Large Terrestrial Bird Adapting Behavior in an Urbanized Zone
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
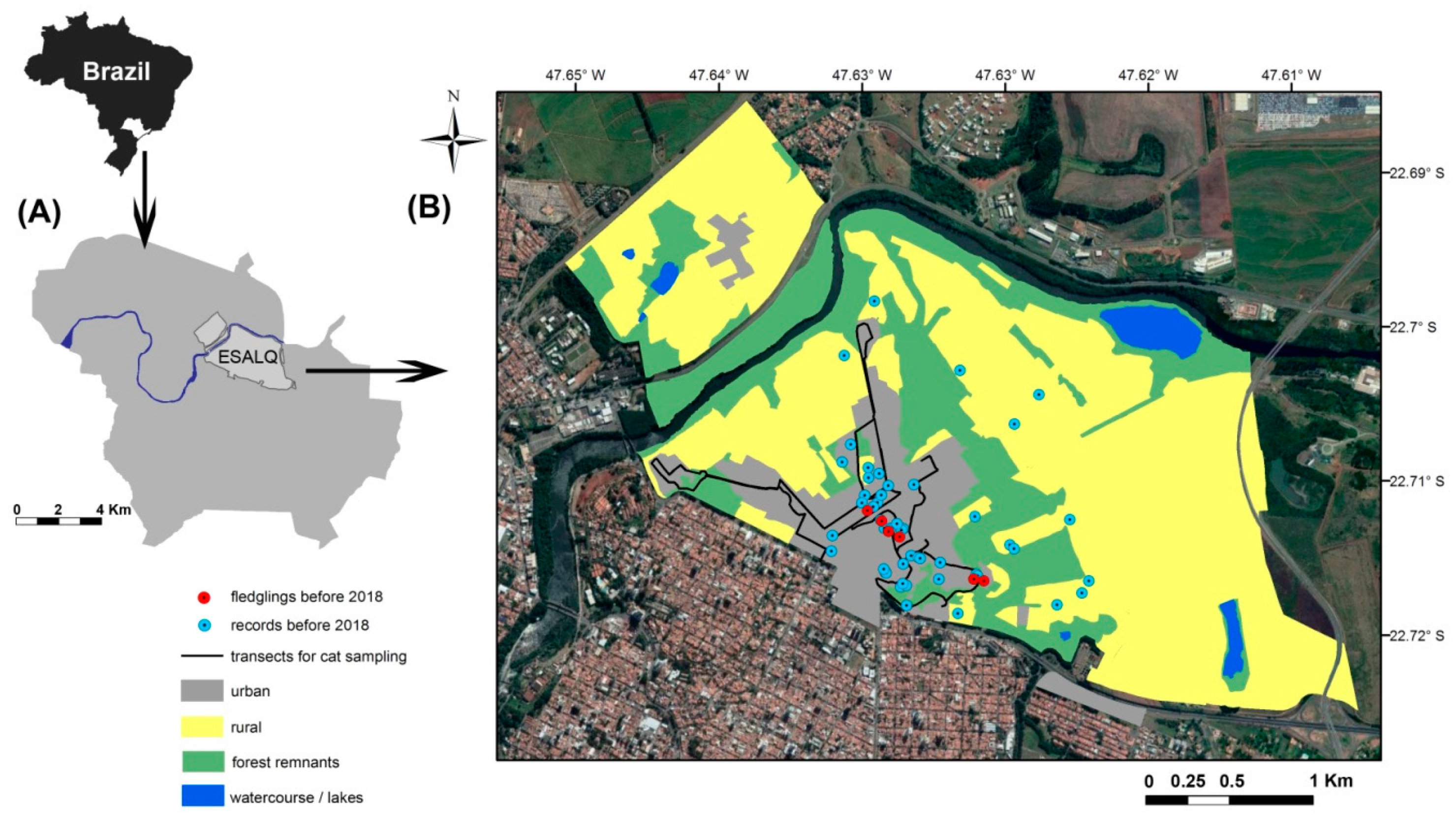
2.1. Study Area
2.2. Domestic Cat Sampling
2.3. Historical Records of Red-legged Seriema at Luiz de Queiroz Campus
2.4. Monitoring of Red-legged Seriema Individuals
2.5. Data Analysis
2.5.1. Red-legged Seriema Behavior Classification
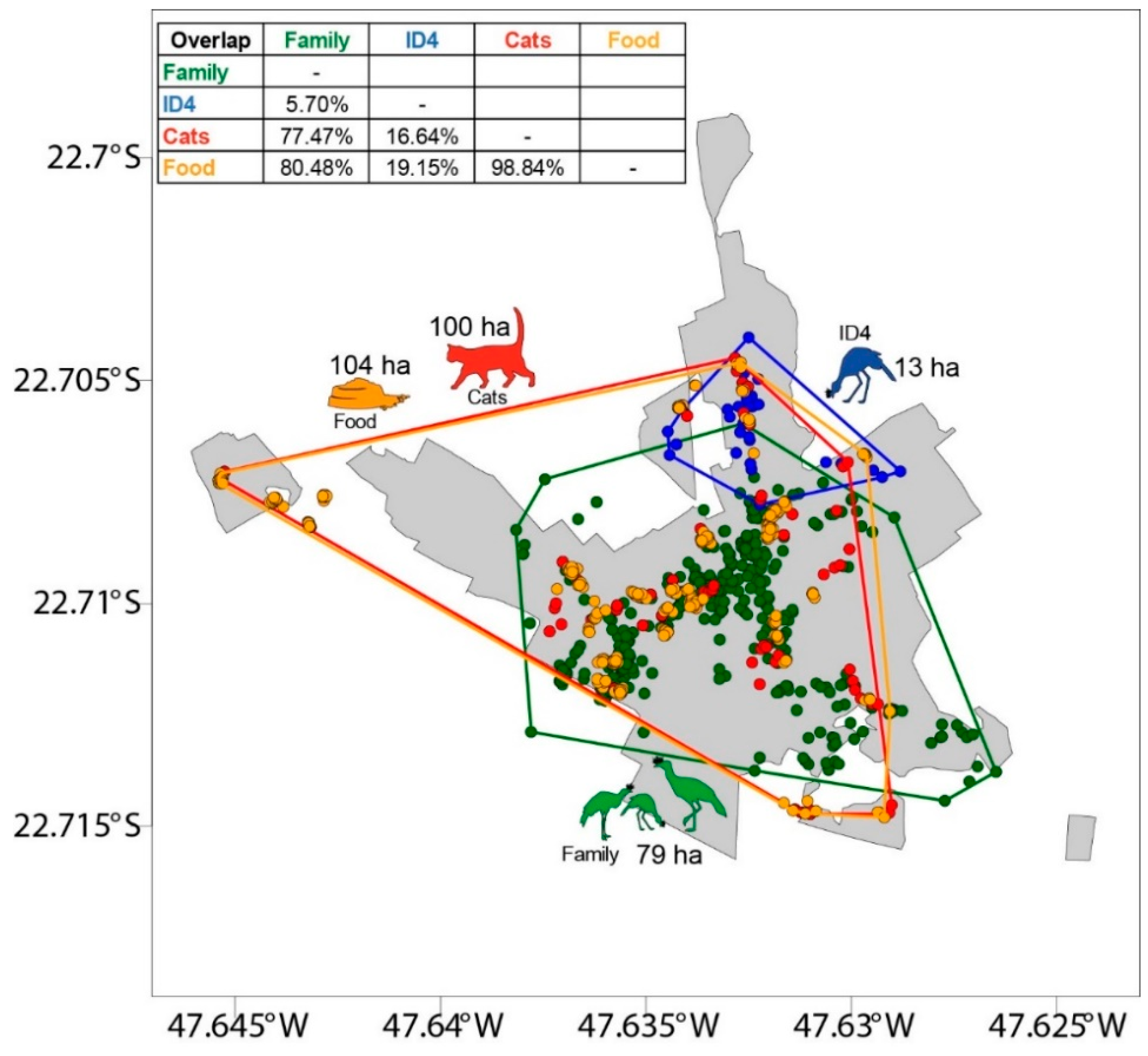
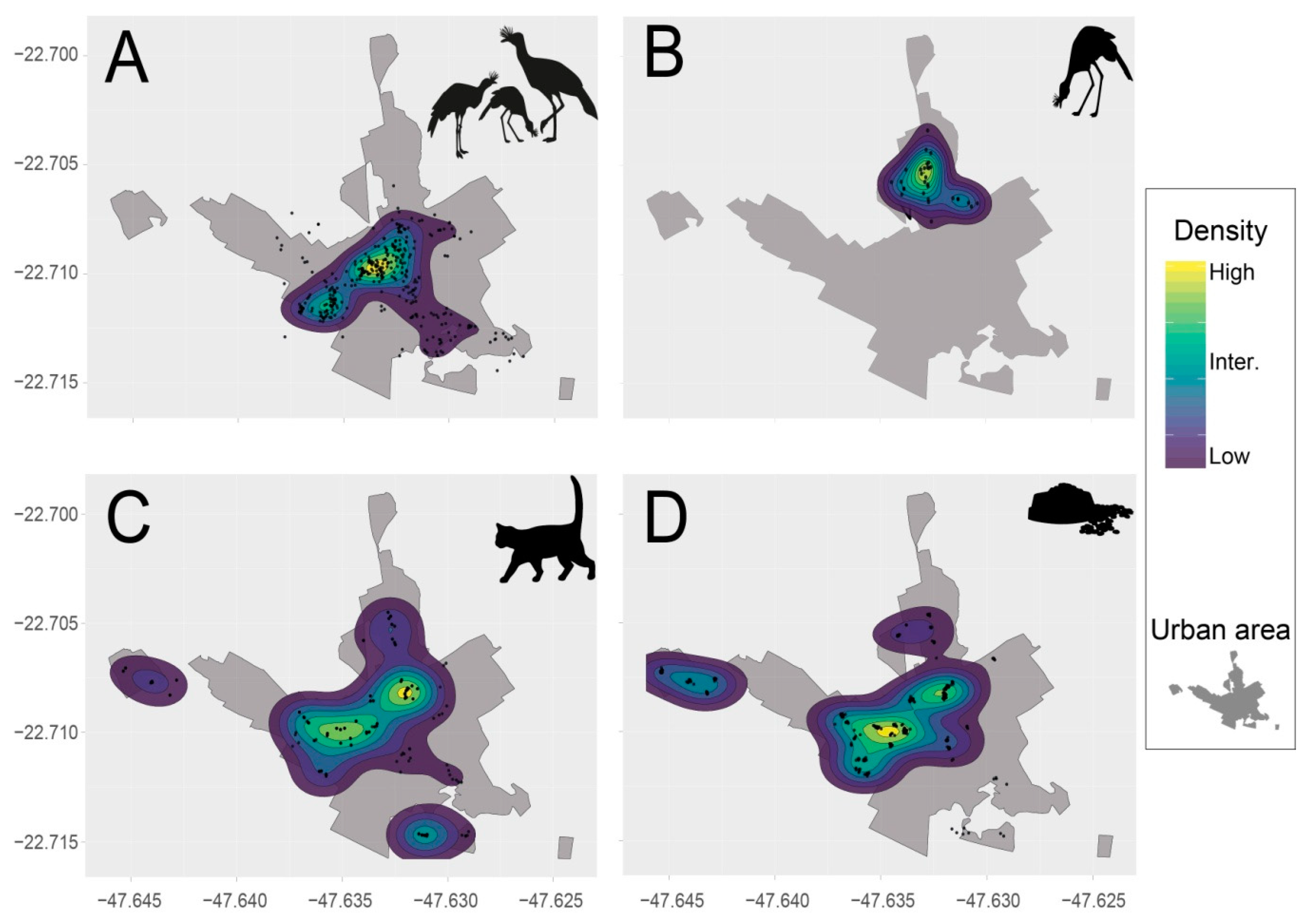
2.5.2. Spatial Analysis: Red-legged Seriema, Cats, and Cat-feeding Points
3. Results
3.1. Red-legged Seriema, Cats, and the Provision of Cat Food
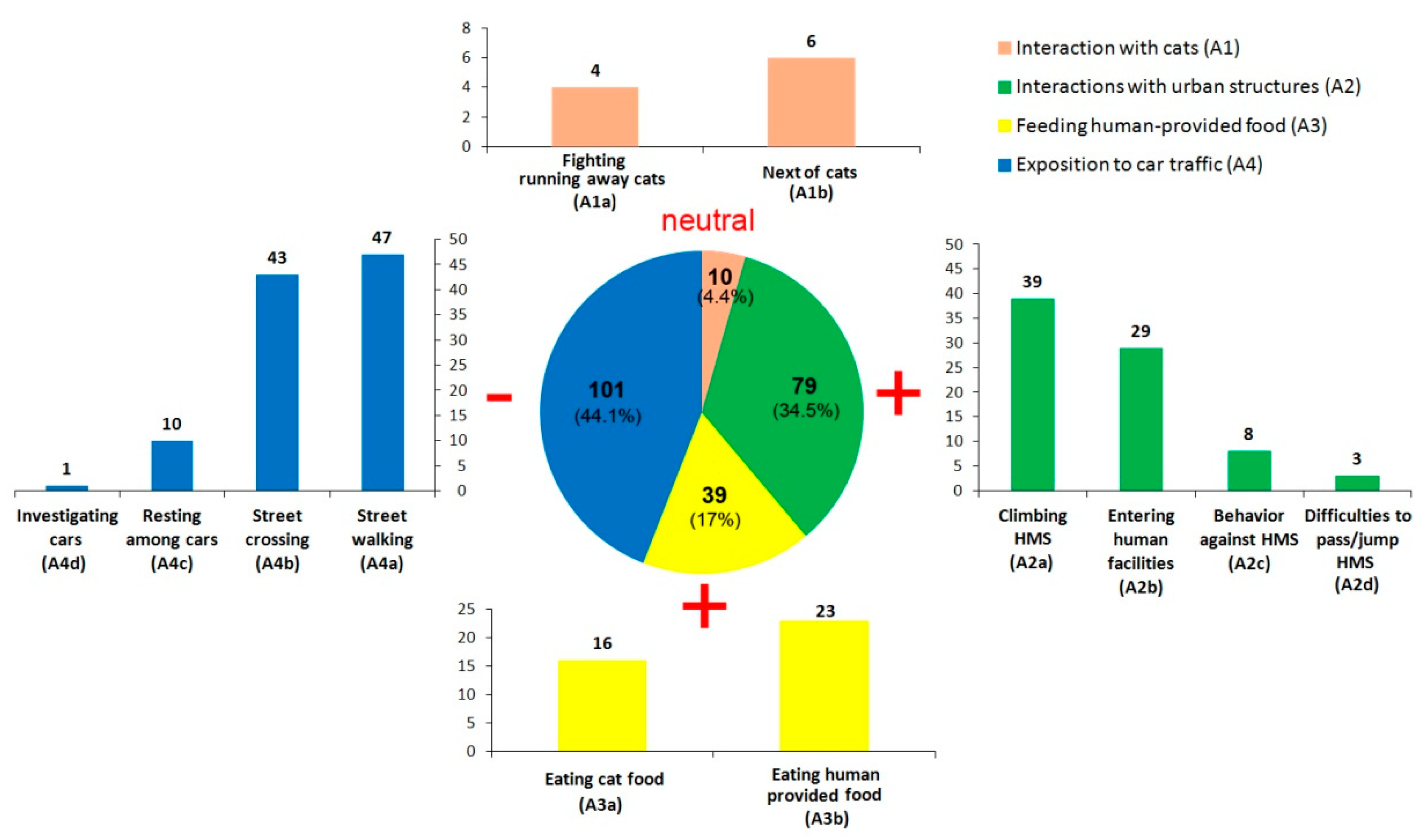
3.2. Red-legged Seriema Behaviors
4. Discussion
4.1. Space Sharing between Red-legged Seriema, Cats, and Humans
4.2. Red-legged Seriema Adaptation to Urbanization
4.3. Red-legged Seriema and Free-ranging Cats—Future Conflicts?
4.4. Individual Bird Monitoring Based on Citizen Science in an Urban Ecosystem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, O.L. The Ecology of Urban Habitats, 1st ed.; Chapman and Hall: New York, NY, USA, 1989. [Google Scholar]
