Simple Summary
Only limited information is available on the use of stick water in aquafeed, even though this could confer benefits by providing cost-effective nutrition to aquaculture as well as reducing waste effluents. Therefore, the optimal replacement of protein from fish meal by stick water in the diet of Nile tilapia was investigated in the current study. An 8 month trial was conducted in floating baskets, mimicking the market stage Nile tilapia production in Thailand. Based on the overall results, 20% protein replacement of fish meal by stick water was near optimal, providing superior traits of overall observed parameters relative to the baseline fish meal-based diet. Findings from the current study suggest that stick water is a suitable alternative ingredient for sex-reversed Nile tilapia. The pursuit of higher replacement levels while maintaining the carcass quality of reared fish could be a topic for further study.
Abstract
The effects of replacing fish meal (FM) protein with stick water (SW) were investigated during the market stage of sex-reversed Nile tilapia, Oreochromis niloticus (18.49 ± 0.31 g initial body weight). The FM protein was replaced with SW for 10% (10SW), 20% (20SW), 30% (30SW) and 50% (50SW) of the FM. The completely randomized design was conducted in outdoor 15 floating baskets (1.5 × 1.5 × 2 m), comprising three replications with 50 fish each, over an 8 month trial. At the end of the experiment, no differences in survival, growth performance or feed utilization were observed across the dietary treatments (p > 0.05). A significant change in lipase-specific activity was caused by the replacement, without changes to trypsin, chymotrypsin or amylase activities. The fish in all dietary groups exhibited normal liver histopathology, but the fish fed a diet containing SW showed higher numbers of cells accumulating lipids as compared to fish fed the baseline 0SW dietary treatment. Hematological parameters were similar across the five dietary groups. Only fish fed the 20SW diet had superior carcass quality compared to the baseline 0SW group, in terms of crude protein and lipids, but lower or higher replacement levels had negative effects on carcass quality. Findings from the current study support the replacement of FM protein with SW at a level of 20% in the diet of sex-reversed Nile tilapia reared to the market stage. Higher replacement levels might be possible with the supplementation of fatty acids.
1. Introduction
Nile tilapia (Oreochromis niloticus) is among the most important fish species in economies around the world. The production of tilapia (all species) increased from 2.5 million tonnes in 2010 to 4.2 million tonnes in 2016 [1]. Despite the high demand for these fish, their rearing is negatively impacted not only by the escape of this highly invasive fish into natural waters but also by fluctuations in the cost of feedstuffs used in their diet preparation. Fish meal (FM) is the most important protein source in aquafeed production. Increasing demand for supporting global aquaculture has caused increases in FM prices in the recent years. The replacement of protein from the costly FM with other alternative sources has been optimized in order to reduce the cost of feed production. However, such replacements at suboptimal levels may cause unbalanced amino acids, resulting in low feed utilization efficiency and poor growth [2,3,4,5].
Stick water (SW) or steam condensate is a byproduct from the marine canned seafood industry, sometimes also obtained from the manufacturing of FM and fish oil. It is prepared by cooking raw materials with steam, pressing them to squeeze out moisture and then evaporating the liquid extracts to obtain viscous, fast-decomposing and evil-smelling liquor. Generally, SW contains large amounts of protein, lipid and ash [6,7]. In applications to aquaculture, SW has been used as an attractant in aquafeed [8]. Only limited information is available on the use of SW in aquafeed, though it use could confer benefits by providing cost-effective nutrition to aquaculture as well as reducing waste effluents [7,9]. However, the replacement of protein from FM with SW may have negative effects on feed utilization due to the difference in amino acid profiles [7] or possibly due to the unsuitable composition of fatty acids.
In order to assess the performance of aquafeed with replaced ingredients, the nutritional responses through digestive enzyme activities have been reported in several prior studies [5,6,7,10,11,12,13]. In the current study, the optimal replacement of protein from FM with SW in the diet of Nile tilapia was investigated. Mono-sex males were used since they provide desirable characteristics for culture such as fast growth and high fillet quality, relative to mixed-sex or all-female fish [14,15]. An 8 month trial was conducted in floating baskets, mimicking the market stage Nile tilapia production in Thailand. Carcass composition is an important criterion in the evaluation of the quality of consumed fish. Long-term side effects were also investigated by hematological parameters and liver histopathology. Findings from the current study suggest an optimal protein replacement of FM with raw SW without any supplementation.
