Using 87Sr/86Sr LA-MC-ICP-MS Transects within Modern and Ancient Calcite Crystals to Determine Fluid Flow Events in Deep Granite Fractures
Abstract
1. Introduction
2. Geological Setting
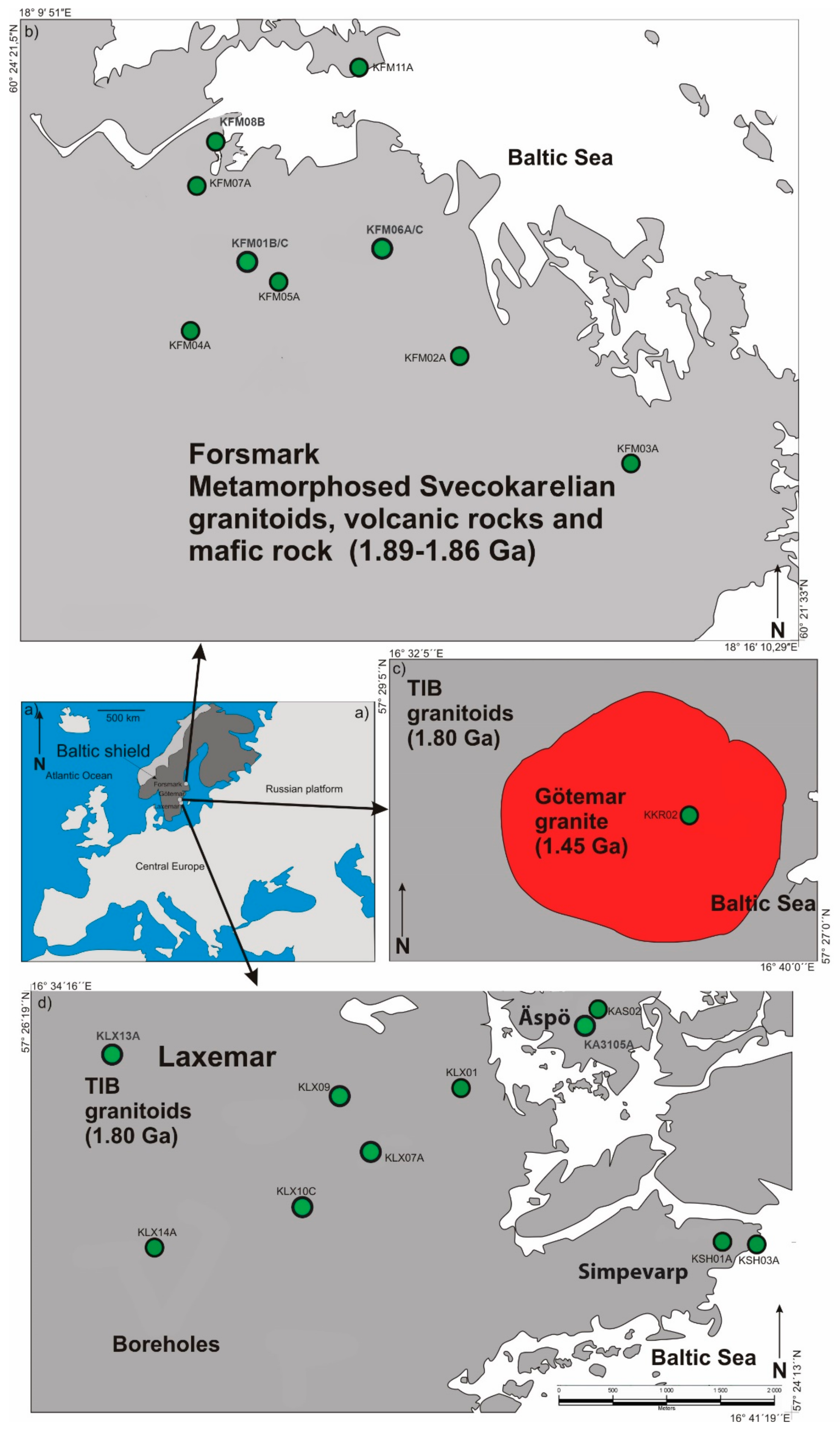
2.1. Äspö Hard Rock Laboratory—Site for Sampling of Modern Calcite and Water
2.2. Forsmark and Laxemar-Äspö—Sites for Sampling of Ancient Calcite and Modern Water
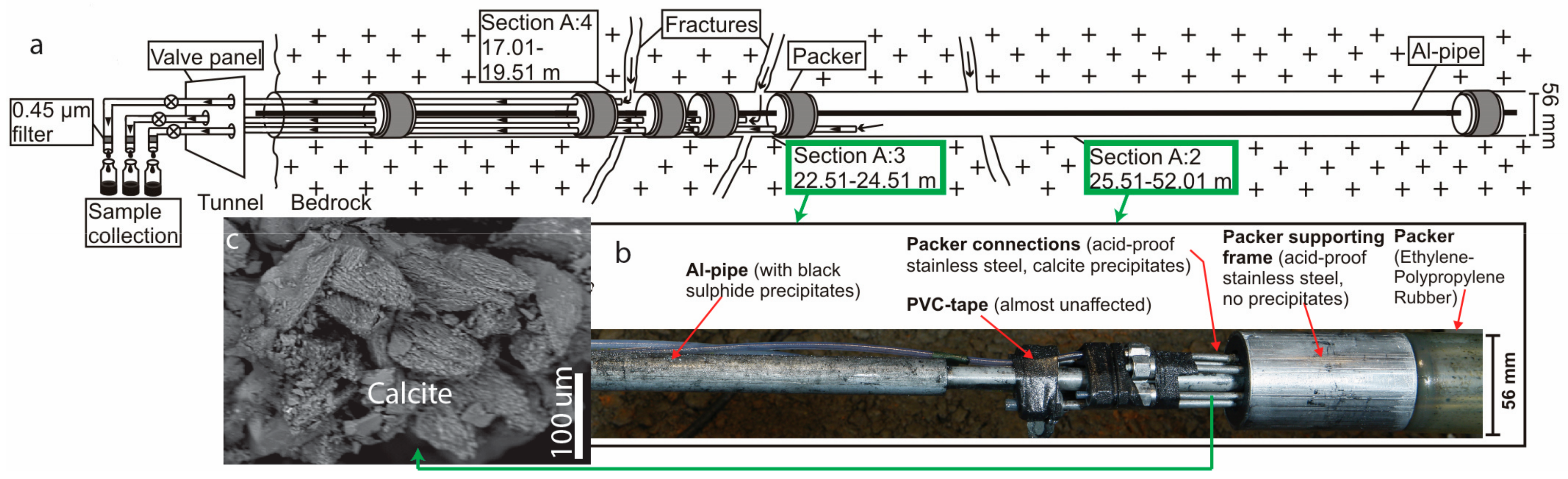
3. Materials and Methods
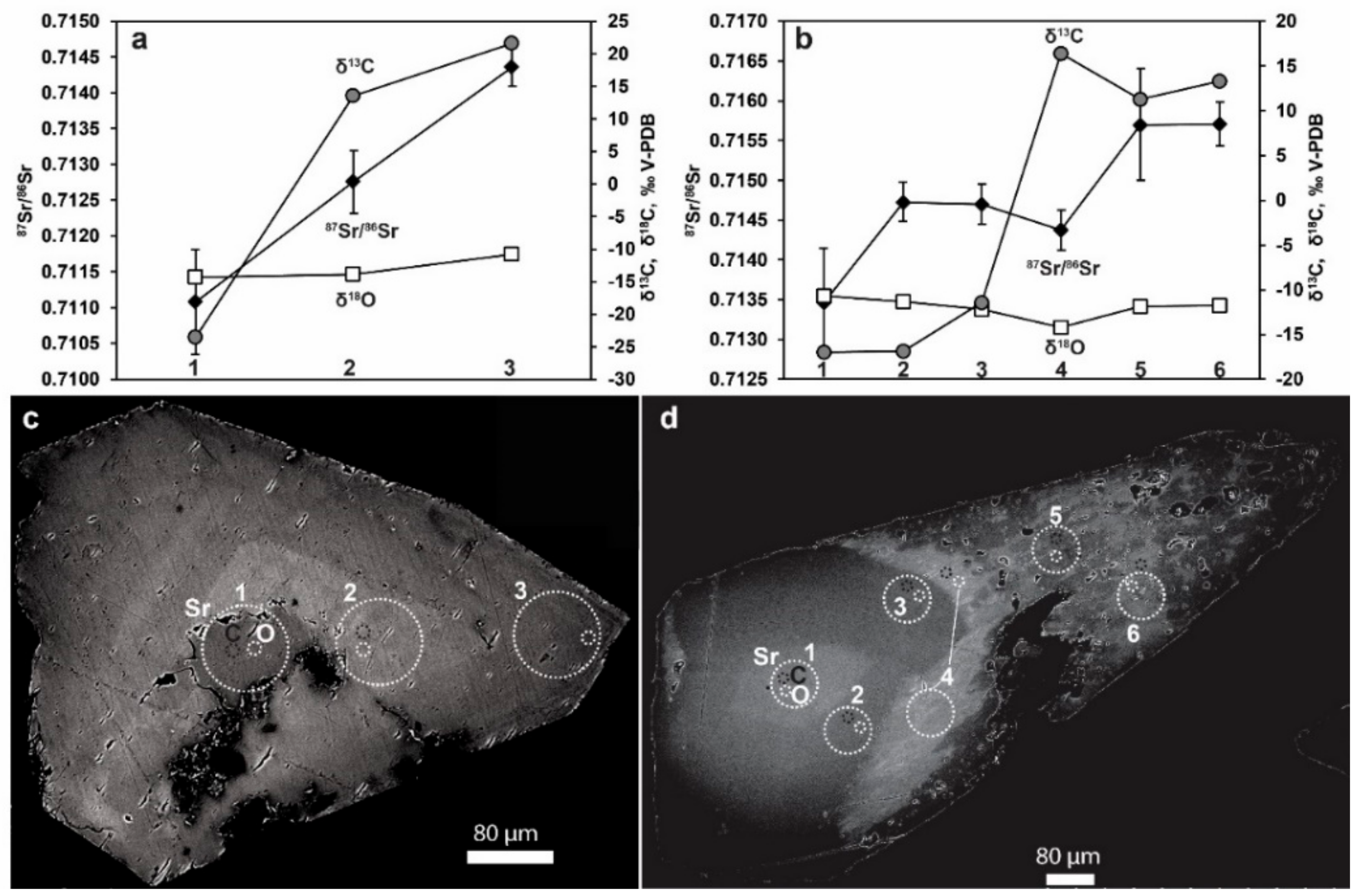
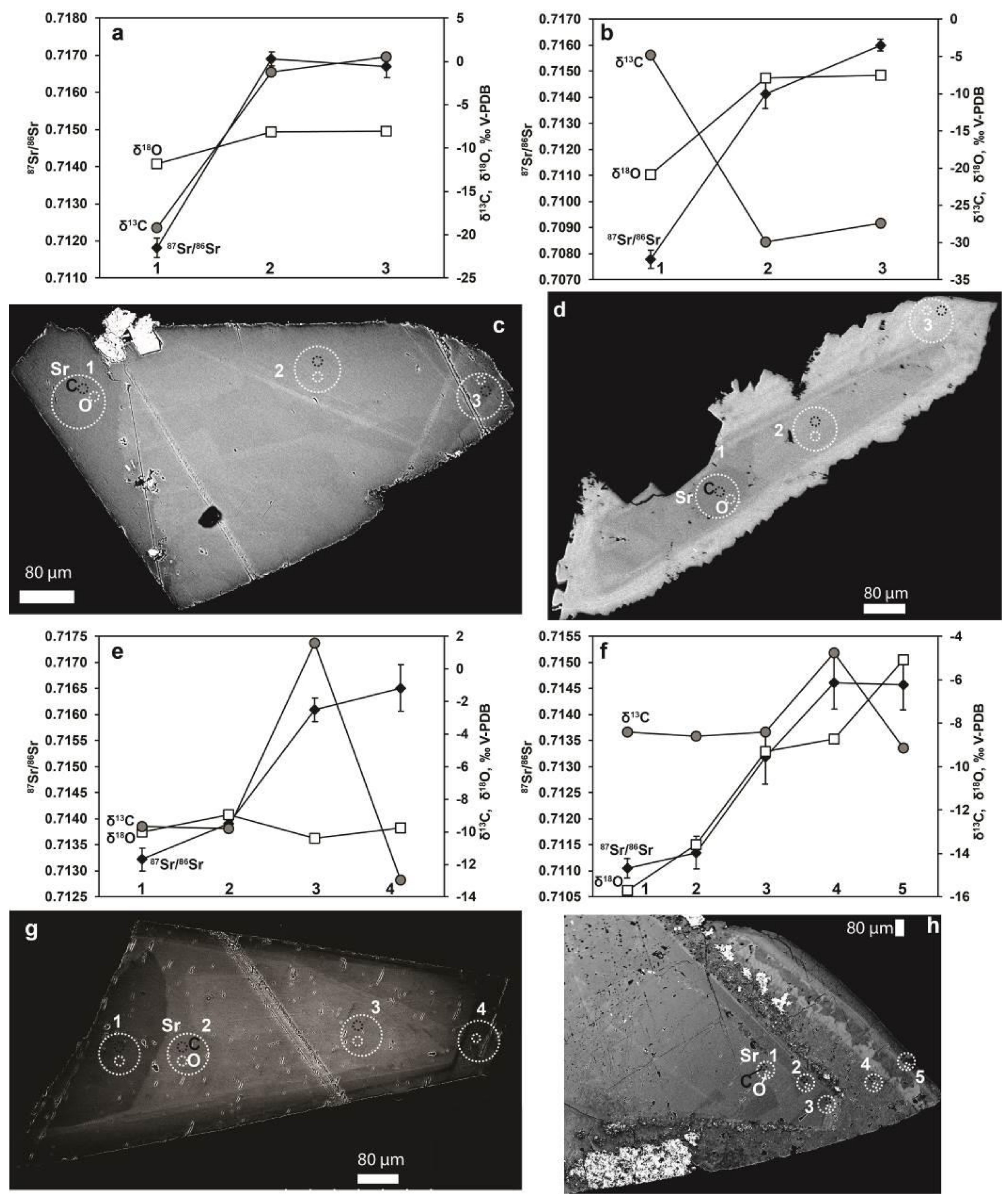
4. Results
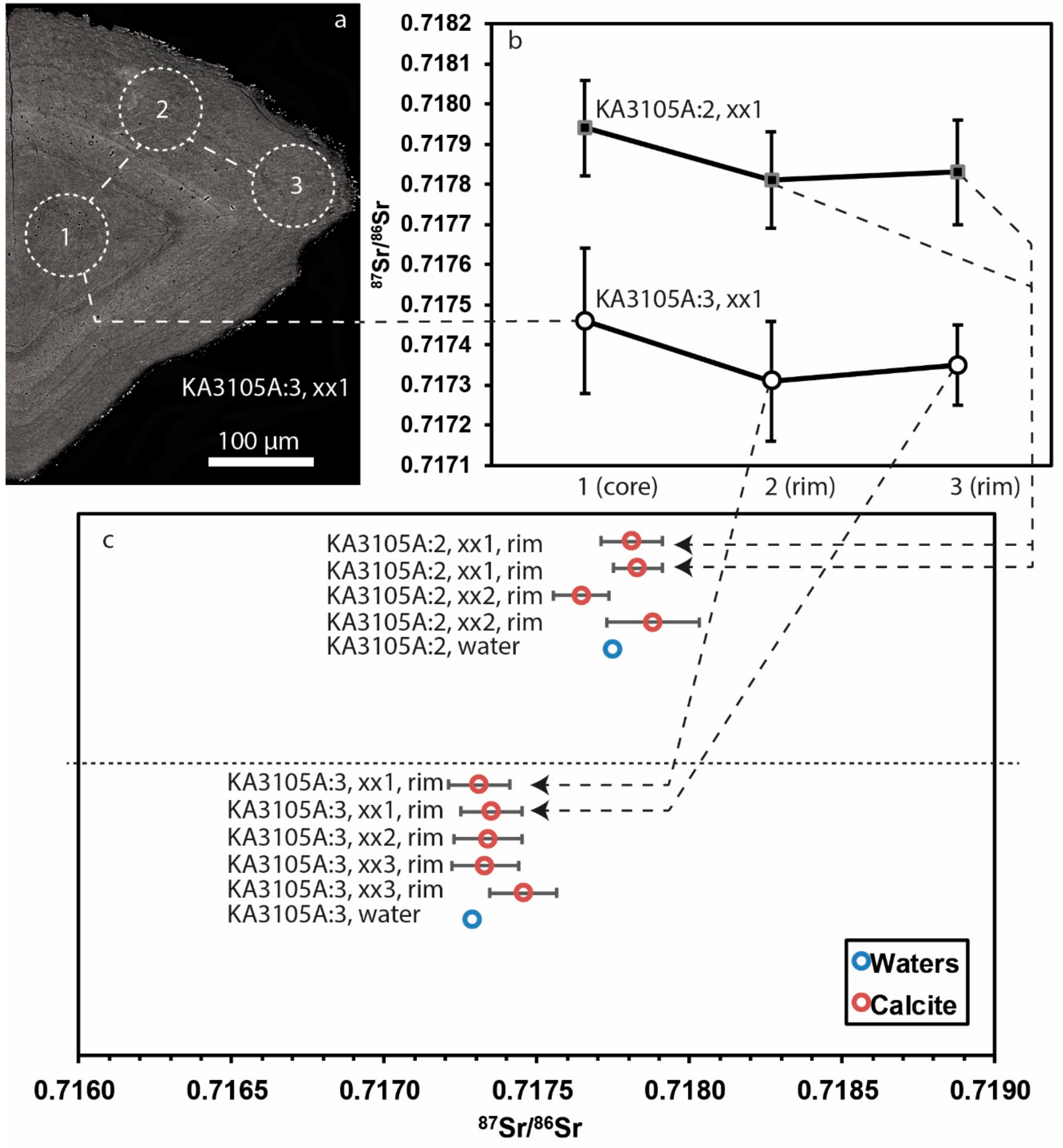
4.1. Modern Calcite and Waters
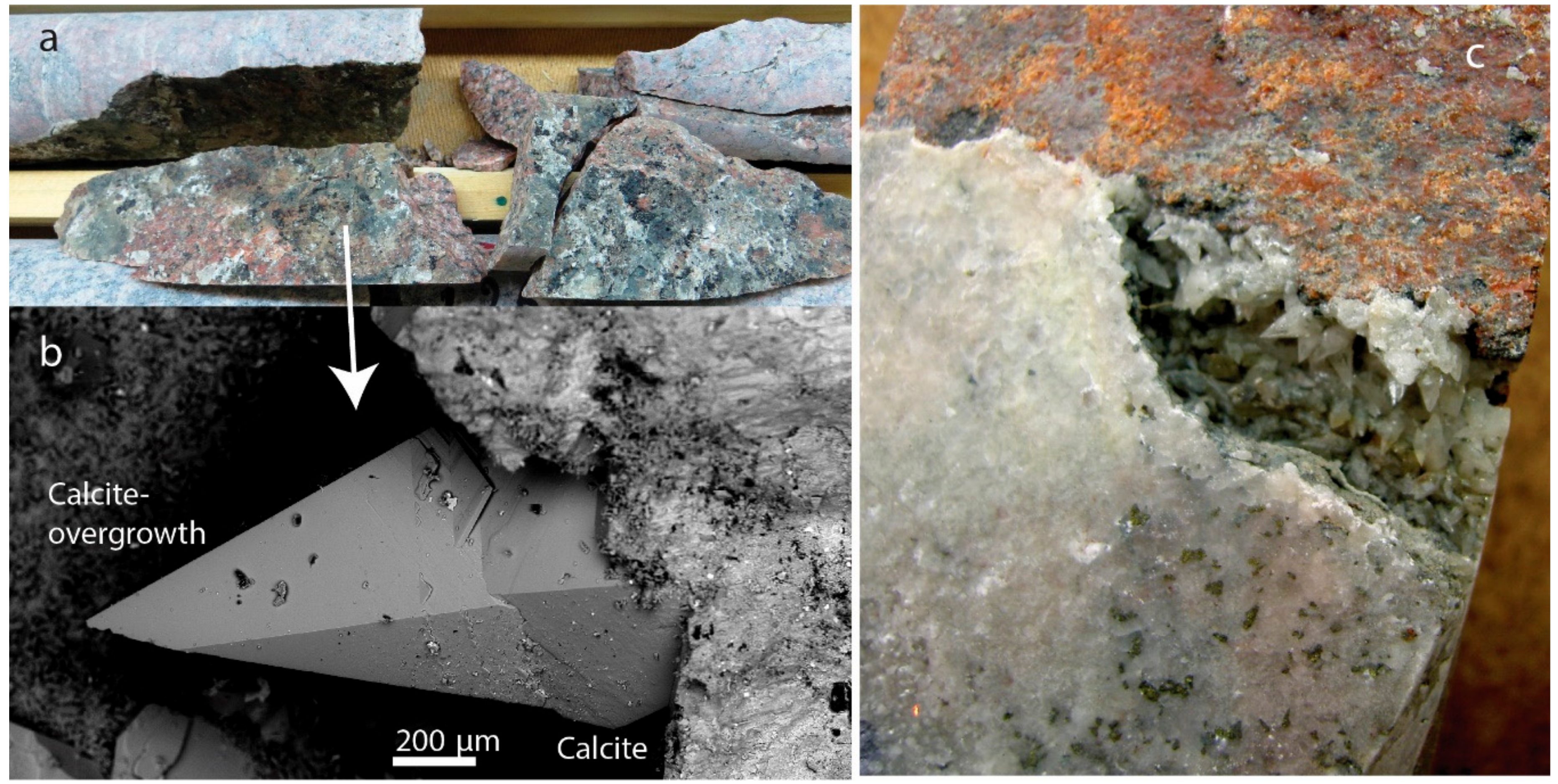
4.2. Fracture Coating Calcite
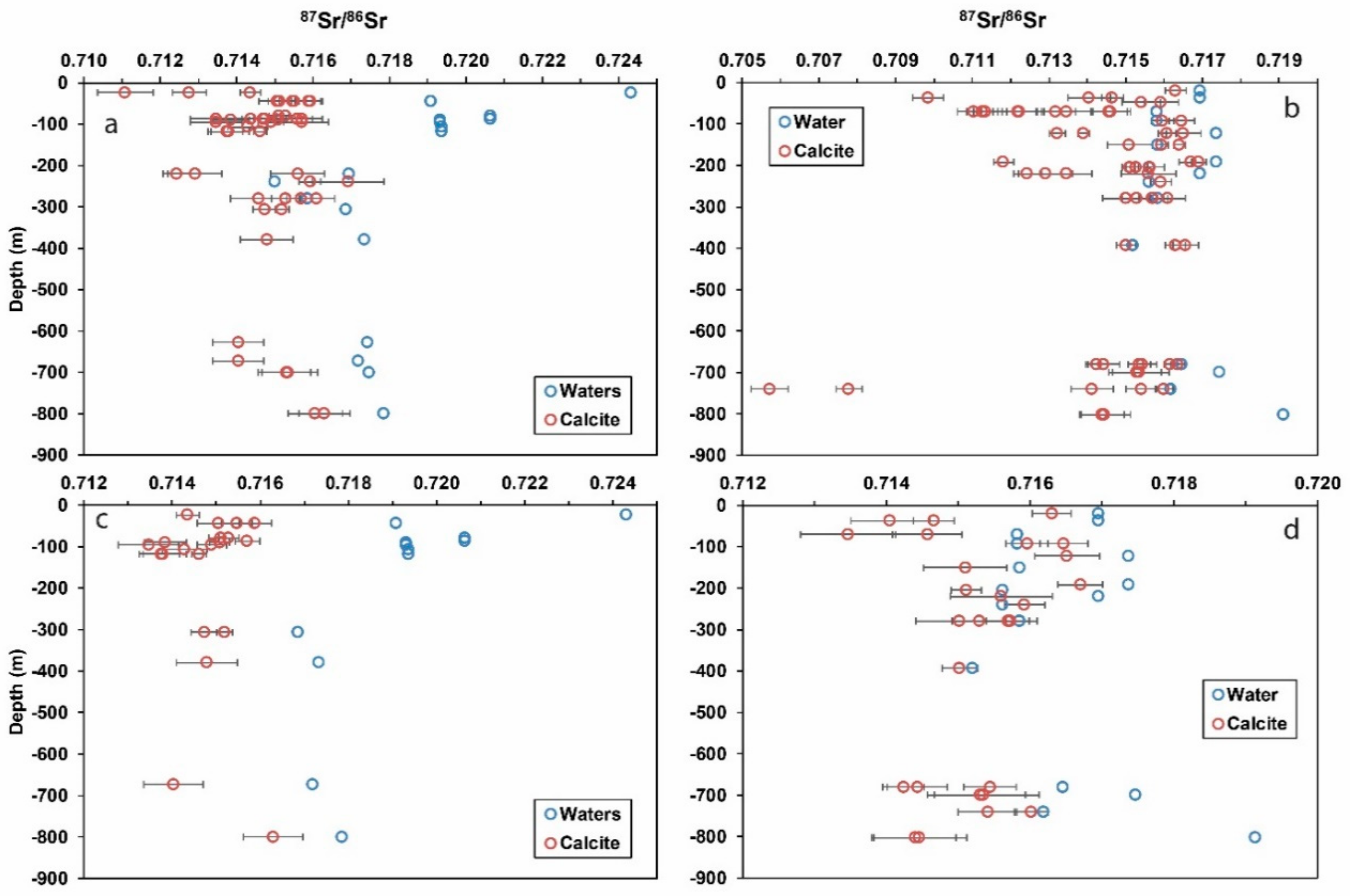
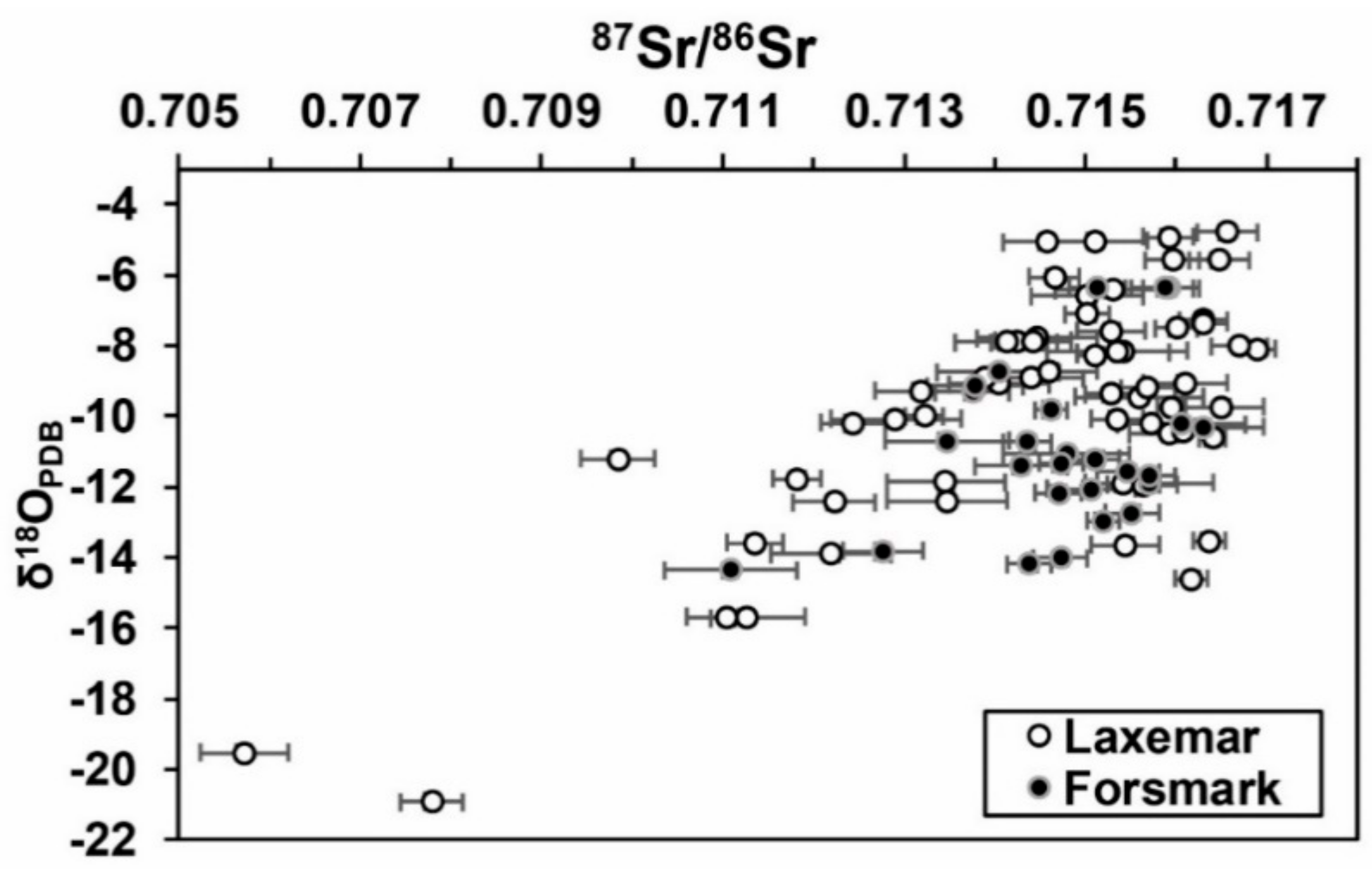
5. Discussion
5.1. 87Sr/86Sr in Calcite as a Hydrochemical Marker
5.2. Calcite-Water Comparisons in Water Conductive Fractures
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Négrel, P.; Fouillac, C.; Brach, M. A strontium isotopic study of mineral and surface waters from the Cézallier (Massif Central, France): Implications for mixing processes in areas of disseminated emergences of mineral waters. Chem. Geol. 1997, 135, 89–101. [Google Scholar] [CrossRef]
- Luís, A.T.; Durães, N.; da Silva, E.F.; Ribeiro, S.; Silva, A.J.F.; Patinha, C.; Almeida, S.F.P.; Azevedo, M.R. Tracking multiple Sr sources through variations in 87Sr/86Sr ratios of surface waters from the Aljustrel massive sulphide mining area: Geological versus anthropogenic inputs. Appl. Geochem. 2019, 102, 108–120. [Google Scholar] [CrossRef]
- Brenot, A.; Petelet-Giraud, E.; Gourcy, L. Insight from surface water-groundwater interactions in an alluvial aquifer: Contributions of δ2H and δ18O of water, δ34SSO4 and δ18OSO4 of sulfates, 87Sr/86Sr ratio. Procedia Earth Planet. Sci. 2015, 13, 84–87. [Google Scholar] [CrossRef][Green Version]
- Schmidt, G.; AlNajem, S.; Isenbeck-Schröter, M.; Freundt, F.; Kraml, M.; Eichstädter, R.; Aeschbach, W. Ascending deep fluids into shallow aquifer at hydraulically active segments of the western boundary fault of the Rhine Graben, Germany: Constraints from 87Sr/86Sr ratios. Procedia Earth Planet. Sci. 2017, 17, 81–84. [Google Scholar] [CrossRef]
- Baublys, K.A.; Hamilton, S.K.; Hofmann, H.; Golding, S.D. A strontium (87Sr/86Sr) isotopic study on the chemical evolution and migration of groundwaters in a low-rank coal seam gas reservoir (Surat Basin, Australia). Appl. Geochem. 2019, 101, 1–18. [Google Scholar] [CrossRef]
- Peterman, Z.E.; Wallin, B. Synopsis of strontium isotope variations in groundwater at Äspö, southern Sweden. Appl. Geochem. 1999, 14, 939–951. [Google Scholar] [CrossRef]
- Negrel, P.; Casanova, J.; Blomqvist, R.; Kaija, J.; Frape, S. Strontium isotopic characterization of the Palmottu hydrosystem (Finland): Water–rock interaction and geochemistry of groundwaters. Geofluids 2003, 3, 161–175. [Google Scholar] [CrossRef]
- McNutt, R.H.; Gascoyne, M.; Kamineni, D.C. 87Sr/86Sr values in groundwaters of the East Bull Lake pluton, superior province, Ontario, Canada. Appl. Geochem. 1987, 2, 93–101. [Google Scholar] [CrossRef]
- Widerlund, A.; Andersson, P.S. Late Holocene freshening of the Baltic Sea derived from high-resolution strontium isotope analyses of mollusk shells. Geology 2011, 39, 187–190. [Google Scholar] [CrossRef]
- van Geldern, R.; Joachimski, M. Carbon, oxygen and strontium isotope records of Devonian brachiopod shell calcite. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 47–67. [Google Scholar] [CrossRef]
- Adams, C.J.; Campbell, H.J.; Griffin, W.L. Isotopic microanalysis of seawater strontium in biogenic calcite to assess subsequent rehomogenisation during metamorphism. Chem. Geol. 2005, 220, 67–82. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef]
- Capo, R.C.; DePaolo, D.J. Seawater strontium isotopic variations from 2.5 million years ago to the present. Science 1990, 249, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Veizer, J. Trace elements and isotopes in sedimentary carbonates. Rev. Mineral. Geochem. 1983, 11, 265–299. [Google Scholar]
- McArthur, J.M.; Rio, D.; Massari, F.; Castradori, D.; Bailey, T.R.; Thirlwall, M.; Houghton, S. A revised pliocene record for marine-87Sr/86Sr used to date an interglacial event recorded in the Cockburn Island Formation, Antarctic Peninsula. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 242, 126–136. [Google Scholar] [CrossRef]
- McNutt, R.H.; Frape, S.K.; Fritz, P.; Jones, M.G.; MacDonald, I.M. The 87Sr/86Sr values of canadian shield brines and fracture minerals with applications to groundwater mixing, fracture history, and geochronology. Geochim. Cosmochim. Acta 1990, 54, 205–215. [Google Scholar] [CrossRef]
- Frape, S.K.; Blyth, A.; Blomqvist, R.; McNutt, R.H.; Gascoyne, M. 5.17-Deep fluids in the continents: II. crystalline rocks. In Treatise on Geochemistry; Pergamon: Oxford, UK, 2003; pp. 541–580. [Google Scholar]
