinans Cockerell, 1925 | Pg(Pc) 66–55.8 | [39,83] |
| 120 | Protoplatycera† laticornis Wickham, 1914 | Pg(Eo-Flor) 37.2–33.9 | [28,39] |
| 121 | Tagenopsis† brevicornis Heer, 1865 | Ng(Mi) 12.7–11.6 | [20,70,71,75] |
| 122 | Eocallidium† rugulosum Haupt, 1950, transferred from Cerambycidae to Tenebrionidae by Vitali [84] | Pg(Eo-Geis) 48.6–40.4 | [30,85] |
| Coleoptera, family incertae sedis | |||
| 1 | Cistelites† insignis Heer, 1865 | Mz(Ju) 201.6–196.5 | [20,59] |
| 2 | Cistelites† sachalinensis Heer, 1878 | Mz(Cr) 85.8–84.9 | [22,39,59,65] |
| 3 | Cistelites† minor Heer, 1883 | Pg(Pc) 61.7–58.7 | [22,39,65] |
| 4 | Cistelites† punctulatus Heer, 1883 | Pg(Pc) 61.7–58.7 | [22,39,65] |
| 5 | Cistelites† longipes (Hong, 1985) (Procarabus), transferred to Cistelites by Zhang [36] | Ng(Mi-Shan) 20.4–16 | [35,36,39] |
| 6 | Cistelites† spectabilis Heer, 1847 | Ng(Mi) 12.7–11.6 | [19,39,65] |
| 7 | “Helops” atticus Redtenbacher in Unger, 1867 | Ng(Mi-Kumi) 20.4–16 | [39,44,84] |
| No. | Hypothetical Lagrioid Ancestor * | Oldest Fossil Tenebrionoid Darkling Beetle (Alleculinae) |
|---|---|---|
| 1 | Simple trichoid antennal sensilla | Mixed, compound, and simple trichoid antennal sensilla |
| 2 | Longitudinal labrum | Transverse labrum |
| 3 | Internally and probably externally open procoxal cavities | Procoxal cavities closed externally and (at least in extant taxa) internally |
| 4 | Elytra with ten full striae | Elytra with nine striae and short scutellary striola |
| 5 | Wings with medial fleck | Unknown for fossil species. Extant Alleculinae without median fleck, and Tenebrioninae without or with |
| 6 | Groove along the outside edges of the abdominal ventrites, into which the elytral edges could fit to produce a sealed subelytral cavity | |
| 7 | Ovipositor with four coxites and terminal styli | Ovipositor with four coxites and lateral styli |
| 8 | Female genital tract with the primary bursa copulatrix and without spermatheca | Alleculinae: unvisible in fossils Extant taxa: female genital tract with primary or secondary bursa copulatrix and without spermatheca |
| Alphitobiini: unvisible in fossils Extant taxa: female genital tract without bursa copilatrix, with spermatheca | ||
| 9 | Aedeagus without alae (extensions of the apical piece) | Aedeagus with alae (at least in Platycteniopus diversoculatus) |
| 10 | Defensive glands are absent | Defensive glands are presented (well visible intersegmental membranes between abdominal ventrites 3–5) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabozhenko, M.V. The Fossil Record of Darkling Beetles (Insecta: Coleoptera: Tenebrionidae). Geosciences 2019, 9, 514. https://doi.org/10.3390/geosciences9120514
Nabozhenko MV. The Fossil Record of Darkling Beetles (Insecta: Coleoptera: Tenebrionidae). Geosciences. 2019; 9(12):514. https://doi.org/10.3390/geosciences9120514
Chicago/Turabian StyleNabozhenko, Maxim V. 2019. "The Fossil Record of Darkling Beetles (Insecta: Coleoptera: Tenebrionidae)" Geosciences 9, no. 12: 514. https://doi.org/10.3390/geosciences9120514
APA StyleNabozhenko, M. V. (2019). The Fossil Record of Darkling Beetles (Insecta: Coleoptera: Tenebrionidae). Geosciences, 9(12), 514. https://doi.org/10.3390/geosciences9120514