2. Materials and Methods
All feedstuffs were purchased from a private sector in the province of Phatthalung, Thailand. Raw SW was obtained from a canned seafood factory (Kuang Pei San Food Products Public CO., Ltd., Trang, Thailand). This byproduct is a condensate from the steam conditioning of mackerels (including Decapterus macarellus, Rastrelliger brachysoma, R. faughni, R. kanagurta and Scomber australasicus), which is then boiled until constant total soluble solids are obtained (57–60°Bx and ≤400 g kg−1 moisture content). The chemical compositions of the main feedstuffs, including FM, SW, soybean meal (SBM), rice bran, corn meal and broken rice, are shown in Table 1, while the fatty acid composition of the SW is shown in Table 2. The four experimental diets were designed to partly replace the FM protein with SW at levels of 10% (10SW), 20% (20SW), 30% (30SW) and 50% (50SW) (Table 3). The FM-based diet (0SW) was used as a control. All solid feedstuffs were ground, sieved, weighed and then mixed for 10 min. Soybean oil, fish oil and vitamin-mineral premixes were added, followed by 300 g kg−1 water, to obtain a dough-like consistency. The experimental diets (2–4 mm die diameter) were produced after pelleting through a meat mincer. The pellets were then dried at 60 °C for 12 h, cut and sieved. The pellets were packed in polyethylene bags, sealed and stored in a refrigerator (4 °C) until they were used in feeding.

Table 1.
Proximate compositions (g kg−1 on fresh weight) of the main feedstuffs in the experimental diets. The analyses were performed in triplicate.

Table 2.
Fatty acid contents in SW (g kg−1 dry weight). The values are averaged from triplicate determinations.

Table 3.
Ingredients and proximate compositions (g kg−1 of fresh weight) of the experimental diets used for rearing sex-reversed Nile tilapia.
The proximate chemical compositions of the main feedstuffs and all experimental diets were analyzed according to standard methods of AOAC [16]. Nitrogen-free extract (NFE, g kg−1) and gross energy (GE, kcal kg−1) were calculated from 1000−(CP + crude lipid + crude ash + crude fiber) and (CP × 5.6) + (crude lipid × 9.44) + (crude fiber × 4.1) + (NFE × 4.1), respectively. For the SW component, its fatty acid profile was also determined. The fatty acid methyl esters were prepared using BF3 in methanol, and the fatty acid profile was analyzed using a gas chromatograph (Agilent 6890; Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a flame ionization detector. The split injection temperature was set at 270 °C. The detector temperature was maintained at 250 °C, the hydrogen flow at 40 mL min−1 and the air flow at 450 mL min−1. High-purity helium was used as the carrier gas at 2 mL min−1. Menhaden oil methyl esters (National Marine Fisheries Service, Seattle, WA, USA) were used as internal standards.
Seed production of Nile tilapia was conducted at Phatthalung Inland Fisheries Research and Development Center, Phatthalung, Thailand. The fish fry were fed the diet containing 40 mg kg−1 17-α-methyltestosterone for three weeks to induce mono-sex males, ≥99% based on acetocarmine staining [17]. After that, sex-reversed fish were transported to the Department of Fisheries Technology, Faculty of Sciences and Fisheries Technology, Rajamangala University of Technology Srivijaya, Trang, Thailand, where they were acclimatized in a cement pond (1 × 4 × 1 m) for 10 days. They were fed to satiation with the control diet (0SW) at 08.00 and 16.00 h daily under natural light conditions (lights on 06.00 to 18.00 h). Subsequently, fish with similar weight (18.49 ± 0.31 g initial body weight) were distributed into 15 floating baskets (1.5 × 1.5 × 2 m) in a 1600 m2 pond. The baskets were covered by 1 inch black straight seine during the first three months, and these were changed to 1.2 inch size seine until the end of the experiment. Each treatment included a total of 150 fish for each replication. The experiment was conducted for 8 months. The fish were fed ad libitum, and the uneaten feed was collected 1 h after feeding, dried at 60 °C until constant weight and the determined weight was used to calculate the feed intake (FI), the feed conversion ratio (FCR) and the protein efficiency ratio (PER). The measured growth and the feed utilization were summarized monthly. At the end of the experiment, all fish were starved for 24 h prior to measuring body weight (BW) and length, followed by collecting samples of whole carcass, intestine and blood. All uses of animals in the current study conformed to the “Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes”, National Research Council, Thailand (Application No. U1-06514-2560).