- McNutt, R.H. Strontium Isotopes. In Environmental Tracers in Subsurface Hydrology; Cook, P., Herczeg, A.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 233–260. [Google Scholar]
- Négrel, P. Geochemical study of a granitic area—The margeride mountains, France: Chemical element behavior and 87Sr/86Sr constraints. Aquat. Geochem. 1999, 5, 125–165. [Google Scholar] [CrossRef]
- Tullborg, E.-L.; Drake, H.; Sandström, B. Palaeohydrogeology: A methodology based on fracture mineral studies. Appl. Geochem. 2008, 23, 1881–1897. [Google Scholar] [CrossRef]
- Horton, T.W.; Blum, J.D.; Craw, D.; Koons, P.O.; Chamberlain, C.P. Oxygen, carbon, and strontium isotopic constraints on timing and sources of crustal fluids in an active orogen: South Island, New Zealand. N. Z. J. Geol. Geophys. 2003, 46, 457–471. [Google Scholar] [CrossRef]
- Templeton, A.S.; Chamberlain, C.P.; Koons, P.O.; Craw, D. Stable isotopic evidence for mixing between metamorphic fluids and surface-derived waters during recent uplift of the Southern Alps, New Zealand. Earth Planet. Sci. Lett. 1998, 154, 73–92. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L. Episodic fluid migration in the Fennoscandian Shield recorded by stable isotopes, rare earth elements and fluid inclusions in fracture minerals at Forsmark, Sweden. Chem. Geol. 2009, 266, 126–142. [Google Scholar] [CrossRef]
- Clauer, N.; Frape, S.K.; Fritz, B. Calcite veins of the Stripa granite (Sweden) as records of the origin of the groundwaters and their interactions with the granitic body. Geochim. Cosmochim. Acta 1989, 53, 1777–1781. [Google Scholar] [CrossRef]
- Vaselli, L.; Cortecci, G.; Tonarini, S.; Ottria, G.; Mussi, M. Conditions for veining and origin of mineralizing fluids in the Alpi Apuane (NW Tuscany, Italy): Evidence from structural and geochemical analyses on calcite veins hosted in Carrara marbles. J. Struct. Geol. 2012, 44, 76–92. [Google Scholar] [CrossRef]
- Uysal, I.T.; Feng, Y.-X.; Zhao, J.-X.; Bolhar, R.; Işik, V.; Baublys, K.A.; Yago, A.; Golding, S.D. Seismic cycles recorded in late Quaternary calcite veins: Geochronological, geochemical and microstructural evidence. Earth Planet. Sci. Lett. 2011, 303, 84–96. [Google Scholar] [CrossRef]
- Milodowski, A.E.; Bath, A.; Norris, S. Palaeohydrogeology using geochemical, isotopic and mineralogical analyses: Salinity and redox evolution in a deep groundwater system through Quaternary glacial cycles. Appl. Geochem. 2018, 97, 40–60. [Google Scholar] [CrossRef]
- Milodowski, A.E.; Tullborg, E.L.; Buil, B.; Gomez, P.; Turrero, M.-J.; Haszeldine, S.; England, G.; Gillespie, M.R.; Torres, T.; Ortiz, J.; et al. Application of Mineralogical, Petrological and Geochemical Tools for Evaluating the Palaeohydrogeological Evolution of the PADAMOT Study Sites. PADAMOT Project Technical Report WP2. 2005. Available online: http://nora.nerc.ac.uk/id/eprint/11494/ (accessed on 20 November 2008).
- Bath, A.; Milodowski, A.; Ruotsalainen, P.; Tullborg, E.-L.; Ruiz, A.C.; Aranyossy, J.-F. Evidences from mineralogy and geochemistry for the evolution of groundwater systems during the quaternary for use in radioactive waste repository safety assessment (EQUIP project). In EUR Report 19613; European Commission: Luxembourg, 2000. [Google Scholar]
- Frape, S.K.; Blyth, A.R.; Jones, M.G.; Blomqvist, R.; Tullborg, E.-L.; Mcnutt, R.H.; Mcdermott, F.; Ivanovich, M. A comparison of calcite fracture mineralogy and geochemistry for the Canadian and Fennoscandian shields. In Proceedings of the 7th International Symposium on Water-Rock Interaction; Kharaka, Y.K., Maest, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 1992; pp. 787–791. [Google Scholar]
- Drake, H.; Tullborg, E.-L.; Hogmalm, K.J.; Åström, M.E. Trace metal distribution and isotope variations in low-temperature calcite and groundwater in granitoid fractures down to 1 km depth. Geochim. Cosmochim. Acta 2012, 84, 217–238. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L. Paleohydrogeological events recorded by stable isotopes, fluid inclusions and trace elements in fracture minerals in crystalline rock, Simpevarp area, SE Sweden. Appl. Geochem. 2009, 24, 715–732. [Google Scholar] [CrossRef]
- Maskenskaya, O.M.; Drake, H.; Broman, C.; Hogmalm, J.K.; Czuppon, G.; Åström, M.E. Source and character of syntaxial hydrothermal calcite veins in Paleoproterozoic crystalline rocks revealed by fine-scale investigations. Geofluids 2014, 14, 495–511. [Google Scholar] [CrossRef]
- Drake, H.; Heim, C.; Roberts, N.M.W.; Zack, T.; Tillberg, M.; Broman, C.; Ivarsson, M.; Whitehouse, M.J.; Åström, M.E. Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet. Sci. Lett. 2017, 470, 108–118. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.E.; Heim, C.; Broman, C.; Åström, J.; Whitehouse, M.; Ivarsson, M.; Siljeström, S.; Sjövall, P. Extreme 13C-depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat. Commun. 2015, 6, 7020. [Google Scholar] [CrossRef]
