Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Animal Ethical Code
2.3. Measurements and Analysis
Dry Matter Intake and Feed Analysis
2.4. Feces Sampling, Digestible Energy Intake and Apparent Nutrient Digestibility
2.5. Average Daily Gain and Feed Conversion Efficiency
2.6. Rumen Sampling
2.7. Blood Sampling
2.8. Statistical Analysis
3. Results
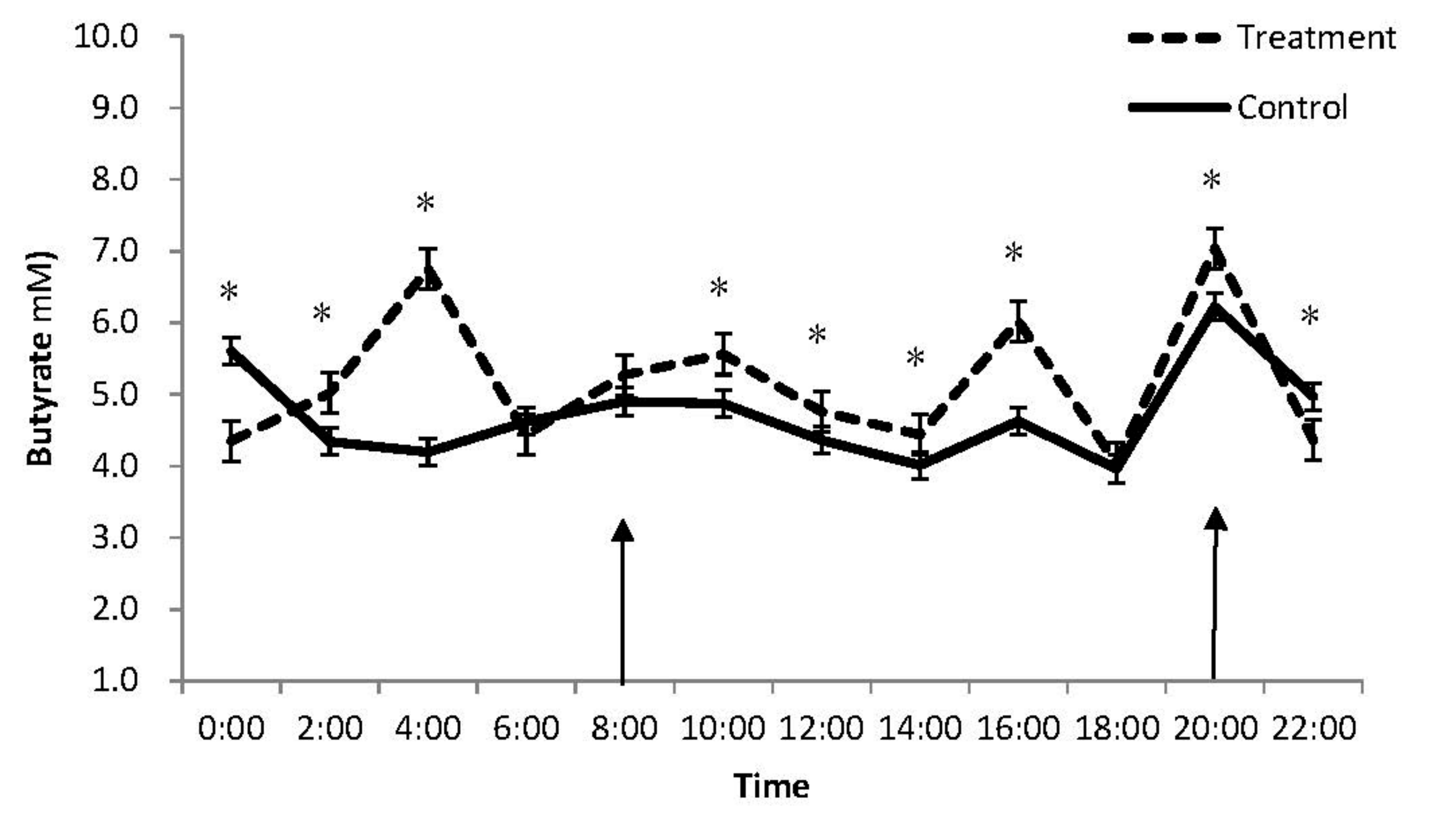
3.1. Rumen pH and Volatile Fatty Acid Concentration
3.2. Blood Indices
3.3. Chemical Composition, Intake, Average Daily Gain and Apparent Nutrient Digestibility
4. Discussion
4.1. Rumen Fermentation Characteristics
4.2. Blood Indices
4.3. Animal Performance and Apparent Nutrient Digestibility
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86 (Suppl. 14), E140–E148. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Xiang, H.; Cheng, L.; Zhao, C.Z.; Wang, F.; Zhao, X.L.; Fang, Y. Effects of Feeding Garlic Powder on Growth Performance, Rumen Fermentation, and the Health Status of Lambs Infected by Gastrointestinal Nematodes. Animals 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Fujita, K.; Katakura, T.; Utsunomiya, T.; Pollard, R.B.; Suzuki, F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res. 2002, 22, 4053–4058. [Google Scholar] [PubMed]
- Sedghi, M.; Golian, A.; Kermanshahi, H.; Ahmadi, H. Effect of dietary supplementation of licorice extract and a prebiotic on performance and blood metabolites of broilers. S. Afr. J. Anim. Sci. 2010, 40, 371–380. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.D.; Zhou, L.; Xiong, Y.L. Inhibition of lipid oxidation and rancidity in precooked pork patties by radical-scavenging licorice (Glycyrrhiza glabra) extract. J. Food Sci. 2013, 78, C1686–C1694. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, H.; Liu, K.; Jia, H.; Chen, Y.; Wang, Z. Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci. 2015, 105, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, R.; Solati, A.A.; Khodaei Motlagh, M.; Kazemi Bonchenari, M. Immune responses and some blood metabolite responses of female Holstein calves to dietary supplementation with licorice root (Glycyrrhiza glabra). Iran. J. Anim. Sci. Appl. 2014, 4, 505–508. [Google Scholar]
- Kim, D.H.; Kim, K.H.; Nam, I.S.; Lee, S.S.; Choi, C.W.; Kim, W.Y.; Kwon, E.G.; Lee, K.Y.; Lee, M.J.; Oh, Y.K. Effect of indigenous herbs on growth, blood metabolites and carcass characteristics in the late fattening period of Hhanwoo steers. Asian-Aust. J. Anim. Sci. 2013, 26, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Liu, J.F.; Sun, L.B.; Gao, J.; Zhang, S.J. Effects of licorice extracts on rumen fermentation and methane yield of sheep in vitro. Chin. J. Anim. Nutr. 2012, 8, 1548–1556. [Google Scholar]
- NY/T 816-2004, Ministry of Agriculture, MOA, PRC. Feeding Standard of Meat-Producing Sheep and Goats; China Agriculture Press: Beijing, China, 2004.
- Tian, M.; Yan, H.; Row, K.H. Extraction of glycyrrhizic acid and glabridin from licorice. Int. J. Mol. Sci. 2008, 9, 571–577. [Google Scholar] [CrossRef] [PubMed]