- Adams, L.W. Urban Wildlife Habitats: A Landscape Perspective, 3nd ed.; University of Minnesota Press: Minneapolis, MN, USA, 1994. [Google Scholar]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Ewing, K. Restoration of fragmented landscapes for the conservation of birds: A general framework and specific recommendations for urbanizing landscapes. Restor. Ecol. 2001, 9, 280–292. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, biodiversity and conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Marchini, S.; Ferraz, K.M.P.M.B.; Zimmermann, A.; Guimarães-Luiz, T.; Morato, R.; Correa, P.L.P.; McDonald, D.W. Planning for coexistence in a complex human-dominated world. In Human-Wildlife Interactions: Turning Conflict into Coexistence; Frank, B., Glikman, J.A., Marchini, S., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 414–438. [Google Scholar]
- Chace, J.F.; Walsh, J.J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Alexandrino, E.R.; Bovo, A.A.A.; Luz, D.T.A.; Costa, J.C.; Betini, G.S.; Ferraz, K.M.P.M.B.; Couto, H.T.Z. Aves do campus “Luiz de Queiroz” (Piracicaba, SP) da Universidade de São Paulo: Mais de 10 anos de observações neste ambiente antrópico. Atual. Ornitol. 2013, 173, 40–52. [Google Scholar]