- Tillberg, M.; Drake, H.; Zack, T.; Kooijman, E.; Whitehouse, M.J.; Åström, M.E. In situ Rb-Sr dating of slickenfibres in deep crystalline basement faults. Sci. Rep. 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.; Whitehouse, M.J.; Heim, C.; Reiners, P.W.; Tillberg, M.; Hogmalm, K.J.; Dopson, M.; Broman, C.; Åström, M.E. Unprecedented 34S-enrichment of pyrite formed following microbial sulfate reduction in fractured crystalline rocks. Geobiology 2018, 16, 556–574. [Google Scholar] [CrossRef] [PubMed]
- Tillberg, M.; Maskenskaya, O.M.; Drake, H.; Hogmalm, J.K.; Broman, C.; Fallick, A.E.; Åström, M.E. Fractionation of rare earth elements in greisen and hydrothermal veins related to a-type magmatism. Geofluids 2019, 20. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L.; Larson, S.Å.; Page, L. Brittle tectonothermal evolution in the Forsmark area, central Fennoscandian Shield, recorded by paragenesis, orientation and 40Ar/39Ar geochronology of fracture minerals. Tectonophysics 2009, 478, 158–174. [Google Scholar] [CrossRef]
- Ying, Y.-C.; Chen, W.; Simonetti, A.; Jiang, S.-Y.; Zhao, K.-D. Significance of hydrothermal reworking for REE mineralization associated with carbonatite: Constraints from in situ trace element and C-Sr isotope study of calcite and apatite from the Miaoya carbonatite complex (China). Geochim. Cosmochim. Acta 2020, 280, 340–359. [Google Scholar] [CrossRef]
- Weber, M.; Wassenburg, J.A.; Jochum, K.P.; Breitenbach, S.F.M.; Oster, J.; Scholz, D. Sr-isotope analysis of speleothems by LA-MC-ICP-MS: High temporal resolution and fast data acquisition. Chem. Geol. 2017, 468, 63–74. [Google Scholar] [CrossRef]
- Drake, H.; Roberts, N.M.W.; Heim, C.; Whitehouse, M.J.; Siljeström, S.; Kooijman, E.; Broman, C.; Ivarsson, M.; Åström, E. Timing and origin of natural gas accumulation in the Siljan impact structure, Sweden. Nat. Commun. 2019, 10, 4736. [Google Scholar] [CrossRef]
- Campos-Alvarez, N.O.; Samson, I.M.; Fryer, B.J.; Ames, D.E. Fluid sources and hydrothermal architecture of the Sudbury Structure: Constraints from femtosecond LA-MC-ICP-MS Sr isotopic analysis of hydrothermal epidote and calcite. Chem. Geol. 2010, 278, 131–150. [Google Scholar] [CrossRef]
- Stanfors, R.; Rhen, I.; Tullborg, E.L.; Wikberg, P. Overview of geological and hydrogeological conditions of the Äspo Hard Rock Laboratory site. Appl. Geochem. 1999, 14, 819–834. [Google Scholar] [CrossRef]
- Bäckblom, G.; Stanfors, R.; Gustafson, G.; Rhen, I.; Wikberg, P.; Olsson, O.; Thegerström, C. Äspö Hard Rock Laboratory—Research, development and demonstration for deep disposal of spent nuclear fuel. Tunn. Undergr. Space Technol. 1997, 12, 385–406. [Google Scholar] [CrossRef]
- Mathurin, F.A.; Åström, M.E.; Laaksoharju, M.; Kalinowski, B.E.; Tullborg, E.-L. Effect of tunnel excavation on source and mixing of groundwater in a coastal granitoidic fracture network. Environ. Sci. Technol. 2012, 46, 12779–12786. [Google Scholar] [CrossRef] [PubMed]
- Louvat, D.; Michelot, J.L.; Aranyossy, J.F. Origin and residence time of salinity in the Äspö groundwater system. Appl. Geochem. 1999, 14, 917–925. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Tullborg, E.-L.; Wikberg, P.; Wallin, B.; Smellie, J. Hydrogeochemical conditions and evolution at the Äspo HRL, Sweden. Appl. Geochem. 1999, 14, 835–859. [Google Scholar] [CrossRef]
- Mahara, Y.; Igarashi, T.; Hasegawa, T.; Miyakawa, K.; Tanaka, Y.; Kiho, K. Dynamic changes in hydrogeochemical conditions caused by tunnel excavation at the Aspo Hard Rock Laboratory (HRL), Sweden. Appl. Geochem. 2001, 16, 291–315. [Google Scholar] [CrossRef]
- Nilsson, A.-C.; Gimeno, M.J.; Tullborg, E.-L.; Mathurin, F.; Smellie, J. Hydrogeochemical Data Report. Site Descriptive Modelling Äspö SDM. SKB Report R-13-26; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2013. [Google Scholar]
- Drake, H.; Tullborg, E.-L.; Sandberg, B.; Blomfeldt, T.; Åström, M.E. Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in Deep groundwater systems. Geochim. Cosmochim. Acta 2015, 161, 1–18. [Google Scholar] [CrossRef]
- Yu, C.; Drake, H.; Lopez-Fernandez, M.; Whitehouse, M.; Dopson, M.; Åström, M.E. Micro-scale isotopic variability of low-temperature pyrite in fractured crystalline bedrock—A large Fe isotope fractionation between Fe(II)aq/pyrite and absence of Fe-S isotope co-variation. Chem. Geol. 2019, 522, 192–207. [Google Scholar] [CrossRef]
- Drake, H.; Mathurin, F.A.; Zack, T.; Schäfer, T.; Roberts, N.M.W.; Whitehouse, M.; Karlsson, A.; Broman, C.; Åström, M.E. Incorporation of metals into calcite in a deep anoxic granite aquifer. Environ. Sci. Technol. 2018, 52, 493–502. [Google Scholar] [CrossRef]
- Curti, E. Coprecipitation of radionuclides with calcite: Estimation of partition coefficients based on a review of laboratory investigations and geochemical data. Appl. Geochem. 1999, 14, 433–445. [Google Scholar] [CrossRef]