Growth and feed utilization parameters of reared fish were calculated as follows:
Survival (%) = 100 × [final fish number/initial fish number],
Condition factor (CF, g cm−3) = 100 × [live BW (g)/total body length (cm)3],
Stomasomatic index (SSI, %) = 100 × [wet weight of stomach (g)/wet BW (g)],
Intestosomatic index (ISI, %) = 100 × [wet weight of intestine (g)/wet BW (g)],
Specific growth rate (SGR, % BW day−1) = 100 × [(lnWt − lnW0)/(t − t0)],
where Wt = mean weight (g) at day t and W0 = mean weight (g) at day t0.
FI (g day−1) = F/(W0 + W1/2)(N0 + N1/2)t,
where F = dry feed fed (g), W0 = average initial BW (g),
W1 = average final BW (g), N0 = initial fish number, N1 = final fish number and t = rearing period (day).
FCR (g feed g gain−1) = dry feed consumed (g)/wet weight gain (g),
PER (g gain g protein−1) = wet weight gain (g)/protein intake (g).
The quality of water was measured every other week. The temperature, pH, total alkalinity and total ammonia were determined according to standard methods of APHA, AWWA and WPCF [18]. Nitrite was determined according to the method of Strickland and Parsons [19]. Dissolved oxygen was determined using a Multiparameter Display System (YSI 650MDS, YSI Incorporated, Yellow Springs, OH, USA). The water quality parameters during the experiment were in the ranges of 28.91 ± 0.30 °C, pH 7.15 ± 0.08, 6.72 ± 0.19 mg L−1 dissolved oxygen, 98.95 ± 1.25 mg L−1 alkalinity, 0.41 ± 0.02 mg L−1 ammonia and 0.24 ± 0.02 mg L−1 nitrite.
The fish were anesthetized with tricainemethanesulfonate (MS-222). The blood samples were collected (n = 3 per replication) with a 2 mL syringe from the caudal vessel. Plasma protein was determined according to the Lowry method [20]. Red (RBC) and white (WBC) blood cell counts from diluted samples were determined [21]. Hemoglobin (Hb) and hematocrit (Hct) were assayed as described in the work of Larsen and Snieszko [22]. Blood indices in terms of mean cell volume (MCV), mean cell hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC) were calculated as follows [23]:
MCV (mm3) = [Hct (%)/RBC (×106 µL)] × 10,
MCH (pg cell−1) = [Hb (g dL−1)/RBC (×106 µL)] × 10,
MCHC (g L−1) = Hb (g dL−1)/Hct (%).
The liver samples (n = 3 per replication) were gently rinsed with cold water, fixed in 10% phosphate-buffer formalin, dehydrated by standard procedures, embedded in paraffin, sliced to sections with a rotary microtome (Shandon AS325 Retraction; Life Sciences International, Cheshire, UK) and stained with hematoxylin and eosin (H&E). Histopathological examination was conducted under a light microscope at 400× magnification.