- Bovo, A.A.A.; Magioli, M.; Percequillo, A.R.; Kruszynski, C.; Alberici, V.; Mello, M.A.; Correa, L.S.; Gebin, J.C.Z.; Ribeiro, Y.G.G.; Costa, F.B.; et al. Human-modified landscape acts as refuge for mammals in Atlantic Forest. Biota Neotrop. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Williams, P.H.; Burgess, N.D.; Rahbek, C. Flagship species, ecological complementarity and conserving the diversity of mammals and birds in sub-Saharan Africa. In Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2000; Volume 3, pp. 249–260. [Google Scholar]
- Lorimer, J. Nonhuman charisma. Environ. Plann. D 2007, 25, 911–932. [Google Scholar] [CrossRef]
- Orams, M.B. Feeding wildlife as a tourism attraction: A review of issues and impacts. Tourism Manag. 2002, 23, 281–293. [Google Scholar] [CrossRef]
- Reynolds, S.J.; Galbraith, J.A.; Smith, J.A.; Jones, D.N. Garden bird feeding: Insights and prospects from a north-south comparison of this global urban phenomenon. Front. Ecol. Evol. 2017, 5, 1–24. [Google Scholar] [CrossRef]
- Auman, H.J.; Meathrel, C.E.; Richardson, A. Supersize me: Does anthropogenic food change the body condition of Silver Gulls? A comparison between urbanized and remote, non-urbanized areas. Waterbirds 2008, 31, 122–126. [Google Scholar] [CrossRef]
