Effects of CO2 on the Mechanical Properties of Hanna Sandstone
Abstract
1. Introduction
2. Materials and Methods
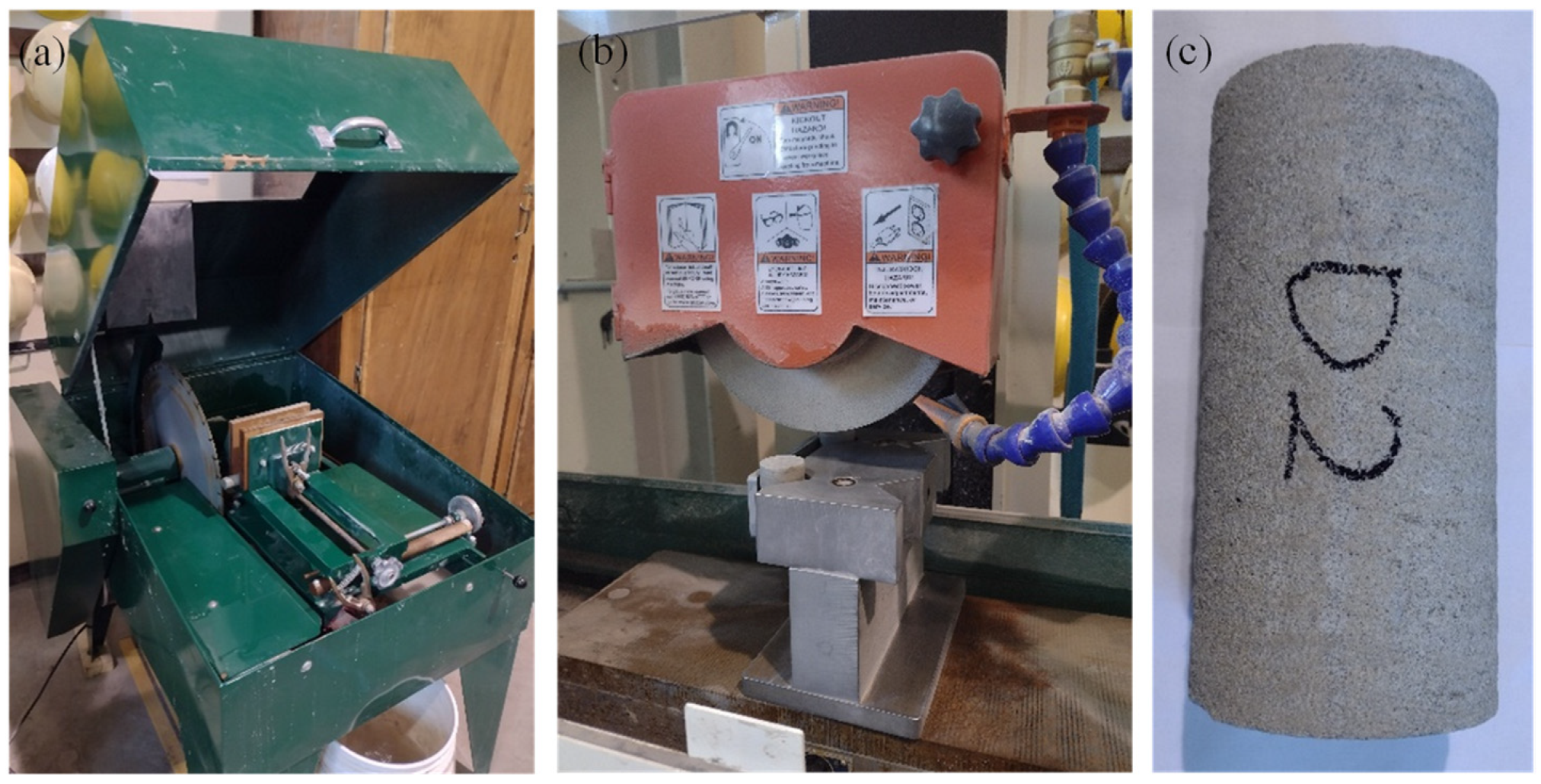
2.1. Specimen Preparation
2.2. Treatment of Rock Specimens
2.3. Rock Characterization via XRD and SEM Analyses
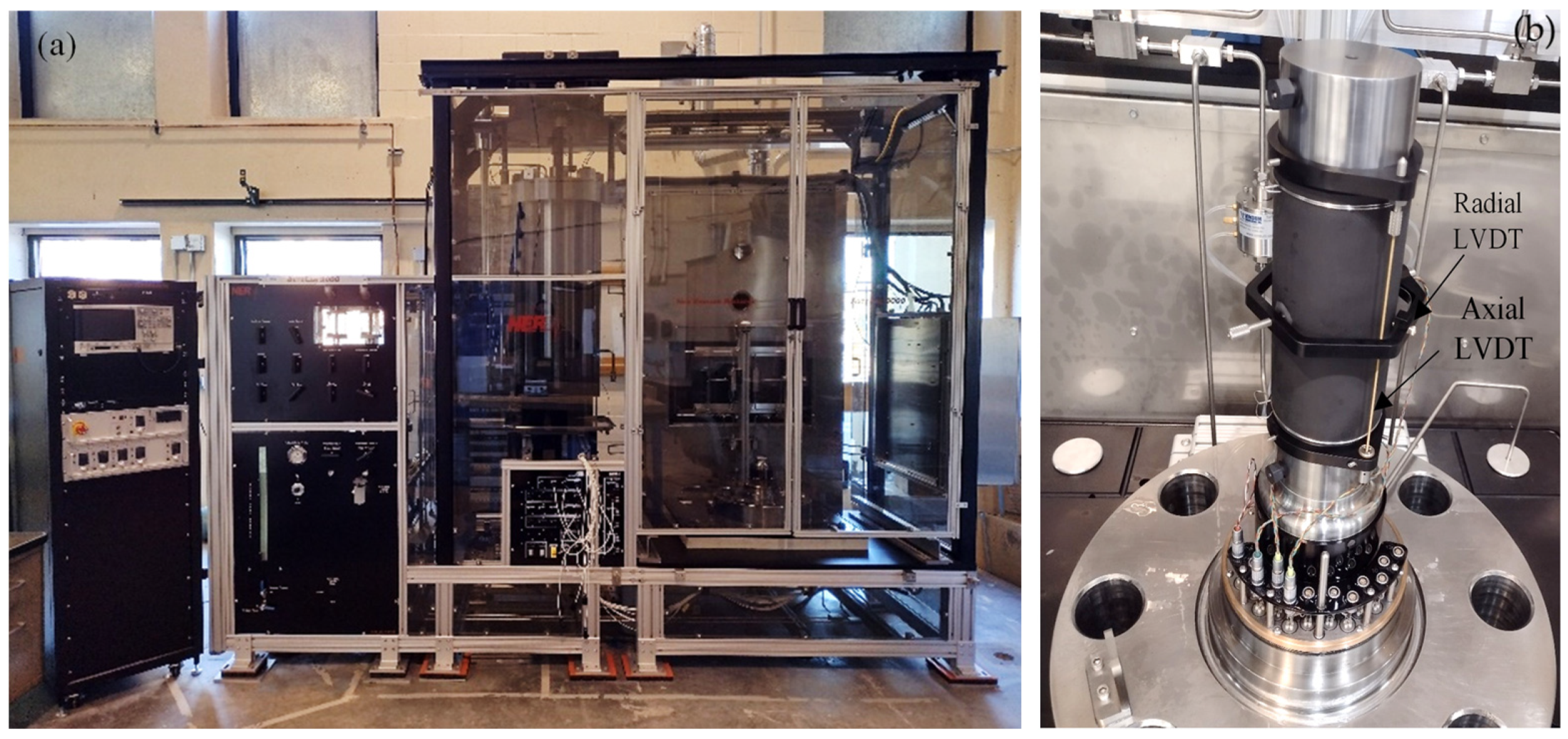
2.4. Rock Strength Determined Using Triaxial Compression (TC) Tests
3. Results and Discussion
3.1. Characterisation
3.1.1. Mineral Changes from XRD Analysis
3.1.2. Changes in Pore Structure from SEM Analysis
3.2. Experimental Results from the Confinement Stage
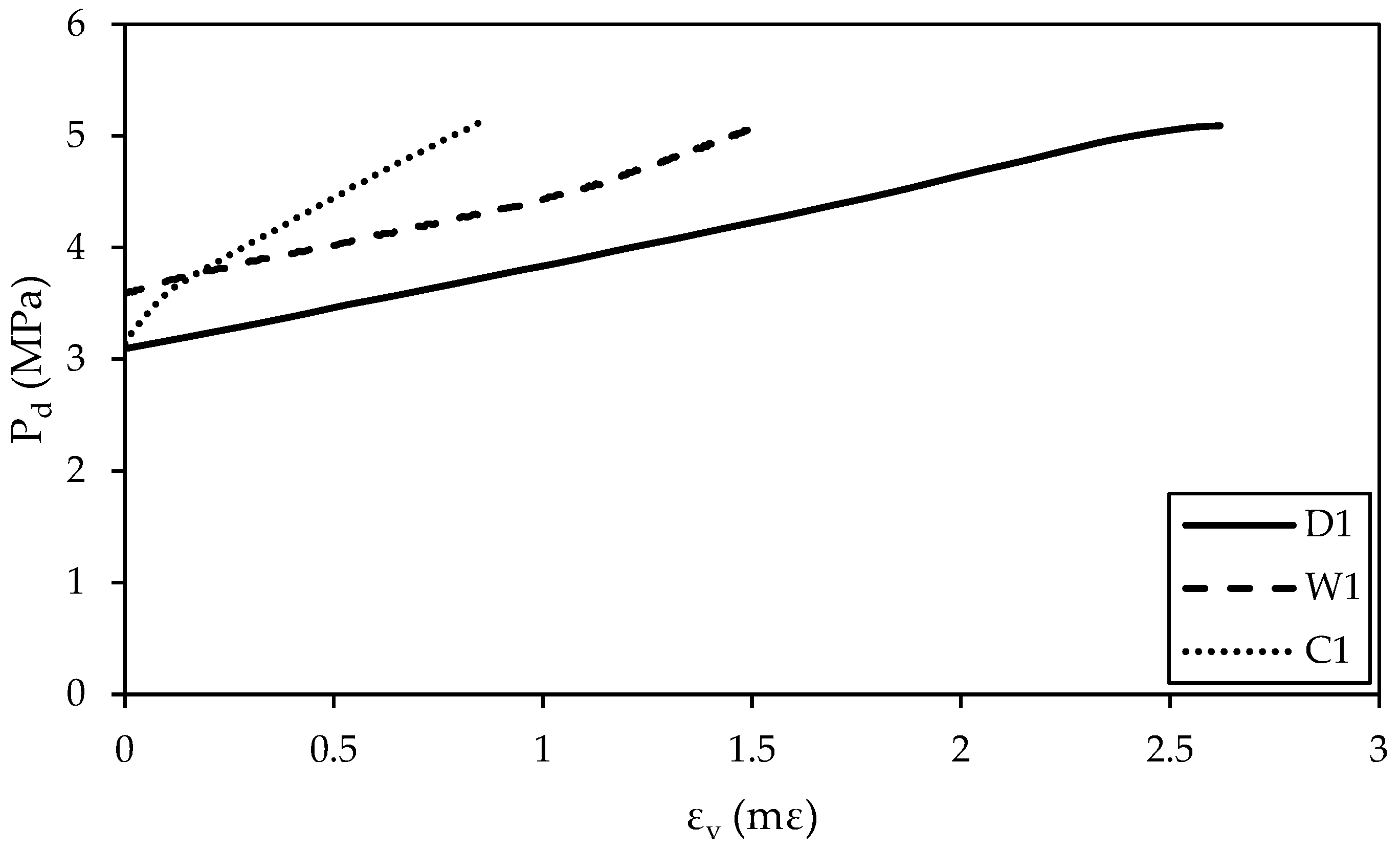
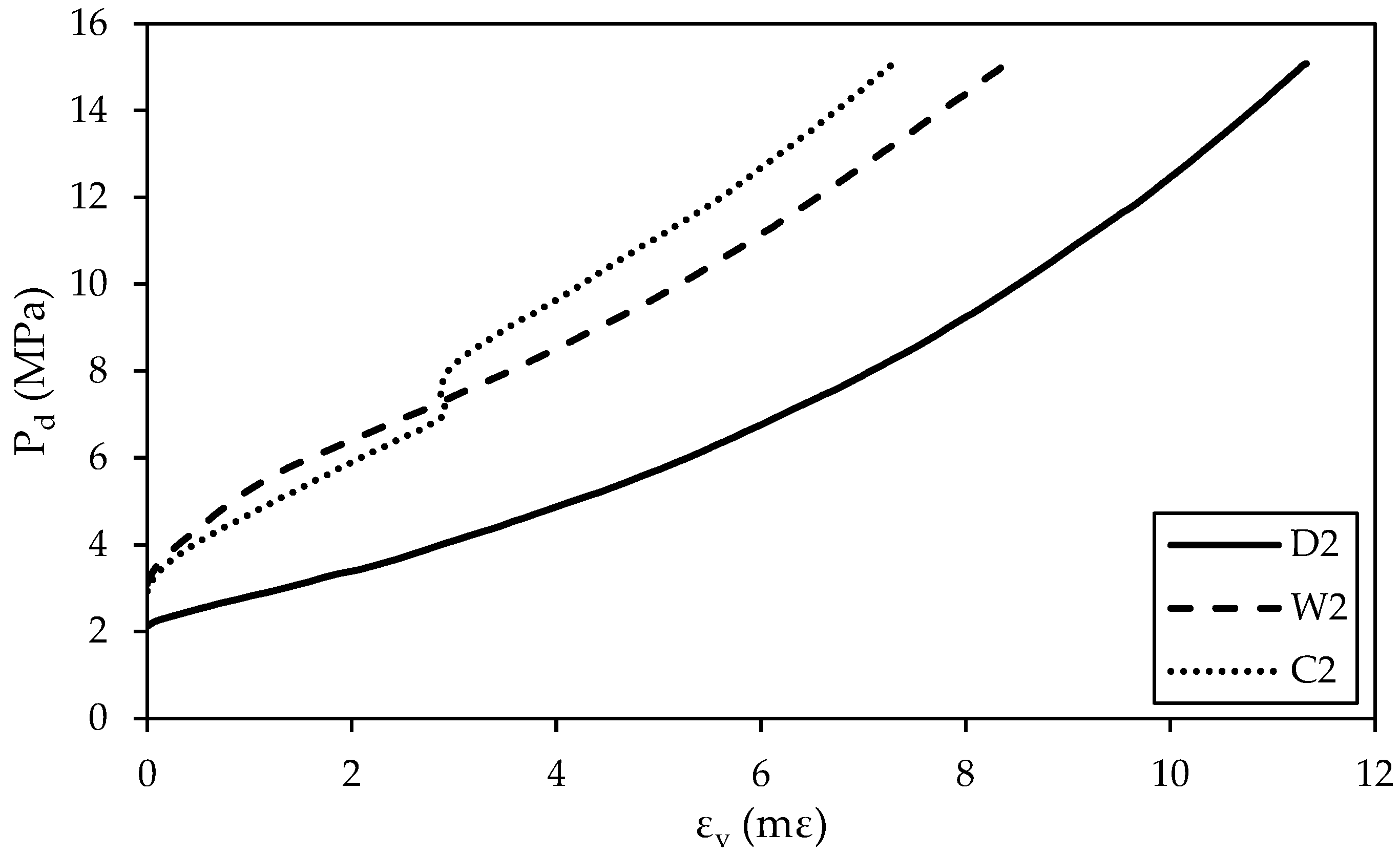
