A First Attempt to Produce Proteins from Insects by Means of a Circular Economy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing under Laboratory Conditions
2.2. Insect Mass Rearing
2.3. Light Microscopy
2.4. Drying Process
2.5. Mass Grinding and Defatting
2.6. Microbiological Analyses
2.7. Experimental Diets and Fish Feeding Trial
2.8. Nutrient and Mineral Analysis
2.9. Earthworm Rearing
2.10. Substrate Analysis before and after Earthworm Rearing
2.11. Statistical Analysis
3. Results
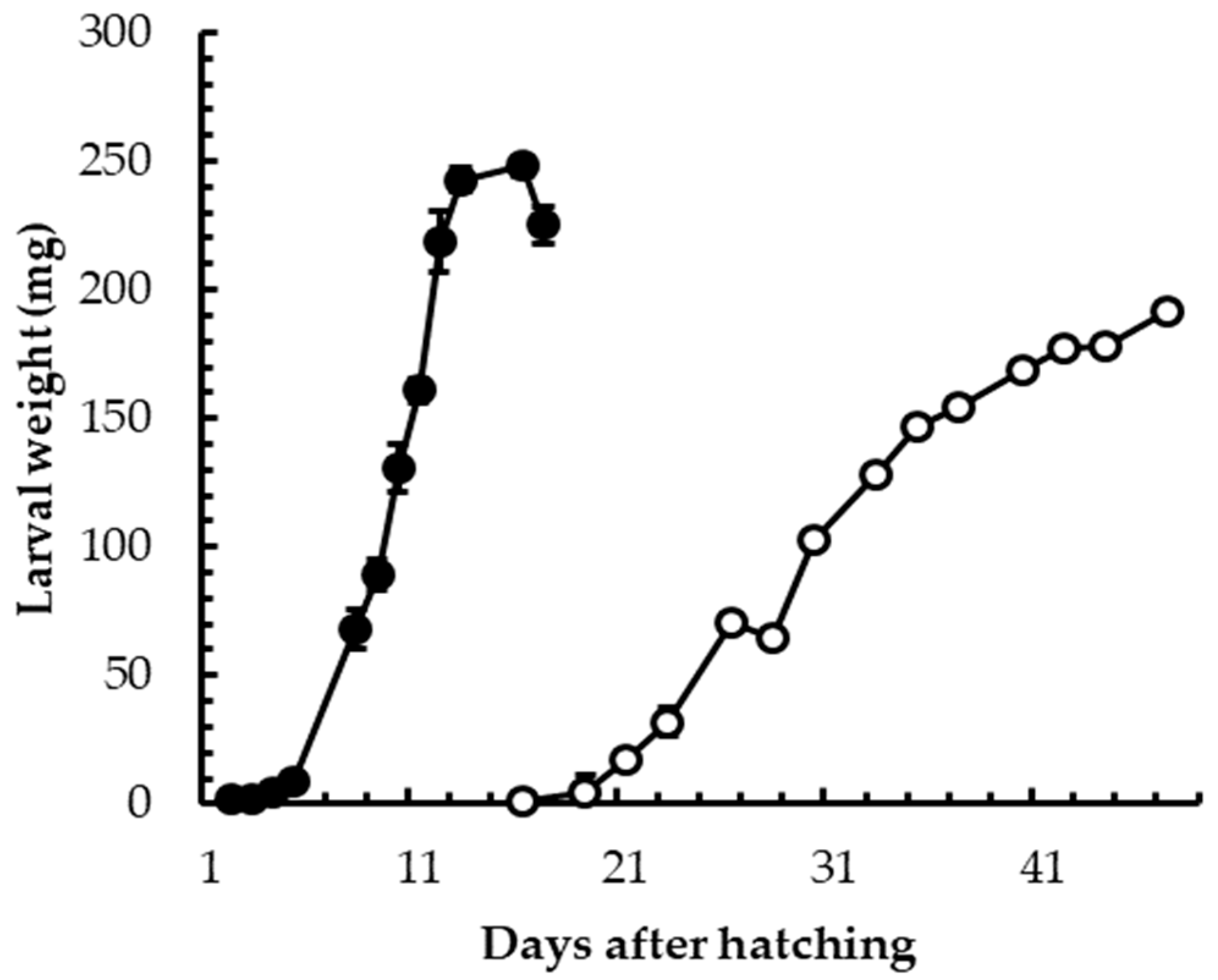
3.1. Insect Rearing
3.2. Insect Welfare and Health Status
3.3. Insect Processing
3.3.1. Set-Up of Drying Conditions at a Laboratory Scale
3.3.2. Mass Drying and Oil Extraction Yields
3.4. Microbiological Analyses
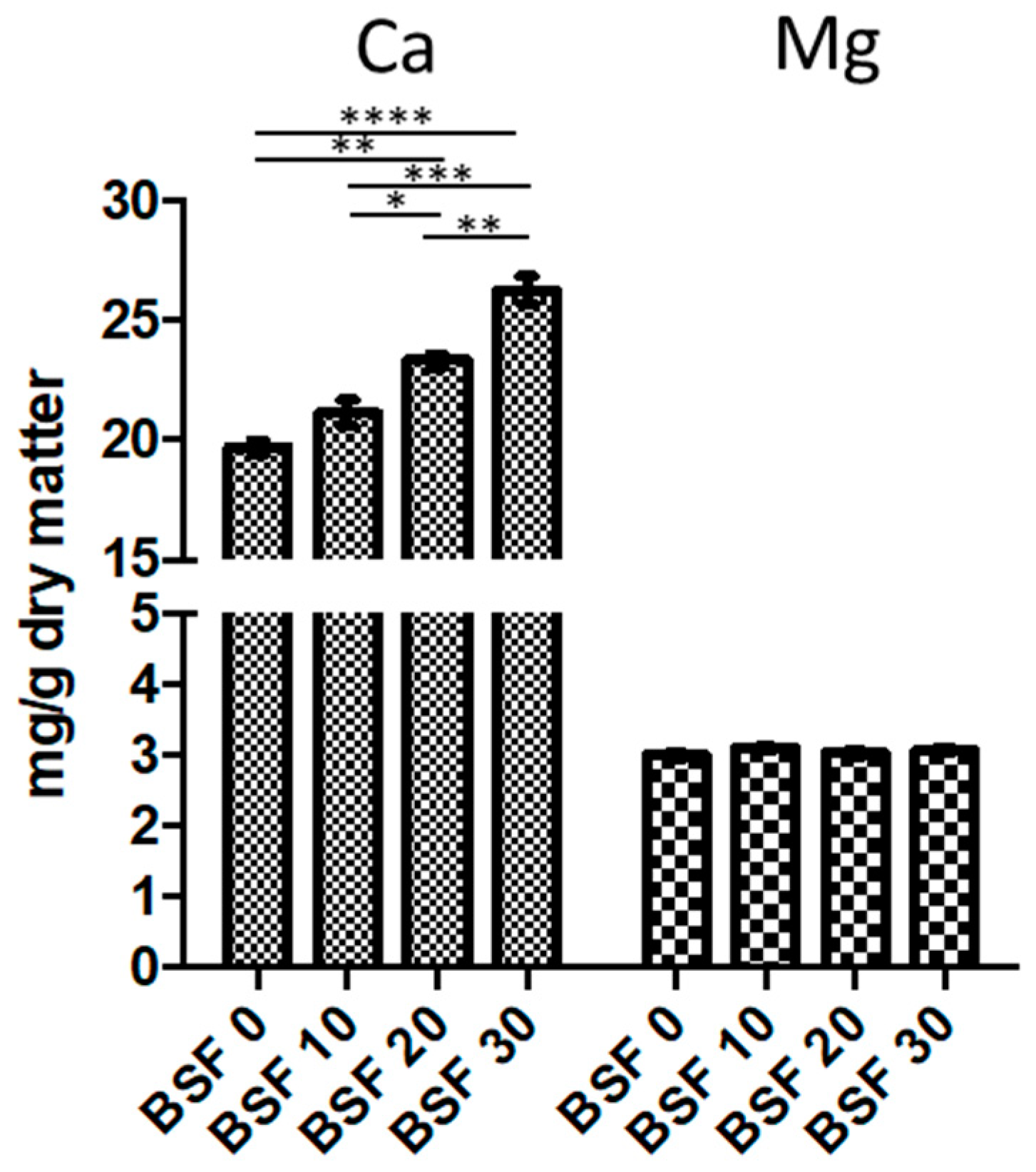
3.5. Nutritional and Chemical Analysis
3.6. Fish Growth Performances
3.7. Earthworm Growth Performances
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Scientific Opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials. Off. J. Eur. Union 2013, L 29, 1–64. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011; pp. 4–9. [Google Scholar]
- Stenmarck, A.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels; European Commission: Stockholm, Sweden, 2016; pp. 19–23. [Google Scholar]
- European Parliament. European Parliament resolution of 19th January 2012 on how to avoid food wastage: Strategies for a more efficient food chain in the EU (2011/2175(INI)). Off. J. Eur. Union 2013, 227, 25–32. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Closing the Loop—An EU Action Plan for the Circular Economy; COM (2015)614 Final; EU Publications: Brussels, Belgium, 2015; pp. 1–21. [Google Scholar]
- International Platform of Insect for Food and Feed. Guide on Good Hygiene Practices for European Union (EU) Producers of Insects as Feed and Food. Available online: http://ipiff.org/wp-content/uploads/2019/03/IPIFF_Guide_A4_2019-v5-separate.pdf (accessed on 13 May 2019).