- Chapman, R.; Jones, D.N. Foraging by native and domestic ducks in urban lakes: Behavioural implications of all that bread. Corella 2011, 35, 101–106. [Google Scholar]
- Loss, S.R.; Will, T.; Loss, S.S.; Marra, P.P. Bird-building collisions in the United States: Estimates of annual mortality and species vulnerability. Condor 2014, 116, 8–23. [Google Scholar] [CrossRef]
- Brisque, T.; Campos-Silva, L.A.; Piratelli, A.J. Relationship between bird-of-prey decals and bird-window collisions on a Brazilian university campus. Zoologia 2017, 34, e13729. [Google Scholar] [CrossRef]
- Carvalho, N.C.; Bordignon, M.O.; Shapiro, J.T. Fast and furious: A look at the death of animals on the highway MS-080, southwestern Brazil. Iheringia Ser. Zool. 2014, 10, 43–49. [Google Scholar] [CrossRef]
- Carvalho, C.F.; Iannini Custódio, A.E.; Marçal Júnior, O. Wild vertebrates roadkill aggregations on the BR-050 highway, state of Minas Gerais, Brasil. Biosci. J. 2015, 31, 951–959. [Google Scholar] [CrossRef]
- Magioli, M.; Bovo, A.A.A.; Huijser, M.P.; Abra, F.D.; Miotto, R.A.; Andrade, V.H.V.P.; Nascimento, A.M.; Martins, M.Z.A.; Ferraz, K.M.P.M.B. Short and narrow roads cause substantial impacts on wildlife. Oecologia Aust. 2019, 23, 99–111. [Google Scholar] [CrossRef]
- Campos, C.B.; Esteves, C.F.; Ferraz, K.M.P.M.B.; Crawshaw, P.G.; Verdade, L.M. Diet of free-ranging cats and dogs in a suburban and rural environment, south-eastern Brazil. J. Zool. 2007, 273, 14–20. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1396. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.D.; Aryal, A.; Pawley, M.D.; Ji, W. So much for the city: Urban-rural song variation in a widespread Asiatic songbird. Integr. Zool. 2018, 13, 194–205. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef]
- Sol, D.; Lapiedra, O.; González-Lagos, C. Behavioural adjustments for a life in the city. Anim. Behav. 2013, 85, 1101–1112. [Google Scholar] [CrossRef]
- Miranda, A.C. Mechanisms of behavioural change in urban animals: The role of microevolution and phenotypic plasticity. In Ecology and Conservation of Birds in Urban Environments; Springer: Cham, Switzerland, 2017; pp. 113–132. [Google Scholar]
- Sick, H. Ornitologia Brasileira [Brazilian Ornithology], 2nd ed.; Nova Fronteira: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Cruz, J.L. Photograph Record WA1183847, Cariama cristata (Linnaeus, 1766). 2013. Available online: http://www.wikiaves.com/1183847 (accessed on 5 May 2019).