- Stephens, M.B.; Fox, A.; Paul, L.P.; Simeonov, A.; Isaksson, H.; Hermanson, J.; Oehman, J. Geology Forsmark. Site Descriptive Modelling Forsmark Stage 2.2; SKB-R-07-45; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2007. [Google Scholar]
- Wahlgren, C.-H.; Hermanson, J.; Forssberg, O.; Triumf, C.A.; Drake, H.; Tullborg, E.L. Geological Description of Rock Domains and Deformation Zones in the Simpevarp and Laxemar Subareas. Preliminary Site Description Laxemar Subarea—Version 1.2 SKB Report R-05-69; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2006. [Google Scholar]
- Saintot, A.; Stephens, M.B.; Viola, G.; Nordgulen, O. Brittle tectonic evolution and paleostress field reconstruction in the southwestern part of the Fennoscandian Shield, Forsmark, Sweden. Tectonics 2011, 30. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Tillberg, M.; Whitehouse, M.; Kooijman, E. Ancient microbial activity in deep hydraulically conductive fracture zones within the forsmark target area for geological nuclear waste disposal, sweden. Geosciences 2018, 8, 211. [Google Scholar] [CrossRef]
- Viola, G.; Ganerod, G.V.; Wahlgren, C.-H. Unravelling 1.5 Gyr of brittle deformation history in the Laxemar-Simpevarp area, SE Sweden: A contribution to the Swedish site investigation study for the disposal of highly radioactive nuclear waste. Tectonics 2009, 28, TC5007. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L.; Page, L. Distinguished multiple events of fracture mineralisation related to far-field orogenic effects in Paleoproterozoic crystalline rocks, Simpevarp area, SE Sweden. Lithos 2009, 110, 37–49. [Google Scholar] [CrossRef]
- Wallin, B.; Peterman, Z. Calcite fracture fillings as indicators of palaeohydrogeology at Laxemar at the Äspö Hard Rock Laboratory, southern Sweden. Appl. Geochem. 1999, 14, 953–962. [Google Scholar] [CrossRef]
- Drake, H.; Sandström, B.; Tullborg, E.-L. Mineralogy and Geochemistry of Rocks and Fracture Fillings from Forsmark and Oskarshamn: Compilation of Data for SR-Can; SKB Report R-06-109; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2006. [Google Scholar]
- Laaksoharju, M.; Smellie, J.A.T.; Tullborg, E.-L.; Wallin, B.; Drake, H.; Gascoyne, M.; Gimeno, M.; Gurban, I.; Hallbeck, L.; Molinero, J.; et al. Bedrock Hydrogeochemistry Laxemar. Site Descriptive Modelling SDM-Site Laxemar. SKB Report R-08-93; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Laaksoharju, M.; Smellie, J.; Tullborg, E.-L.; Gimeno, M.; Molinero, J.; Gurban, L.; Hallbeck, L. Hydrogeochemical evaluation and modelling performed within the Swedish site investigation programme. Appl. Geochem. 2008, 23, 1761–1795. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Smellie, J.; Tullborg, E.-L.; Gimeno, M.; Hallbeck, L.; Molinero, J.; Waber, N. Bedrock Hydrogeochemistry Forsmark. Site Descriptive Modelling. SDM-Site Forsmark; SKB Report R-08-47; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2008. [Google Scholar]
- Gómez, J.B.; Gimeno, M.J.; Auqué, L.F.; Acero, P. Characterisation and modelling of mixing processes in groundwaters of a potential geological repository for nuclear wastes in crystalline rocks of Sweden. Sci. Total Environ. 2014, 468–469, 791–803. [Google Scholar] [CrossRef]
- Gimeno, M.J.; Auqué, L.F.; Acero, P.; Gómes, J.B. Hydrogeochemical characterisation and modelling of groundwaters in a potential geological repository for spent nuclear fuel in crystalline rocks (Laxemar, Sweden). Appl. Geochem. 2014, 45, 50–71. [Google Scholar] [CrossRef]
- Emo, R.B.; Smit, M.A.; Schmitt, M.; Kooijman, E.; Scherer, E.E.; Sprung, P.; Bleeker, W.; Mezger, K. Evidence for evolved Hadean crust from Sr isotopes in apatite within Eoarchean zircon from the acasta gneiss complex. Geochim. Cosmochim. Acta 2018, 235, 450–462. [Google Scholar] [CrossRef]
- Kiel, S.; Glodny, J.; Birgel, D.; Bulot, L.G.; Campbell, K.A.; Gaillard, C.; Graziano, R.; Kaim, A.; Lazăr, L.; Sandy, M.R.; et al. The paleoecology, habitats, and stratigraphic range of the enigmatic cretaceous brachiopod peregrinella. PLoS ONE 2014, 9, e109260. [Google Scholar] [CrossRef]
- Mokadem, F.; Parkinson, I.J.; Hathorne, E.C.; Anand, P.; Allen, J.T.; Burton, K.W. High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: Limits on glacial–interglacial variations in continental weathering. Earth Planet. Sci. Lett. 2015, 415, 111–120. [Google Scholar] [CrossRef]
- Smellie, J.; Tullborg, E.-L. Quality assurance and categorisation of groundwater samples from the Laxemar-Simpevarp area. In Background Complementary Hydrogeochemical Studies, Site Descriptive Modelling, SDM-Site Laxemar, SKB Report R-08-111, R-08-111; Kalinowski, B.E., Ed.; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2009; pp. 163–347. [Google Scholar]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Drake, H.; Hallbeck, L.; Rosdahl, A.; Tullborg, E.-L.; Wallin, B.; Sandberg, B.; Blomfeldt, T. Investigation of Sulphide Production in Core-Drilled Boreholes in Äspö Hard Rock Laboratory. Boreholes KA3110A, KA3385A and KA3105A. SKB Report TR-13-12; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2013. [Google Scholar]