The intestine with food content (n = 3 per replication) was removed onto ice, dissected and then homogenized in 0.2 M Na2HPO4-NaH2PO4 buffer (pH 8, 1:3 w/v). Centrifugation of tissue homogenate was performed at 15,000× g, at 4 °C for 30 min to obtain the supernatant. This crude enzyme extract was kept in aliquots at −20 °C until use. The protein concentration of a crude enzyme extract was determined using the Lowry method [20]. Bovine serum albumin (BSA) was used to construct the standard curve. The activities of trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) were assayed using N-benzoyl-L-Arg-p-nitroanilide (BAPNA) and N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (SAPNA) as the substrates, respectively [24]. Amylase activity (EC 3.2.1.1) was assayed using starch soluble as the substrate [25]. Lipase activity (EC 3.1.1.3) was assayed using p-nitrophenyl palmitate as a substrate [26].The measured products of these four enzymes were referenced to the concentrations of p-nitroanilide (A410), p-nitroanilide (A410), maltose (A540) and p-nitrophenol (A410), respectively, to quantify their activities.
The whole bodies (n = 3 per replication), excluding the intestine, were minced and used to determine the proximate chemical compositions. The moisture, CP, crude lipid and ash were analyzed according to standard methods of AOAC [16].
Fifteen experimental units were set under a completely randomized design (five treatments × three replications). All the data are herein expressed as the mean ± standard error of the mean (SEM). All statistical evaluations were made using SPSS Version 14 (SPSS Inc., Chicago, IL, USA). Variables that are percentages were subjected to arcsine transformation. One-way ANOVA was used to check differences between the treatments and then Duncan’s multiple range test was used to confirm significant differences. The significance criterion was set at p < 0.05 in all statistical analyses.
3. Results
3.1. Fatty Acid Profile of SW and Chemical Composition of the Experimental Diets
The raw material, on a dry weight basis, contained 48.31 g kg−1 saturated fatty acids (SFA), 9.62 g kg−1 monounsaturated fatty acids (MUFA), 65.84 g kg−1 polyunsaturated fatty acids (PUFA), 40.60 g kg−1 long chain-PUFA (LC-PUFA), 39.28 g kg−1 n–3 and 25.18 g kg−1 n–6, giving the n–3:n–6 ratio 1.56 (Table 2). All the experimental diets were similar in proximate chemical compositions (Table 3). The experimental diets were mutually isonitrogenous, isolipidic and isoenergetic.
3.2. Survival, Growth and Feed Consumption
Survival (97% on average) of reared fish did not differ across all the dietary treatments (p > 0.05, Table 4). The performances in terms of final BW, total length, CF, SSI, ISI, SGR, FI, FCR and PER were also similar across all treatments.

Table 4.
Survival, growth performance and feed utilization of sex-reversed Nile tilapia fed experimental diets containing various levels of FM protein replaced with SW.
3.3. Hematological Parameters
At the end of the experiment, the levels of plasma protein (8.09 ± 0.20 g% on average), RBC (2.08 ± 0.09 × 106 cells µL−1 on average), WBC (2.31 ± 0.05 × 104 cells µL−1 on average), Hb (6.77 ± 0.12 g dL−1 on average), Hct (27.07 ± 0.49% on average), MCV (131.09 ± 5.77 mm3 on average), MCH (32.78 ± 1.44 pg cell−1 on average) and MCHC (250.04 ± 0.04 g L−1 on average) did not differ across the five dietary treatments.
3.4. Liver Histoarchitecture
Fish from all treatments exhibited normal-shaped hepatocytes with regular gross morphology and clearly located cell nuclei (Figure 1). Compared to the control FM-based diet (Figure 1a), the number of cells accumulating lipids (blue arrows) appeared to increase in density with the increasing replacement level of FM protein by SW (Figure 1b–e). However, there were no signs of any kind of necrosis or inflammation in the liver histoarchitecture with any of the alternative dietary treatments.
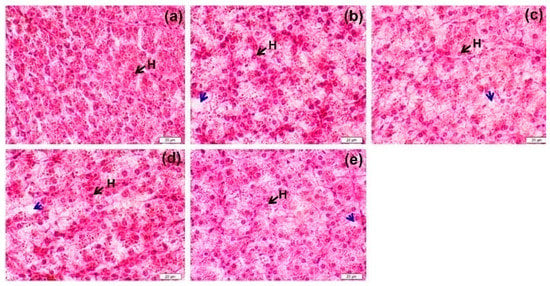
Figure 1.