- Maimone, N.M. Photograph Record WA1550233, Cariama cristata (Linnaeus, 1766). 2014. Available online: http://www.wikiaves.com/1550233 (accessed on 5 May 2019).
- Ortiz, L.F. Photograph Record WA1593694, Cariama cristata (Linnaeus, 1766). 2015. Available online: http://www.wikiaves.com/1593694 (accessed on 5 May 2019).
- Vallese, A.L. Photograph Record WA2833206, Cariama cristata (Linnaeus, 1766). 2017. Available online: http://www.wikiaves.com/2833206 (accessed on 5 May 2019).
- Silva, A.N.; Nunes, R.; Estrela, D.C.; Malafaia, G.; Castro, A.L. Behavioral repertoire of the poorly known Red-legged Seriema, Cariama cristata (Cariamiformes: Cariamidae). Rev. Bras. Ornitol. 2016, 24, 73–79. [Google Scholar]
- IUCN: International Union for Conservation of Nature. Available online: https://www.iucn.org/ (accessed on 10 April 2019).
- Willis, E.O.; Oniki, Y. Aves do Estado de São Paulo, 1st ed.; Editora Divisa: Rio Claro, Brazil, 2003. [Google Scholar]
- Pereira, K.D.L.; Silva, R. Levantamento da avifauna da área urbana de Anápolis, Goiás. Ensaios e Ciência: C. Biológicas, Agrárias e da Saúde 2009, 13, 2009. [Google Scholar]
- González-Lagos, C.; Quesada, J. Stay or leave? Avian behavioral responses to urbanization in Latin America. In Avian Ecology in Latin American Cityscapes; Springer: Cham, Switzerland, 2017; pp. 99–123. [Google Scholar]
- Ferraz, M.S. Piracicaba e sua Escola Agrícola; Typographia Brazil de Rothschild & Cia: São Paulo, Brazil, 1916. [Google Scholar]
- Romero, J.P. 1901–2001 Escola Superior de Agricultura “Luiz de Queiroz”. Notáveis, Docentes e Filhos Nobres: Uma Cronologia de Fatos Relevantes; Agronômica Ceres: São Paulo, Brazil, 2002. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.; Leonardo, J.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Kronka, F.J.; Matsukuma, C.K.; Nalon, M.A. Inventário florestal do estado de São Paulo. In Inventário Florestal do Estado de São Paulo; Instituto Florestal: São Paulo, Brazil, 1993. [Google Scholar]
- Tinbergen, N. On aims and methods of ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- WikiAves. Available online: https://www.wikiaves.com.br/ (accessed on 5 May 2019).
- Alexandrino, E.R.; Navarro, A.B.; Paulete, V.F.; Camolesi, M.; Lima, V.G.R.; Green, A.; Conto, T.; Ferraz, K.M.P.M.B.; Couto, H.T.Z. Challenges in engaging birdwatchers in bird monitoring in a forest patch: Lessons for future citizen science projects in agricultural landscapes. Citizen Sci. Theory Pract. 2019, 4, 1–14. [Google Scholar]
- Braghieri, V. Photograph Record WA3159704, Cariama cristata (Linnaeus, 1766). 2018. Available online: http://www.wikiaves.com/3159704 (accessed on 5 May 2019).
- Redford, K.H.; Peters, G. Notes on the biology and song of the red-legged seriema (Cariama cristata). J. Field Ornithol. 1986, 57, 261–269. [Google Scholar]
- Braghieri, V. Photograph Record WA3158196, Cariama cristata (Linnaeus, 1766). 2018b. Available online: http://www.wikiaves.com/3158196 (accessed on 5 May 2019).
- Almeida, A.C.C. Notas sobre a biologia reprodutiva da Seriema Cariama cristata (Linnaeus, I766) (Gruiformes-Cariamidae). Revista Nordestina de Biologia 1994, 9, 49–59. [Google Scholar]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Yao, A.C.C. A lower bound to finding convex hulls. J. Assoc. Comput. Mach. 1981, 28, 780–787. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bivand, R.; Lewin-Koh, N. Maptools: Tools for Handling Spatial Objects. R Package Version 0.9-5. 2019. Available online: https://CRAN.R-project.org/package=maptools (accessed on 20 April 2019).
- Bivand, R.S.; Pebesma, E.J.; Gómez-Rubio, V.; Pebesma, E.J. Applied Spatial Data Analysis with R, 2nd ed.; Springer: New York, USA, 2013. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the ‘Geospatial‘ Data Abstraction Library. R Package Version 1.4-3. 2019. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 20 April 2019).