- GB 14925-2001. Laboratory Animal-Requirements of Environment and Housing Facilities; Standardization Administration of China: Beijing, China, 2001. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Chen, H.M.; Lifschitz, C.H. Preparation of fecal samples for assay of volatile fatty acids by gasliquid chromatography and high-performance liquid chromatography. Clin. Chem. 1989, 35, 74–76. [Google Scholar]
- Feng, Y.L. (Ed.) Ruminant Nutrition; Science Press: Beijing, China, 2004; pp. 131–135. (In Chinese) [Google Scholar]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets. I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Lila, Z.A.; Mohammed, N.; Kanda, S.; Kamada, S.; Kurihara, M.; Itabashi, H. Ssarsaponin effects on ruminal fermentation and microbes, methane production, digestibility and blood metabolites in steers. Asian-Aust. J. Anim. Sci. 2005, 18, 1746–1751. [Google Scholar] [CrossRef]
- Santoso, B.; Kilmaskossu, A.; Sambodo, P. Effects of saponin from Biophytum petersianum Klotzsch on ruminal fermentation, microbial protein synthesis and nitrogen utilization in goats. Anim. Feed Sci. Technol. 2007, 137, 58–68. [Google Scholar] [CrossRef]
- Wallace, R.J.; Arthaud, L.; Newbold, C.J. Influence of Yucca shidigera extract on ruminal ammonia concentrations and ruminal microorganisms. Appl. Environ. Microbiol. 1994, 60, 1762–1767. [Google Scholar]
- Das, T.K.; Banerjee, D.; Chakraborty, D.; Pakhira, M.C.; Shrivastava, B.; Kuhad, R.C. Saponin: Role in animal system. Vet. World 2012, 5, 248–254. [Google Scholar] [CrossRef]
- Bird, S.H.; Leng, R.A. Further studies on the effects of the presence or absence of protozoa in the rumen on live-weight gain and wool growth of sheep. Br. J. Nutr. 1984, 52, 607–611. [Google Scholar] [CrossRef]
- Qin, W.Z.; Li, C.Y.; Kim, J.K.; Ju, J.G.; Song, M.K. Effects of defaunation on fermentation characteristics and methane production by rumen microbes in vitro when incubated with starchy feed sources. Asian-Aust. J. Anim. Sci. 2012, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Zhang, X.M.; Ungerfeld, E.M.; Long, D.L.; Mao, H.X.; Jiao, J.Z.; Beauchemin, K.A.; Tan, Z.L. Molecular hydrogen generated by elemental magnesium supplementation alters rumen fermentation and microbiata in goats. Br. J. Nutri. 2017, 118, 401–410. [Google Scholar] [CrossRef]
- Sun, H.X.; Pan, H.J. Immunological adjuvant effect of Glycyrrhiza uralensis saponins on the immune responses to ovalbumin in mice. Vaccine 2006, 24, 1914–1920. [Google Scholar] [CrossRef]
- Gordon, M.H.; An, J. Antioxidant activity of flavonoids isolated from licorice. J. Agric. Food Chem. 1995, 43, 1784–1788. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Soni, B.; Dalwadi, N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. Int. J. Food Sci. Nutr. 2009, 60, 135–149. [Google Scholar] [CrossRef]
- Jugl-Chizzola, M.; Ungerhofer, E.; Gabler, C.; Hagmüller, W.; Chizzola, R.; Zitterl-Eglseer, K.; Franz, C. Testing of the palatability of Thymus vulgaris L. and Origanum vulgare L. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berl. Munch. Tierarztl. Wochenschr. 2006, 119, 238–243. [Google Scholar]