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomidae) larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Hogsette, J.A. New diets for production of house-flies and stable flies (Diptera, Muscidae) in the laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Bonelli, M.; Bruno, D.; Caccia, S.; Sgambeterra, G.; Cappellozza, S.; Jucker, C.; Tettamanti, G.; Casartelli, M. Structural and functional characterization of Hermetia illucens larval midgut. Front. Physiol. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.C.; Montali, A.; Bruno, D.; Tettamanti, G. Metabolic adjustment of the larval fat body in Hermetia illucens to dietary conditions. J. Asia Pac. Entomol. 2017, 20, 1307–1313. [Google Scholar] [CrossRef]
- Franzetti, E.; Romanelli, D.; Caccia, S.; Cappellozza, S.; Congiu, T.; Rajagopalan, M.; Grimaldi, A.; de Eguileor, M.; Casartelli, M.; Tettamanti, G. The midgut of the silkmoth Bombyx mori is able to recycle molecules derived from degeneration of the larval midgut epithelium. Cell Tissue Res. 2015, 361, 509–528. [Google Scholar] [CrossRef]
- ISO 4833-1. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 21528-2. Microbiology of Food and Animal Feeding Stuffs Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Method; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- ISO 16649-2. Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide; International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- ISO 21527-1. Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- Mucchetti, G.; Ghiglietti, R.; Locci, F.; Francolino, S.; Bonvini, B.; Remagni, M.C.; Zago, M.; Iezzi, R.; Carminati, D. Technological, microbiological and chemical characteristics of Pannerone, a traditional Italian raw milk cheese. Dairy Sci. Technol. 2009, 89, 419–436. [Google Scholar] [CrossRef]
- ISO 7937. Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Clostridium perfringens-Colony-Count Technique; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- ISO 6579-1. Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Terova, G.; Rimoldi, S.; Ascione, C.; Ceccotti, C.; Gini, E.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 152/2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 1–130. [Google Scholar]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoobiology 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clerq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 5th ed.; European Directorate for the Quality of Medicines: Strasbourg, France, 2004. [Google Scholar]
- Commission Directive. Establishing Community methods of analysis for the determination of vitamin A, vitamin E and tryptophan in feedingstuffs. Off. J. Eur. Communities 2000, 174, 32–50. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; 2nd rev.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2003. [Google Scholar]
- ANPA. Metodi di Analisi del Compost; Manuali e Linee Guida: Roma, Italy, 2001. [Google Scholar]
- Sweeney, R.A. Generic combustion method for determination of crude protein in feeds: Collaborative study. J. AOAC Int. 1989, 72, 770–774. [Google Scholar]
- Bonelli, M.; Bruno, D.; Gianfranceschi, N.; Brilli, M.; Caccia, S.; Casartelli, M.; Tettamanti, G. The feeding substrate affects morphological and functional features of the Hermetia illucens larval midgut. Front. Physiol. 2019. submitted. [Google Scholar]
- Boehm, A.B.; Sassoubre, L.M. Enterococci as indicators of environmental fecal contamination. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Finke, M.D. Complete nutrient content of four species of feeder Insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D.; Oonincx, D. Insects as food for insectivores. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, J., Rojas, G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2013; pp. 583–616. [Google Scholar]
- Domínguez, J.; Briones, M.J.I.; Mato, S. Effect of the diet on growth and reproduction of Eisenia andrei (Oligochaeta, Lumbricidae). Pedobiologia 1997, 41, 566–577. [Google Scholar]
- Domínguez, J.; Edwards, C.A.; Webster, M. Vermicomposting of sewage sludge: Effect of bulking materials on the growth and reproduction of the earthworm Eisenia Andrei. Pedobiologia 2000, 44, 24–32. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Dominguez, J. C to N ratio strongly affects population structure of Eisenia fetida in vermicomposting system. Eur. J. Soil Biol. 2006, 42, 127–131. [Google Scholar] [CrossRef]
- Carbon to Nitrogen Ratios in Cropping Systems. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcseprd331820.pdf (accessed on 1 March 2019).