- ESRI. ArcGIS Desktop: Release 10.1; Environmental Systems Research Institute: Redlands, CA, USA, 2013. [Google Scholar]
- Souza, D.C.; Vieira, L.D.; Castro, A.L.S. Territoriality and home range of the red-legged Seriema (Cariama cristata). Ornitol. Neotrop. 2018, 29, 101–105. [Google Scholar]
- Bogoni, J.A.; Pires, J.S.R.; Graipel, M.E.; Peroni, N.; Peres, C.A. Wish you were here: How defaunated is the Atlantic Forest biome of its medium-to large-bodied mammal fauna? PLoS ONE 2018, 13, e0204515. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Peres, C.A.; Bogoni, J.A.; Cassano, C.R. Use of agroecosystem matrix habitats by mammalian carnivores (Carnivora): A global-scale analysis. Mammal Rev. 2018, 48, 312–327. [Google Scholar] [CrossRef]
- Orme, C.D.L.; Mayor, S.; Anjos, L.; Develey, P.F.; Hatfield, J.H.; Morante-Filho, J.C.; Tylianakis, J.M.; Uezu, A.; Banks-Leite, C. Distance to range edge determines sensitivity to deforestation. Nat. Ecol. Evol. 2019, 3, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.W.S. The Behaviour of the Domestic Cat; CABI Publishing: Wallingford, UK, 1992. [Google Scholar]
- Collins, S. Breeding the red-legged seriema. AFA Watchbird 1998, 25, 50–51. [Google Scholar]
- AZA. Red-Legged Seriema (Cariama cristata) Care Manual; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2013. [Google Scholar]
- Cazalis, V.; Prévot, A.C. Are protected areas effective in conserving human connection with nature and enhancing pro-environmental behaviours? Biol. Conserv. 2019, in press. [Google Scholar] [CrossRef]
- Toomey, A.H.; Domroese, M.C. Can citizen science lead to positive conservation attitudes and behaviors. Hum. Ecol. Rev. 2013, 20, 50–62. [Google Scholar]
- Frigerio, D.; Pipek, P.; Kimmig, S.; Winter, S.; Melzheimer, J.; Diblíková, L.; Wachter, B.; Richter, A. Citizen science and wildlife biology: Synergies and challenges. Ethology 2018, 124, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Marten, K.; Marler, P. Sound transmission and its significance for animal vocalization. Behav. Ecol. Sociobiol. 1977, 2, 271–290. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; White, P.C. Human-wildlife interactions in urban areas: A review of conflicts, benefits and opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Baker, P.J.; Dowding, C.V.; Molony, S.E.; White, P.C.; Harris, S. Activity patterns of urban red foxes (Vulpes vulpes) reduce the risk of traffic-induced mortality. Behav. Ecol. 2007, 18, 716–724. [Google Scholar] [CrossRef]
- Nicolaus, M.; Tinbergen, J.M.; Bouwman, K.M.; Michler, S.P.M.; Ubels, R.; Both, C.; Kempenaers, B.; Dingemanse, N.J. Experimental evidence for adaptive personalities in a wild passerine bird. Proc. R. Soc. B 2012, 279, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Loyd, K.A.T.; Devore, J.L. An evaluation of feral cat management options using a decision analysis network. Ecol. Soc. 2010, 15, 10–27. [Google Scholar] [CrossRef]
- Murray, M.H.; Becker, D.J.; Hall, R.J.; Hernandez, S.M. Wildlife health and supplemental feeding: A review and management recommendations. Biol. Conserv. 2016, 204, 163–174. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Szabo, J.K. A behavioural ecology approach to understand volunteer surveying for citizen science datasets. Emu-Austral Ornithol. 2012, 112, 313–325. [Google Scholar] [CrossRef] [Green Version]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.C.; Evans, D.M.; French, R.A.; Parrish, J.K.; Phillips, T.B.; et al. Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef] [Green Version]
| General Behavior | Specific Behavior | |
|---|---|---|
| Code | Description | |