The liver histoarchitecture (longitudinal section) for sex-reversed Nile tilapia fed 0SW (a), 10SW (b), 20SW (c), 30SW (d) and 50SW (e) for 8 months. Tissues were stained with hematoxylin and eosin (H&E) and the images were photographed at 400× magnification. H = hepatocyte, blue arrows = cells accumulating lipids.
3.5. Digestive Enzyme-Specific Activities
There were no effects of protein replacement in FM by SW on the specific activities of trypsin (Figure 2a), chymotrypsin (Figure 2b) or amylase (Figure 2c). The fish fed the 50SW diet had the highest lipase-specific activity, while the remaining treatments were mutually rather similar, with a significant difference only between the 10SW and the 30SW groups (Figure 2d).

Figure 2.
The specific activities of trypsin (a), chymotrypsin (b), amylase (c) and lipase (d) in sex-reversed Nile tilapia fed experimental diets containing various levels of FM protein replaced with SW. The data are the mean ± SEM (n = 3 per replication). Different superscripts are significantly different between groups (p < 0.05).
3.6. Carcass Composition
The moisture and ash contents were unaffected by the alternative dietary treatments (Table 5). The CP was lowest in the fish fed the 30SW diet, while the other treatments gave mutually similar levels. The significantly decreased lipid content was found in fish fed the 10SW and 50SW diets relative to control FM-based diet.

Table 5.
Carcass proximate composition (g kg−1 wet weight) of the sex-reversed Nile tilapia fed experimental diets containing various replacement levels of FM protein by SW.
4. Discussion
The water consumption by the precooking step in a canned seafood factory is about 0.5 m3 tonne−1 of fish raw material [27]. The generated wastewater is then evaporated to obtain a viscous, fast-decomposing and evil-smelling liquor, SW. Although SW contains large amounts of moisture, it is relatively high in CP, crude lipid and ash, while the crude fiber and NFE contents are very low [7,8]. The contents of essential amino acids (EAA) in SW are inferior as compared to FM, except for histidine [7], leading to different ratios of EAA to non-essential amino acids. Also, fatty acids are present in relatively lower amounts in SW compared to FM [28]. This deficiency in amino acids and fatty acids might not satisfy the nutritional requirements of fish, so the optimization of the inclusion level, or alternatively supplementation with synthetic nutrient sources, is necessary.
All the water quality parameters in the current study were within the standards for tilapia aquaculture [29]. The various replacement levels of protein in FM by SW had no effect on growth or feed consumption. The SGR (1.03% BW day−1 on average) may seem slightly low and the FCR (2.68 g feed g gain−1 on average) slightly high in the current study, relative to typical values reported. This is due to the current trial being long-term, with the larger fish having a higher feed consumption than the smaller fish. Generally, FM protein can be replaced by SW at levels of 40–50% in aquafeed, and in a 350–400 g kg−1 CP diet for giant freshwater prawn and striped snakehead [6,7]. Based on the survival, growth performance or feed utilization of reared fish in the current study, SW could replace FM protein up to the maximum value tested (50SW in a 300 g kg−1 CP diet).
Hematological examination could be used to trace the physiological or pathological changes of reared fish [30,31]. For Nile tilapia, there were no differences in the observed hematological parameters across the five dietary treatments, in contrast to changes in hematological parameters in fishes fed diets containing SBM-derived protein [4,5]. The observations following SBM inclusion might be caused by anti-nutritional compounds, such as phytohemagglutinins, affecting blood functionality [32]. The blood profiles observed in the current study are in agreement with the stable liver histoarchitectures observed, when fish fed diets containing SW were compared to the 0SW group. Similarly, a high replacement level of FM protein by SW can induce the accumulation of lipids in cells [6], while it does not affect body morphometry (CF) or visceral organ indices (SSI and ISI). This phenomenon is in agreement with use of animal protein blend instead of FM [3]. Since there were no changes in hematological parameters or liver histoarchitecture and survival was unaffected, this indicates a lack of toxic dietary effects from the protein replacement levels of the current study, during the long-term trial over 8months.