- Schone, F.; Vetter, A.; Hartung, H.; Bergmann, H.; Biertumpfel, A.; Richter, G.; Muller, S.; Breitschuh, G. Effects of essential oils from fennel (Foeniculi aetheroleum) and caraway (Carvi aetheroleum) in pigs. J. Anim. Physiol. & Anim. Nutr. (Berl). 2006, 90, 500–510. [Google Scholar]
- Lovett, D.K.; Stack, L.; Lovell, S.; Callan, J.; Flynn, B.; Hawkins, M.; Mara, F.P.O. Effect of feeding Yucca schidigera extract on performance of lactating dairy cows and ruminal fermentation parameters in steers. Livest. Sci. 2006, 102, 23–32. [Google Scholar] [CrossRef]
- Pen, B.; Sar, C.; Mwenya, B.; Kuwaki, K.; Morikawa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci. Technol. 2006, 129, 175–186. [Google Scholar] [CrossRef]

| Control | Supplemented | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Treatment | Time | Treatment–Time | ||||
| Rumen pH | 6.6 | 6.6 | 0.01 | 0.537 | <0.001 | <0.001 |
| Acetate (mM) | 31.4 | 27.8 | 0.39 | <0.001 | 0.002 | 0.597 |
| Propionate (mM) | 13.0 | 14.2 | 0.21 | 0.002 | <0.001 | 0.130 |
| Butyrate (mM) | 4.7 | 5.2 | 0.07 | 0.038 | <0.001 | 0.011 |
| Total volatile fatty acid (mM) | 49.1 | 47.2 | 0.49 | 0.019 | <0.001 | 0.424 |
| Acetate: propionate ratio | 2.52 | 1.96 | 0.17 | <0.001 | 0.302 | 0.422 |
| Control | Supplemented | SEM | p-Value | |
|---|---|---|---|---|
| Total protein (g L−1) | 86.4 | 86.8 | 2.29 | 0.905 |
| Aspartate aminotransferase (U L−1) | 131.1 | 131.7 | 11.62 | 0.803 |
| Alanine aminotransferase (U L−1) | 16.2 | 16.1 | 0.41 | 0.333 |
| Immunoglobulin A (ng mL−1) | 4.3 | 9.1 | 0.12 | <0.001 |
| Immunoglobulin G (ng mL−1) | 44.6 | 82.2 | 1.05 | <0.001 |
| Total antioxidant capacity (U mL−1) | 7.3 | 12.9 | 0.34 | <0.001 |
| Total superoxide dismutase (U mL−1) | 87.5 | 105.5 | 1.61 | <0.001 |
| Control | Supplemented | SEM | p-Value | |
|---|---|---|---|---|
| Initial live weight (kg) | 30.43 | 30.38 | 0.243 | 0.739 |
| Dry matter intake (kg day−1) | 0.89 | 0.85 | 0.011 | 0.013 |
| Digestible energy intake (MJ day−1) | 8.4 | 8.2 | 0.09 | 0.176 |
| Average daily gain (g day−1) | 86.7 | 71.1 | 21.77 | 0.702 |
| Feed conversion efficiency (g kg−1) | 93.3 | 86.2 | 26.08 | 0.878 |
| Apparent Nutrient Digestibility (%) | ||||
| Dry matter | 48.9 | 50.9 | 0.69 | 0.067 |
| Crude protein | 47.5 | 49.5 | 0.74 | 0.092 |
| Neutral detergent fiber | 39.8 | 42.1 | 0.76 | 0.054 |
| Acid detergent fiber | 37.3 | 39.8 | 0.87 | 0.073 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Cheng, L.; Liu, J.; Zhang, S.; Sun, X.; Al-Marashdeh, O. Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep. Animals 2019, 9, 279. https://doi.org/10.3390/ani9050279
Guo X, Cheng L, Liu J, Zhang S, Sun X, Al-Marashdeh O. Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep. Animals. 2019; 9(5):279. https://doi.org/10.3390/ani9050279
Chicago/Turabian StyleGuo, Xuefeng, Long Cheng, Junfeng Liu, Sujiang Zhang, Xuezhao Sun, and Omar Al-Marashdeh. 2019. "Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep" Animals 9, no. 5: 279. https://doi.org/10.3390/ani9050279
APA StyleGuo, X., Cheng, L., Liu, J., Zhang, S., Sun, X., & Al-Marashdeh, O. (2019). Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep. Animals, 9(5), 279. https://doi.org/10.3390/ani9050279