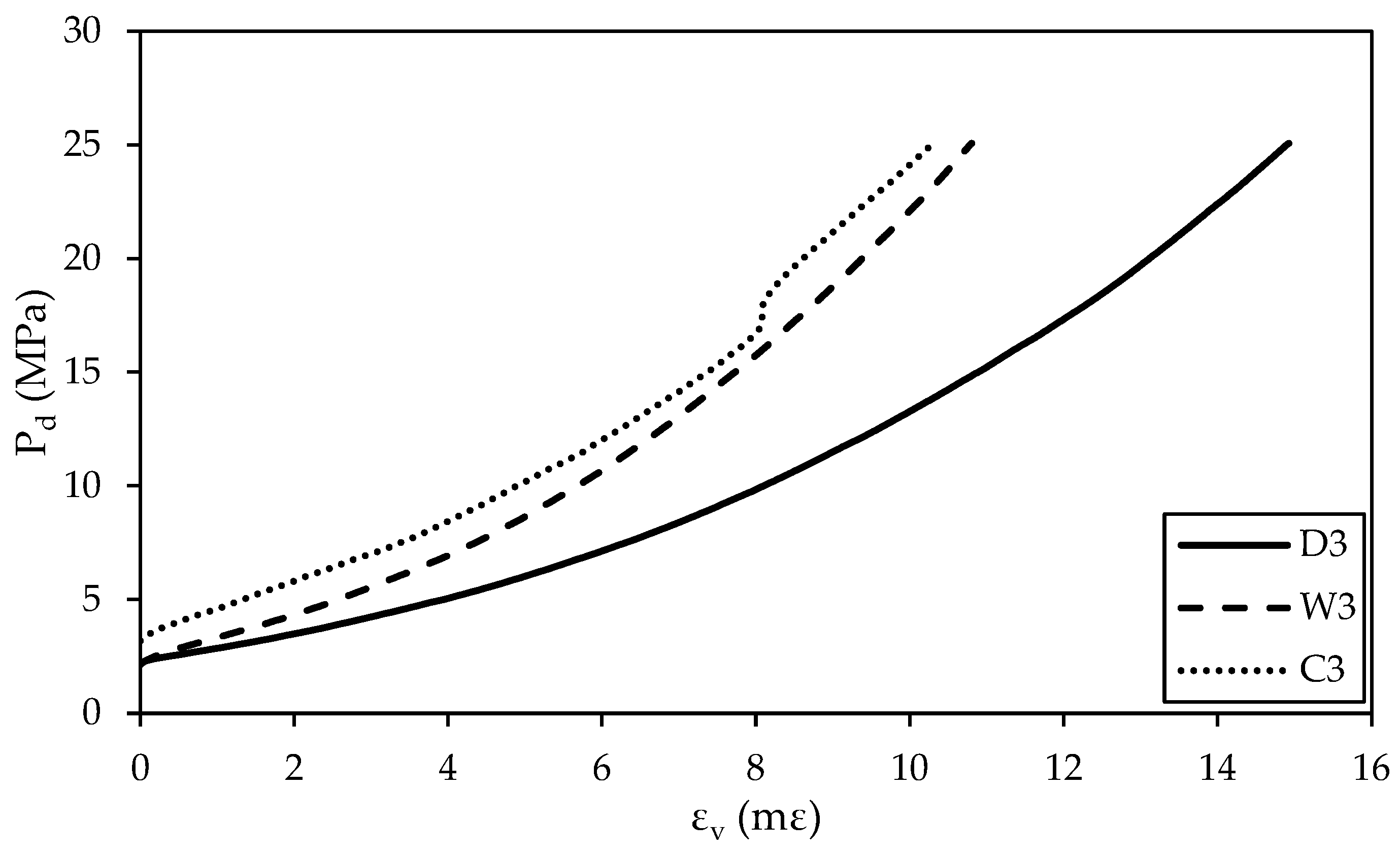
3.2.1. Volumetric Stress–Strain Behavior
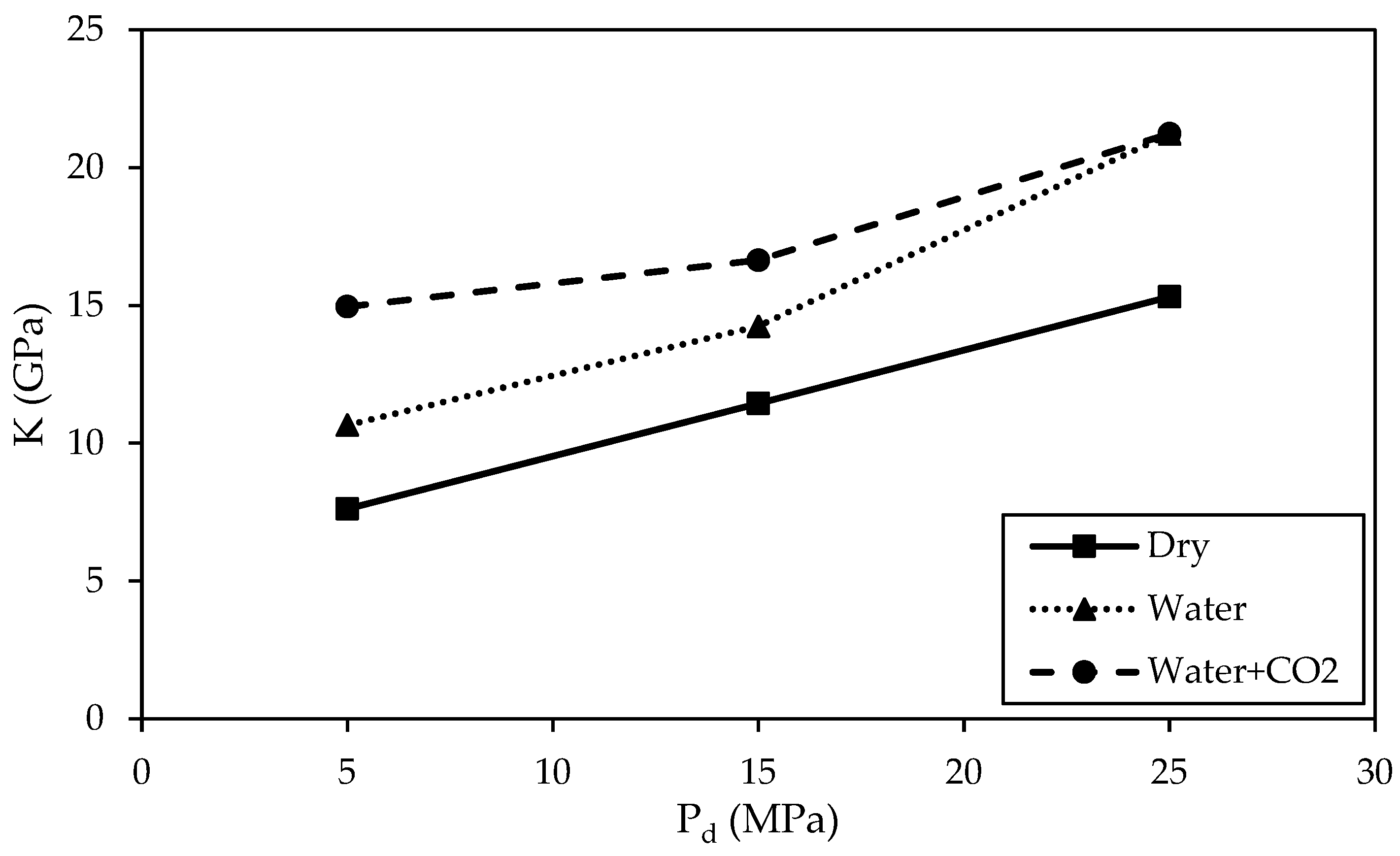
3.2.2. Comparison of Bulk Modulus
3.3. Experimental Results from Axial Loading Stage
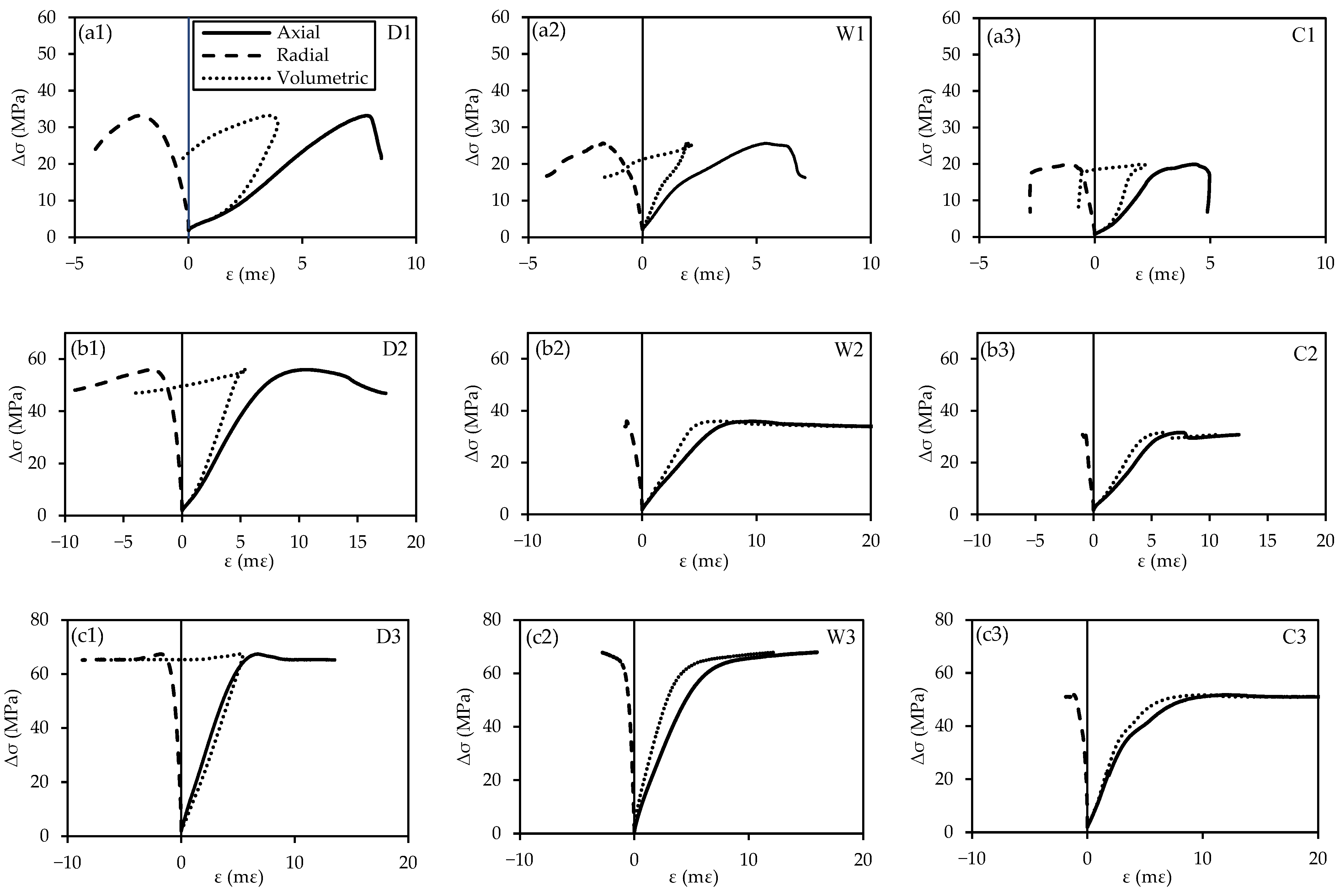
3.3.1. Axial Stress–Strain Behavior
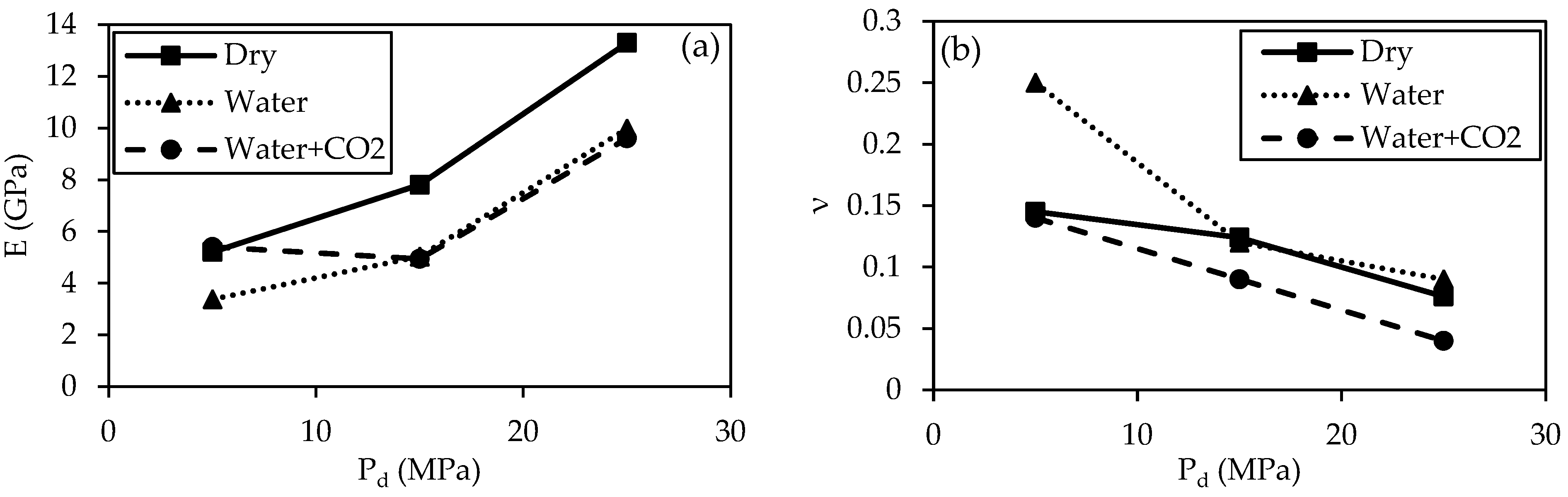
3.3.2. Elastic Properties
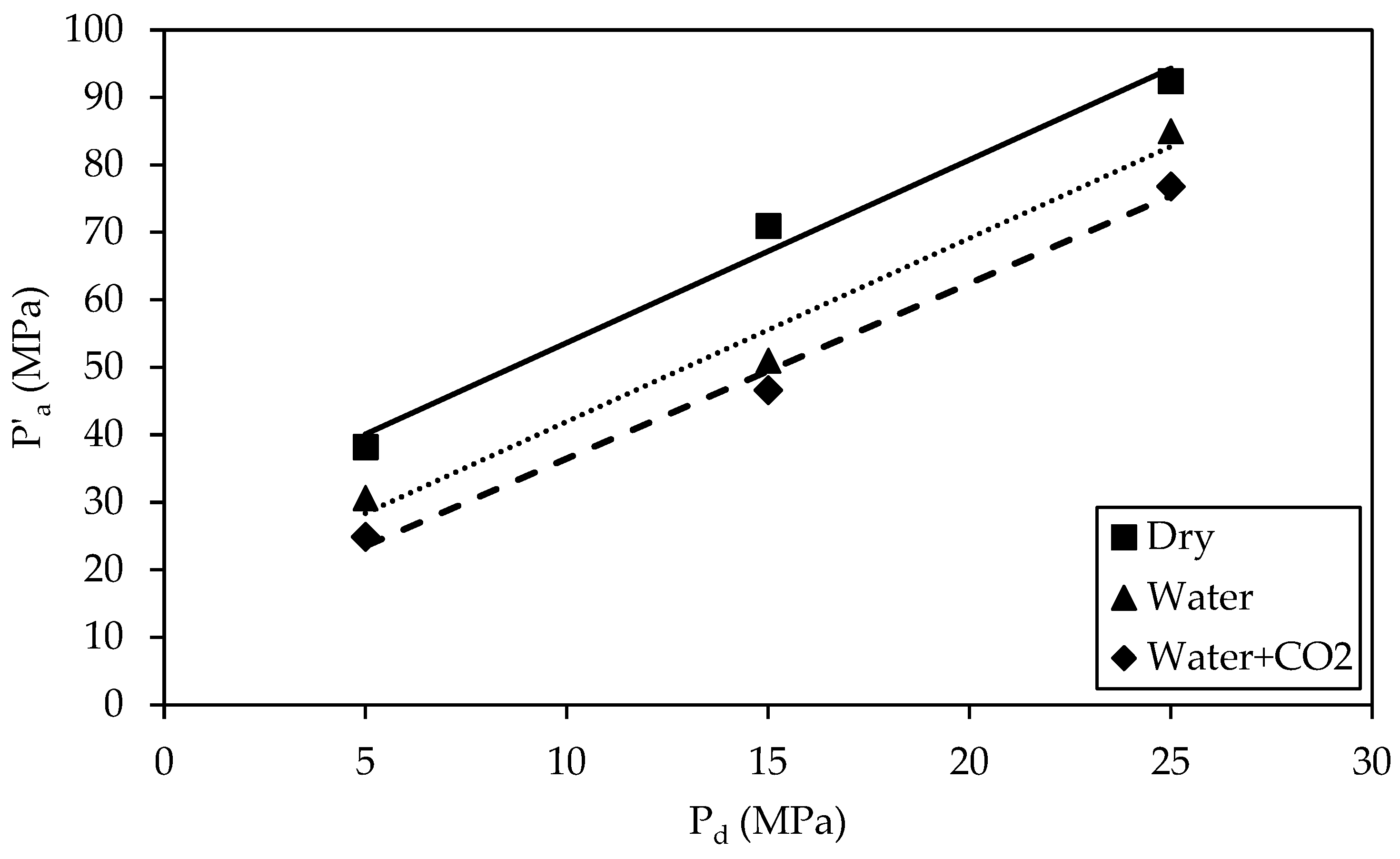
3.3.3. Major Effective Principal Stress
3.3.4. Shear Strength Parameters
3.4. Study Evaluation
4. Conclusions
- XRD results show that the CO2 treatment significantly reduces the calcite content. SEM images show the dissolution of calcite and the increase in the number and size of pores in the CO2 treatment.