- Faure, G.; Mensing, T.M. Isotopes: Principles and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Faure, G. Stable Isotope Geochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1986; p. 589. [Google Scholar]
- Smellie, J.; Tullborg, E.-L.; Nilsson, A.-C.; Sandstroem, B.; Waber, N.; Gimeno, M.; Gascoyne, M. Explorative Analysis of Major Components and Isotopes. SDM-Site Forsmark; Report SKB R-08-84; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2008. [Google Scholar]
- Selroos, J.-O.; Follin, S. Overview of hydrogeological site-descriptive modeling conducted for the proposed high-level nuclear waste repository site at Forsmark, Sweden. Hydrogeol. J. 2014, 22, 295–298. [Google Scholar] [CrossRef]
- Andersson, J.; Skagius, K.; Winberg, A.; Lindborg, T.; Ström, A. Site-descriptive modelling for a final repository for spent nuclear fuel in Sweden. Environ. Earth Sci. 2013, 69, 1045–1060. [Google Scholar] [CrossRef]
- Follin, S.; Hartley, L.; Jackson, P.; Roberts, D.; Marsic, N. Hydrogeological Conceptual Model Development and Numerical Modeling Using CONNECTFLOW, Forsmark Modeling Stage 2.3. SKB R-08-23; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2008. [Google Scholar]
- Hartley, L.; Hunter, F.; Jackson, P.; McCarthy, R.; Gylling, B.; Marsic, N. Regional Hydrogeological Simulations Using CONECTFLOW. Preliminary Site Description. Laxemar Sub Area—Version 1.2; SKB-R--06-23; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2006. [Google Scholar]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P.; Whitehouse, M. Biogenic processes in crystalline bedrock fractures indicated by carbon isotope signatures of secondary calcite. Appl. Geochem. 2016, 67, 30–41. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P. Indications for the past redox environments in deep groundwaters from the isotopic composition of carbon and oxygen in fracture calcite, Olkiluoto, SW Finland. Isot. Environ. Health Stud. 2010, 46, 370–391. [Google Scholar] [CrossRef] [PubMed]
- Blyth, A.R.; Frape, S.K.; Tullborg, E.L. A review and comparison of fracture mineral investigations and their application to radioactive waste disposal. Appl. Geochem. 2009, 24, 821–835. [Google Scholar] [CrossRef]
- Blyth, A.; Frape, S.; Blomqvist, R.; Nissinen, P. Assessing the past thermal and chemical history of fluids in crystalline rock by combining fluid inclusion and isotopic investigations of fracture calcite. Appl. Geochem. 2000, 15, 1417–1437. [Google Scholar] [CrossRef]
- Åberg, G. Precambrian geochronology of south-eastern Sweden. Geol. Fören. Stockh. Förh. 1978, 100, 125–154. [Google Scholar] [CrossRef]
- Sandström, B.; Page, L.; Tullborg, E.-L. Forsmark Site Investigation. 40Ar/39Ar (Adularia) and Rb-Sr (Adularia, Prehnite, Calcite) Ages of Fracture minerals; Report P-06-213; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2006. [Google Scholar]
| Calcite | Water | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borehole, Section | Crystal | Crystal Part | Sampling Time | 87Sr/86Sr | 2SE | Prop. 2SE 1 | 84Sr/86Sr | 2SE | 87Rb/86Sr 2 | 2SE | Total Sr-Beam | 87Sr/86Sr | Sr (mg/L) |
| KA3105A:3 | 1 | rim | 34.8 | 0.71731 | 0.00014 | 0.00014 | 0.05666 | 0.00015 | 0.000017 | 0.000008 | 7.48 | 0.71729 | 5.47 |
| KA3105A:3 | 1 | rim | 47.3 | 0.71735 | 0.00011 | 0.00011 | 0.05660 | 0.00010 | 0.000034 | 0.000009 | 7.07 | ||
| KA3105A:3 | 2 | rim | 26.0 | 0.71734 | 0.00014 | 0.00014 | 0.05656 | 0.00011 | 0.000025 | 0.000010 | 7.40 | ||
| KA3105A:3 | 3 | rim | 32.8 | 0.71733 | 0.00016 | 0.00016 | 0.05657 | 0.00007 | 0.000036 | 0.000007 | 9.25 | ||
| KA3105A:3 | 3 | rim | 45.5 | 0.71746 | 0.00010 | 0.00010 | 0.05651 | 0.00007 | 0.000053 | 0.000008 | 6.47 | ||
| KA3105A:2 | 1 | rim | 43.6 | 0.71781 | 0.00012 | 0.00012 | 0.05658 | 0.00010 | 0.000075 | 0.000013 | 6.23 | 0.717749 | 8.34 |
| KA3105A:2 | 1 | rim | 44.5 | 0.71783 | 0.00013 | 0.00013 | 0.05655 | 0.00010 | 0.000081 | 0.000016 | 5.85 | ||
| KA3105A:2 | 2 | rim | 46.0 | 0.71765 | 0.00008 | 0.00008 | 0.05665 | 0.00010 | 0.000031 | 0.000008 | 6.57 | ||
| KA3105A:2 | 2 | rim | 46.0 | 0.71788 | 0.00011 | 0.00011 | 0.05661 | 0.00008 | 0.000096 | 0.000022 | 6.21 | ||
| KA3105A:3 | 1 | inner | 43.1 | 0.71746 | 0.00010 | 0.00010 | 0.05660 | 0.00018 | <DL | <DL | 7.43 | ||
| KA3105A:3 | 2 | inner | 36.0 | 0.71756 | 0.00015 | 0.00015 | 0.05651 | 0.00009 | 0.000028 | 0.000008 | 6.39 | ||
| KA3105A:2 | 1 | inner | 48.0 | 0.71794 | 0.00012 | 0.00012 | 0.05653 | 0.00005 | 0.000031 | 0.000005 | 10.97 |