| Interaction with cats (A1) | A1a | Fighting with cats or running away from them. |
| A1b | Staying next to cats but without negative or positive interaction. | |
| Interactions with urban structures (A2) | A2a | Climbing human-made structures (e.g., buildings, walls, poles, etc.) and performing other natural behaviors up there. |
| A2b | Entering (or trying) into human facilities (e.g., buildings and other walled/gated spaces) | |
| A2c | Agonistic behavior against human-made structures (e.g., fighting/playing with a pole, interacting with own reflection in windows or mirrors). | |
| A2d | Difficulties to pass through or jump human-made structures (e.g., fences, gates, walls). | |
| Feeding human-provided food (A3) | A3a | Eating cat food provided by humans. |
| A3b | Consuming any other food provided intentionally or not by humans (e.g., waste, bread, fruits, water from the tap). | |
| Exposition to car traffic (A4) | A4a | Calmly walking in the streets. |
| A4b | Crossing the street, walking in front of, or through, cars in movement. | |
| A4c | Resting among parked cars or below them. | |
| A4d | Investigating parked cars. | |
| Behavior Category | n. | % | Specific Behavior | n. | % | |
|---|---|---|---|---|---|---|
| Code | Description | |||||
| Resting (1) | 173 | 33.2 | 1a | Observing | 152 | 29.2 |
| 1b | Resting | 21 | 4.0 | |||
| 1c | Sleeping in the nest | |||||
| 1d | Sleeping on a branch | |||||
| 1e | Hiding | |||||
| Locomotion (2) | 221 | 42.4 | 2a | Walking | 190 | 36.5 |
| 2b | Short flight | 12 | 2.3 | |||
| 2c | Long flight | 4 | 0.8 | |||
| 2d | Short run | 8 | 1.5 | |||
| 2e | Long run | 2 | 0.4 | |||
| 2f | Climbing on a branch | 5 | 1.0 | |||
| Ingest/excretory (3) | 72 | 13.8 | 3a | Drinking | 7 | 1.3 |
| 3b | Eating | 62 | 11.9 | |||
| 3c | Eating crouched | 2 | 0.4 | |||
| 3d | Defecating | 1 | 0.2 | |||
| Comfort/maintenance (4) | 21 | 4.0 | 4a | Preening the chest feathers | 3 | 0.6 |
| 4b | Preening the wing feathers | 4 | 0.8 | |||
| 4c | Preening the thigh feathers | 2 | 0.4 | |||
| 4d | Preening the tail | |||||
| 4e | Preening the cloaca | |||||
| 4f | Preening the dorsum | 2 | 0.4 | |||
| 4g | Preening the abdomen | |||||
| 4h | Dust bathing | 1 | 0.2 | |||
| 4i | Scratching the head | 1 | 0.2 | |||
| 4j | Scratching the neck | 1 | 0.2 | |||
| 4k | Scratching the beak | |||||
| 4l | Ruffling | 5 | 1.0 | |||
| 4m | Repositioning the wings | 1 | 0.2 | |||
| 4n | Stretching | 1 | 0.2 | |||
| Social behavior (5) | 16 | 3.1 | 5a | Agonistic interspecific interaction | 4 | 0.8 |
| 5b | Agonistic intraspecific interaction | 5 | 1.0 | |||
| 5c | Air contact | 3 | 0.6 | |||
| 5d | Juvenile chasing | 4 | 0.8 | |||
| Vocalization (6) | 19 | 3.6 | 6a | Short vocalization | 1 | 0.2 |
| 6b | Agonistic vocalization | 4 | 0.7 | |||
| 6c | Full vocalization | 14 | 2.7 | |||
| TOTAL | 522 | 522 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrino, E.R.; Bogoni, J.A.; Navarro, A.B.; Bovo, A.A.A.; Gonçalves, R.M.; Charters, J.D.; Domini, J.A.; Ferraz, K.M.P.M.B. Large Terrestrial Bird Adapting Behavior in an Urbanized Zone. Animals 2019, 9, 351. https://doi.org/10.3390/ani9060351
Alexandrino ER, Bogoni JA, Navarro AB, Bovo AAA, Gonçalves RM, Charters JD, Domini JA, Ferraz KMPMB. Large Terrestrial Bird Adapting Behavior in an Urbanized Zone. Animals. 2019; 9(6):351. https://doi.org/10.3390/ani9060351
Chicago/Turabian StyleAlexandrino, Eduardo R., Juliano A. Bogoni, Ana B. Navarro, Alex A. A. Bovo, Rafael M. Gonçalves, Jacob D. Charters, Juan A. Domini, and Katia M. P. M. B. Ferraz. 2019. "Large Terrestrial Bird Adapting Behavior in an Urbanized Zone" Animals 9, no. 6: 351. https://doi.org/10.3390/ani9060351