- Regardless of the treatment conditions, an increase in the effective confining pressure (Pd) increases the maximum volumetric strain (εv). For the same Pd, dry specimens exhibit the largest maximum εv, followed by water-treated specimens and CO2-treated specimens. CO2 treatment decreases the maximum εv and reduces the compressibility of Hanna sandstone specimens during the confining stage.
- Bulk modulus (K) values increase with the increase in Pd. Specimens treated with water + CO2 exhibit large K values, and the difference in K values of the specimens at three treatment conditions diminishes at the largest Pd of 25 MPa.
- At three Pd values, dry specimens exhibit the highest compressive strength, followed by specimens treated with water, while specimens treated with water + CO2 exhibit the lowest compressive strength. Compared to dry specimens, the compressive strengths of specimens treated with water + CO2 are 40, 43, and 23% lower at Pd = 5, 15, and 25 MPa, respectively. At the lowest Pd = 5 MPa, all specimens exhibit a similar brittle post-failure behavior. Increasing the Pd to 15 and 25 MPa induces a more ductile failure behavior of specimens.
- Young’s modulus (E) values generally increase with the increase in Pd, while Poisson’s ratio (ν) values of specimens decrease at higher Pd. Dry specimens have the highest E values, while the E values of specimens treated with water and water + CO2 are comparable except at Pd = 5 MPa. CO2 treatment does not have much effect on the E of specimens, and the effect of treatment conditions becomes insignificant at a higher Pd. CO2 treatment decreases the ν of the specimens for all Pd.
- As Pd rises from 5 MPa to 25 MPa, the major principal effective stress (P′a) values for all specimens increase. Specimens treated with CO2 show reduced P′a values.