| Sr Isotopes | C and O Isotopes in Calcite (SIMS) | Modern Water Data | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borehole | Length(m) | Depth (m) | Crystal | Sampling Time | 87Sr/86Sr | 2SE | Prop. 2SE 1 | 84Sr/86Sr | 2SE | 87Rb/86Sr 2 | 2SE | Total Sr-Beam | δ13CPDB (SIMS) | ±σext | δ18OPDB (SIMS) | ±σext | 87Sr/86Sr 3 |
| KFM01B | 24 | –24 | 1 | 41.6 | 0.71108 | 0.00073 | 0.00074 | 0.0579 | 0.0019 | 0.01371 | 0.00330 | 0.27 | –23.6 | 0.5 | –14.4 | 0.2 | 0.724317 |
| KFM01B | 24 | –24 | 1 | 33.2 | 0.71276 | 0.00044 | 0.00044 | 0.0574 | 0.0011 | 0.00464 | 0.00090 | 0.54 | 13.6 | 0.5 | –13.8 | 0.2 | 0.724317 |
| KFM01B | 24 | –24 | 1 | 44.8 | 0.71436 | 0.00027 | 0.00027 | 0.0569 | 0.0005 | 0.05777 | 0.00469 | 0.84 | 21.6 | 0.5 | –10.7 | 0.2 | 0.724317 |
| KFM01C | 90 | –80 | 1 | 37.3 | 0.71511 | 0.00027 | 0.00028 | 0.0570 | 0.0006 | 0.03837 | 0.01073 | 1.09 | 21.9 | 0.4 | –11.2 | 0.2 | 0.720640 |
| KFM01C | 90 | –80 | 2 | 50.5 | 0.71529 | 0.00023 | 0.00023 | 0.0565 | 0.0004 | 0.00039 | 0.00006 | 1.31 | n.a. | n.a. | 0.720640 | ||
| KFM02A | 107 | –107 | 1 | 33.9 | 0.71429 | 0.00051 | 0.00051 | 0.0572 | 0.0009 | 0.00275 | 0.00069 | 0.48 | 16.2 | 0.7 | –11.4 | 0.2 | 0.719362 |
| KFM02A | 118 | –118 | 1 | 45.8 | 0.71462 | 0.00017 | 0.00018 | 0.0568 | 0.0004 | 0.00015 | 0.00004 | 1.25 | 10.7 | 0.7 | –9.8 | 0.2 | 0.719362 |
| KFM02A | 118 | –118 | 1 | 40.1 | 0.71375 | 0.00041 | 0.00041 | 0.0576 | 0.0014 | 0.01404 | 0.00378 | 0.42 | 9.2 | 0.6 | –9.3 | 0.2 | 0.719362 |
| KFM02A | 118 | –118 | 1 | 47.5 | 0.71379 | 0.00053 | 0.00053 | 0.0569 | 0.0016 | 0.06375 | 0.01525 | 0.30 | 8.4 | 0.6 | –9.2 | 0.2 | 0.719362 |
| KFM03A | 380 | –380 | 1 | 43.1 | 0.71479 | 0.00069 | 0.00069 | 0.0564 | 0.0017 | 0.00357 | 0.00129 | 0.28 | –8.0 | 0.5 | –11.1 | 0.2 | 0.717339 |
| KFM04A | 306 | –306 | 1 | 47.0 | 0.71519 | 0.00018 | 0.00019 | 0.0569 | 0.0005 | 0.00008 | 0.00004 | 1.44 | –14.8 | 0.6 | –13.0 | 0.2 | 0.716865 |
| KFM04A | 306 | –306 | 2 | 41.8 | 0.71473 | 0.00030 | 0.00030 | 0.0575 | 0.0010 | 0.00015 | 0.00008 | 0.68 | –46.3 | 0.5 | –14.0 | 0.2 | 0.716865 |
| KFM05A | 110 | –87 | 1 | 49.5 | 0.71347 | 0.00068 | 0.00068 | 0.0590 | 0.0025 | 0.00040 | 0.00015 | 0.30 | –16.9 | 0.5 | –10.7 | 0.1 | 0.720640 |
| KFM05A | 110 | –87 | 1 | 27.1 | 0.71473 | 0.00025 | 0.00025 | 0.0569 | 0.0004 | 0.00050 | 0.00019 | 2.00 | –16.8 | 0.4 | –11.3 | 0.2 | 0.720640 |
| KFM05A | 110 | –87 | 1 | 46.9 | 0.71470 | 0.00025 | 0.00025 | 0.0568 | 0.0007 | 0.00046 | 0.00007 | 0.81 | –11.4 | 0.4 | –12.2 | 0.2 | 0.720640 |
| KFM05A | 110 | –87 | 1 | 44.8 | 0.71438 | 0.00025 | 0.00025 | 0.0566 | 0.0008 | 0.00052 | 0.00009 | 0.79 | 16.4 | 0.4 | –14.2 | 0.2 | 0.720640 |
| KFM05A | 110 | –87 | 1 | 56.0 | 0.71570 | 0.00070 | 0.00070 | 0.0564 | 0.0010 | 0.01251 | 0.00259 | 1.23 | 11.3 | 0.4 | –11.9 | 0.2 | 0.720640 |
| KFM05A | 110 | –87 | 1 | 41.5 | 0.71571 | 0.00028 | 0.00028 | 0.0568 | 0.0004 | 0.00011 | 0.00003 | 1.63 | 13.3 | 0.4 | –11.7 | 0.2 | 0.720640 |
| KFM06A | 110 | –96 | 1 | 49.5 | 0.71347 | 0.00068 | 0.00068 | 0.0590 | 0.0025 | 0.00040 | 0.00015 | 0.30 | –22.2 | 0.4 | n.a. | 0.719319 | |
| KFM06A | 110 | –96 | 1 | 39.3 | 0.71490 | 0.00033 | 0.00033 | 0.0569 | 0.0009 | 0.00219 | 0.00095 | 0.69 | –19.5 | 0.4 | n.a. | 0.719319 | |
| KFM06C | 103 | –90 | 1 | 51.6 | 0.71508 | 0.00022 | 0.00022 | 0.0569 | 0.0004 | 0.00011 | 0.00004 | 1.30 | n.a. | n.a. | 0.719319 | ||
| KFM06C | 103 | –90 | 2 | 45.3 | 0.71385 | 0.00047 | 0.00047 | 0.0578 | 0.0013 | 0.00041 | 0.00014 | 0.40 | n.a. | n.a. | 0.719319 | ||
| KFM07A | 968 | –800 | 1 | 49.0 | 0.71606 | 0.00071 | 0.00071 | 0.0567 | 0.0019 | 0.00099 | 0.00032 | 0.30 | –23.1 | 0.4 | –10.2 | 0.3 | 0.717855 |
| KFM07A | 968 | –800 | 1 | 40.0 | 0.71630 | 0.00067 | 0.00067 | 0.0563 | 0.0021 | 0.00129 | 0.00046 | 0.29 | –33.3 | 0.4 | –10.3 | 0.3 | 0.717855 |
| KFM08B | 44 | –44 | 1 | 17.0 | 0.71551 | 0.00030 | 0.00030 | 0.0570 | 0.0007 | 0.00020 | 0.00008 | 1.14 | –21.7 | 0.4 | –12.7 | 0.2 | 0.719092 |
| KFM08B | 44 | –44 | 1 | 45.3 | 0.71506 | 0.00048 | 0.00048 | 0.0566 | 0.0012 | 0.00039 | 0.00013 | 0.41 | –67.2 | 0.4 | –12.0 | 0.2 | 0.719092 |
| KFM08B | 44 | –44 | 1 | 27.3 | 0.71547 | 0.00035 | 0.00035 | 0.0565 | 0.0005 | 0.00158 | 0.00029 | 1.14 | –62.1 | 0.4 | –11.6 | 0.2 | 0.719092 |
| KFM08B | 44 | –44 | 2 | 21.5 | 0.71592 | 0.00028 | 0.00029 | 0.0566 | 0.0004 | 0.00011 | 0.00009 | 1.08 | –35.4 | 0.4 | –6.4 | 0.2 | 0.719092 |
| KFM08B | 44 | –44 | 3 | 21.1 | 0.71513 | 0.00031 | 0.00032 | 0.0561 | 0.0006 | 0.00014 | 0.00006 | 1.07 | –35.4 | 0.4 | –6.4 | 0.2 | 0.719092 |
| KFM08B | 44 | –44 | 4 | 32.3 | 0.71588 | 0.00037 | 0.00037 | 0.0561 | 0.0006 | 0.00091 | 0.00042 | 1.16 | –35.4 | 0.4 | –6.4 | 0.2 | 0.719092 |
| KFM11A | 793 | –673 | 1 | 46.0 | 0.71404 | 0.00067 | 0.00068 | 0.0580 | 0.0017 | 0.00013 | 0.00017 | 0.29 | 28.4 | 0.6 | –8.8 | 0.2 | 0.717188 |