Trypsin and chymotrypsin are the main protein-digesting enzymes in the intestine of fish. Only trypsin can activate itself as well as other zymogens, such as chymotrypsinogen to chymotrypsin, contributing 40–50% of the overall protein digestion [33]. The activities of both these enzymes are important in the physiological responses to feed protein stressors [34]. Therefore, the stable activities of both these enzymes indicate unchanged protein digestion in the intestine, while the PER indicates steady protein utilization. Thus, tilapia appear to have the capacity to adjust their digestive proteases to a range of SW inclusion levels. Similar observations were reported when the protein in FM was replaced by SW in the range of 10–60% in the diet of freshwater prawns [7].
Carbohydrate utilization by aquatic animals has been widely studied by assessing the activity of α-amylase. This enzyme activity can be changed according to diet replacement in some aquaculture animals [11,12]. The finding of no difference in amylase activity between the treatments in the current study is in agreement with observations of hybrid tilapia fed various levels of SBM as a replacement of FM protein [10]. This suggests that tilapia may have the capacity to utilize carbohydrates in response to changes of inclusion level in the diet.
Lipase-specific activity increased linearly with the protein replacement level, suggesting that the low levels of digestible protein in the SW diet lead to protein sparing [35]. This assumption is based on three observations: the high CP and lipid contents in the SW diet; comparable CP but low lipid in fish carcasses that were fed a higher dose of SW; and lipase-specific activity.
No negative effect on carcass composition was observed only in tilapia fed 20SW, compared to the 0SW dietary baseline, while higher replacement levels exhibited significant effects on either CP or lipid levels. These findings match well with the significantly reduced accumulation of lipids in the carcass of striped snakehead that were fed diets with some protein replacement levels of FM by SW (20% and 40% CP) [6]. The optimal protein replacement level in the diet for tilapia (20SW in ca. 300 g kg−1 CP diet), based on the overall observations in the current study, is clearly below those reported in prior studies. The omnivorous feeding habit of Nile tilapia appears to affect its ability to utilize the nutrients in SW, with results different from the carnivorous striped snakehead. The findings from lipase-specific activity and the composition of carcass lipids in the current study indicate unbalanced lipid composition profiles, especially in terms of the fatty acids, due to the protein replacement of FM by SW. The supplementation of some select fatty acids with increasing protein replacement levels should be further investigated, even a few months prior to the catch, in order to improve the carcass compositions. In addition, from an economic viewpoint, the current prices of SW are very inexpensive in comparison to FM (0.45 vs. 0.84 USD kg−1); therefore, the 20SW diet (1.58 USD kg fish−1) compares well against the 0SW baseline diet (1.68 USD kg fish−1). This aspect supports the use of the low cost agro-industrial byproduct SW in the diet for sex-reversed Nile tilapia.
5. Conclusions
The replacement of FM protein by SW from 10 to 50% gave similar results in terms of the survival, growth and feed utilization of Nile tilapia, and had no negative effect on hematological or histopathological examinations over an 8 month dietary treatment at the market stage. Specific activities of trypsin, chymotrypsin and amylase indicated no change in the catabolism of protein or carbohydrate within this replacement range. However, changes in the specific activity of lipase and the carcass lipid and carcass protein contents significantly depended the replacement level. Based on the overall results, the 20SW diet was near optimal, maintaining carcass quality relative to the 0SW baseline diet. Findings from the current study suggest that SW is a suitable alternative ingredient for sex-reversed Nile tilapia. The pursuit of higher replacement levels while maintaining the carcass quality of reared fish could be a topic for further study. Additionally, the development of containment measures or escapement control to prevent this invasive fish from entering natural waters should be of interest.
Author Contributions
Conceptualization, U.W., W.W. and K.T.; methodology, U.W., W.W. and K.T.; investigation and laboratory analysis, U.W., W.W. and K.T.; resources, U.W. and W.W.; data curation, W.W. and K.T.; writing—original draft preparation, K.T.; writing—review and editing, U.W., W.W. and K.T.; supervision, K.T.; project administration, W.W. and K.T.; funding acquisition, W.W.