- Compared to that of dry specimens, CO2 treatment reduces the effective friction angle (φ′) value by 4% and the effective cohesion (c′) value by 62%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedi, B.; Orujov, A.; Dabbaghi, E.; Ng, K.; Ackerman, J.; Aryana, S.A. Containment Strategy for Subsurface H2 Storage Based on Time-Dependent Soft Solids. Int. J. Hydrogen Energy 2024, 82, 1001–1014. [Google Scholar] [CrossRef]
- Peter, A.; Yang, D.; Eshiet, K.I.-I.I.; Sheng, Y. A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process. Geosciences 2022, 12, 168. [Google Scholar] [CrossRef]
- Dabbaghi, E.; Ng, K.; Kou, Z.; Copeland, G.; Alvarado, V. Experimental Investigation to Understand the Effect of Fracturing Fluid on the Geomechanical Behavior of Mowry Shale. J. Pet. Explor. Prod. Technol. 2024, 14, 1505–1519. [Google Scholar] [CrossRef]
- Zhao, D.; Yin, D. Effect of Fluid/Rock Interactions on Physical Character of Tight Sandstone Reservoirs during CO2 Flooding. Geofluids 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, C.P.; Ma, Z.Y.; Zhou, J.P.; Zhang, D.C.; Liu, X.F.; Chen, H.; Ranjith, P.G. Experimental Study of Micromechanical Properties Alterations of Shale Matrix Treated by ScCO2-Water Saturation Using Nanoindentation Tests. Energy 2022, 242, 122965. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Xing, H.; Zou, Y. CO2–Brine–Rock Interactions Altering the Mineralogical, Physical, and Mechanical Properties of Carbonate-Rich Shale Oil Reservoirs. Energy 2022, 256, 124608. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Gunter, W.D.; Perkins, E.H. Geochemical Monitoring of Fluid-Rock Interaction and CO2 Storage at the Weyburn CO2-Injection Enhanced Oil Recovery Site, Saskatchewan, Canada. Energy 2004, 29, 1393–1401. [Google Scholar] [CrossRef]
- He, Y.; Ji, B.; Yang, S.; Liu, X.; Zhao, S.; Zhou, Y. Water–Rock–CO2 Interactions and CO2 Storage of Honghe Tight Oil Reservoirs: An Experimental and Simulation Study. Greenh. Gases Sci. Technol. 2019, 9, 703–718. [Google Scholar] [CrossRef]
- An, Q.; Zhang, Q.; Li, X.; Yu, H.; Yin, Z.; Zhang, X. Accounting for Dynamic Alteration Effect of SC-CO2 to Assess Role of Pore Structure on Rock Strength: A Comparative Study. Energy 2022, 260, 125125. [Google Scholar] [CrossRef]
- Kim, K.; Makhnenko, R.Y. Short- and Long-Term Responses of Reservoir Rock Induced by CO2 Injection. Rock. Mech. Rock. Eng. 2022, 55, 6605–6625. [Google Scholar] [CrossRef]
- Prakash, R.; Mahgoub, S.A.; Abedi, S. Chemo-Mechanical Alteration of Silicate-Rich Shale Rock after Exposure to CO2-Rich Brine at High Temperature and Pressure. Rock. Mech. Rock. Eng. 2023, 57, 5317–5333. [Google Scholar] [CrossRef]
- Dabbaghi, E.; Ng, K. Effects of CO2 on the Mineralogy, Mechanical, and Transport Properties of Rocks. Renew. Sustain. Energy Rev. 2024, 199, 114519. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Haque, A.; Lashin, A.; Al Arifi, N.; Chandrasekharam, D.; Yang, S.; Xu, T.; Wang, S.; et al. CO2-Induced Mechanical Behaviour of Hawkesbury Sandstone in the Gosford Basin: An Experimental Study. Mater. Sci. Eng. A 2015, 641, 123–137. [Google Scholar] [CrossRef]
- Zandvakili, A.; Rahbar Shahrouzi, J.; Tabatabaei-Nejad, S.A.; Khodapanah, E. Experimental Investigation of CO2–Rock–Brine Interaction for Injection of CO2 in an Iranian Oil Reservoir as an EOR Method. Environ. Earth Sci. 2020, 79, 480. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yang, S.-Q.; Li, W.-P.; Hall, M.R. Influence of Super-Critical CO2 on the Strength and Fracture Behavior of Brine-Saturated Sandstone Specimens. Rock. Mech. Rock. Eng. 2020, 53, 653–670. [Google Scholar] [CrossRef]
- Lamy-Chappuis, B.; Angus, D.; Fisher, Q.J.; Yardley, B.W.D. The Effect of CO2-Enriched Brine Injection on the Mechanical Properties of Calcite-Bearing Sandstone. Int. J. Greenh. Gas. Control 2016, 52, 84–95. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, L.; Haljasmaa, I.; Harbert, W.; Sanguinito, S.; Tkach, M.; Goodman, A.; Tsotsis, T.T.; Jessen, K. Impact of Brine/CO2 Exposure on the Transport and Mechanical Properties of the Mt Simon Sandstone. J. Pet. Sci. Eng. 2019, 177, 295–305. [Google Scholar] [CrossRef]
- Prasad, M.; Glubokovskikh, S.; Daley, T.; Oduwole, S.; Harbert, W. CO2 Messes with Rock Physics. Lead. Edge 2021, 40, 424–432. [Google Scholar] [CrossRef]
- Nooraiepour, M.; Bohloli, B.; Park, J.; Sauvin, G.; Skurtveit, E.; Mondol, N.H. Effect of Brine-CO2 Fracture Flow on Velocity and Electrical Resistivity of Naturally Fractured Tight Sandstones. GEOPHYSICS 2018, 83, WA37–WA48. [Google Scholar] [CrossRef]
- Vanorio, T.; Nur, A.; Ebert, Y. Rock Physics Analysis and Time-Lapse Rock Imaging of Geochemical Effects Due to the Injection of CO2 into Reservoir Rocks. Geophysics 2011, 76, O23–O33. [Google Scholar] [CrossRef]
- Wang, L.; Yao, B.; Xie, H.; Winterfeld, P.H.; Kneafsey, T.J.; Yin, X.; Wu, Y.-S. CO2 Injection-Induced Fracturing in Naturally Fractured Shale Rocks. Energy 2017, 139, 1094–1110. [Google Scholar] [CrossRef]
- Rinehart, A.J.; Dewers, T.A.; Broome, S.T.; Eichhubl, P. Effects of CO2 on Mechanical Variability and Constitutive Behavior of the Lower Tuscaloosa Formation, Cranfield Injection Site, USA. Int. J. Greenh. Gas. Control 2016, 53, 305–318. [Google Scholar] [CrossRef]