| Sr Isotopes | C and O Isotopes in Calcite (SIMS) | Modern Water Data | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borehole | Length (m) | Depth (m) | Crystal | Sampling Time | 87Sr/86Sr | 2SE | Prop. 2SE 1 | 84Sr/86Sr | 2SE | 87Rb/86Sr 2 | 2SE | Total Sr-Beam | δ13CPDB (SIMS) | ±σext | δ18OPDB (SIMS) | ±σext | 87Sr/86Sr 3 |
| KKR02 | 48 | −48 | 1 | 44.7 | 0.71593 | 0.00044 | 0.00044 | 0.0573 | 0.0009 | 0.00020 | 0.00009 | 0.62 | −70.9 | 0.4 | −10.5 | 0.2 | n.a. |
| KKR02 | 48 | −48 | 1 | 47.0 | 0.71541 | 0.00051 | 0.00051 | 0.0570 | 0.0011 | 0.00059 | 0.00015 | 0.39 | −20.9 | 0.4 | −8.2 | 0.2 | n.a. |
| KAS02 | 802 | −802 | 1 | 21.6 | 0.71446 | 0.00066 | 0.00066 | 0.0565 | 0.0009 | 0.00012 | 0.00012 | 0.61 | −77.9 | 0.4 | −7.8 | 0.2 | 0.719128 |
| KAS02 | 802 | −802 | 1 | 30.7 | 0.71440 | 0.00057 | 0.00057 | 0.0575 | 0.0010 | 0.00019 | 0.00008 | 0.70 | −76.8 | 0.3 | −8.9 | 0.2 | 0.719128 |
| KSH01A | 206 | −204 | 1 | 19.7 | 0.71563 | 0.00038 | 0.00038 | 0.0578 | 0.0013 | 0.00025 | 0.00011 | 0.66 | 19.1 | 0.4 | −12.0 | 0.2 | 0.715614 |
| KSH01A | 206 | −204 | 1 | 32.0 | 0.71528 | 0.00028 | 0.00028 | 0.0572 | 0.0007 | 0.00007 | 0.00006 | 0.95 | −25.8 | 0.4 | −9.4 | 0.1 | 0.715614 |
| KSH01A | 206 | −204 | 1 | 48.5 | 0.71511 | 0.00021 | 0.00021 | 0.0570 | 0.0005 | 0.00006 | 0.00004 | 1.21 | −20.7 | 0.4 | −8.3 | 0.2 | 0.715614 |
| KSH01A | 242 | −240 | 1 | 45.3 | 0.71592 | 0.00028 | 0.00028 | 0.0575 | 0.0010 | 0.02761 | 0.00681 | 0.68 | −88.6 | 0.5 | −4.9 | 0.3 | 0.715614 |
| KSH03A | 863 | −680 | 1 | 44.6 | 0.71636 | 0.00018 | 0.00018 | 0.0562 | 0.0004 | 0.00011 | 0.00004 | 1.33 | −49.6 | 0.4 | −13.5 | 0.2 | 0.716456 |
| KSH03A | 863 | −680 | 1 | 41.8 | 0.71424 | 0.00029 | 0.00029 | 0.0562 | 0.0007 | 0.00029 | 0.00008 | 0.80 | −58.8 | 0.4 | −7.9 | 0.2 | 0.716456 |
| KSH03A | 863 | −680 | 2 | 44.2 | 0.71617 | 0.00018 | 0.00019 | 0.0567 | 0.0004 | 0.00011 | 0.00004 | 1.36 | −13.1 | 0.4 | −14.6 | 0.2 | 0.716456 |
| KSH03A | 863 | −680 | 2 | 29.5 | 0.71544 | 0.00037 | 0.00038 | 0.0571 | 0.0008 | 0.00016 | 0.00017 | 0.74 | −47.7 | 0.5 | −13.6 | 0.1 | 0.716456 |
| KSH03A | 863 | −680 | 2 | 26.5 | 0.71535 | 0.00029 | 0.00030 | 0.0572 | 0.0007 | 0.00436 | 0.00075 | 0.95 | −11.1 | 0.4 | −10.1 | 0.2 | 0.716456 |
| KSH03A | 863 | −680 | 2 | 41.4 | 0.71443 | 0.00042 | 0.00042 | 0.0577 | 0.0012 | 0.00526 | 0.00099 | 0.80 | −65.9 | 0.4 | −7.9 | 0.2 | 0.716456 |
| KLX01 | 20 | −20 | 1 | 31.2 | 0.71630 | 0.00027 | 0.00027 | 0.0560 | 0.0005 | 0.00031 | 0.00008 | 1.32 | −92.4 | 0.5 | −7.3 | 0.2 | 0.716951 |
| KLX01 | 37 | −37 | 1 | 43.8 | 0.70985 | 0.00041 | 0.00041 | 0.0574 | 0.0009 | 0.00031 | 0.00008 | 0.71 | −11.4 | 0.5 | −11.2 | 0.2 | 0.716951 |
| KLX01 | 37 | −37 | 1 | 36.0 | 0.71405 | 0.00055 | 0.00055 | 0.0571 | 0.0013 | 0.01065 | 0.00210 | 0.45 | 0.9 | 0.5 | −9.1 | 0.2 | 0.716951 |
| KLX01 | 37 | −37 | 2 | 29.5 | 0.71466 | 0.00028 | 0.00028 | 0.0571 | 0.0006 | 0.00379 | 0.00239 | 0.97 | 0.6 | 0.5 | −6.1 | 0.2 | 0.716951 |
| KLX01 | 220 | −220 | 1 | 27.8 | 0.71346 | 0.00066 | 0.00066 | 0.0560 | 0.0012 | 0.05454 | 0.01729 | 0.57 | −50.4 | 0.4 | −11.9 | 0.2 | 0.716951 |
| KLX01 | 220 | −220 | 2 | 45.6 | 0.71243 | 0.00035 | 0.00035 | 0.0571 | 0.0009 | 0.00276 | 0.00169 | 0.68 | −48.3 | 0.4 | −10.2 | 0.2 | 0.716951 |
| KLX01 | 220 | −220 | 3 | 10.8 | 0.71291 | 0.00071 | 0.00071 | 0.0575 | 0.0014 | 0.01495 | 0.00929 | 0.73 | −46.2 | 0.3 | −10.1 | 0.2 | 0.716951 |
| KLX01 | 220 | −220 | 3 | 27.1 | 0.71560 | 0.00071 | 0.00071 | 0.0572 | 0.0014 | 0.03222 | 0.00495 | 0.45 | −11.6 | 0.4 | −9.5 | 0.2 | 0.716951 |
| KLX07A | 193 | −150 | 1 | 39.9 | 0.71640 | 0.00015 | 0.00015 | 0.0563 | 0.0003 | 0.00010 | 0.00003 | 2.40 | −13.8 | 0.5 | −10.6 | 0.2 | 0.715849 |
| KLX07A | 193 | −150 | 1 | 45.5 | 0.71595 | 0.00016 | 0.00016 | 0.0567 | 0.0002 | 0.00027 | 0.00008 | 2.32 | −16.7 | 0.5 | −9.7 | 0.2 | 0.715849 |
| KLX07A | 193 | −150 | 1 | 21.7 | 0.71510 | 0.00058 | 0.00058 | 0.0568 | 0.0004 | 0.00027 | 0.00007 | 2.03 | 0.0 | 0.5 | −5.1 | 0.2 | 0.715849 |
| KLX07A | 356 | −280 | 1 | 42.9 | 0.71569 | 0.00030 | 0.00030 | 0.0565 | 0.0008 | 0.00440 | 0.00107 | 0.65 | −6.6 | 0.5 | −9.2 | 0.4 | 0.715849 |
| KLX07A | 356 | −280 | 2 | 44.2 | 0.71610 | 0.00046 | 0.00046 | 0.0573 | 0.0009 | 0.11668 | 0.01445 | 0.61 | −6.7 | 0.5 | −9.1 | 0.4 | 0.715849 |
| KLX07A | 356 | −280 | 2 | 45.0 | 0.71529 | 0.00038 | 0.00038 | 0.0572 | 0.0010 | 0.00664 | 0.00163 | 0.53 | −6.4 | 0.5 | −7.6 | 0.3 | 0.715849 |
| KLX07A | 356 | -280 | 3 | 29.1 | 0.71502 | 0.00061 | 0.00061 | 0.0572 | 0.0015 | 0.00517 | 0.00170 | 0.39 | −12.1 | 0.5 | −6.6 | 0.2 | 0.715849 |
| KLX07A | 356 | −280 | 3 | 47.5 | 0.71572 | 0.00038 | 0.00038 | 0.0559 | 0.0012 | 0.00035 | 0.00012 | 0.50 | −32.3 | 0.5 | −10.2 | 0.2 | 0.715849 |
| KLX07A | 883 | -700 | 1 | 17.3 | 0.71530 | 0.00063 | 0.00063 | 0.0576 | 0.0012 | 0.00015 | 0.00013 | 0.67 | −93.1 | 0.4 | −6.4 | 0.4 | 0.717460 |
| KLX07A | 883 | −700 | 2 | 8.8 | 0.71535 | 0.00078 | 0.00078 | 0.0577 | 0.0012 | 0.00007 | 0.00018 | 0.63 | −88.5 | 0.4 | −8.2 | 0.4 | 0.717460 |