Funding
This research was funded by the National Research Council of Thailand, grant number 2556NRCT53485.
Acknowledgments
We acknowledge Seppo Karrila and the Publication Clinic, Research and Development Office, Prince of Songkla University, for advice on manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. Available online: http://www.fao.org/fishery/culturedspecies/Oreochromis_niloticus/en (accessed on 1 June 2019).
- Ai, Q.; Mai, K.; Tan, B.; Xu, W.; Duan, Q.; Ma, H.; Zhang, L. Replacement of fish meal by meat and bone meal in diets for large yellow croaker, Pseudosciaena crocea. Aquaculture 2006, 260, 255–263. [Google Scholar] [CrossRef]
- Hu, L.; Yun, B.; Xue, M.; Wang, J.; Wu, X.; Zheng, Y.; Han, F. Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus). Aquaculture 2013, 372–375, 52–61. [Google Scholar] [CrossRef]
- Haghbayan, S.; Mehrgan, M.S. The effect of replacing fish meal in the diet with enzyme-treated soybean meal (HP310) on growth and body composition of rainbow trout fry. Molecules 2015, 20, 21058–21066. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Mozanzadeh, M.T.; Marammazi, J.G.; Safari, O.; Gisbert, E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar] [CrossRef]
- Wattanakul, W.; Wattanakul, U.; Thongprajukaew, K.; Muenpo, C. Fish condensate as effective replacer of fish meal protein in diet for striped snakehead, Channa striata (Bloch). Fish Physiol. Biochem. 2017, 43, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wattanakul, W.; Wattanakul, U.; Thongprajukaew, K.; Muenpo, C. Optimal protein replacement of fish meal by mackerel condensate in diet for giant freshwater prawn (Macrobrachium rosenbergii). Aquac. Res. 2017, 48, 697–710. [Google Scholar] [CrossRef]
- Somboon, S.; Semachai, W. Using Fish Soluble Sludge as Feed Flavored in Hybrid Catfish (Clarias macrocephalus × C. gariepinus) Diets. In Proceedings of the 42nd Kasetsart University Annual Conference, Bangkok, Thailand, 3–6 February 2004. [Google Scholar]
- Is-Haak, J.; Koydon, S. Utilization of precooking concentrated from production of tuna canning factory to replace fish meal for protein source in feeds on white meat Pangasius culture. Rajamangala Univ. Technol. Thanyaburi. J. 2010, 14, 65–71. [Google Scholar]
- Lin, S.; Luo, L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia. Oreochromis niloticus × O. aureus. Anim. Feed Sci. Technol. 2011, 168, 80–87. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Wang, C.A.; Zhao, Z.G.; Luo, L. Effects of replacement of fish meal by soy protein isolate on the growth, digestive enzyme activity and serum biochemical parameters for juvenile Amur sturgeon (Acipenser schrenckii). Asian Australas. J. Anim. Sci. 2012, 25, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.S.S.; Kohli, M.P.S.; Kumar, S.; Sundaray, J.K.; Roy, S.D.; Venkateshwarlu, G.; Sinha, A.; Pailan, G.H. Effect of dietary supplementation of biofloc on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 2014, 418–419,, 108–115. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Belal, I.E.H.; Seenivasan, C.; Muralisankar, T.; Bhavan, P.S. Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquac. Rep. 2016, 3, 35–44. [Google Scholar] [CrossRef]
- Damien, D.; Eddy, G.; Marie, C.H.; Charles, M.; Pierre, B.; Jean, F.B. Production of a high percentage of male offspring with a natural androgen, 11h-hydroxyandrostenedione (11hOHA4), in Florida red tilapia. Aquaculture 2003, 216, 55–65. [Google Scholar]
- Little, D.C.; Edwards, P. Impact of nutrition and season on pond culture performance of mono-sex and mixed-sex Nile tilapia (Oreochromis niloticus). Aquaculture 2004, 232, 279–292. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 69–90. [Google Scholar]