- Ng, K.; Yu, H.; Wang, H.; Kaszuba, J.; Alvarado, V.; Grana, D.; Campbell, E. The Effect of CO2 on the Mechanical Properties of the Reservoir Sandstone under a Low Differential Pressure. In Proceedings of the ARMA US Rock Mechanics/Geomechanics Symposium, ARMA, Seattle, WA, USA, 17–20 June 2018; p. ARMA-2018. [Google Scholar]
- Foroutan, M.; Ghazanfari, E.; Amirlatifi, A. Variation of Failure Properties, Creep Response and Ultrasonic Velocities of Sandstone upon Injecting CO2-Enriched Brine. Geomech. Geophys. Geo-Energy Geo-Resour. 2021, 7, 27. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, S.; Li, X.; Shi, L. Preliminary Experimental Study of Effect of CO2-H2O Biphasic Fluid on Mechanical Behavior of Sandstone under True Triaxial Compression. Int. J. Geomech. 2019, 19, 06018036. [Google Scholar] [CrossRef]
- Tarokh, A.; Makhnenko, R.Y.; Kim, K.; Zhu, X.; Popovics, J.S.; Segvic, B.; Sweet, D.E. Influence of CO2 Injection on the Poromechanical Response of Berea Sandstone. Int. J. Greenh. Gas. Control 2020, 95, 102959. [Google Scholar] [CrossRef]
- Li, H.; Xie, L.; Ren, L.; He, B.; Liu, Y.; Liu, J. Influence of CO2–Water–Rock Interactions on the Fracture Properties of Sandstone from the Triassic Xujiahe Formation, Sichuan Basin. Acta Geophys. 2021, 69, 135–147. [Google Scholar] [CrossRef]
- Song, W.; Ni, H.; Tang, P.; Zhang, S.; Gao, J.; Zhang, J.; Shen, B. Simulation of Supercritical Carbon Dioxide Fracturing in Shale Gas Reservoir. J. Therm. Sci. 2021, 30, 1444–1451. [Google Scholar] [CrossRef]
- Choi, C.-S.; Song, J.-J. Swelling and Mechanical Property Change of Shale and Sandstone in Supercritical CO2. J. Korean Soc. For. Rock. Mech. 2012, 22, 266–275. [Google Scholar] [CrossRef]
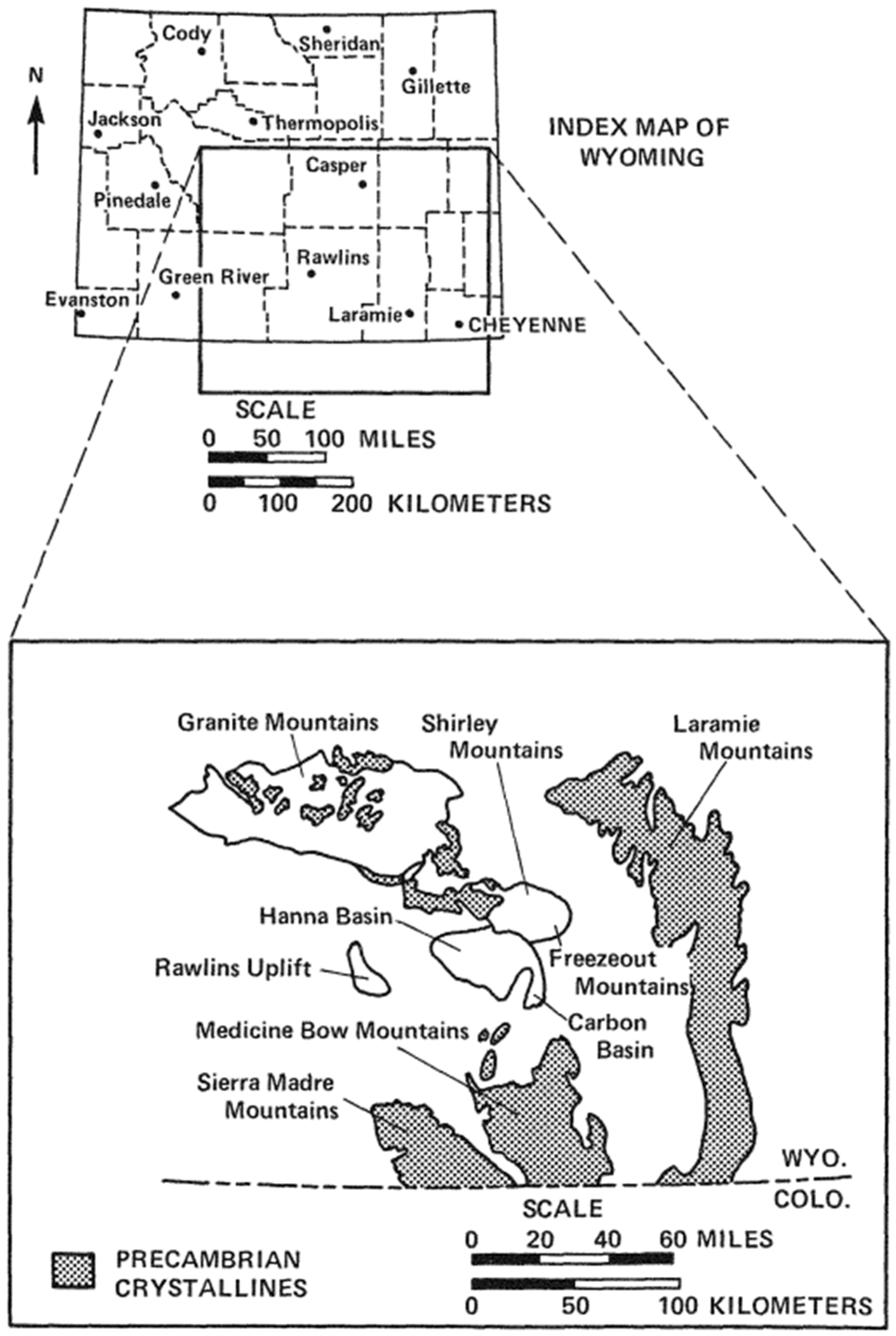
- Bowen, C.F. Stratigraphy of the Hanna Basin, Wyoming; US Government Printing Office: Washington, DC, USA, 1918.
- Oliver, R.L.; Youngberg, A.D. Geology of the Hanna Formation, Hanna Underground Coal Gasification Site, Hanna, Wyoming; Associated Western Universities, Inc.: Salt Lake, UT, USA, 1984. [Google Scholar]
- Alomari, E.M.; Ng, K.W.; Khatri, L.; Wulff, S.S. Effect of Physical Properties on Mechanical Behaviors of Sandstone under Uniaxial and Triaxial Compressions. Materials 2023, 16, 4867. [Google Scholar] [CrossRef]
- ASTM. ASTM D4543-19; Standard Practices for Preparing Rock Core as Cylindrical Test Specimens and Verifying Conformance to Dimensional and Shape Tolerances. ASTM International: West Conshohocken, PA, USA, 2019.
- Dabbaghi, E.; Ng, K.; Brown, T.C.; Yu, Y. Experimental Study on the Effect of Hydrogen on the Mechanical Properties of Hulett Sandstone. Int. J. Hydrogen Energy 2024, 60, 468–478. [Google Scholar] [CrossRef]
- Dabbaghi, E.; Ng, K. Investigation of the Mechanical Properties of Hanna Sandstone Using Newly Installed High Pressure and High Temperature Polyaxial Equipment. In Proceedings of the ARMA US Rock Mechanics/Geomechanics Symposium, ARMA, Atlanta, GA, USA, 25–28 June 2023. [Google Scholar]
- Sun, Y.; Wei, L.; Dai, C.; Yu, Z.; Xin, Y. The Carbonic Acid-Rock Reaction in Feldspar/Dolomite-Rich Tightsand and Its Impact on CO2-Water Relative Permeability during Geological Carbon Storage. Chem. Geol. 2021, 584, 120527. [Google Scholar] [CrossRef]
- Lin, R.; Yu, Z.; Zhao, J.; Dai, C.; Sun, Y.; Ren, L.; Xie, M. Experimental Evaluation of Tight Sandstones Reservoir Flow Characteristics under CO2–Brine–Rock Multiphase Interactions: A Case Study in the Chang 6 Layer, Ordos Basin, China. Fuel 2022, 309, 122167. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Wang, B.; Yi, Y.; Zhao, C.; Tian, G. Effect of Supercritical CO2-Water/Brine-Rock Interaction on Microstructures and Mechanical Properties of Tight Sandstone. Transp. Porous Media 2023, 149, 87–115. [Google Scholar] [CrossRef]
- Fauziah, C.A.; Al-Yaseri, A.; Al-Khdheeawi, E.; Jha, N.K.; Abid, H.R.; Iglauer, S.; Lagat, C.; Barifcani, A. Effect of CO2 Flooding on the Wettability Evolution of Sand-Stone. Energies 2021, 14, 5542. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Kamali, F.; Othman, F.; Wang, Y.; Le-Hussain, F. Wettability of Sandstone Rocks and Their Mineral Components during CO2 Injection in Aquifers: Implications for Fines Migration. J. Nat. Gas. Sci. Eng. 2020, 73, 103050. [Google Scholar] [CrossRef]
- Hussain, A.; Haughton, P.D.W.; Shannon, P.M.; Turner, J.N.; Pierce, C.S.; Obradors-Latre, A.; Barker, S.P.; Martinsen, O.J. High-resolution X-ray Fluorescence Profiling of Hybrid Event Beds: Implications for Sediment Gravity Flow Behaviour and Deposit Structure. Sedimentology 2020, 67, 2850–2882. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Lu, Z.; Pan, Z.; Zeng, M.; Du, X.; Xiao, S. The Effect of Subcritical and Supercritical CO2 on the Pore Structure of Bituminous Coals. J. Nat. Gas. Sci. Eng. 2021, 94, 104132. [Google Scholar] [CrossRef]
- Hangx, S.; van der Linden, A.; Marcelis, F.; Bauer, A. The Effect of CO2 on the Mechanical Properties of the Captain Sandstone: Geological Storage of CO2 at the Goldeneye Field (UK). Int. J. Greenh. Gas. Control 2013, 19, 609–619. [Google Scholar] [CrossRef]