| KLX09 | 192 | −192 | 1 | 48.5 | 0.71182 | 0.00026 | 0.00026 | 0.0571 | 0.0006 | 0.00005 | 0.00006 | 1.01 | −19.2 | 0.4 | −11.8 | 0.2 | 0.717363 |
| KLX09 | 192 | −192 | 1 | 40.9 | 0.71690 | 0.00019 | 0.00019 | 0.0562 | 0.0005 | 0.00010 | 0.00005 | 1.35 | -1.2 | 0.4 | −8.1 | 0.2 | 0.717363 |
| KLX09 | 192 | −192 | 1 | 29.2 | 0.71670 | 0.00031 | 0.00031 | 0.0566 | 0.0006 | 0.00159 | 0.00056 | 1.15 | 0.6 | 0.4 | −8.0 | 0.2 | 0.717363 |
| KLX09 | 740 | −740 | 1 | 37.9 | 0.70779 | 0.00034 | 0.00034 | 0.0573 | 0.0010 | 0.00029 | 0.00008 | 0.58 | −4.8 | 0.4 | −20.9 | 0.2 | 0.716186 |
| KLX09 | 740 | −740 | 1 | 43.1 | 0.71413 | 0.00056 | 0.00056 | 0.0580 | 0.0016 | 0.02299 | 0.00770 | 0.36 | −29.9 | 0.4 | −7.9 | 0.2 | 0.716186 |
| KLX09 | 740 | −740 | 1 | 50.7 | 0.71601 | 0.00023 | 0.00023 | 0.0567 | 0.0004 | 0.00038 | 0.00014 | 1.20 | −27.4 | 0.4 | −7.5 | 0.2 | 0.716186 |
| KLX09 | 740 | −740 | 2 | 37.5 | 0.70572 | 0.00049 | 0.00049 | 0.0577 | 0.0014 | 0.00303 | 0.00162 | 0.35 | −5.4 | 0.4 | −19.5 | 0.2 | 0.716186 |
| KLX09 | 740 | −740 | 2 | 50.4 | 0.71541 | 0.00041 | 0.00041 | 0.0573 | 0.0008 | 0.00024 | 0.00008 | 0.61 | −16.6 | 0.4 | −11.9 | 0.2 | 0.716186 |
| KLX10C | 122 | −122 | 1 | 49.1 | 0.71322 | 0.00022 | 0.00022 | 0.0569 | 0.0004 | 0.00018 | 0.00005 | 1.36 | −9.7 | 0.4 | −10.0 | 0.2 | 0.717363 |
| KLX10C | 122 | −122 | 1 | 44.4 | 0.71390 | 0.00015 | 0.00015 | 0.0567 | 0.0002 | 0.00011 | 0.00003 | 3.21 | −9.8 | 0.4 | −8.9 | 0.2 | 0.717363 |
| KLX10C | 122 | −122 | 1 | 18.1 | 0.71609 | 0.00023 | 0.00023 | 0.0567 | 0.0005 | 0.00158 | 0.00007 | 1.70 | 1.6 | 0.4 | −10.4 | 0.2 | 0.717363 |
| KLX10C | 122 | −122 | 1 | 24.2 | 0.71651 | 0.00045 | 0.00045 | 0.0567 | 0.0006 | 0.00527 | 0.00118 | 1.23 | −13.0 | 0.4 | −9.7 | 0.2 | 0.717363 |
| KLX13A | 393 | −393 | 1 | 41.6 | 0.71630 | 0.00026 | 0.00026 | 0.0571 | 0.0006 | 0.00027 | 0.00005 | 1.02 | −24.6 | 0.5 | −7.4 | 0.4 | 0.715201 |
| KLX13A | 393 | −393 | 2 | 33.7 | 0.71656 | 0.00033 | 0.00033 | 0.0573 | 0.0008 | 0.01996 | 0.00389 | 0.86 | −119.2 | 0.5 | −4.8 | 0.4 | 0.715201 |
| KLX13A | 393 | −393 | 2 | 31.1 | 0.71502 | 0.00025 | 0.00025 | 0.0571 | 0.0008 | 0.00019 | 0.00008 | 0.84 | −15.5 | 0.5 | −7.1 | 0.4 | 0.715201 |
| KLX14A | 80 | −70 | 1 | 45.2 | 0.71105 | 0.00019 | 0.00019 | 0.0568 | 0.0004 | 0.00315 | 0.00058 | 1.39 | −8.4 | 0.4 | −15.7 | 0.2 | 0.715818 |
| KLX14A | 80 | −70 | 1 | 40.5 | 0.71135 | 0.00031 | 0.00031 | 0.0570 | 0.0009 | 0.00059 | 0.00011 | 0.57 | −8.6 | 0.4 | −13.6 | 0.2 | 0.715818 |
| KLX14A | 80 | −70 | 1 | 26.2 | 0.71318 | 0.00051 | 0.00051 | 0.0576 | 0.0012 | 0.01035 | 0.00220 | 0.58 | −8.4 | 0.4 | −9.3 | 0.2 | 0.715818 |
| KLX14A | 80 | −70 | 1 | 45.2 | 0.71461 | 0.00051 | 0.00051 | 0.0571 | 0.0016 | 0.01759 | 0.00528 | 0.38 | −4.7 | 0.4 | −8.7 | 0.2 | 0.715818 |
| KLX14A | 80 | −70 | 1 | 22.7 | 0.71457 | 0.00048 | 0.00048 | 0.0573 | 0.0010 | 0.00017 | 0.00009 | 0.84 | −9.1 | 0.4 | −5.1 | 0.3 | 0.715818 |
| KLX14A | 80 | −70 | 2 | 50.0 | 0.71220 | 0.00066 | 0.00066 | 0.0556 | 0.0013 | 0.00101 | 0.00016 | 0.42 | −13.7 | 0.5 | −13.9 | 0.4 | 0.715818 |
| KLX14A | 80 | −70 | 2 | 48.0 | 0.71223 | 0.00045 | 0.00045 | 0.0561 | 0.0012 | 0.00094 | 0.00014 | 0.42 | −13.2 | 0.5 | −12.4 | 0.4 | 0.715818 |
| KLX14A | 80 | −70 | 2 | 49.0 | 0.71126 | 0.00065 | 0.00065 | 0.0572 | 0.0017 | 0.00156 | 0.00020 | 0.29 | −6.0 | 0.5 | −15.7 | 0.4 | 0.715818 |
| KLX14A | 80 | −70 | 2 | 46.1 | 0.71347 | 0.00066 | 0.00066 | 0.0567 | 0.0012 | 0.00237 | 0.00043 | 0.45 | −7.6 | 0.5 | −12.4 | 0.4 | 0.715818 |
| KLX14A | 92 | −92 | 1 | 41.6 | 0.71596 | 0.00029 | 0.00029 | 0.0571 | 0.00071 | 0.13288 | 0.02007 | 0.80 | −60.5 | 0.6 | −5.6 | 0.2 | 0.715818 |
| KLX14A | 92 | −92 | 1 | 47.4 | 0.71647 | 0.00033 | 0.00033 | 0.0570 | 0.00067 | 0.11757 | 0.01518 | 0.69 | −60.5 | 0.6 | −5.6 | 0.2 | 0.715818 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drake, H.; Kooijman, E.; Kielman-Schmitt, M. Using 87Sr/86Sr LA-MC-ICP-MS Transects within Modern and Ancient Calcite Crystals to Determine Fluid Flow Events in Deep Granite Fractures. Geosciences 2020, 10, 345. https://doi.org/10.3390/geosciences10090345
Drake H, Kooijman E, Kielman-Schmitt M. Using 87Sr/86Sr LA-MC-ICP-MS Transects within Modern and Ancient Calcite Crystals to Determine Fluid Flow Events in Deep Granite Fractures. Geosciences. 2020; 10(9):345. https://doi.org/10.3390/geosciences10090345
Chicago/Turabian StyleDrake, Henrik, Ellen Kooijman, and Melanie Kielman-Schmitt. 2020. "Using 87Sr/86Sr LA-MC-ICP-MS Transects within Modern and Ancient Calcite Crystals to Determine Fluid Flow Events in Deep Granite Fractures" Geosciences 10, no. 9: 345. https://doi.org/10.3390/geosciences10090345
APA StyleDrake, H., Kooijman, E., & Kielman-Schmitt, M. (2020). Using 87Sr/86Sr LA-MC-ICP-MS Transects within Modern and Ancient Calcite Crystals to Determine Fluid Flow Events in Deep Granite Fractures. Geosciences, 10(9), 345. https://doi.org/10.3390/geosciences10090345