- Norris, M.J. Group effects on feeding in adult males of the desert locust, Schistocerca gregaria (Forsk.), in relation to sexual maturation. Bull. Entomol. Res. 1961, 51, 731–753. [Google Scholar] [CrossRef]
- Gere, G. Investigations into the laws governing the growth of Hyphantria cunea Drury caterpillars. Acta Biol. Hung. 1956, 7, 43–72. [Google Scholar]
- Long, D.B. Effects of population density on larvae of Lepidoptera. Trans. R. Entomol. Soc. Lond. 1953, 104, 543–584. [Google Scholar] [CrossRef]
- Parra Paz, A.S.; Carrejo, N.S.; Gomez Rodriguez, C.H. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste Biomass Valoriz. 2015, 6, 1059–1065. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management-system using the Black Soldier Fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquac. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; De Filippis, F.; Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The intestinal microbiota of Hermetia illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Appl. Environ. Microbiol. 2018, 85, e01864-18. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the microbiota of black soldier fly Larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb. Ecol. 2018, 77, 913–930. [Google Scholar] [CrossRef]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological spoilage of fruits and vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages, 1st ed.; Sperber, W.H., Doyle, M.P., Eds.; Springer Science: New York, NY, USA, 2009; pp. 135–183. [Google Scholar]
- Grabowski, N.T.; Jansen, W.; Klein, G. Microbiological status of edible insects sold as pet feed in Germany. In Insects to Feed the World Conference, Summary Report; Vantomme, P., Münke, C., van Huis, A., van Itterbeeck, J., Hackman, A., Eds.; Wageningen University and Research Centre: Ede, The Netherlands, 2014; p. 48. [Google Scholar]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Sci. Technol. Int. 2017, 23, 17–23. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) 1441/2007, amending Regulation (EC) 2073/2005, on microbiological criteria for foodstuffs. Off. J. Eur. Union 2007, L 322, 12–29. [Google Scholar]
- Grabowski, N.T.; Klein, G. Bacteria encountered in raw insect, spider, scorpion, and centipede taxa including edible species, and their significance from the food hygiene point of view. Trends Food Sci. Technol. 2017, 63, 80–90. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef]
- NVWA. Netherlands Food and Consumer Product Safety Authority. In Advisory Report on the Risks Associated with the Consumption of Mass-Reared Insects; NVWA/BuRO/2014/2372; NVWA: Utrecht, The Netherlands, 2014. [Google Scholar]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Li, S.L.; Ji, H.; Zhang, B.X.; Tian, J.J.; Zhou, J.S.; Yu, H.B. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Surendraa, K.C.; Olivierb, R.; Tomberlinc, J.K.; Jhad, R.; Khanala, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Lock, E.; Arsiwalla, T.; Waagbo, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Ziniu, Y.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef]
- Pathak, R.; Sharma, S.; Prasad, R. Study on the occurrence of black soldier fly larvae in composting of kitchen waste. Int. J. Res. Biosci. 2015, 4, 38–45. [Google Scholar]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar Enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef]
- Clive, E.A.; Fletcher, K.E. Interactions between earthworms and microorganisms in organic-matter breakdown. Agric. Ecosyst. Environ. 1988, 24, 235–247. [Google Scholar]
- Decreto Legislativo n. 75. Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 luglio 2009, n. 88. Gazzetta Ufficiale della Repubblica Italiana, 2010. Available online: https://www.gazzettaufficiale.it/eli/id/2010/05/26/010G0096/sg (accessed on 28 May 2018).