- Yenpoeng, T.; Wattanamahard, T.; Chainon, J.; Kanhasura, S. Seed Production of Sex Reversal of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758), in Fiberglass Tanks Using Water Circulation System at Different Stocking Densities; Mahasarakham Inland Fisheries Research and Development Bureau: Mahasarakham, Thailand, 2011; pp. 4–6. [Google Scholar]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Pollution Control Federation: Richmond, VA, USA, 1998; pp. 2.24–4.159. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; pp. 77–80. [Google Scholar]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Blaxhall, P.C.; Daisley, K.W. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Larsen, H.N.; Snieszko, S.F. Comparison of various methods of determination of haemoglobin in trout blood. Prog. Fish Cult. 1961, 23, 8–17. [Google Scholar] [CrossRef]
- Dacie, J.V.; Lewis, S.M. Practical Haematology. In Practical Haematology, 9th ed.; Lewis, S.M., Bain, B.J., Bates, I., Eds.; Churchill Livingstone: London, UK, 2001; pp. 444–451. [Google Scholar]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbo, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef]
- Areekijseree, M.; Engkagul, A.; Kovitvadhi, U.; Thongpan, A.; Mingmuang, M.; Pakkong, P.; Rungruangsak-Torrissen, K. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 2004, 234, 575–587. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar]
- Uttamangkabovorn, M.; Prasertsan, P.; Kittikun, A.H. Water conservation in canned tuna (pet food) plant in Thailand. J. Clean. Prod. 2005, 13, 547–555. [Google Scholar] [CrossRef]
- Gümüş, E. Fatty acid composition of fry mirror carp (Cyprinus carpio) fed graded levels of sand smelt (Atherinaboyeri) meal. Asian-Australas. J. Anim. Sci. 2011, 24, 264–271. [Google Scholar]
- Sansuwan, K.; Kovitvadhi, S.; Thongprajukaew, K.; Ozório, R.O.A.; Somsueb, P.; Kovitvadhi, U. Microwave irradiation and pelleting method affected feed chemical composition, and growth performance and feed utilization of sex-reversed Nile tilapia, Oreochromis niloticus (L.). Aquac. Res. 2017, 48, 1836–1848. [Google Scholar] [CrossRef]
- De Pedro, N.; Guijarro, A.E.; Lopez-Patino, M.A.; Marinez-Alvarez, R.; Delgado, M. Daily and seasonal variations in haematological and blood biochemical parameters in the tench, Tinca tinca. Aquac. Res. 2005, 36, 85–96. [Google Scholar] [CrossRef]
- Kikuchi, K.; Furuta, T.; Honda, H. Utilization of soybean meal as a protein source in the diet of juvenile Japanese flounder. Aquac. Sci. 1994, 42, 601–604. [Google Scholar]
- Sindhu, A.A.; Khan, M.A.; Nisa, M.U.; Sarwar, M. Agro-industrial by-products as a potential source of livestock feed. Int. J. Agric. Biol. 2002, 4, 307–310. [Google Scholar]
- Eshel, A.; Lindner, P.; Smirnoff, P.; Newton, S.; Harpaz, S. Comparative study of proteolytic enzymes in the digestive tracts of the European sea bass and hybrid striped bass reared in freshwater. Comp. Biochem. Physiol. 1993, 106, 627–634. [Google Scholar] [CrossRef]
- Córdova-Murueta, J.H.; García-Carreño, F.L.; Navarrete-del-Toro, M.A. Effects of stressors on shrimp digestive enzymes from assays of feces: An alternate method of evaluation. Aquaculture 2004, 233, 439–449. [Google Scholar] [CrossRef]
- Bureau, D.P. Factors affecting metabolic waste outputs in fish. In Proceedings of the Avances en Nutricion Acuicola VII. Memorias del VII Simposium Internacional de Nutricion Acuicola, 16–19 November 2004; Cruz Suarez, L.E., Ricque Marie, D., Nieto Lopez, M.G., Villarreal, D., Scholz, U., Gonzalez, M., Eds.; Universidad de Sonora: Hermosillo, Mexico, 2004; pp. 2–7. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).