- Curtis, M.; Mamoudou, S.; Cruz, F.; Dang, S.T.; Rai, C.; Devegowda, D. Effects of CO2 Exposure on Unconventional Reservoir Rock Microstructure. In Proceedings of the Proceedings of the 11th Unconventional Resources Technology Conference, Online, 13–15 June 2023; American Association of Petroleum Geologists: Tulsa, OK, USA, 2023. [Google Scholar]
- Richart, F.E.; Brandtzæg, A.; Brown, R.L. A Study of the Failure of Concrete under Combined Compressive Stresses; University of Illinois, Engineering Experiment Station, Bulletin: Urbana, IL, USA, 1928; No. 185. [Google Scholar]
- Cook, N.G.W. An Experiment Proving That Dilatancy Is a Pervasive Volumetric Property of Brittle Rock Loaded to Failure. Rock. Mech. 1970, 2, 181–188. [Google Scholar] [CrossRef]
- Zhang, Z.-X. Environmental Effects on Rock Fracture. In Rock Fracture and Blasting; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–153. [Google Scholar]
- Greshnyakov, V.A.; Belenkov, E.A. Technique for Calculating the Bulk Modulus. Russ. Phys. J. 2014, 57, 731–737. [Google Scholar] [CrossRef]
- Guo, S.; Pu, H.; Yang, M.; Liu, D.; Sha, Z.; Xu, J. Study of the Influence of Clay Minerals on the Mechanical and Percolation Properties of Weakly Cemented Rocks. Geofluids 2022, 2022, 1712740. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, W.; Chen, Y.; Li, X.; Hu, S. Mechanical Behavior of Sandstone Pressurized with Supercritical CO2 and Water under Different Confining Pressure Conditions. Int. J. Geomech. 2021, 21, 04021100. [Google Scholar] [CrossRef]
- Debasis, D.E.B.; Kumar, V.A. Fundamentals and Applications of Rock Mechanics; PHI Learning Pvt. Ltd.: Delhi, India, 2016; ISBN 8120351827. [Google Scholar]
- Marbler, H.; Erickson, K.P.; Schmidt, M.; Lempp, C.; Pöllmann, H. Geomechanical and Geochemical Effects on Sandstones Caused by the Reaction with Supercritical CO2: An Experimental Approach to in Situ Conditions in Deep Geological Reservoirs. Environ. Earth Sci. 2013, 69, 1981–1998. [Google Scholar] [CrossRef]
- Sivakugan, N.; Das, B.M.; Lovisa, J.; Patra, C.R. Determination of c and φ of Rocks from Indirect Tensile Strength and Uniaxial Compression Tests. Int. J. Geotech. Eng. 2014, 8, 59–65. [Google Scholar] [CrossRef]
- Wang, S.R.; Hagan, P.; Li, Y.C.; Zhang, C.G.; Liu, X.L. Experimental Study on Deformation and Strength Characteristics of Sandstone with Different Water Contents. J. Eng. Sci. Technol. Rev. 2017, 10, 199–203. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J.; Peach, C.J. Mechanical Behavior of Anhydrite Caprock and Implications for CO2 Sealing Capacity. J. Geophys. Res. Solid. Earth 2010, 115, B07402. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Jung, H.; Major, J.R.; Sun, Z.; Ramos, M.J.; Eichhubl, P.; Balhoff, M.T.; Choens, R.C.; Dewers, T.A. CO2 Charged Brines Changed Rock Strength and Stiffness at Crystal Geyser, Utah: Implications for Leaking Subsurface CO2 Storage Reservoirs. Int. J. Greenh. Gas. Control 2018, 73, 16–28. [Google Scholar] [CrossRef]
- Zhang, X.; Ranjith, P.G.; Lu, Y.; Ranathunga, A.S. Experimental Investigation of the Influence of CO2 and Water Adsorption on Mechanics of Coal under Confining Pressure. Int. J. Coal Geol. 2019, 209, 117–129. [Google Scholar] [CrossRef]





| Specimen Identifications | Treatment Specifications | Confining Pressure (MPa) | Pore Pressure (MPa) | Effective Confining Pressure (MPa) |
|---|---|---|---|---|
| D1 | Dry | 5 | 0 | 5 |
| D2 | 15 | 0 | 15 | |
| D3 | 25 | 0 | 25 | |
| W1 | Submerged in water for one week | 10 | 5 | 5 |
| W2 | 20 | 5 | 15 | |
| W3 | 30 | 5 | 25 | |
| C1 | Immersed in water for a week, then subjected to water + CO2 for the following week | 10 | 5 | 5 |
| C2 | 20 | 5 | 15 | |
| C3 | 30 | 5 | 25 |
| Treatment | Quartz | Calcite | Dolomite | Siderite | Aegirine | Albite | Microcline | Muscovite | Kaolinite |
|---|---|---|---|---|---|---|---|---|---|
| Untreated | 30.6 | 14.1 | 1.5 | 0.7 | 2.6 | 11.2 | 16.6 | 5.6 | 17.1 |
| Water + CO2 | 35.1 | 0.6 | 1.5 | 1.2 | 1.9 | 11.1 | 16.8 | 7.6 | 24.2 |
| Specimen | Pd (MPa) | K (GPa) | E (GPa) | ν | P′a (MPa) | φ′ (°) | c′ (MPa) | ||
|---|---|---|---|---|---|---|---|---|---|
| D1 | 5 | 2.62 | 7.6 | 33.2 | 5.2 | 0.15 | 38.2 | 27.4 | 8.1 |
| D2 | 15 | 11.3 | 11.4 | 56 | 7.8 | 0.12 | 71 | ||
| D3 | 25 | 14.92 | 15.3 | 67.4 | 13.3 | 0.08 | 92.4 | ||
| W1 | 5 | 1.51 | 10.7 | 25.7 | 3.4 | 0.25 | 30.7 | 27.5 | 4.5 |
| W2 | 15 | 8.44 | 14.2 | 36 | 5 | 0.12 | 51 | ||
| W3 | 25 | 10.8 | 21.2 | 60 | 10 | 0.09 | 85 | ||
| C1 | 5 | 0.87 | 15 | 19.9 | 5.4 | 0.14 | 24.9 | 26.4 | 3.1 |
| C2 | 15 | 7.31 | 16.6 | 31.7 | 4.9 | 0.09 | 46.7 | ||
| C3 | 25 | 10.32 | 21.2 | 51.8 | 9.6 | 0.04 | 76.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabbaghi, E.; Ng, K. Effects of CO2 on the Mechanical Properties of Hanna Sandstone. Geosciences 2024, 14, 279. https://doi.org/10.3390/geosciences14100279
Dabbaghi E, Ng K. Effects of CO2 on the Mechanical Properties of Hanna Sandstone. Geosciences. 2024; 14(10):279. https://doi.org/10.3390/geosciences14100279
Chicago/Turabian StyleDabbaghi, Ehsan, and Kam Ng. 2024. "Effects of CO2 on the Mechanical Properties of Hanna Sandstone" Geosciences 14, no. 10: 279. https://doi.org/10.3390/geosciences14100279
APA StyleDabbaghi, E., & Ng, K. (2024). Effects of CO2 on the Mechanical Properties of Hanna Sandstone. Geosciences, 14(10), 279. https://doi.org/10.3390/geosciences14100279