| SD | VMD | Mass Rearing VMD | |
|---|---|---|---|
| Development time (day) | 17 ± 0 c | 45 ± 1 a | 31 ± 0 b |
| Maximum weight before prepupal stage (mg) | 225.4 ± 7.3 | 183.3 ± 6.6 | 230.8 ± 26.9 |
| Overall degradation (D) | 0.46 ± 0.01 c | 0.52 ± 0.01 b | 0.96 ± 0.00 a |
| Waste reduction index (WRI) | 3.06 ± 0.06 a | 1.78 ± 0.07 c | 2.35 ± 0.00 b |
| Larvae | Nobs | Intercept (a) | Slope (b) | r | R2adj | SEE | MAE |
|---|---|---|---|---|---|---|---|
| Estimates | Estimates | ||||||
| SD | 16 | 95.09 ± 3.43 | −3.65 ± 0.28 | −0.96 | 91.07 | 6.71 | 5.60 |
| VMD1 | 30 | 95.78 ± 2.27 | −3.86 ± 0.17 | −0.97 | 94.69 | 5.68 | 4.88 |
| VMD2 | 30 | 92.51 ± 2.68 | −3.81 ± 0.20 | −0.96 | 92.58 | 6.71 | 5.66 |
| Drying Time (min) | SD | VMD1 | VMD2 | ANOVA |
|---|---|---|---|---|
| p-Value | ||||
| 90 | 54.6 ± 0.12 | 56.4 ± 0.72 | 52.6 ± 3.91 | 0.218 |
| 120 | 45.9 ± 0.58 | 47.1 ± 0.29 | 43.9 ± 3.47 | 0.286 |
| 180 | 38.4 ± 0.57 | 36.4 ± 0.87 | 34.0 ± 3.52 | 0.195 |
| 240 | 36.4 ± 0.35 a | 30.7 ± 1.19 b | 28.6 ± 3.41 b | 0.034 |
| 300 | 35.9 ± 0.24 a | 28.1 ± 1.12 b | 26.1 ± 3.16 b | 0.009 |
| 360 | 35.7 ± 0.13 a | 26.7 ± 0.89 b | 24.9 ± 2.84 b | 0.004 |
| Products | Total Viable Aerobic Count | Enterobacteriaceae | E. coli | Enterococci | Sulfite-Reducing Clostridia Spores | Yeasts | Molds | Salmonella (in 25 g) |
|---|---|---|---|---|---|---|---|---|
| BSF rearing substrate (VMD) | 6.55 ± 0.57 | 1.70 ± 0.71 | <1.00 | 3.88 ± 1.64 | 2.15 ± 0.75 | 2.48 ± 0.76 | <2.00 | Absence |
| Dried BSF biomass | 5.05 ± 0.06 | <1.00 | <1.00 | <1.00 | 2.50 ± 0.14 | 2.30 ± 0.43 | <2.00 | Absence |
| Defatted BSF meal | 4.35 ± 0.49 | <1.00 | <1.00 | <1.00 | 1.54 ± 0.51 | <2.00 | <2.00 | Absence |
| Experimental fish feed | ||||||||
| BSF 0 (Control) | 4.23 ± 0.24 | 2.51 ± 0.63 | <1.00 | 2.20 ± 0.17 | <1.00 | <2.00 | <2.00 | Absence |
| BSF 10 (10% BSF meal) | 4.43 ± 0.38 | 2.76 ± 0.49 | <1.00 | 1.30 ± 0.43 | 1.35 ± 0.49 | 2.15 ± 0.21 | 2.48 ± 0.67 | Absence |
| BSF 20 (20% BSF meal) | 4.25 ± 0.13 | 1.00 ± 0.00 | <1.00 | 1.24 ± 0.34 | 1.15 ± 0.21 | <2.00 | <2.00 | Absence |
| BSF 30 (30% BSF meal) | 4.22 ± 0.45 | <1.00 | <1.00 | <1.00 | <1.00 | <2.00 | <2.00 | Absence |
| % d.m. | Mean ± SD |
|---|---|
| Dry matter | 97.00 ± 0.31 |
| Crude protein | 39.42 ± 0.16 |
| Ash | 7.08 ± 0.00 |
| Fat | 35.62 ± 0.27 |
| Chitin | 4.02 ± 0.02 |
| Nitrogen-free extracts | 13.86 ± 0.14 |
| Starch | 1.82 ± 0.05 |
| Glucose | 0.30 ± 0.00 |
| Amino Acids | Mean ± SD | Amino Acids | Mean ± SD |
|---|---|---|---|
| Aspartate | 37.89 ± 0.43 | Cysteine | 0.46 ± 0.02 |
| Glutamic acid | 64.87 ± 0.36 | Valine | 24.68 ± 0.57 |
| Serine | 17.94 ± 0.15 | Methionine | 17.62 ± 0.83 |
| Histidine | 12.36 ± 0.74 | Phenylalanine | 18.84 ± 0.06 |
| Glycine | 22.90 ± 0.01 | Isoleucine | 15.86 ± 0.09 |
| Threonine | 21.53 ± 0.69 | Leucine | 26.98 ± 0.13 |
| Arginine | 48.30 ± 1.04 | Lysine | 19.78 ± 0.31 |
| Alanine | 38.85 ± 0.11 | Proline | 14.56 ± 0.32 |
| Tyrosine | 20.46 ± 0.32 | Tryptophan | 4.27 ± 0.08 |
| Diet | BSF0 | BSF10 | BSF20 | BSF30 |
|---|---|---|---|---|
| IBW(g) | 67.01 ± 1.71 | 66.38 ± 2.51 | 65.63 ± 0.42 | 66.95 ± 2.31 |
| FBW(g) | 223.20 ± 23.67 | 220.34 ± 29.60 | 216.97± 26.16 | 221.74 ± 22.25 |
| WG(g) | 156.86 ± 4.33 | 154.20 ± 6.04 | 146.89 ± 8.03 | 152.30 ± 10.18 |
| BSF Leftovers | VMD | Peat Moss | |
|---|---|---|---|
| Initial earthworm number | 10 | 10 | 10 |
| Initial earthworm weight (g) | 3.17 ± 0.32 | 2.90 ± 0.15 | 2.90 ± 0.62 |
| Final earthworm number | 21 ± 7 | 41 ± 21 | 8 ± 3 |
| Final earthworm weight (g) | 3.69 ± 1.12 a | 3.10 ± 0.25 a | 1.58 ± 1.73 b |
| Number increase | 11 ± 7 a | 31 ± 21 a | −1.33 ± 0.78 b |
| Weight increase (g) | 0.52 ± 0.33 a | 0.20 ± 0.33 a | −1.32 ± 1.38 b |
| Dry Matter (%) | Ash * | N * (TKN) | EE * | CF * | A * | Nitrates § | N * (CNS) | C * (CNS) | C/N |
|---|---|---|---|---|---|---|---|---|---|
| Peat moss (63.74) * | 3.05 | 0.99 | 0.96 | 32.33 | 0.022 | 39.74 | 1.22 | 51.92 | 42.56 |
| VMD (19.78) * | 5.18 | 1.18 | 2.67 | 30.63 | 0.056 | N.D. | 5.50 | 51.93 | 32.25 |
| BSF leftovers (90.70) * | 9.77 | 2.25 | 3.12 | 34.68 | 0.127 | 5.73 | 2.45 | 47.46 | 19.37 |
| Peat moss + Ef (19.63) * | 4.10 | 1.07 | 1.14 | 33.36 | 0.017 | 61.56 | 1.22 | 52.33 | 42.89 |
| VMD + Peat moss + Ef (13.90) * | 4.75 | 1.30 | 0.88 | 31.99 | 0.095 | 2035.26 | 1.50 | 51.34 | 34.23 |
| BSF + Peat moss + Ef (18.36) * | 7.07 | 1.84 | 0.85 | 30.55 | 0.228 | 8185.37 | 2.06 | 49.86 | 24.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A First Attempt to Produce Proteins from Insects by Means of a Circular Economy. Animals 2019, 9, 278. https://doi.org/10.3390/ani9050278
Cappellozza S, Leonardi MG, Savoldelli S, Carminati D, Rizzolo A, Cortellino G, Terova G, Moretto E, Badaile A, Concheri G, et al. A First Attempt to Produce Proteins from Insects by Means of a Circular Economy. Animals. 2019; 9(5):278. https://doi.org/10.3390/ani9050278
Chicago/Turabian StyleCappellozza, Silvia, Maria Giovanna Leonardi, Sara Savoldelli, Domenico Carminati, Anna Rizzolo, Giovanna Cortellino, Genciana Terova, Enzo Moretto, Andrea Badaile, Giuseppe Concheri, and et al. 2019. "A First Attempt to Produce Proteins from Insects by Means of a Circular Economy" Animals 9, no. 5: 278. https://doi.org/10.3390/ani9050278
APA StyleCappellozza, S., Leonardi, M. G., Savoldelli, S., Carminati, D., Rizzolo, A., Cortellino, G., Terova, G., Moretto, E., Badaile, A., Concheri, G., Saviane, A., Bruno, D., Bonelli, M., Caccia, S., Casartelli, M., & Tettamanti, G. (2019). A First Attempt to Produce Proteins from Insects by Means of a Circular Economy. Animals, 9(5), 278. https://doi.org/10.3390/ani9050278










